Abstract
In a phase 2 study, 62 patients with relapsed and refractory acute myeloid leukemia (AML; n = 31), myelodysplastic syndrome (MDS; n = 8), chronic myeloid leukemia in blastic phase (CMLBP; n = 11), and acute lymphocytic leukemia (ALL; n = 12) received 40 mg/m2 clofarabine intravenously over 1 hour daily for 5 days, every 3 to 6 weeks. Twenty patients (32%) achieved complete response (CR), 1 had a partial response (PR), and 9 (15%) achieved CR but without platelet recovery (CRp), for an overall response rate of 48%. In AML, responses were noted in 2 (18%) of 11 patients in first salvage with short first CR (≤ 12 months), in 7 (87%) of 8 patients with longer first CR, and in 8 (67%) of 12 patients in second or subsequent salvage. Responses were observed in 4 of 8 patients with high-risk MDS (50%), in 7 (64%) of 11 with CML-BP, and in 2 (17%) of 12 with ALL. Severe reversible liver dysfunction was noted in 15% to 25%. After the first clofarabine infusion, responders accumulated more clofarabine triphosphate in blasts compared with nonresponders (median 18 vs 10 μM; P = .03). This increased only in responders (median, 1.8-fold; P = .008) after the second clofarabine infusion. In summary, clofarabine is active in acute leukemias and MDS; cellular pharmacokinetics may have prognostic significance. (Blood. 2003;102:2379-2386)
Introduction
Nucleoside analogs have been a rich source of many highly effective agents in the treatment of leukemias and other hematologic cancers.1-8 Deoxyadenosine analogues, such as fludarabine, cladribine, or deoxycoformycin, have been active against lymphoproliferative disorders including chronic lymphocytic leukemia (CLL), indolent lymphomas, Waldenstrom macroglobulinemia, and hairy cell leukemia without untoward toxicity.7,8 Of particular interest is the activity of these agents in acute leukemia, albeit at dose levels associated with prohibitive extramedullary toxicities.9-11 These were dose limited by frequent serious renal and neurologic toxicities.
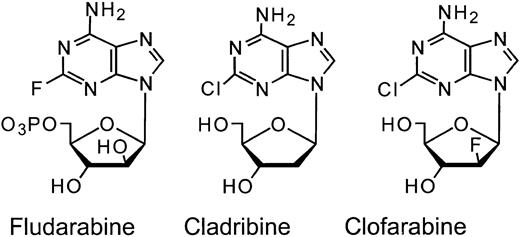
A series of 2-halo-2′-halo-2′-arabinosyladenine analogues including clofarabine (Cl-F-ara-A; 2-chloro-2′-fluoro-deoxy-9-β-D-arabinofuranosyladenine) was developed as a rational extension of the experience with fludarabine and chlorodeoxyadenosine, in an attempt to improve the drug efficacy and minimize extramedullary toxicities12 (Figure 1). The 2-(F, Cl, or Br)-2′-F-ara-A analogues (but not the 2′-Cl or 2′-Br analogues) were found to be potent inhibitors of cell growth. These analogues were synthesized because of the noted antitumor activity of 2-halodeoxyadenosine analogues, and the appearance of 2-F-adenine (a toxic compound with no antitumor selectivity) in animals treated with fludarabine,13,14 which resulted from cleavage of the glycosidic bond by the bacterial purine nucleoside phosphorylase.15,16 Substitution of a fluorine at the 2′ carbon in the arabino configuration, made these derivatives highly resistant to phosphorolysis by bacterial purine nucleoside phosphorylase.17,18 Similar to fludarabine and cladribine, clofarabine was resistant to deamination by adenosine deaminase and required intracellular phosphorylation by deoxycytidine kinase to the cytotoxic triphosphate form.18,19 Both fludarabine and cladribine inhibit DNA synthesis; however, fludarabine triphosphate does this by terminal incorporation into DNA whereas cladribine triphosphate particularly inhibited ribonucleotide reductase. Clofarabine triphosphate has the favorable properties of both congeners and affects both DNA elongation and ribonucleotide reductase.18,20-23
Structures of fludarabine, cladribine, and clofarabine.
Structures of fludarabine, cladribine, and clofarabine.
Although clofarabine had been synthesized in the 1980s, there was little interest in its development by pharmaceutical companies because of the availability of other nucleoside analogues, its perceived small market potential, and lack of compelling evidence for activity outside the lymphoproliferative disorders. In 1993, we initiated the development of clofarabine at MD Anderson Cancer Center. Clofarabine was produced in quantities large enough for animal toxicology studies and for phase 1 human studies. Studies in mice established the limiting dose that kills 10% (LD10) of clofarabine at 75 mg/kg per day equal to 225 mg/m2 intravenously over 1 hour daily times 7 days; the dose-limiting toxicity (DLT) was gastrointestinal. Limited dog toxicology studies demonstrated lack of toxicities at clofarabine doses of 7.5 mg/kg intravenously over 1 hour daily times 5 days equal to 150 mg/m2 intravenously over 1 hour daily times 5 days (unpublished data). This resulted in the initiation of a phase 1 study of clofarabine in solid and hematologic cancers in 1998. The maximum tolerated dose (MTD) of clofarabine on a 5-day schedule was 2 mg/m2/day in solid tumors and 4 mg/m2/day in lymphoproliferative disorders with myelosuppression as DLT. In acute leukemia, reversible severe hepatotoxicity defined the nonmyelosuppressive DLT, and suggested a clofarabine dose of 40 mg/m2 daily for 5 days as a reasonable schedule for phase 2 acute leukemia studies. Among the 32 evaluable patients in this group, 2 with a complete response (CR) and 3 with a marrow CR were observed.24
The encouraging clinical response rate in this heavily pretreated study group, tolerable toxicity profile, and associated pharmacokinetic and pharmacodynamic parameters24 led us to investigate clofarabine in acute leukemia. In the current report, we summarize our clinical and pharmacologic experience in the phase 2 study of clofarabine in patients with acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS), acute lymphocytic leukemia (ALL), and chronic myelogenous leukemia in blastic phase (CML-BP).
Patients and methods
Study group
Patients with a diagnosis of AML, MDS, ALL, or CML-BP whose disease relapsed or was refractory to frontline and/or salvage therapy were eligible. Informed consent was obtained according to institutional guidelines. Other eligibility criteria included (1) age 15 years or older; (2) performance status 0 to 2 (Eastern Cooperative Oncology Group [ECOG] scale); and (3) adequate renal (creatinine 2 mg/dL or less), hepatic (bilirubin 2 mg/dL or less), and cardiac functions. Approval for the study was obtained from the institutional review board (IRB) of the University of Texas MD Anderson Cancer Center. All patients signed an informed consent approved by the IRB; the study was conducted in accordance with the Declaration of Helsinki.
Drugs and other chemicals
Clofarabine for clinical use was initially prepared by Ash Stevens (Detroit, MI) and formulated for injection by the University of Iowa Pharmaceutical Services (Ames, IA). Subsequently, production of the drug in bulk was conducted by Delmar Chemicals (Lasalle, QC, Canada). For cellular pharmacokinetics, high-pressure liquid chromatography (HPLC) standards, clofarabine 5′-triphosphate was synthesized by Sierra Biochemicals (Tucson, AZ). All other chemicals were reagent grade.
Therapy
Clofarabine was administered intravenously at 40 mg/m2 over 1 hour daily for 5 days every 3 to 6 weeks depending on marrow antileukemic response and recovery of counts. Subsequent courses of induction chemotherapy were given at the same dose schedule if toxicity was grade 1 or less, at 1 dose-level reduction (30 mg/m2) if grade 2 extramedullary toxicity was noted, and at 2 dose-level reductions (50% of dose, 20 mg/m2) if grade 3 to 4 extramedullary toxicity was observed and associated with favorable response. Dose-level reductions were also implemented for severe (-1 level) and life-threatening infections (-2 levels). Patients who did not achieve an objective response after 2 courses of induction therapy were taken off the study.
The pretreatment evaluation included history, physical exam, complete blood counts (CBC), differentials and platelets counts, and marrow aspiration and cytogenetic analysis. Follow-up studies included CBC, differential and platelet counts 2 to 3 times weekly, and serum chemistries weekly until response. Marrow aspiration was done on day 14 to day 21 of therapy then every 1 to 2 weeks as indicated to document response. Patients with marrow studies showing no evidence of leukemia but with persistent cytopenias were allowed time to recover their counts to document the best response. Growth factors were allowed as indicated by the clinical condition. In patients with persistent or increasing marrow leukemic infiltrates on serial studies, a subsequent induction course was given even with persistent cytopenia. Patients received routine antiemetic prophylaxis during chemotherapy. Antibiotic prophylaxis was given (usually ciprofloxacin or levaquin, and fluconazole) at the discretion of the treating physician.
Patients achieving remission were offered consolidation therapy at 1 dose-level reduction or the last tolerable induction dose schedule, whichever was lower. Up to 6 courses of consolidation chemotherapy could be offered every 28 days upon recovery of myelosuppression. However, consolidation therapy could be modified at the discretion of the treating physician as judged to be in the best interest of the patient.
Response criteria
A CR required normalization of the marrow (5% or less blasts in a normocellular marrow) and peripheral counts, a granulocyte count above 109/L, and a platelet count of 100 × 109/L or above. A partial response (PR) was as for CR but with persistence of 6% to 25% marrow blasts. A hematologic improvement (HI) had similar criteria as that for CR, but without recovery of the platelet counts to 100 × 109/L or above. This is also called marrow CR with incomplete platelet recovery, or CRp.
A partial response in MDS required improvement in at least 2 parameters: reduction of marrow blasts by 50% or more and to less than 10%; increase of platelet counts by 100% and to above 100 × 109/L; increase of granulocyte count by 100% and to above 0.5 × 109/L.
In CML-BP, a complete hematologic response (CHR) was similar to CR. A partial hematologic response (PHR) was as for CHR but with persistence of few immature peripheral cells (< 5% myelocytes and metamyelocytes) or with persistent but 50% or more reduced splenomegaly or thrombocytosis. A return to second chronic phase (second CP) required disappearance of accelerated or blastic phase features.
Clinical pharmacology
Blood samples were obtained from 29 patients (CML-BP = 10, ALL = 6, AML = 12, MDS = 1) who agreed and signed the written informed consent for blood drawing for pharmacologic determinations. Samples were collected from patients on the basis of 3 criteria: signed consent from patients, number of circulating leukemia cells, and laboratory preparedness. Samples were obtained prior to therapy for baseline values, at the end of the initial infusion and at several times thereafter, including 24 hours after start of therapy. For some patients, samples were also obtained after the second infusion and daily for 5 days prior to and at the end of clofarabine infusion in some patients. All samples (10 mL) were collected in green stopper vacutainer tubes containing heparin and 1 μM deoxycoformycin (obtained from the National Cancer Institute [NCI], Bethesda, MD) to inhibit potential deamination of clofarabine by adenosine deaminase. The tubes were immediately placed in an ice water bath and transported to the laboratory. Control studies have demonstrated that normal and leukemia cells are stable under these conditions with respect to size and membrane integrity. The cellular nucleotide content is stable for at least 15 hours under these conditions.25
Plasma pharmacology
The plasma was removed after centrifugation of peripheral blood samples and stored at -70°C until analyses were done. Human plasma samples (100 μL) containing clofarabine were spiked with cladribine as the internal standard. Samples were precipitated, evaporated, and reconstituted with mobile phase. The samples were analyzed by reversed-phase HPLC using a tandem quadrupole mass spectrometer by a modified previously described procedure26 and analyzed at MicroConstants (San Diego, CA). Authentic clofarabine standard was used for assay validation, and for identification and quantitation of the nucleoside in plasma. The assay has a sensitivity range from 10 to 5000 ng/mL and the coefficient of variability (CV) for accuracy and precision was less than 7%.
Cellular pharmacology
Cell pellets from blood samples were diluted with phosphate-buffered saline (PBS), and mononuclear cells were isolated using Ficoll-Hypaque density gradient step-gradient centrifugation procedures described previously.26 A Coulter electronics channelizer (Coulter, Hialeah, FL) was used to determine the mean cell volume. After being washed with PBS, cells were processed for nucleotide extraction. Normal nucleotides and clofarabine triphosphate were extracted from cells using standard procedures with HClO4. Triphosphates were separated on an anion-exchange Partisil-10 SAX column (Waters, Milford, MA) using HPLC as previously described.27 The intracellular concentration was calculated and expressed as the quantity of nucleotides contained in the extract from a given number of cells of a determined mean volume. This calculation assumes that nucleotides are uniformly distributed in total cell water. In general, the lower limit of quantitation of this assay was about 1 pmol in an extract of 2 × 107 cells, corresponding to a cellular concentration of about 0.2 μM. For many samples as much as 8 × 107 cell equivalents was analyzed to detect and quantitate the peak.
Statistical considerations
Survival was calculated from the date of start of therapy until death. Remission duration was calculated from the date of initial response until disease recurrence. Survival and remission duration were plotted by the method of Kaplan and Meier. Toxicity was graded according to the NCI Common Toxicity Criteria, Version 2.0 (National Cancer Institute, Bethesda, MD).
Exponential decay of clofarabine in plasma was calculated using the Prism software program (GraphPad Software, San Diego, CA). Comparison of clofarabine triphosphate values in responders and nonresponders was done by Student unpaired 1-tailed t test.
Results
Study group
A total of 62 patients were treated in this study. Their characteristics are shown in Table 1. The median age was 54 years (range, 19 to 82 years). There were 39 patients with AML or high-risk MDS; 25 of them were in first salvage: first CR duration of less than 12 months in 17 patients, and more than 12 months in 8 patients. Karyotypic studies showed unfavorable chromosomal abnormalities in 9. There were 11 patients with Ph+ CML in blastic phase, and 12 with ALL.
Characteristics of the study group (62 patients)
Parameter/category . | No. (%) . |
|---|---|
| Age, 60 y or older | 23 (37) |
| Diagnosis | |
| AML | 31 (50) |
| Salvage 1 | |
| CRD1 less than 12 mo | 11 (18) |
| CRD1 12 mo or longer | 8 (13) |
| Second or subsequent salvage | 12 (19) |
| MDS | 8 (13) |
| Salvage 1, CRD1 less than 12 mo | 6 (10) |
| Second or subsequent salvage | 2 (3) |
| CML, blastic phase | 11 (18) |
| Salvage 1 | 7 (11) |
| Second or subsequent salvage | 4 (6) |
| ALL | 12 (19) |
| Salvage 1 | 4 (6) |
| Second or subsequent salvage | 8 (13) |
| Karyotype | |
| AML-MDS | |
| Favorable | 1 (2) |
| Diploid | 20 (32) |
| Unfavorable | 18 (29) |
| CML | |
| −Ph only | 4 (6) |
| Ph + other | 7 (11) |
| ALL | |
| Ph ± other | 2 (3) |
| Diploid | 1 (2) |
| Other | 9 (15) |
Parameter/category . | No. (%) . |
|---|---|
| Age, 60 y or older | 23 (37) |
| Diagnosis | |
| AML | 31 (50) |
| Salvage 1 | |
| CRD1 less than 12 mo | 11 (18) |
| CRD1 12 mo or longer | 8 (13) |
| Second or subsequent salvage | 12 (19) |
| MDS | 8 (13) |
| Salvage 1, CRD1 less than 12 mo | 6 (10) |
| Second or subsequent salvage | 2 (3) |
| CML, blastic phase | 11 (18) |
| Salvage 1 | 7 (11) |
| Second or subsequent salvage | 4 (6) |
| ALL | 12 (19) |
| Salvage 1 | 4 (6) |
| Second or subsequent salvage | 8 (13) |
| Karyotype | |
| AML-MDS | |
| Favorable | 1 (2) |
| Diploid | 20 (32) |
| Unfavorable | 18 (29) |
| CML | |
| −Ph only | 4 (6) |
| Ph + other | 7 (11) |
| ALL | |
| Ph ± other | 2 (3) |
| Diploid | 1 (2) |
| Other | 9 (15) |
AML indicates acute myeloid leukemia; CRD1, duration of first complete response; MDS, myelodysplastic syndrome; CML, chronic myeloid leukemia; and ALL, acute lymphoid leukemia.
Response and outcome
Overall, 20 patients (32%) achieved CR, 1 had a PR (2%), and 9 (15%) had hematologic improvement or CRp, for an overall objective response rate of 48% (Table 2). Responses by disease category and by subset analysis are shown in Table 3. In AML, 13 (42%) of 31 patients achieved CR and 4 had CRp (13%) for an overall objective response rate of 55%. Response rates were higher in patients with longer first CR durations. In MDS, 4 (50%) of 8 patients had objective responses (2 CR, 2 CRp). Among the 39 patients with AML or MDS, the objective response rate by karyotype was as follows: diploid or favorable, 9 CR and 2 CRp of 21 (52%); unfavorable, 6 CR and 4 CRp of 18 (56%). Among the 15 patients with AML or MDS achieving CR, 7 had chromosomal abnormalities at the start of therapy (patients 1, 4, 6, 11, 12, 13, and 19; Table 4). Six of them were analyzed at CR by cytogenetic studies: all 6 showed reappearance of a normal karyotype. In CML blastic phase, 7 patients (58%) achieved objective response: 4 CHR, 2 CRp, 1 PR. Suppression of Ph+ cells to 0% was also noted in 3 patients, lasting for 1 to 3 months. Pertinent details of responding patients are shown in Table 4. Patient 7 (Table 4) had previously received clofarabine on the phase 1 study and achieved a CR lasting 9 months. Upon relapse, the patient was offered clofarabine therapy again on this phase 2 study, and achieved a CR lasting 5.5 months.
Overall response (62 patients)
Response . | No. (%) . |
|---|---|
| Complete response | 20 (32) |
| Partial response | 1 (2) |
| Hematologic improvement (CRp) | 9 (15) |
| Early death (within 2 weeks) | 2 (3) |
| Induction death | 3 (5) |
| Primary resistance | 11 (18) |
| Secondary resistance | 16 (26) |
Response . | No. (%) . |
|---|---|
| Complete response | 20 (32) |
| Partial response | 1 (2) |
| Hematologic improvement (CRp) | 9 (15) |
| Early death (within 2 weeks) | 2 (3) |
| Induction death | 3 (5) |
| Primary resistance | 11 (18) |
| Secondary resistance | 16 (26) |
CRp indicates complete response with incomplete platelet recovery.
Response by disease category, salvage number, and duration of first complete response
. | No. treated . | No. (%) . | . | . | ||
|---|---|---|---|---|---|---|
| Disease . | . | CR . | CRp/PR . | Overall . | ||
| AML | ||||||
| First salvage | ||||||
| CRD1, 12 mo or longer | 11 | 2 (18) | 0 | 2 (18) | ||
| CRD1, less than 12 mo | 8 | 6 (75) | 1 (13) | 7 (87) | ||
| Second or subsequent salvage | ||||||
| CRD1, 12 mo or longer | 4 | 2 (50) | 1 (25) | 3 (75) | ||
| CRD1, less than 12 mo | 8 | 3 (38) | 2 (25) | 5 (63) | ||
| MDS | ||||||
| First salvage | 6 | 2 (33) | 1 (17) | 3 (50) | ||
| Second or subsequent salvage | 2 | 0 (0) | 1 (50) | 1 (50) | ||
| CML, blastic phase | ||||||
| First salvage | 7 | 3 (43) | 1 (14) | 4 (57) | ||
| Second or subsequent salvage | 4 | 1 (25) | 1/1 (50) | 3 (75) | ||
| ALL | 12 | 1 (8) | 1 (8) | 2 (17) | ||
. | No. treated . | No. (%) . | . | . | ||
|---|---|---|---|---|---|---|
| Disease . | . | CR . | CRp/PR . | Overall . | ||
| AML | ||||||
| First salvage | ||||||
| CRD1, 12 mo or longer | 11 | 2 (18) | 0 | 2 (18) | ||
| CRD1, less than 12 mo | 8 | 6 (75) | 1 (13) | 7 (87) | ||
| Second or subsequent salvage | ||||||
| CRD1, 12 mo or longer | 4 | 2 (50) | 1 (25) | 3 (75) | ||
| CRD1, less than 12 mo | 8 | 3 (38) | 2 (25) | 5 (63) | ||
| MDS | ||||||
| First salvage | 6 | 2 (33) | 1 (17) | 3 (50) | ||
| Second or subsequent salvage | 2 | 0 (0) | 1 (50) | 1 (50) | ||
| CML, blastic phase | ||||||
| First salvage | 7 | 3 (43) | 1 (14) | 4 (57) | ||
| Second or subsequent salvage | 4 | 1 (25) | 1/1 (50) | 3 (75) | ||
| ALL | 12 | 1 (8) | 1 (8) | 2 (17) | ||
CRD1 indicates duration of first complete response; CR, complete response; CR-p, complete response without platelet recovery; and PR, partial response. Other abbreviations are explained in Table 1.
Characteristics of responders
Patient . | Age, y/sex . | Diagnosis . | Salvage . | CRD1; CRD2; CRD3, mo . | Prior therapy . | Karyotype . | Response/duration in months . |
|---|---|---|---|---|---|---|---|
| 1 | 63/M | AML | 2 | 3.5;5 | Thalidomide × 2 | Deletion 5q− | CR/7.5+ |
| 2 | 61/M | AML | 1 | 10.5 | CAT + ATRA | Diploid | CR/5 |
| 3 | 73/F | AML | 1 | 13.5 | IA | Diploid | CR/2 |
| 4 | 53/M | AML | 1 | 0 | FA + mylotarg | Multiple | CR/4 |
| 5 | 32/F | AML | 1 | 29 | CAT + GCSF | Diploid | CR/10.5+ |
| 6 | 65/M | AML | 1 | 32 | CAT + GCSF | t(10;11) (p13;q21) | CR/5.5 |
| 7 | 75/F | AML | 3 | 0; 5; 9 | CAT; mylotarg; clofarabine | Diploid | CR/5.5 |
| 8 | 60/M | AML | 1 | 16 | IA | Diploid | CR/8+ |
| 9 | 22/M | AML | 1 | 30 | IA | Diploid | CR/11+ |
| 10 | 58/M | AML | 2 | 18; 18 | CAT + GCSF troxatyl + topotecan | Diploid | CR/4 |
| 11 | 46/M | AML | 2 | 24; 22 | IA; FAM; MUD SCT in CR | t(11;19) + other | CR/3.5+ |
| 12 | 74/M | AML | 2 | 11; 0 | IA; gelonin | t(9;11), deletion 7q−, trisomy 8 | CR/7 |
| 13 | 58/M | AML | 1 | 32.5 | IA | Trisomy 8 + other | CR/2.5+ |
| 14 | 34/M | CML-BP | 1 | 7 | imatinib* | Ph + other | CR/7 (died in CR) |
| 15 | 46/M | CML-BP | 1 | NA | IFN + LDAC*; imatinib | Double Ph, trisomy 8, isochromosome 17 | CR/3 (died in CR) |
| 16 | 67/M | CML-BP | 2 | 3 | HCVAD + imatinib | Ph | CR/7 |
| 17 | 46/M | CML-BP | 1 | NA | None | Ph | CR/6+ |
| 18 | 61/F | MDS | 1 | First Rx for MDS | COAP†; IFN + LDAC; 9NC | Diploid | CR/5+ |
| 19 | 70/M | MDS | 1 | 12 | IA | t(5; 12) | CR/2 |
| 20 | 61/F | AML | 2 | 8;7 | IA;mylotarg + ara-C | t(8;21) | CRp/3.5 |
| 21 | 57/F | AML | 2 | 27;30 | CAT + ATRA; lipo DNR + HDAC | t(3;21) + other | CRp/2 |
| 22 | 28/F | AML | 2 | 12; 6.5 | IA; HDAC; MUD in CR | Diploid | CRp/4.5 |
| 23 | 76/M | AML | 1 | 20.5 | IA | Trisomy 8; 20q- | CRp/1 |
| 24 | 39/M | CML-BP | 2 | 0 | IFN + hydrea*; allo SCT; IA + imatinib | Ph; trisomy 8 | CRp/5 |
| 25 | 37/F | CML-BP | 1 | NA | PEG IFN + LDAC*; imatinib; Lonafarnib | Ph + other | CRp/5 |
| 26 | 60/F | MDS | 2 | 0 | DNR + HDAC; mylotarg | Chromosome 5 and 7 abnormalities | CRp/1.3 |
| 27 | 82/F | MDS | 1 | 9 | IA | Chromosome 5 and 7 abnormalities | CRp/2 |
| 28 | 31/M | ALL | 1 | 0 | VAD | Ph, monosomy 7 | CR/4 |
| 29 | 23/M | ALL | 3 | 20.5; 3; 0 | CALGB ALL therapy; allo SCT; IA;, asparaginase + VP | Multiple | CRp/1 |
| 30 | 42/M | CML-BP | 2 | 14 | imatinib | Ph | PR/1 |
Patient . | Age, y/sex . | Diagnosis . | Salvage . | CRD1; CRD2; CRD3, mo . | Prior therapy . | Karyotype . | Response/duration in months . |
|---|---|---|---|---|---|---|---|
| 1 | 63/M | AML | 2 | 3.5;5 | Thalidomide × 2 | Deletion 5q− | CR/7.5+ |
| 2 | 61/M | AML | 1 | 10.5 | CAT + ATRA | Diploid | CR/5 |
| 3 | 73/F | AML | 1 | 13.5 | IA | Diploid | CR/2 |
| 4 | 53/M | AML | 1 | 0 | FA + mylotarg | Multiple | CR/4 |
| 5 | 32/F | AML | 1 | 29 | CAT + GCSF | Diploid | CR/10.5+ |
| 6 | 65/M | AML | 1 | 32 | CAT + GCSF | t(10;11) (p13;q21) | CR/5.5 |
| 7 | 75/F | AML | 3 | 0; 5; 9 | CAT; mylotarg; clofarabine | Diploid | CR/5.5 |
| 8 | 60/M | AML | 1 | 16 | IA | Diploid | CR/8+ |
| 9 | 22/M | AML | 1 | 30 | IA | Diploid | CR/11+ |
| 10 | 58/M | AML | 2 | 18; 18 | CAT + GCSF troxatyl + topotecan | Diploid | CR/4 |
| 11 | 46/M | AML | 2 | 24; 22 | IA; FAM; MUD SCT in CR | t(11;19) + other | CR/3.5+ |
| 12 | 74/M | AML | 2 | 11; 0 | IA; gelonin | t(9;11), deletion 7q−, trisomy 8 | CR/7 |
| 13 | 58/M | AML | 1 | 32.5 | IA | Trisomy 8 + other | CR/2.5+ |
| 14 | 34/M | CML-BP | 1 | 7 | imatinib* | Ph + other | CR/7 (died in CR) |
| 15 | 46/M | CML-BP | 1 | NA | IFN + LDAC*; imatinib | Double Ph, trisomy 8, isochromosome 17 | CR/3 (died in CR) |
| 16 | 67/M | CML-BP | 2 | 3 | HCVAD + imatinib | Ph | CR/7 |
| 17 | 46/M | CML-BP | 1 | NA | None | Ph | CR/6+ |
| 18 | 61/F | MDS | 1 | First Rx for MDS | COAP†; IFN + LDAC; 9NC | Diploid | CR/5+ |
| 19 | 70/M | MDS | 1 | 12 | IA | t(5; 12) | CR/2 |
| 20 | 61/F | AML | 2 | 8;7 | IA;mylotarg + ara-C | t(8;21) | CRp/3.5 |
| 21 | 57/F | AML | 2 | 27;30 | CAT + ATRA; lipo DNR + HDAC | t(3;21) + other | CRp/2 |
| 22 | 28/F | AML | 2 | 12; 6.5 | IA; HDAC; MUD in CR | Diploid | CRp/4.5 |
| 23 | 76/M | AML | 1 | 20.5 | IA | Trisomy 8; 20q- | CRp/1 |
| 24 | 39/M | CML-BP | 2 | 0 | IFN + hydrea*; allo SCT; IA + imatinib | Ph; trisomy 8 | CRp/5 |
| 25 | 37/F | CML-BP | 1 | NA | PEG IFN + LDAC*; imatinib; Lonafarnib | Ph + other | CRp/5 |
| 26 | 60/F | MDS | 2 | 0 | DNR + HDAC; mylotarg | Chromosome 5 and 7 abnormalities | CRp/1.3 |
| 27 | 82/F | MDS | 1 | 9 | IA | Chromosome 5 and 7 abnormalities | CRp/2 |
| 28 | 31/M | ALL | 1 | 0 | VAD | Ph, monosomy 7 | CR/4 |
| 29 | 23/M | ALL | 3 | 20.5; 3; 0 | CALGB ALL therapy; allo SCT; IA;, asparaginase + VP | Multiple | CRp/1 |
| 30 | 42/M | CML-BP | 2 | 14 | imatinib | Ph | PR/1 |
M indicates male; F, female; AML, acute myeloid leukemia; CML-BP, chronic myeloid leukemia in blastic phase; MDS, myelodysplastic syndrome; ALL, acute lymphoid leukemia; CRD1, duration of first complete response; CAT, cyclophosphamide, ara-C, topotecan; ATRA, all transretinoic acid; IA, idarubicin + high-dose ara-C; BID FA, fludarabine + ara-C; GCSF, granulocyte colony-stimulating factor; FAM, fludarabine, ara-C, mitoxantrone; MUD SCT, matched unrelated donor stem cell transplantation; IFN, interferon alpha; LD AC, low-dose ara-C; HCVAD, hyperfractionated cyclophosphamide, vincristine, adriamycin, dexamethasone; allo SCT, allogeneic stem cell transplantation; DNR, daunorubicin; and VP, vincristine + prednisone.
Therapy for chronic phase CML.
Prior history of Ph− CML.
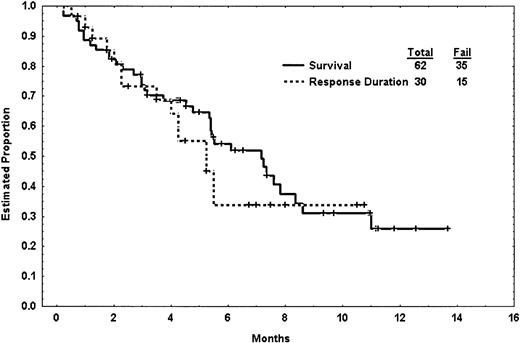
Response duration and survival are shown in Figure 2. Among the 20 patients achieving CR, 16 achieved CR after 1 course and 4 required 2 or more courses to achieve CR. Among the 10 patients achieving PR or CRp, 1 required 2 courses to achieve the response.
Side effects
Common side effects with clofarabine have included transient liver dysfunction, skin rashes and palmoplantar erythrodyesthesia, and mucositis. Side effects by grade are shown in Table 5 and Table 6. Although grade 3 to 4 liver dysfunction was common, it was transient, starting around day 5 to day 6 of therapy and subsiding by day 10 to day 15.
Nonhematologic side effects (62 patients)
Side effect . | Grades 1-2 no. (%) . | Grades 3-4 no. (%) . |
|---|---|---|
| Liver dysfunction | ||
| Elevated bilirubin | 22 (35) | 9 (15) |
| Elevated transaminases | 31 (50) | 15 (24) |
| Skin rashes | 23 (37) | 6 (10) |
| Palmoplantar erythrodyesthesia | 5 (8) | 7 (11) |
| Mucositis | 9 (15) | 0 |
| Diarrhea | 13 (21) | 0 |
| Nausea/vomiting | 40 (65) | 2 (3) |
| Other | 42 (68) | 7 (11) |
Side effect . | Grades 1-2 no. (%) . | Grades 3-4 no. (%) . |
|---|---|---|
| Liver dysfunction | ||
| Elevated bilirubin | 22 (35) | 9 (15) |
| Elevated transaminases | 31 (50) | 15 (24) |
| Skin rashes | 23 (37) | 6 (10) |
| Palmoplantar erythrodyesthesia | 5 (8) | 7 (11) |
| Mucositis | 9 (15) | 0 |
| Diarrhea | 13 (21) | 0 |
| Nausea/vomiting | 40 (65) | 2 (3) |
| Other | 42 (68) | 7 (11) |
Hematologic side effects (62 patients)
Characteristic . | . |
|---|---|
| Median time to recovery for 20 patients in CR, d | |
| Platelet count greater than 100 × 109/L | 31 |
| Granulocyte count greater than 109/L | 28 |
| No. febrile episodes (%) | |
| Unknown origin | 19 (31) |
| Documented infections | |
| Bacterial/sepsis | 16 (26) |
| Fungal | 3 (5) |
| Pneumonia, including fungal | 12 (19) |
| Fixed | 3 (5) |
| Minor/other | 4 (7) |
Characteristic . | . |
|---|---|
| Median time to recovery for 20 patients in CR, d | |
| Platelet count greater than 100 × 109/L | 31 |
| Granulocyte count greater than 109/L | 28 |
| No. febrile episodes (%) | |
| Unknown origin | 19 (31) |
| Documented infections | |
| Bacterial/sepsis | 16 (26) |
| Fungal | 3 (5) |
| Pneumonia, including fungal | 12 (19) |
| Fixed | 3 (5) |
| Minor/other | 4 (7) |
Myelosuppression-associated complications included febrile episodes in 50 (81%) of 62 patients: fever of unknown origin in 19 patients (31%), and documented infections in 31 patients (50%). Some patients had multiple febrile episodes in any particular course. Although 5 patients died during induction (2 early deaths, 3 induction deaths; Table 2), none of the deaths were related to drug toxicities. All deaths were attributed to leukemia or to myelosuppression-associated complications.
Plasma pharmacokinetics
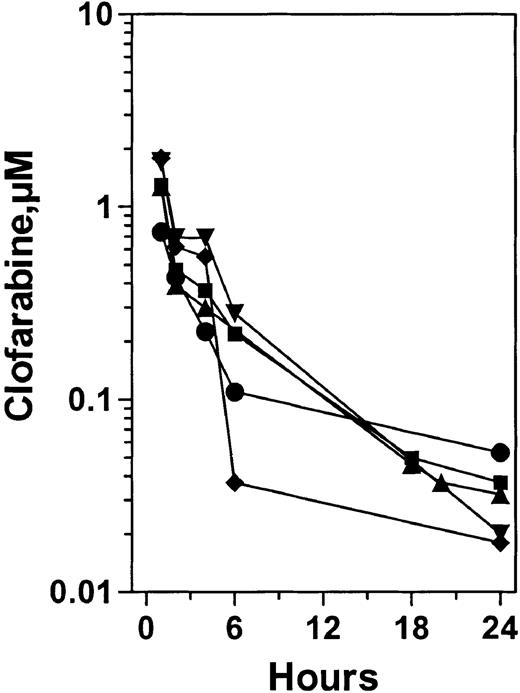
Our previous phase 1 study suggested that there was a dose proportionality between plasma concentration of clofarabine at a dose range of 2 mg/m2 to 55 mg/m2.24 At 40 mg/m2, the median plasma clofarabine level was 1.5 μM (n = 7). Among the 29 patients studied in the current trial, data after the end of first infusion of clofarabine were available from 25 patients. There was heterogeneity for accumulation of plasma clofarabine; the median value was 1.0 μM (range, 0.26μM-1.94 μM). To determine the variability in elimination kinetics of the drug from plasma, clofarabine levels were quantified at different time points after infusion. As presented in Figure 3, for 5 patients clofarabine appeared to be eliminated in a biphasic manner with faster kinetics during the first 6 hours followed by slower kinetics up to 24 hours. Because the data points are sparse, elimination half-lives were not calculated. Nonetheless, there was a measurable clofarabine concentration at 24 hours. Among the 26 patients, the median concentration of plasma clofarabine at 24 hours was 0.038 mM (range, 0.013 mM-0.11 mM). When applied to leukemia cell lines in vitro such concentrations caused more than 50% loss of clonogenicity.22
Plasma pharmacokinetics after first infusion of clofarabine from 5 patients. Blood samples were collected prior to and at the end of infusion, and at indicated times on the first day of therapy. Clofarabine levels were measured in plasma as described. Symbols represent individual patients.
Plasma pharmacokinetics after first infusion of clofarabine from 5 patients. Blood samples were collected prior to and at the end of infusion, and at indicated times on the first day of therapy. Clofarabine levels were measured in plasma as described. Symbols represent individual patients.
Cellular pharmacokinetics
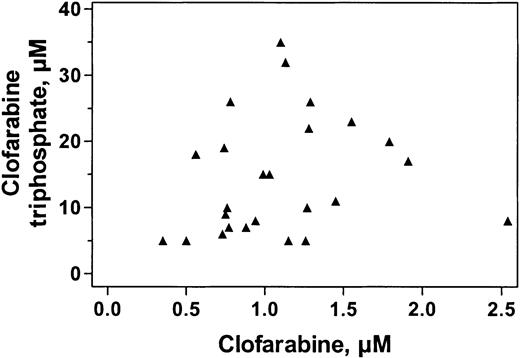
Similar to plasma clofarabine levels, the concentration of clofarabine triphosphate (the cellular cytotoxic metabolite) measured in circulating leukemia blasts varied among patients; the median value was 15 μM with a range of less than 1 μM to 44 μM (n = 29). The variability in the level of clofarabine triphosphate appeared similar in different diagnoses, with a less than 1 μM to 18 μM range in ALL (n = 6), a 6 μM to 26 μM range in CML (n = 10), and a 5 μM to 44 μM range in AML (n = 12). The concentration of cellular clofarabine triphosphate is a combination of the phosphorylation of the drug to its triphosphate level and the cellular retention of the triphosphate. Therefore, we sought to determine if there was a relationship between the levels of plasma clofarabine and the concentration of the triphosphate (Figure 4). As shown in Figure 4, there was heterogeneity for both the cellular levels of clofarabine triphosphate and plasma levels of clofarabine. However, linear and nonlinear regression analyses suggested no relationship between these 2 parameters.
Relationship between plasma levels of clofarabine and cellular accumulation of clofarabine triphosphate at the end of first infusion. Blood samples obtained at the end of infusion from 25 patients were processed to analyze both plasma levels of clofarabine and clofarabine triphosphate in leukemia cells.
Relationship between plasma levels of clofarabine and cellular accumulation of clofarabine triphosphate at the end of first infusion. Blood samples obtained at the end of infusion from 25 patients were processed to analyze both plasma levels of clofarabine and clofarabine triphosphate in leukemia cells.
Retention of clofarabine triphosphate was measured after the end of the first infusion. The intracellular clofarabine triphosphate value was increased about 10% in each case for up to 2 hours (Figure 5), suggesting that the cells were still phosphorylating the available plasma clofarabine (Figure 3). The limited data points 4 hours after the end of infusion indicated that clofarabine triphosphate was maintained in the leukemia cells with an elimination half-life of 24 hours or more.
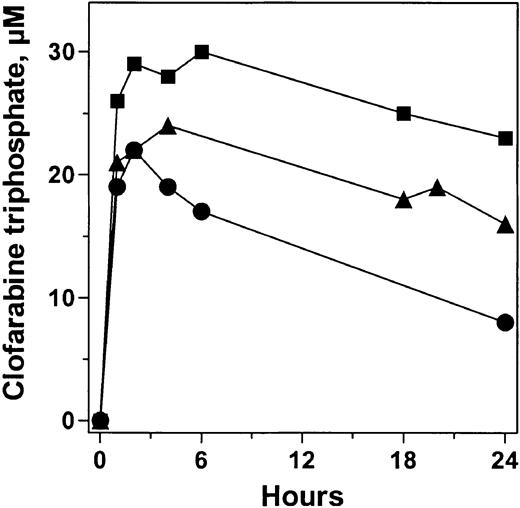
Accumulation and elimination profiles of clofarabine triphosphate in 3 patients. Blood samples obtained at different times after first infusion of clofarabine were processed to isolate leukemia cells and to analyze the concentration of clofarabine triphosphate in these cells. Symbols represent 3 individual patients.
Accumulation and elimination profiles of clofarabine triphosphate in 3 patients. Blood samples obtained at different times after first infusion of clofarabine were processed to isolate leukemia cells and to analyze the concentration of clofarabine triphosphate in these cells. Symbols represent 3 individual patients.
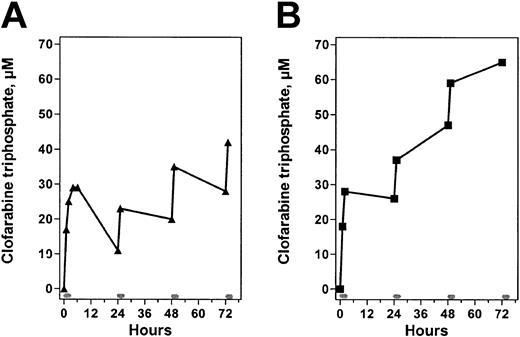
These elimination profiles indicated that, in many patients, there would be residual triphosphate at 24 hours when a second infusion of clofarabine was administered. In such situations, the intracellular clofarabine triphosphate value would increase with each infusion. To test this, clofarabine triphosphate values were quantified after each infusion of clofarabine for 3 to 4 days (Figure 6). As shown in 2 patients, after the first infusion, the intracellular level of clofarabine triphosphate accumulated to about 30 μM. Because of a differential in elimination profiles of the analog triphosphate, the levels of clofarabine triphosphate following subsequent daily infusions varied in each patient. In 1 patient, levels increased marginally and, after a total of 4 infusions, the value was 40 μM (Figure 6A). In contrast, the elimination kinetics of clofarabine triphosphate was slower in the next patient (Figure 6B) resulting in about 65 μM of the analog triphosphate after 3 infusions. These data suggested an incremental increase in clofarabine triphosphate concentration that was related to its rate of elimination.
Incremental accumulation of clofarabine triphosphate in 2 representative patients. Pretreatment and end-of-infusion samples were obtained from 2 patients after several infusions of clofarabine to quantitate intracellular levels of triphosphate. Rectangular bars on the abscissa indicate clofarabine infusions. (A) Nonresponder; (B), responder.
Incremental accumulation of clofarabine triphosphate in 2 representative patients. Pretreatment and end-of-infusion samples were obtained from 2 patients after several infusions of clofarabine to quantitate intracellular levels of triphosphate. Rectangular bars on the abscissa indicate clofarabine infusions. (A) Nonresponder; (B), responder.
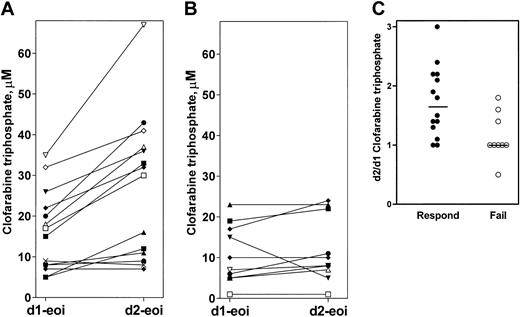
Because the 2 patients referred to in Figure 6 responded differently to clofarabine treatment, nonresponder (Figure 6A) and achiever of complete remission (Figure 6B), we studied whether there were differences in the intracellular pharmacokinetics of clofarabine triphosphate in responders versus nonresponders. Of the 29 patients studied for pharmacokinetic analyses, 16 were responders (CR or CRp), 11 were nonresponders, and 2 were early deaths. First, the median triphosphate value at the end of infusion (eoi) of clofarabine on the first day (1 hour after start of therapy; day-1 eoi in Figure 7A-B) was higher in responders (18 μM; range, 5 μM-44 μM; n = 16) compared with nonresponders (10 μM; range, < 1-23 μM; n = 11; P = .032). Second, the 24-hour value (23 hours after the end of the first clofarabine infusion or just prior to the second infusion) in patients who responded to therapy was higher in responders (11 μM; range, < 1-44 μM; n = 15) than in nonresponders (5 μM; range, < 1-20 μM; n = 10; P = .039). Third, as illustrated for individual patients, there was a trend for an increase in the end of infusion value from days 1 to 2 in responders (Figure 7A) compared with nonresponders (Figure 7B). Overall, the median concentration of clofarabine triphosphate at the end of second infusion (25 hours after start of therapy) was 30 μM (range, < 1-67 μM; n = 15) for patients who responded to therapy and 9 μM (range, < 1-23 μM; n = 10) for those who failed the treatment (P = .015). Finally, when plotted for the fold increase in clofarabine triphosphate value, responders had a median 1.8-fold increase compared with nonresponders who did not increase the concentration of clofarabine triphosphate from day 1 to day 2 (Figure 7C, P = .008). Taken together, these data suggested an overall greater accumulation, longer duration of analog triphosphate retention, and incremental increase in clofarabine triphosphate during the 5-day therapy in patients who benefited from clofarabine treatment.
Clofarabine triphosphate pharmacokinetics in responders and nonresponders. The analog triphosphate levels were compared at the end of infusion (eoi) on day 1 (d1-eoi) and day 2 (d2-eoi) in patients who were responders (A) or nonresponders (B). Each bar represents an individual patient. The increase in clofarabine triphosphate value was determined in these patients after calculating and plotting the ratio of clofarabine triphosphate on day 2 versus day 1 (C). Horizontal bars represent median values.
Clofarabine triphosphate pharmacokinetics in responders and nonresponders. The analog triphosphate levels were compared at the end of infusion (eoi) on day 1 (d1-eoi) and day 2 (d2-eoi) in patients who were responders (A) or nonresponders (B). Each bar represents an individual patient. The increase in clofarabine triphosphate value was determined in these patients after calculating and plotting the ratio of clofarabine triphosphate on day 2 versus day 1 (C). Horizontal bars represent median values.
Discussion
In this phase 2 study of clofarabine, given at 40 mg/m2 daily for 5 days per course in acute leukemia, we observed a significant antileukemic activity in AML, MDS, and CML-BP, and modest activity in ALL. Overall, 30 (48%) of the 62 patients achieved either CR, CRp, or PR. In AML and high-risk MDS, the CR rates were 42% and 25%, respectively, with an objective response rate of 54%. In CML-BP, 7 (64%) of 11 patients treated experienced objective responses. In ALL, 2 (17%) of 12 patients responded. The experience in AML was particularly impressive with response rates of 87% and 67% respectively in patients with long first CR duration or those treated with clofarabine as second or subsequent salvage therapy.
In addition to these encouraging response rates, the toxicity profile of clofarabine was noteworthy. Side effects were tolerable and mostly reversible. Severe but transient liver dysfunction was noted in 15% to 25%, while skin rashes and hand-foot syndrome were observed in 10% to 15% of the patient population. Myelosuppression-associated complications were similar to other salvage programs in leukemia. Of importance is the activity of clofarabine in acute leukemia at doses not associated with severe extramedullary side effects, particularly neurotoxicity.
In the phase 1 study of fludarabine in acute leukemia,9,10 doses of 125 mg/m2 or more given intravenously over 1 hour daily for 5 days were associated with severe neurotoxicities including mental changes, coma, and blindness. Interestingly, 4 of 9 patients with acute leukemia treated at these doses achieved complete remission.9 However, because of the severe and fatal extramedullary toxicities, fludarabine was subsequently developed at dose schedules of 20 mg/m2 to 30 mg/m2 intravenously daily for 5 days in phase 2 studies and demonstrated marked activity in CLL and other lymphoproliferative disorders. Similar renal and nervous system toxicities were observed with cladribine11 in the acute leukemia studies in adults, although this treatment is efficacious in pediatric acute leukemias.28 Our results showed that, in contrast to these other deoxyadenosine analogues, clofarabine was active in acute leukemia at doses well tolerated by patients.
Cellular pharmacokinetic studies suggested that the accumulation and especially retention of clofarabine triphosphate were favorable in leukemia cells of responding patients. For instance, there was a 1.8-fold increase in the level of clofarabine triphosphate in the blasts of responders after the second infusion (Figure 7). This demonstrates that leukemia blasts have an additional capacity to accumulate the active triphosphate. Indeed, after 5 days of therapy, a gradual and daily increase of the clofarabine triphosphate was observed in the blasts of a responder (Figure 6). Prospective validation of this relationship between clinical response and cellular pharmacokinetics may form a rational basis for the design of alternative dose schedules and combinations with other active agents.
Purine nucleoside analogs have been highly effective in indolent lymphocytic leukemias.5-7 In these hematologic malignancies, there was also a prolonged retention of analog triphosphates. For example, the elimination half-lives of ara-G triphosphate, fludarabine triphosphate, and cladribine triphosphate were more than 24 hours, more than 24 hours, and 10 hours, respectively.6,29,30 This positive feature may in part be associated with the favorable response in indolent leukemias. However, in circulating leukemia blasts of patients with relapsed acute leukemias, the elimination half-life of fludarabine triphosphate was much faster (t1/2 = 7 hours).31 The peak level of triphosphate at the maximum tolerated dose was a median 40 μM.32 With these kinetic profiles, the pharmacodynamic effect will be greatly diminished prior to the subsequent fludarabine dose. For cladribine triphosphate, the information in acute leukemia was very limited. Nonetheless, the accumulation of triphosphate in blasts either after continuous infusion or bolus administration was less than 5 μM.33,34 Thus, incremental daily increases of clofarabine triphosphate and its prolonged retention, in comparison to other deoxyadenosine nucleoside analogues, may account in part for its beneficial clinical effect in acute leukemias.
In a phase 1 pediatric study,35 22 evaluable children were treated at our institution: 5 (23%) achieved CR and 8 (36%) had an objective response. Phase 2 multi-institutional studies are currently ongoing in both adult and pediatric acute leukemia.36,37 The results from these studies should be available in final forms in the near future.
An important extension of the current trial is the rational future combination of clofarabine based on laboratory results and clinical experience. Both clofarabine and cytarabine are active in adult acute leukemia, and target 2 different DNA sites.38 The pharmacokinetic profile of clofarabine is favorable compared with other deoxyadenosine analogs, and in the preclinical settings, pretreatment with clofarabine increases the ability of cells to accumulate ara-cellular triphosphate (CTP) through a biochemical modulation strategy.39 Hence, one promising approach is to sequentially combine clofarabine with cytarabine, as had been done with fludarabine and cladribine.33,35 We are currently testing the biochemical modulation strategy in circulating leukemic cells from patients receiving such combination therapy with clofarabine and cytarabine. Another approach is to use clofarabine as an inhibitor of excision DNA repair elicited by DNA damaging agents.40 This could be done in a combination of clofarabine with anthracyclines or alkylating agents.
In summary, clofarabine is the first deoxyadenosine analog that has promising activity in adult acute leukemia at doses that are well tolerated. A favorable pharmacokinetic profile of clofarabine triphosphate was associated with response. Mechanism-based combinations of clofarabine with other active agents are planned based on its toxicity profile, pharmacokinetic properties, and clinical activity.
Prepublished online as Blood First Edition Paper, June 5, 2003; DOI 10.1182/blood-2003-03-0925.
Supported in part by grants CA57629, CA32839, and FD-R-00212 from the National Cancer Institute, Department of Health and Human Services, and FDR-002127 from the Food and Drug Administration.
Two of the authors (Z.S., A.C.) are employed by a company (Ilex) whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal