Abstract
Whether hematopoietic stem cells (HSCs) home selectively to bone marrow (BM) early after transplantation remains an issue of debate. Better understanding of homing mechanisms may benefit BM transplantation protocols in cases of limited graft cell number or nonmyeloablative conditioning regimens. Using flow cytometry and serial transplantation to stringently identify HSCs, trafficking patterns of long-term engrafting cells were mapped between BM and spleen early after transplantation. Low-density BM cells were tracked in irradiated or nonirradiated mice 1, 3, 6, and 20 hours after transplantation, at which time recipient BM and spleen were analyzed for recovery of primitive donor cells by phenotype and adhesion molecule expression. In addition, phenotypically defined HSC-enriched or HSC-depleted grafts were tracked 20 hours after transplantation in recipient BM and spleen and analyzed for recovery and long-term repopulating potential in mice undergoing serial transplantation. Regardless of irradiation status, recovery of donor Sca-1+ lin- cells was higher at most time points in recipient BM than in spleen, while recovery of total Sca-1+ cells was variable. A significantly higher percentage of BM-homed donor Sca-1+ cells expressed CD43, CD49e, and CD49d 20 hours after transplantation than spleen-homed cells, which contained significantly more non-HSC phenotypes. Furthermore, BM-homed cells were significantly enriched for cells capable of secondary multilineage hematopoiesis in mice undergoing serial transplantation compared with spleen-homed cells. These results support the notion of specific homing of HSCs to BM by 20 hours after transplantation and provide a basis for the enhanced engraftment potential afforded some Sca-1+ lin- cells subfractionated on the basis of adhesion molecule expression.
Introduction
Engraftment and reconstitution of normal hematopoiesis following transplantation of hematopoietic stem cells (HSCs) rely on the ability of these cells to lodge within specialized bone marrow niches where the process of proliferation and self-renewal of stem cell functions commence for lifelong hematopoiesis. The ability to manipulate this process may hold clinical benefits in cases of limited graft cell number or compromised homing ability of ex vivo–manipulated cells. However, whether transplanted cells home to bone marrow (BM) by a coordinated series of events based on their potential or whether homing represents a nonspecific process whereby these cells are retained in BM based on the relative size of this organ remains undefined. Evidence exists to support both specific and nonspecific lodging of transplanted HSCs in BM,1-13 and data are beginning to suggest that homing mechanisms may be more functional in nonablated hosts.14-18 Knowledge of the specificity and kinetics of homing is an essential first step in the design of methods aimed at increasing the efficiency of transplantation.
Results from homing studies that tracked unfractionated cells or progenitors in recipient BM or spleen1-5,9,14 may not signify the trafficking of target stem cell populations. More direct homing assays have focused on tracking phenotypically defined populations of transplanted cells in host tissues by flow cytometry6,10 or transplanting prospectively isolated purified stem cell grafts.7,11,12,14,16,18 To directly examine HSC trafficking, investigators have more recently focused on isolation and retransplantation of tracked BM- or spleen-homed cells in long-term reconstitution assays,5,7,12,18 given that hematopoietic reconstitution is regarded as the only true test of HSC function. These studies have documented the presence of repopulating cells in both BM and spleen 3 hours after transplantation5 but exclusive presence in BM 48 hours later.7 In these studies, repopulating cells present in spleen 3 hours after transplantation provided more rapid reconstitution than BM-homed cells,5 suggesting the possibility of hierarchic differences in the hematopoietic potential of BM-homed versus spleen-homed cells. Functional studies of BM-versus spleen-homed cells in serial transplantation studies may more precisely define kinetics of BM homing and trafficking patterns of HSCs.
In the current investigation, trafficking patterns of transplanted stem cell grafts were determined in recipient BM and spleen using several different approaches. In the first, grafts of low-density BM (LDBM) were tracked 1 to 20 hours after transplantation by phenotypic characterization of BM- and spleen-homed donor Sca-1+ cells possessing a complex phenotype of adhesion molecules and other markers associated with repopulating cells. In the second approach, HSC-enriched and HSC-depleted grafts were tracked in vivo. Non-HSC grafts were significantly enriched in spleen tissue, while BM recovery of either group of cells was similar. However, serial transplantation studies revealed the exclusive presence of long-term repopulating cells in BM rather than spleen by 20 hours after transplantation. Collectively, these studies support the notion of selective homing or survival of HSCs in BM 20 hours after transplantation.
Materials and methods
Mice
C57BL/6 mice (CD45.2 allele) were purchased from Jackson Laboratories (Bar Harbor, ME) at 8 to 10 weeks of age and allowed to acclimate for 1 to 2 weeks prior to their use in these studies. B6.SJL-PtrcaPep3b/BoyJ (B6.BoyJ) congenic mice (CD45.1 allele) were either purchased from Jackson Laboratories or maintained in our breeding colony and used between 8 to 12 weeks of age. All studies were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Primary short-term (1°ST) cell tracking
BM grafts consisted of LDBM cells or purified Sca-1+ lin– cells subfractionated on the basis of CD49e or CD62L expression into fractions enriched for long-term engraftment potential (ENG: Sca-1+ lin– CD49e+ or Sca-1+ lin– CD62L–) or those devoid of such potential (non-ENG: Sca-1+ lin– CD49e– or Sca-1+ lin– CD62L+) as previously documented.19 Adhesion molecules CD49e and CD62L were chosen in these studies so that grafts would consist of phenotypes where both positive expression and negative expression enriches for long-term repopulating cells (CD49e+ and CD62L–) and nonrepopulating cells (CD49e– and CD62L+), as previously shown.19 Donor LDBM, ENG, and non-ENG cells were isolated from C57BL/6 or B6.BoyJ mice and tracked in vivo with PKH26, PKH2 (Sigma ImmunoChemicals, St Louis, MO), or carboxyfluorescein diacetate succinimidyl ester-1 (CFSE) (Molecular Probes, Eugene, OR) as previously described.18 When possible, congenic donor cells were not labeled with any dye and were identified by appropriate CD45.1 or CD45.2 expression by flow cytometry. The different tracking methods were equally effective in detecting donor cells. Recipient C57BL/6 or B6.BoyJ mice between 10 and 12 weeks of age were lethally irradiated with 950 cGy administered in a single dose from a 137Cs γ irradiator (GammaCell 40; Nordion International, Kanata, ON, Canada) 17.5 to 20 hours prior to transplantation (mean, 18.5 hours). Irradiated or nonirradiated mice underwent transplantation via tail-vein injections with 1 × 107 to 1 × 108 LDBM donor cells or 4 × 104 to 3 × 105 ENG or non-ENG cells. Mice that received LDBM grafts were killed 1, 3, 6, or 20 to 24 hours later, while mice receiving ENG and non-ENG transplants were killed 3 or 20 hours later. For simplicity, the 20- to 24-hour time point is referred to as 20 hours hereafter. BM, spleen, and peripheral blood (PB) were collected from individual mice at the time points indicated above, and single cell suspensions were prepared and lysed. Lysed cells from LDBM transplants were analyzed for recovery of primitive phenotypes and adhesion molecule expression, while cells from ENG and non-ENG transplants were analyzed for recovery. BM- and spleen-homed donor cells from 20-hour ENG transplants were further analyzed for engraftment potential in serial transplantation studies. In experiments where homed cells were used in serial transplantation studies, 1°ST recipients received at least 1 × 105 ENG cells.
Flow cytometric analysis of harvested donor cells
Harvested BM and spleen cells from LDBM recipients were stained with Sca-1 and biotinylated antibodies to either CD11a (clone 2D7), CD43 (clone S7), CD44 (clone IM7), CD49d (clone 9C10), CD49e (clone 5H10-27; MFR5), CD62L (clone MEL-14), or CD3 and CD45R/B220 (B220). Biotinylated antibodies were developed with streptavidin-allophycocyanin (APC) (Molecular Probes). PB samples were stained only with Sca-1 and CD3 and B220. In some experiments cells were stained with biotinylated anti–Sca-1 and adhesion molecule antibodies conjugated to phycoerythrin (PE), followed by streptavidin-APC. All antibodies were from BD Pharmingen (San Diego, CA). Donor cells were gated to include lymphocytes and large granular cells for analysis of the recovery of Sca-1+ and Sca-1+ CD3–/B220– cells (referred to throughout as Sca-1+ lin– cells), or lymphocytes only for adhesion molecule analysis. Cells were analyzed using a FACScan or FACSCalibur (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA), and 1 × 104 to 5 × 105 events were acquired per sample. BM, spleen, and PB from 1°ST recipients of ENG and non-ENG transplants were analyzed for presence of donor cells by flow cytometric analysis of data files containing 2 × 105 to 1 × 106 events. No further phenotyping was performed in ENG/non-ENG experiments. In all experiments, donor cells were distinguished from recipient by PKH2, PKH26, CFSE, or appropriate CD45.1 or CD45.2 staining as described in “Primary short-term (1°ST) cell tracking.”
Calculation of recovery of primitive donor cells
The frequency of donor cells falling within a light scatter gate including lymphocytes and large granular cells was determined for each harvested tissue based on the background fluorescence of cells from control mice, as previously described.18 Control mice were subjected to the same treatment as experimental groups, including radiation and injection of vehicle. Frequency of Sca-1+ cells among donor cells and frequency of lin– cells among donor Sca-1+ cells were likewise determined and used in conjunction with tissue cellularity to calculate recovery of primitive phenotypes after LDBM transplants. Frequencies were multiplied by the total number of cells in each tissue and then divided by the number of cells in the original graft to calculate the recovery of transplanted cells. The number of BM cells harvested from both tibias and femurs was considered to represent 18.7% of total murine marrow.20 The total volume of PB was assumed to be 2 mL per mouse.21 A sample calculation of recovery of Sca-1+ cells in nonirradiated BM is as follows: No. BM cells in whole mouse (210 × 106) × Frequency of donor cells in BM (2%) = No. BM-homed donor cells (4.2 × 106) × Frequency of Sca-1+ cells among donor cells (20%) = No. BM-homed Sca-1+ donor cells (0.84 × 106)/No. Sca-1+ cells injected (6.4 × 106) = 0.13 × 100 = % Recovery of Sca-1+ donor cells in BM (13%). Similar calculations were then used to obtain the recovery of lin– Sca-1+ donor cells in BM. Due to the low frequency of detectable donor cells when ENG or non-ENG cells were injected, the frequency of donor cells was calculated manually using event count of saved fluorescence-activated cell sorted (FACS) files.
Serial transplantation studies
BM- and spleen-homed donor cells from irradiated or nonirradiated 1°ST recipients of ENG cells were isolated 20 hours after transplantation by flow cytometric cell sorting using CD45.1-PE and CD45.2–fluorescein isothiocyanate (CD45.2-FITC). Equal numbers of sorted donor cells (range, 50-200 cells per mouse), along with 1 × 105 competitor LDBM cells of recipient origin, were transplanted into primary long-term (1°LT) congenic recipients within 3 hours of receiving lethal irradiation in a split dose of 700 cGy plus 350 cGy 4 hours apart. Recipient mice were bled from the tail vein monthly until 6 months for analysis of donor-derived hematopoiesis. In 3 experiments (1 irradiated 1°ST and 2 nonirradiated 1°ST), 1°LT recipients were killed 6 months after transplantation, and 1 × 106 to 5 × 106 LDBM cells were transplanted into lethally irradiated (700 cGy plus 350 cGy) secondary LT (2°LT) recipients without competition. 2°LT recipients were bled from the tail vein monthly until 6 months for analysis of donor-derived hematopoiesis. Donor-derived cells from some 1°LT and 2°LT recipients were analyzed 6 months after transplantation for the percentage of lineage cells using PE-conjugated antibodies specific for CD3+, B220+,Gr-1+, and Mac-1+ cells.
Statistical analysis
Data are expressed as the mean ± SEM where applicable. Differences between groups were analyzed using an unpaired 2-sided t test. Differences in chimerism were analyzed by repeated measures analysis of variance using an arcsine transformation. A probability value of less than .05 was considered significant for all tests.
Results
Primitive phenotype of BM- and spleen-homed donor cells
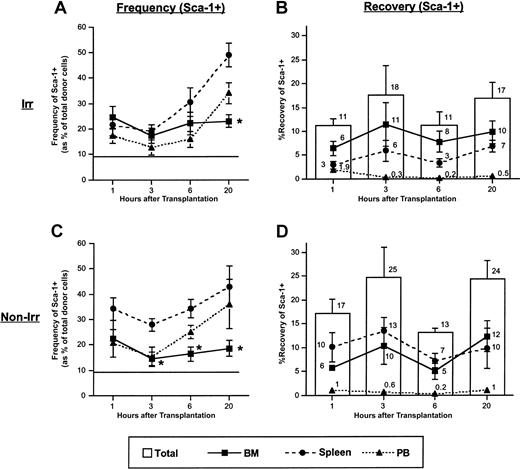
To investigate trafficking patterns of primitive hematopoietic progenitor cells (HPCs), irradiated or nonirradiated mice underwent transplantation with LDBM and were killed 1, 3, 6, or 20 hours later. Donor cells harvested from BM or spleen or circulating in PB were phenotyped for expression of primitive markers. In irradiated mice, the frequency of Sca-1+ donor cells in BM and spleen was similar 1 and 3 hours after transplantation but increased significantly in spleen by 20 hours (Figure 1A). The frequency of Sca-1+ donor cells in nonirradiated mice was significantly higher in spleen than BM at 3, 6, and 20 hours after transplantation (Figure 1C). Cellularity of BM, spleen, and PB from irradiated mice ranged from 24 × 106 to 55 × 106, 3 × 106 to 8 × 106, and 1 × 106 to 4 × 106, respectively, depending on the time after irradiation that mice were killed.18 Mean cellularity of BM, spleen, and PB from nonirradiated mice was 212 × 106,72 × 106, and 14 × 106, respectively.18 Data on cellularity are reproduced from Plett and colleagues.18 Due to the higher cellularity of BM compared with spleen, recoveries of Sca-1+ cells did not differ significantly between BM and spleen in either transplantation group (Figure 1B,D).
Frequency and recovery of Sca-1+ donor cells in BM, spleen, and PB for times up to 20 hours following transplantation of low-density BM cells in irradiated and nonirradiated recipients. Mice underwent transplantation with 1 × 107 to 1 × 108 LDBM cells, were killed at the indicated time points, and were analyzed for frequency and recovery of donor Sca-1+ cells in BM, spleen, and PB as described in “Materials and methods.” Data in panels A and C are expressed as the mean ± SEM percent of donor cells falling within a light scatter gate including lymphocytes and large granular cells and that express Sca-1. Horizontal lines in panels A and C represent the mean percentage of Sca-1 in the original graft. Using a similar light scatter gate, recoveries of Sca-1+ donor cells were calculated and are shown in panels B and D. Bars in panels B and D represent mean ± SEM recovery of Sca-1+ donor cells (sum of recoveries in BM plus spleen plus PB), and lines represent mean ± SEM recoveries in BM, spleen, or PB. Numbers above each bar represent mean recoveries. n = 6-13 in panel A; n = 4-11 in panel B; n = 4-5 in panel C; n = 3-5 in panel D. *P < .05 when compared with spleen of the same time point. To provide an SEM value for total recovery at each time point, total recoveries were not calculated by adding the means of the recoveries from each tissue but rather by averaging the total recoveries from each individual experiment.
Frequency and recovery of Sca-1+ donor cells in BM, spleen, and PB for times up to 20 hours following transplantation of low-density BM cells in irradiated and nonirradiated recipients. Mice underwent transplantation with 1 × 107 to 1 × 108 LDBM cells, were killed at the indicated time points, and were analyzed for frequency and recovery of donor Sca-1+ cells in BM, spleen, and PB as described in “Materials and methods.” Data in panels A and C are expressed as the mean ± SEM percent of donor cells falling within a light scatter gate including lymphocytes and large granular cells and that express Sca-1. Horizontal lines in panels A and C represent the mean percentage of Sca-1 in the original graft. Using a similar light scatter gate, recoveries of Sca-1+ donor cells were calculated and are shown in panels B and D. Bars in panels B and D represent mean ± SEM recovery of Sca-1+ donor cells (sum of recoveries in BM plus spleen plus PB), and lines represent mean ± SEM recoveries in BM, spleen, or PB. Numbers above each bar represent mean recoveries. n = 6-13 in panel A; n = 4-11 in panel B; n = 4-5 in panel C; n = 3-5 in panel D. *P < .05 when compared with spleen of the same time point. To provide an SEM value for total recovery at each time point, total recoveries were not calculated by adding the means of the recoveries from each tissue but rather by averaging the total recoveries from each individual experiment.
In contrast to total Sca-1+ cells, frequencies and recoveries of the more primitive Sca-1+ lin– donor cells in irradiated and nonirradiated recipients tended to be higher in BM than spleen, although these data were not significant at all time points (Figure 2).
Frequency and recovery of lineage-negative cells among Sca-1+ donor cells in BM, spleen, and PB for times up to 20 hours following transplantation of low-density BM cells in irradiated and nonirradiated recipients. Mice underwent transplantation with 1 × 107 to 1 × 108 LDBM cells, were killed at the indicated time points, and were analyzed for frequency and recovery of donor Sca-1+ lin– cells in BM, spleen, and PB as described in “Materials and methods.” Data in panels A and C are expressed as the mean ± SEM percent of donor Sca-1+ cells falling within a light scatter gate including lymphocytes and large granular cells and that lack lineage expression. Horizontal lines in panels A and C represent the mean percentage of lineage-negative donor Sca-1+ cells in the original graft. Using a similar light scatter gate, recoveries of Sca-1+ lin– donor cells were calculated and are shown in panels B and D. Bars in panels B and D represent mean ± SEM recovery of Sca-1+ lin– donor cells (sum of recoveries in BM plus spleen plus PB), and lines represent mean ± SEM recoveries in BM, spleen, or PB. Numbers above each bar represent mean recoveries. n = 6-12 in panel A; n = 4-11 in panel B; n = 4-5 in panel C; n = 3-5 in panel D. *P < .05 when compared with spleen of the same time point. To provide an SEM value for total recovery at each time point, total recoveries were not calculated by adding the means of the recoveries from each tissue but rather by averaging the total recoveries from each individual experiment.
Frequency and recovery of lineage-negative cells among Sca-1+ donor cells in BM, spleen, and PB for times up to 20 hours following transplantation of low-density BM cells in irradiated and nonirradiated recipients. Mice underwent transplantation with 1 × 107 to 1 × 108 LDBM cells, were killed at the indicated time points, and were analyzed for frequency and recovery of donor Sca-1+ lin– cells in BM, spleen, and PB as described in “Materials and methods.” Data in panels A and C are expressed as the mean ± SEM percent of donor Sca-1+ cells falling within a light scatter gate including lymphocytes and large granular cells and that lack lineage expression. Horizontal lines in panels A and C represent the mean percentage of lineage-negative donor Sca-1+ cells in the original graft. Using a similar light scatter gate, recoveries of Sca-1+ lin– donor cells were calculated and are shown in panels B and D. Bars in panels B and D represent mean ± SEM recovery of Sca-1+ lin– donor cells (sum of recoveries in BM plus spleen plus PB), and lines represent mean ± SEM recoveries in BM, spleen, or PB. Numbers above each bar represent mean recoveries. n = 6-12 in panel A; n = 4-11 in panel B; n = 4-5 in panel C; n = 3-5 in panel D. *P < .05 when compared with spleen of the same time point. To provide an SEM value for total recovery at each time point, total recoveries were not calculated by adding the means of the recoveries from each tissue but rather by averaging the total recoveries from each individual experiment.
Adhesion molecule phenotype of BM-versus spleen-homed donor cells
We previously examined the expression of adhesion molecules on BM-homed Sca-1+ donor cells 1 to 20 hours after transplantation in irradiated or nonirradiated recipients.18 In the present study, we compared concurrently the adhesion molecule expression of BM- and spleen-homed cells in irradiated recipients. Expression of CD43, CD49e, and CD49d significantly decreased on spleenhomed Sca-1+ cells between 1 and 20 hours after transplantation, while remaining relatively constant on BM-homed Sca-1+ cells (Figure 3A-C). By 20 hours after transplantation, expression of these 3 adhesion molecules, previously shown to be present on primitive HPCs from both murine and human sources,8,19,22,23 was significantly greater on BM-homed Sca-1+ cells than on similar spleen-homed cells (Figure 3A-C). Because the cellularity of BM was higher than that of spleen, not only was the frequency of CD49e+, CD43+, and CD49d+ Sca-1+ cells higher in BM than in spleen, but absolute numbers were also higher in BM (4.3-, 2.8-, and 1.7-fold higher at 20 hours after transplantation, respectively). Similar results were observed in nonirradiated mice (data not shown).
Frequency of CD43+, CD49e+, CD49d+, and CD62L+ cells among donor Sca-1+ cells in BM, spleen, and PB at 1, 3, 6, and 20 hours following transplantation of low-density BM cells. Mice were irradiated with 950 cGy, underwent transplantation an average of 18.5 hours later with 1 × 107 to 1 × 108 LDBM cells, were killed at the indicated time points, and donor Sca-1+ cells in BM and spleen were analyzed for expression of CD43 (A), CD49e (B), CD49d (C), and CD62L (D) by flow cytometry as described in “Materials and methods.” All 4 time points were assayed in every experiment. Data are expressed as the mean ± SEM percent of donor Sca-1+ cells with light scatter properties characteristic of primitive hematopoietic cells and that express each adhesion molecule. n = 4; *P < .05 when compared with spleen at the same time points, #P < .05 when compared with earlier time points of same tissue. Data for BM cells are partially reproduced, with permission, from Plett et al.18
Frequency of CD43+, CD49e+, CD49d+, and CD62L+ cells among donor Sca-1+ cells in BM, spleen, and PB at 1, 3, 6, and 20 hours following transplantation of low-density BM cells. Mice were irradiated with 950 cGy, underwent transplantation an average of 18.5 hours later with 1 × 107 to 1 × 108 LDBM cells, were killed at the indicated time points, and donor Sca-1+ cells in BM and spleen were analyzed for expression of CD43 (A), CD49e (B), CD49d (C), and CD62L (D) by flow cytometry as described in “Materials and methods.” All 4 time points were assayed in every experiment. Data are expressed as the mean ± SEM percent of donor Sca-1+ cells with light scatter properties characteristic of primitive hematopoietic cells and that express each adhesion molecule. n = 4; *P < .05 when compared with spleen at the same time points, #P < .05 when compared with earlier time points of same tissue. Data for BM cells are partially reproduced, with permission, from Plett et al.18
The frequency of CD62L+ cells increased among BM- and spleen-homed Sca-1+ cells until approximately 70% of these cells expressed CD62L by 20 hours (Figure 3D). Expression of CD11a and CD44 was not different between BM- or spleen-homed Sca-1+ cells at any time point and ranged from 95% to 100% on donor Sca-1+ cells in all tissues examined (n = 4, data not shown).
Differential trafficking of ENG versus non-ENG cells in irradiated mice
We next examined the in vivo movement of BM cells highly enriched for long-term engraftment potential in comparison to BM cells known to be devoid of such activity. Such a strategy would identify whether long-term engrafting cells possess specific trafficking mechanisms and whether nonengrafting cells lack such mechanisms, which may in turn contribute to their lack of stem cell function. Based on our previously published work identifying cells with long-term engrafting potential,19 purified populations of engrafting cells (ENG) consisting of Sca-1+ lin– CD49e+ or Sca-1+ lin– CD62L– cells or of nonengrafting cells (non-ENG) containing Sca-1+ lin– CD49e– or Sca-1+ lin– CD62L+ cells were isolated prior to transplantation and tracked in vivo. Irradiated 1°ST mice received 4 × 104 to 3 × 105 sorted ENG or non-ENG cells, and then BM, spleen, and PB were analyzed 20 hours later for the presence of donor cells by flow cytometric analysis. Frequency and recovery of donor cells did not differ between non-ENG and ENG grafts in recipient BM (recovery = 4.9% ± 1.4% and 4.2% ± 1.4%, respectively; n = 10-11). However, frequency of non-ENG cells was consistently higher in spleen than that of ENG cells, as shown in the representative experiment in Figure 4A-B. This higher frequency translated to a significantly higher recovery of non-ENG cells in spleen than ENG cells (1.9% ± 0.5% versus 0.8% ± 0.2%, respectively, P < .05; n = 10-11). Recovery of graft cells in PB was similar for both phenotypes (0.1% ± 0.0% and 0.1% ± 0.0%; n = 10-11). Despite the relatively low number of graft cells transplanted, recovery of donor cells in these tissues was similar to that reported for grafts containing 10- to 100-fold more cells.5,10,17,18,24 The 2-fold greater recovery of non-ENG cells from the spleen translated into a significantly greater proportion of recoverable non-ENG cells in spleen compared with the proportion of recoverable ENG cells in spleen (Figure 4C-D). Likewise, the proportion of non-ENG cells in BM was significantly decreased compared with the proportion of ENG cells in BM (Figure 4C-D).
Increased trafficking of nonengrafting Sca-1+ lin- CD62L+ or Sca-1+ lin- CD49e- cells to spleen 20 hours after transplantation. In one representative experiment shown in panels A and B, irradiated mice (950 cGy) underwent transplantation with 8 × 104 CFSE-labeled Sca-1+ lin– CD49e– (non-ENG) or Sca-1+ lin– CD49e+ (ENG) cells, were killed 20 hours later, and CFSE-positive donor cells were detected flow cytometrically as described in “Materials and methods.” Dot plots illustrate increased frequency of non-ENG cells (A) in spleen compared with that of ENG cell transplants (B). (C-D) The distribution of total recovered graft cells in vivo when total recoveries in individual experiments were normalized to 100%. n = 10 in panel C and n = 11 in panel D. *P < .05 when compared with similar tissues in ENG cell transplants.
Increased trafficking of nonengrafting Sca-1+ lin- CD62L+ or Sca-1+ lin- CD49e- cells to spleen 20 hours after transplantation. In one representative experiment shown in panels A and B, irradiated mice (950 cGy) underwent transplantation with 8 × 104 CFSE-labeled Sca-1+ lin– CD49e– (non-ENG) or Sca-1+ lin– CD49e+ (ENG) cells, were killed 20 hours later, and CFSE-positive donor cells were detected flow cytometrically as described in “Materials and methods.” Dot plots illustrate increased frequency of non-ENG cells (A) in spleen compared with that of ENG cell transplants (B). (C-D) The distribution of total recovered graft cells in vivo when total recoveries in individual experiments were normalized to 100%. n = 10 in panel C and n = 11 in panel D. *P < .05 when compared with similar tissues in ENG cell transplants.
To investigate whether increased homing of non-ENG cells to spleen occurs earlier than 20 hours, recoveries of ENG and non-ENG cells were determined in mice killed 3 hours after transplantation. In experiments performed similarly to those at 20 hours, a trend toward higher recovery of non-ENG cells in spleen was observed 3 hours after transplantation, although these data did not reach statistical significance (spleen recovery of non-ENG and ENG = 1.3% ± 0.3% and 0.8% ± 0.3%, respectively; n = 3). Recovery of these 2 groups of cells in BM did not differ 3 hours after transplantation (BM recovery of non-ENG and ENG = 6.1% ± 1.0% and 4.0% ± 1.3%, respectively; n = 3).
Serial transplantation
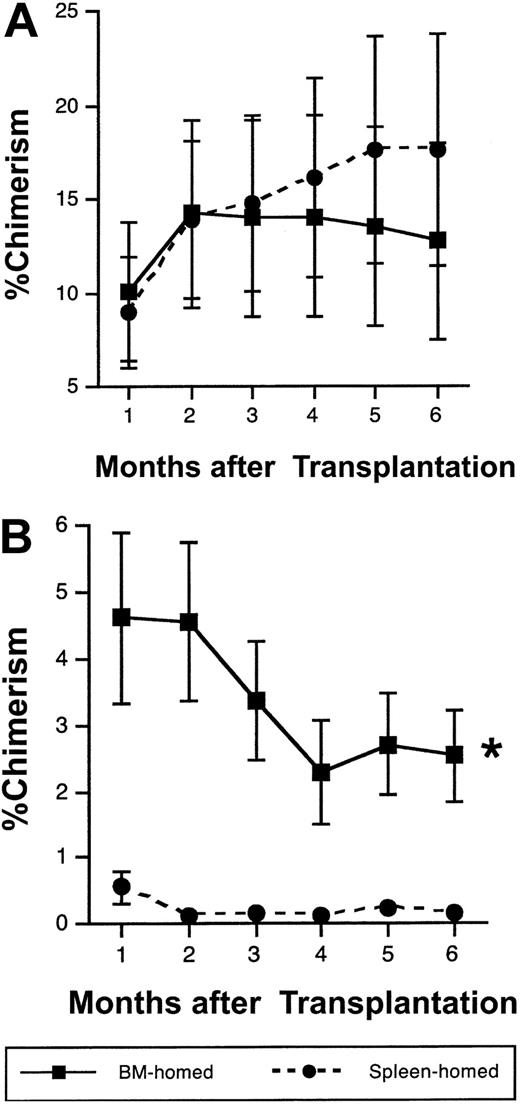
To further investigate whether long-term engrafting cells distribute selectively between BM and spleen early after transplantation, ENG cells homing to BM or spleen at 20 hours were isolated and examined for their long-term repopulating potential in mice undergoing serial transplantation. To this end, 50 to 200 BM- or spleen-homed donor cells were isolated from irradiated or nonirradiated 1°ST recipients 20 hours after transplantation and equal numbers transplanted competitively into 1°LT irradiated recipients. In 8 experiments using a total of 41 mice, no differences were noted in the degree of donor-derived chimerism in 1°LT mice up to 6 months after transplantation of BM-homed or spleen-homed cells, regardless of irradiation status of 1°ST mice (Figure 5A, pooled data from irradiated and nonirradiated 1°ST mice). Six months later, 1 × 106 to 5 × 106 LDBM cells from 1°LT mice in 3 experiments were transplanted into 2°LT irradiated mice without competition. Interestingly, chimerism was significantly higher in mice that received cells initially homed to BM rather than spleen in 1°ST recipients (Figure 5B, pooled data from 1 experiment with an irradiated 1°ST recipient and 2 experiments with nonirradiated 1°ST recipients). In one particular experiment where spleen-homed cells provided 88% donor-derived hematopoiesis in 1°LT recipient BM, 70% of 2°LT recipients undergoing transplantation with this marrow failed to survive beyond 1 month of transplantation, illustrating the inability of spleen-homed cells to support hematopoiesis in secondary recipients.
Cells capable of sustaining secondary long-term hematopoiesis home more efficiently to BM than to spleen by 20 hours after transplantation. Irradiated or nonirradiated 1°ST recipients underwent transplantation with 1 × 105 to 3 × 105 Sca-1+ lin– CD62L– or Sca-1+ lin– CD49e+ ENG cells, were killed 20 hours later, and BM-homed or spleen-homed donor cells were isolated flow cytometrically. Equal numbers of sorted cells (range, 50 to 200 cells per mouse) were transplanted along with 1 × 105 LDBM competitor cells of recipient origin into lethally irradiated 1°LT recipients. Mean ± SEM percent donor-derived chimerism in 1°LT recipients analyzed monthly is shown in panel A; n = 18-22 mice per symbol from 8 separate experiments. In 3 experiments, 1 × 106 to 5 × 106 LDBM cells from 1°LT recipients 6 months after transplantation were transplanted without competition into lethally irradiated 2°LT recipients (B). Data in panel B are pooled data from 1 irradiated and 2 nonirradiated experiments and represent mean ± SEM chimerism; n = 11-14 for BM-homed, and n = 3-7 for spleen-homed donor cells (7 mice in the spleen-homed group failed to survive beyond 1 month of transplantation and therefore are not included in the analysis). Because the relative difference in chimerism between BM-homed and spleen-homed donor cells in 1°LT and 2°LT mice did not differ depending on the conditioning of 1°ST recipients, data from both groups were pooled together. *P < .05 by repeated measures analysis of variance, compared with spleen-homed donor cells.
Cells capable of sustaining secondary long-term hematopoiesis home more efficiently to BM than to spleen by 20 hours after transplantation. Irradiated or nonirradiated 1°ST recipients underwent transplantation with 1 × 105 to 3 × 105 Sca-1+ lin– CD62L– or Sca-1+ lin– CD49e+ ENG cells, were killed 20 hours later, and BM-homed or spleen-homed donor cells were isolated flow cytometrically. Equal numbers of sorted cells (range, 50 to 200 cells per mouse) were transplanted along with 1 × 105 LDBM competitor cells of recipient origin into lethally irradiated 1°LT recipients. Mean ± SEM percent donor-derived chimerism in 1°LT recipients analyzed monthly is shown in panel A; n = 18-22 mice per symbol from 8 separate experiments. In 3 experiments, 1 × 106 to 5 × 106 LDBM cells from 1°LT recipients 6 months after transplantation were transplanted without competition into lethally irradiated 2°LT recipients (B). Data in panel B are pooled data from 1 irradiated and 2 nonirradiated experiments and represent mean ± SEM chimerism; n = 11-14 for BM-homed, and n = 3-7 for spleen-homed donor cells (7 mice in the spleen-homed group failed to survive beyond 1 month of transplantation and therefore are not included in the analysis). Because the relative difference in chimerism between BM-homed and spleen-homed donor cells in 1°LT and 2°LT mice did not differ depending on the conditioning of 1°ST recipients, data from both groups were pooled together. *P < .05 by repeated measures analysis of variance, compared with spleen-homed donor cells.
No differences in chimerism in 1°LT or 2°LT mice were noted when Sca-1+ lin– CD49e+ or Sca-1+ lin– CD62L– cells were used in the initial homing step in 1°ST mice. In addition, BM-homed and spleen-homed cells from 1°ST mice did not apparently differ in their relative contribution to either myeloid or lymphoid lineages (data not shown).
Discussion
In this report, evidence is presented that supports the notion of selective distribution or homing of transplanted HSCs to BM during the first 20 hours after transplantation. Phenotypic evidence documenting increased frequency and recovery of donor Sca-1+ lin– cells in BM relative to spleen, as well as increased frequency and recovery in BM of adhesion molecule phenotypes known to be enriched for HSCs, further support this notion. Significant enrichment of purified non-ENG grafts in spleen 20 hours after transplantation suggests trapping of nonengrafting cells in secondary hematopoietic tissues. Finally, selective homing of HSCs to BM is further supported by serial transplantation studies that revealed the exclusive presence of cells capable of secondary long-term multilineage hematopoiesis in BM by 20 hours after transplantation, while donor cells in spleen at this time failed to provide secondary donor-derived hematopoiesis and, in some cases, failed to provide radioprotective support in 2°LT recipients.
Previous reports have documented the presence of rapid reconstituting ability in spleen-homed donor cells collected 3 hours after transplantation5 but exclusive presence of repopulating cells among BM-homed cells by 48 hours.7 In the present studies, both BM- and spleen-homed donor cells isolated 20 hours after transplantation from both irradiated and nonirradiated 1°ST recipients were capable of supporting hematopoiesis in 1°LT recipients to a similar degree, but BM-homed cells provided a significantly higher degree of chimerism in 2°LT recipients than spleen-homed cells. Of interest was the apparent lack of radioprotective cells in the marrow of some 1°LT recipients of spleen-homed cells, despite high levels (up to 88%) of donor-derived chimerism. These results suggest that 20-hour spleen-homed cells, while capable of sustaining long-term hematopoiesis for one generation, do not contain the most primitive HPCs capable of providing hematopoiesis in secondary recipients and illustrate a hierarchic process that results in efficient homing of the most primitive HPCs to BM rather than to spleen during the first 20 hours following transplantation. These results corroborate those of Szilvassy et al,5 in which this group reported more rapid reconstitution by 3-hour spleen-homed cells than BM-homed, emphasizing the degree of heterogeneity in the stem cell pool and possible presence of more “mature” HSCs in spleen at this time point. The relatively higher recovery in spleen of total Sca-1+ cells (Figure 1) compared with the more primitive lin– fraction that was relatively enriched in BM, especially in nonirradiated mice (Figure 2), further supports the notion that more mature cells home to spleen. Alternatively, it is also possible that transplanted HSCs survive better in BM than spleen during the first 20 hours after transplantation as previously suggested,11 contributing to their engraftment potential.
To eliminate variables influencing stem cell phenotype after transplantation, and to test the possibility that certain classes of cells fail to engraft due to an inability to efficiently home to the BM, we examined the homing of populations of cells highly enriched for HSCs (ENG) and those lacking this potential (non-ENG). Notably, although ENG cells were highly enriched for HSCs,19 this phenotype was still heterogeneous in stem cell potential and in vivo trafficking ability, as revealed in selective homing of long-term repopulating cells to BM while cells of lesser potential segregated to spleen. Homing experiments performed in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice suggest little selectivity in BM-versus spleen-homing of transplanted primitive HPCs.11,12 Whether differences in results obtained in NOD/SCID mice and the present studies are secondary to xenogeneic homing models or differences in stringency of HSC assays requires further investigation.
Interestingly, trafficking of ENG and non-ENG cells to BM was similar, suggesting that defects in homing are not the cause of the reported engraftment failure of non-ENG cells but, rather, limited intrinsic hematopoietic potential. Whether differences in adhesion molecule expression between ENG and non-ENG cells translates to differences in the ability of these 2 groups of cells to communicate effectively with the BM microenvironment for determination of their fate remains an interesting possibility that may explain lack of engraftment potential of non-ENG cells. While several adhesion molecules have been implicated at various steps of homing and egress from the BM,8,23,25-29 a direct role for any adhesion molecule in homing of HSCs has not been documented. Because interactions between CD49e and fibronectin are known to stimulate proliferation and differentiation of primitive HPCs (reviewed by Chan and Watt30 ), it is likely that cells lacking CD49e expression are unable to effectively contribute to BM hematopoiesis. A role for CD62L in hematopoiesis is less well defined, but data are beginning to suggest that this molecule may be more important for progenitor cell function than stem cell function,19,26,31,32 providing a possible reason for the rapid up-regulation of CD62L on BM-homed Sca-1+ cells in the present studies. We and others have previously documented the presence of CD49e19,23 and absence of CD62L19 on murine long-term engrafting cells. We now show that lack of expression of CD49e and expression of CD62L predisposes Sca-1+ lin– cells to sequestration in the spleen, suggesting the direct involvement of these or other, yet unknown, adhesion molecules in directing the trafficking of hematopoietic cells. The percentage of non-ENG cells recovered in spleen was nearly twice that of ENG cells and similar to that previously reported for grafts of LDBM (1.5% ± 0.2%18 ), another source of cells composed largely of nonengrafting cells. Our present studies provide a mechanistic link between phenotypically defined engrafting cells identified previously by our group based on expression of adhesion molecules19 and the potential of these molecules to direct the homing of engrafting cells to the BM.
Increased frequency and recovery of CD43+, CD49e+, and CD49d+ Sca-1+ donor cells in BM compared with spleen was greatest 20 hours after transplantation. These results can potentially be explained by either trafficking of these phenotypes from spleen to BM between 1 and 20 hours, as previously suggested,5,9 or modulation of the expression of these markers on Sca-1+ cells that had already homed to BM or spleen in the first 20 hours (possibly to facilitate anchorage of these cells). Because recovery of total Sca-1+ cells remained fairly constant in spleen while the percentage of CD43+, CD49e+, and CD49d+ Sca-1+ cells declined, and because recovery of ENG cells in BM was similar between 3 and 20 hours (4.0% ± 1.3% and 4.2% ± 1.4%, respectively), it is plausible that phenotypic down-modulation of adhesion markers on spleen-homed cells is responsible for the observed changes. However, trafficking of donor cells between BM and spleen during this time frame cannot be ruled out. It is also possible that BM and spleen act cooperatively to ensure efficient homing of transplanted HSCs to BM; in this case spleen would be a necessary component of the homing process. Manipulation of the trafficking of transplanted HSCs by blocking pathways involved in spleen homing, or via splenectomy, may provide more precise answers to the role of spleen in homing of primitive HPCs to BM.
We have previously documented an enrichment of long-term repopulating cells in BM of nonirradiated recipients compared with irradiated ones.18 This observation may signify a more hospitable environment and more efficient trafficking of cells to nonablated BM compared with ablated BM, where endothelial damage may hinder efficient movement of cells to BM and/or increase nonspecific binding of cells to other sites. Our present studies add to these results by showing that even within an irradiated host, despite endothelial damage and relatively inefficient trafficking, a certain degree of selective trafficking to BM remains. It is possible that host influences elaborated by irradiated tissues promote proliferation and survival of transplanted cells in BM. Whether the dosing or timing of host conditioning prior to transplantation can be manipulated to better preserve a microenvironment that is more conducive for homing warrants further investigation. Nevertheless, these results begin to define a basis for the enhanced engraftment potential afforded some groups of Sca-1+ lin– cells fractionated on adhesion molecule expression and begin to outline distinct in vivo trafficking patterns of transplanted long-term engrafting cells within the first 20 hours of transplantation.
Prepublished online as Blood First Edition Paper, May 29, 2003; DOI 10.1182/blood-2002-12-3742.
Supported in part by National Institutes of Health grant R01 HL62200.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal