Abstract
Severe elevation of red blood cell number is often associated with hypertension and thromboembolism resulting in severe cardiovascular complications. However, some individuals such as high altitude dwellers cope well with an increased hematocrit level. We analyzed adaptive mechanisms to excessive erythrocytosis in our transgenic (tg) mice that, due to hypoxia-independent erythropoietin (Epo) overexpression, reached hematocrit values of 0.8 to 0.9 without alteration of blood pressure, heart rate, or cardiac output. Extramedullar erythropoiesis occurred in the tg spleen, leading to splenomegaly. Upon splenectomy, hematocrit values in tg mice decreased from 0.89 to 0.62. Tg mice showed doubled reticulocyte counts and an increased mean corpuscular volume. In tg mice, plasma volume was not elevated whereas blood volume was up to 25% of the body weight compared with 8% in wild-type (wt) siblings. Although plasma viscosity did not differ between tg and wt mice, tg whole-blood viscosity increased to a lower degree (4-fold) than expected from corresponding hemoconcentrated wt blood (8-fold). This moderate increase in viscosity is explicable by the up to 3-fold higher elongation of tg erythrocytes at physiologic shear rates. Apart from the nitric oxide–mediated vasodilation we reported earlier, adaptation to high hematocrit levels in tg mice involves regulated elevation of blood viscosity by increasing erythrocyte flexibility.
Introduction
High hematocrit levels are observed in patients suffering from erythrocytoses such as polycythemia vera and chronic mountain sickness, as well as in lowlanders at high altitude and erythropoietin (Epo)–abusing athletes. Severe elevation of the hematocrit level is often associated with hypertension and thromboembolism, leading to severe clinical complications and frequently to death.1 However, several reports demonstrate that some individuals can cope with excessive erythrocytosis. One case report describes a Chilean miner working at 5950 m above sea level who reached an hematocrit level of 0.75 without showing impaired health conditions or reduced physical activity.2 In keeping with this, Peruvian miners living and working at extreme altitude and exposed to cobalt (known to induce Epo expression) have been found to reach hematocrit levels of 0.75 to 0.91.3 One should keep in mind, however, that adaptive mechanisms of high altitude dwellers might be population dependent as recently shown by differences in nitric oxide (NO) metabolism of the lung in Tibetans and Bolivian Aymara.4 Excessive erythrocytosis is also found in sports medicine: an endurance athlete with an autosomal dominant erythrocytosis5 resulting in hematocrit levels up to 0.68 has won several Olympic gold medals in the past.6 These and other reports7 indicate that adaptive mechanisms to excessive erythrocytosis exist. Because the blood's flow resistance is regulated mainly by the radius of the vessel and the whole-blood viscosity (reviewed in Pearson and Path8 ) it is conceivable to expect that adaptation to highly increased hematocrit levels involves vasodilation and reduced viscosity. Due to the lack of a suitable model, however, there are few data available on adaptive mechanisms to excessive erythrocytosis in vivo. Thus, we generated a transgenic (tg) mouse line that, due to overexpression of human Epo cDNA, reaches hematocrit values up to 0.9 during the first 8 to 9 postnatal weeks.9,10
Physiologically, Epo is produced in the fetal liver and adult kidney and primarily stimulates proliferation, differentiation, and maturation of erythroid progenitor cells (erythropoiesis).11,12 In addition, Epo is also expressed in neuronal cells,13 can cross the blood-brain barrier,14 and displays a protective effect against cerebral stroke14-16 and light-induced retinal degeneration.17 Reduced oxygen supply increases Epo synthesis through stabilization of the hypoxia-inducible factor-1α (HIF-1α) (reviewed in Hofer et al,18 Semenza,19 and Wenger20 ). Remarkably, this finely controlled regulation of Epo gene expression still occurs after decades of weekly exposure of men to intermittent hypoxia.21 In contrast, in our tg mice an overexpression of human Epo induced excessive erythrocytosis independent of hypoxia and resulted in a 10- to 12-fold elevation of Epo plasma levels.9 Unexpectedly, the blood pressure was not elevated nor was the cardiac output altered. Endothelial nitric oxide synthase levels, NO-mediated endothelium dependent relaxation, and circulating NO levels were markedly increased.9 In turn, despite concomitant activation of the endothelin system observed in our tg mice,22 elevated NO-levels led to a pronounced vasodilation, thereby protecting the transgenic animals from cardiovascular complications. Apart from this, another tg mouse line lacking the receptor tyrosine kinase c-Kit that exhibits hematopoietic defects causing perinatal death survived after the line was bred with our Epo-overexpressing tg mouse.23 Although Epo was found to activate components of oxidative metabolism pathways that could be related to neuroprotective effects of Epo in the brain of our tg mouse,24 we determined markedly elevated cerebral infarction volumes in the tg mice upon experimental stroke.25 Thus, although acute application of Epo is beneficial in humans and mice suffering from stroke14,15 this observation suggests that chronic systemic treatment with Epo might reduce a favorable outcome due to the elevated hematocrit level.
Apart from NO-mediated vasodilation, we hypothesize that adaptive mechanisms to excessive erythrocytosis involves regulation of blood viscosity. Whole-blood viscosity depends not only on hematocrit but also mainly on red blood cell deformability and aggregation as well as on plasma viscosity. The concentration of other components such as platelets and leukocytes is of minor importance due to their far-lower number. We made use of our erythrocytotic tg mouse line to measure total blood viscosity, viscosity-relevant red cell parameters, as well as red cell flexibility in comparison with wt mice.
Materials and methods
Generation and propagation of transgenic mice
The tg mouse line was generated by pronuclear microinjection of the full-length human Epo cDNA driven by the human platelet–derived growth factor (PDGF) B-chain promotor as described.9 The resulting transgenic mouse line TgN(PDGFBEPO)321ZbZ (termed tg6) showed increased Epo levels in plasma and brain25 and was bred by mating hemizygous males to wild-type C57Bl/6 females. Half of the offspring was hemizygous for the transgene while the other half was wild type (wt) and served as a control. Male and female animals were used at the age indicated. All experiments were performed in accordance with the German and Swiss animal protection laws and institutional guidelines.
Determination of cardiovascular parameters
Anesthesia in all surgical procedures was induced with a gas mixture containing 4% halothane, 70% N2O, remainder O2, and was maintained by reducing the inspired halothane concentration to 1% to 1.5%. Body temperatures of all mice were maintained at 37°C using a temperaturecontrolled heating pad. Catheters were inserted into the right femoral artery and vein to enable blood analysis, the injection of Evans blue, 5% glucose solution, and the measurement of arterial blood pressure. A thin catheter (outer diameter: 0.61 mm) was inserted via the left femoral vein into the right atrium to measure the central venous pressure. The diameter of the vena cava caudalis in the mice was 2 mm in wt and 3 mm in tg mice. Thus, the vena cava caudalis was obstructed in the present experiments in wt mice to an extent of 15% and in tg mice to 9%. Since the obstruction of less than 40% of the cross-sectional area of a vessel does not reduce the volume flow nor cause a pressure drop,26,27 it is unlikely that the venous return in the present experiments was negatively affected by the catheter. The correct catheter position was verified by the typical breath-synchronous pattern of pressure oscillations and after each experiment by necropsy.
A Teflon-coated platinum wire (diameter: 0.2 mm) was inserted via the left femoral artery into the aorta to measure cardiac output using the conductivity dilution method.28 A silver-plated copper wire (diameter: 2 mm) inside the rectum served as a reference electrode. Cardiac output was determined in tg mice and in age-matched wt siblings during anesthesia as well as in conscious animals after recovery from anesthesia for one hour. Because there was no statistical difference between the measurements obtained during anesthesia and those obtained during consciousness, for practical reasons, all following experiments were performed during anesthesia. Of note, the animals placed in the mouse restrainer showed no signs of discomfort. Cardiac output was calculated from conductivity dilution curves after a bolus injection of 20 μL 5% glucose solution as described previously.28 The integrals under the conductivity dilution curves were determined with an image analyzing system (MCID, Imaging Research, St Catherines, ON, Canada). All experiments were terminated by euthanizing the animals with an overdose of barbiturate (Inaktin).
Blood analysis
The hematocrit level was determined after centrifugation of heparinized blood at 15 000g for 3 minutes. Platelet counts, hemoglobin concentration, and erythrocyte counts were determined with the automatic blood analyzer Technicon H3 (Bayer, Leverkusen, Germany) and the latter values were used to calculate the mean corpuscular volume (MCV) and the mean corpuscular hemoglobin concentration (MCHC). Reticulocytes were counted in blood smears stained with Brilliant-Kresyl blue. At least 1000 erythrocytes were analyzed per animal.
Plasma volume was calculated from the steady-state plasma concentration of Evans blue after intravenous injection of 10 μL saline containing 1% dye. To this end, 10 μLto 15 μL blood samples were taken 2, 8, 12, 14, 16, 18, and 20 minutes after the injection. The concentrations of Evans blue, measured photometrically in plasma, were correlated with the sampling time and the resulting regression line was used to calculate the steady-state plasma concentration of the dye at the moment of its injection. The blood volume was calculated using plasma volume and hematocrit value.
Whole-blood and plasma viscosity were measured with a rotation viscosimeter (DVIII+ Rheometer, Brookfield Engineering Laboratories, Middlebrow, MA) in heparinized blood at 37°C and shear rates between 2 s–1 and 450 s–1 for whole-blood and between 45 s–1 and 450 s–1 for plasma. Plasma viscosity at lower shear rates could not be measured due to the limitations of the viscosimeter. Wt blood with hematocrit values similar to those found in tg mice was obtained by centrifugation at 2000g for 20 minutes. The supernating plasma was drawn off and the erythrocytes resuspended in the remaining plasma by gentle agitation. The hematocrit level was then adjusted to 0.89 by dilution with the previously withdrawn plasma. In addition, we performed an erythrocyte-plasma tg/wt exchange experiment. After centrifugation at 2000g for 20 minutes, wt erythrocytes were resuspended in tg plasma and tg erythrocytes in wt plasma. This centrifugation/resuspension procedure was repeated twice for each sample of reconstituted blood to wash off the remaining original plasma. Then the hematocrit level was adjusted to 0.44 with plasma. Each blood and plasma sample was measured in triplicate.
To measure erythrocyte elongation, 10 μL transgenic blood and 20 μL wild-type blood were mixed with 2 mL of a solution of a viscosity of 9 mPa · s which was composed of 13.4% Dextran (molecular weight 60 000 kDa; Serva, Heidelberg, Germany) in phosphate-buffered saline. The resulting suspension was transferred to a Laser diffractometer (Rheodyn-SSD; Myrenne, Röttgen, Germany) and the elongation of the red cells was measured at shear stresses between 0.11 and 22.5 Pa.
Serum and plasma were analyzed using 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and subsequent silver staining.29 Band analysis was performed using an image analyzing system (MCID; Imaging Research).
Splenectomy
Necropsy of tg mice revealed severe splenomegaly, suggesting extramedullar erythropoiesis in the spleen. Wet weight of spleens was determined in 7 wt and tg mice. Nine 2- to 3-month-old tg mice were anesthetized with halothane and splenectomized under sterile conditions via a median laparatomy to reduce erythropoiesis. After splenectomy and recovery from the anesthesia the animals were housed under standard conditions. No additional care was necessary since no mice suffered from wound infections or showed any signs of pain. All splenectomized tg mice behaved normally and gained weight like the age-matched control tg mice. Cardiovascular and blood analyses were performed 6 to 7 weeks after splenectomy.
Statistical analysis
The physiologic and hematologic parameters shown in Table 1 were compared among the experimental groups using a 2-tailed Student t test for unpaired samples with Bonferroni correction. The level of statistical significance was set at P < .05.
Cardiovascular parameters in 4- to 7-month-old wild-type (wt), transgenic (tg), and splenectomized tg mice
. | wt . | tg . | Splenectomized tg . |
|---|---|---|---|
| n | 11 | 13 | 8 |
| Mean arterial blood pressure (mmHg) | 99 ± 12 | 100 ± 7 | 110 ± 11 |
| Heart rate (min-1) | 548 ± 44 | 573 ± 31 | 559 ± 39 |
| Cardiac output (mL/min)* | 17.2 ± 3.2 | 14.5 ± 6.9 | 16.5 ± 5.2 |
| Central venous pressure (mmHg) | 3.9 ± 0.8 | 6.6 ± 0.9† | 5.2 ± 1.3‡ |
. | wt . | tg . | Splenectomized tg . |
|---|---|---|---|
| n | 11 | 13 | 8 |
| Mean arterial blood pressure (mmHg) | 99 ± 12 | 100 ± 7 | 110 ± 11 |
| Heart rate (min-1) | 548 ± 44 | 573 ± 31 | 559 ± 39 |
| Cardiac output (mL/min)* | 17.2 ± 3.2 | 14.5 ± 6.9 | 16.5 ± 5.2 |
| Central venous pressure (mmHg) | 3.9 ± 0.8 | 6.6 ± 0.9† | 5.2 ± 1.3‡ |
Values are given as means plus or minus standard deviations.
Cardiac output was measured invasively in conscious as well as in halothane-anesthetized mice.
P < .01 versus wt.
P < .05 versus wt; P < .01 versus tg6.
Results
Increased hematocrit does not alter cardiovascular parameters
Compared with the wt control siblings with an hematocrit value of about 0.42, 4- to 7-month-old Epo-overexpressing tg mice with excessive erythrocytosis developed an average hematocrit value of up to 0.89. Whereas we observed no differences in mean arterial blood pressure and heart rate compared with the age-matched control wt siblings, central venous pressure was increased by about 70% in tg mice, suggesting an elevated preload in the tg heart (Table 1). Moreover, cardiac output as determined invasively in both anesthetized and conscious tg mice did not differ from that of the wt controls. The latter results agree with our previous report in which cardiac output was determined noninvasively by echocardiography in spontaneously breathing anesthetized mice.10
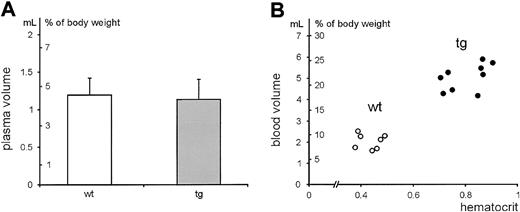
Blood volume but not plasma volume is increased in erythrocytotic tg mice
Blood and plasma volumes were determined in 4- to 7-month-old wt and tg mice. As shown in Figure 1, the plasma volume did not differ in both groups. In contrast, tg mice reached blood volumes as high as one-quarter of the body weight, indicating that the blood volume was directly dependent on the dramatically increased amount of red blood cells. Of note, increased blood volume correlated directly and positively with the central venous pressure.
Relationship between hematocrit value and plasma volume or blood volume. Plasma and blood volumes were determined in 4- to 7-month-old male and female wt (n = 7) and tg (n = 9) mice. The resulting volumes are depicted in milliliters and in percentage of the body weight.
Relationship between hematocrit value and plasma volume or blood volume. Plasma and blood volumes were determined in 4- to 7-month-old male and female wt (n = 7) and tg (n = 9) mice. The resulting volumes are depicted in milliliters and in percentage of the body weight.
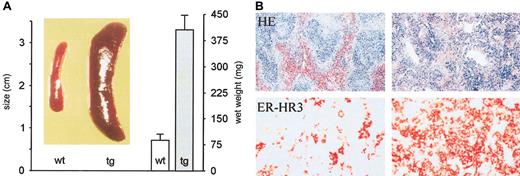
Enhanced extramedullar erythropoiesis in the tg spleen
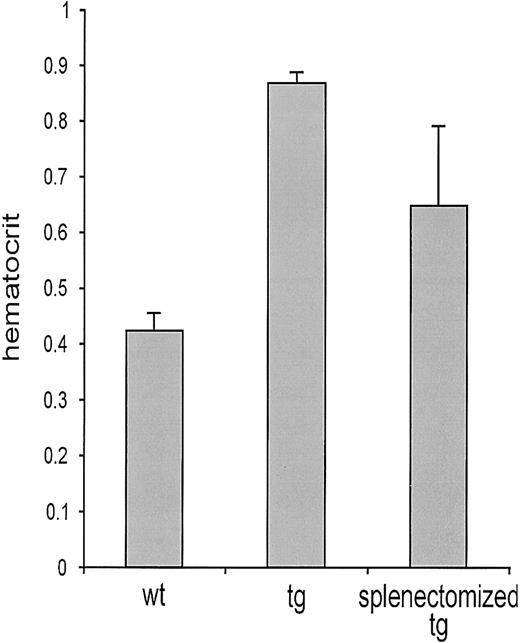
Visual inspection of tg mice at necropsy revealed a dramatically enlarged spleen, suggesting increased extramedullar eythropoiesis (Figure 2). The wet weight of tg spleen was about 5 times higher than that of the wt control (Figure 2). Histologic examination confirmed extramedullar erythropoiesis in the spleen. Whereas a clear separation of white pulp (lymphatic tissue) and red pulp (physiologically degrading senescent erythrocytes) was observed in wt spleens, the tg spleen revealed an increased red pulp area with dissected white pulp structures (Figure 2). To visualize erythropoietic activity, we made use of the erythropoiesis-associated monoclonal antibody ER-HR3.30 The number of these stroma cells was dramatically increased in the tg organ whereas only a few erythropoiesis-associated stroma cells were found in the ER-HR3–stained wt spleen (Figure 2). We splenectomized tg mice to unequivocally prove that extramedullar erythropoiesis occurs in the spleen. Considering that normal mouse erythrocytes persist in the circulation for 45 days,31 we determined the hematocrit value 6 to 7 weeks after surgery. Figure 3 shows the age-matched hematocrit values of wt mice (114 ± 8 days), untreated tg mice (119 ± 8 days), and splenectomized tg animals that were 121 ± 10 days old at the day of the analysis. Splenectomy reduced tg hematocrit levels from 0.89 ± 0.06 to 0.62 ± 0.1. Relief from excessive erythrocytosis in turn reduced the cardiac preload as suggested by the significantly decreased central venous pressure (Table 1, last column). Blood pressure, heart rate, and cardiac output, however, remained unaltered.
Splenomegaly and extramedullary erythropoiesis in tg mice. (A) The spleens from a wt and a tg mouse are shown in the photograph. The wet weights of the spleens are depicted in the histogram. Wt versus tg: 87 ± 18.5 mg vs 407 ± 43 mg (n = 7 in each group; P < .001). (B) Exemplary sections obtained from wt (left images) and tg spleens (right images) were stained either with hematoxylin-eosin (HE; original magnification, × 63) or with the erythropoiesis-associated antibody ER-HR3 (red; original magnification, × 400). The proportion of the red pulp as well as the density of ER-HR3–positive cells is largely increased in tg mice, indicating, together with the 5-fold higher wet weight of the tg spleen, the massive extramedullary erythropoesis in tg mice.
Splenomegaly and extramedullary erythropoiesis in tg mice. (A) The spleens from a wt and a tg mouse are shown in the photograph. The wet weights of the spleens are depicted in the histogram. Wt versus tg: 87 ± 18.5 mg vs 407 ± 43 mg (n = 7 in each group; P < .001). (B) Exemplary sections obtained from wt (left images) and tg spleens (right images) were stained either with hematoxylin-eosin (HE; original magnification, × 63) or with the erythropoiesis-associated antibody ER-HR3 (red; original magnification, × 400). The proportion of the red pulp as well as the density of ER-HR3–positive cells is largely increased in tg mice, indicating, together with the 5-fold higher wet weight of the tg spleen, the massive extramedullary erythropoesis in tg mice.
Reduced hematocrit level upon splenectomy. Hematocrit measurements were performed in wt mice, untreated tg mice, and splenectomized tg animals that were 114 ± 8 days old, 119 ± 8 days old, and 121 ± 10 days old, respectively, when the hematocrit level was determined. Splenectomy was performed 6 to 7 weeks earlier. The hematocrit level was increased 2-fold in tg mice (0.86 ± 0.06) compared with wt mice (0.42 ± 0.04; P < .01). Six to 7 weeks after splenectomy, the hematocrit level in tg mice was 0.62, which is 31% lower (P < .01) but still significantly 50% higher than that in wt mice (P < .01).
Reduced hematocrit level upon splenectomy. Hematocrit measurements were performed in wt mice, untreated tg mice, and splenectomized tg animals that were 114 ± 8 days old, 119 ± 8 days old, and 121 ± 10 days old, respectively, when the hematocrit level was determined. Splenectomy was performed 6 to 7 weeks earlier. The hematocrit level was increased 2-fold in tg mice (0.86 ± 0.06) compared with wt mice (0.42 ± 0.04; P < .01). Six to 7 weeks after splenectomy, the hematocrit level in tg mice was 0.62, which is 31% lower (P < .01) but still significantly 50% higher than that in wt mice (P < .01).
Increase of blood viscosity is regulated in tg mice
We recently showed that NO-mediated vasodilation is one adaptive mechanism to excessive erythrocytosis in our tg mice.9 We hypothesized that regulation of blood viscosity could represent another important, yet uninvestigated, adaptive mechanism.
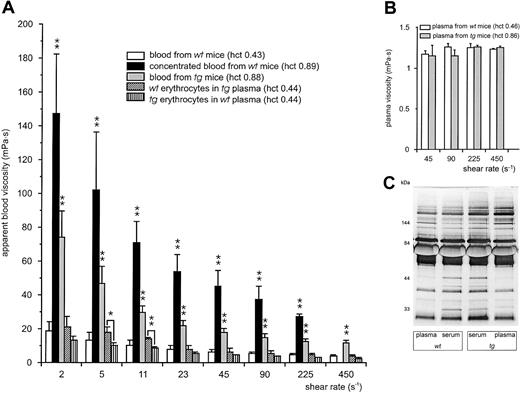
To test this concept, we determined the viscosity of normal wt and tg blood as well as hemoconcentrated wt blood at varying shear rates (Figure 4A). Viscosity increased about 8 times compared with untreated wt blood when wt blood was concentrated to the same hematocrit value as in the tg mice. In contrast, the viscosity of tg blood was only 4 times higher than that of wt blood. In other words, at all shear rates measured, hemoconcentrated blood from wt mice had double the viscosity of tg blood at the same hematocrit value. Reconstituted blood samples consisting of wt erythrocytes and tg plasma or of tg erythrocytes and wt plasma both at an hematocrit value of 0.44 were as viscous as normal wt blood with an hematocrit value of 0.43, thereby indicating that there is no major effect of the tg plasma on the whole-blood viscosity (Figure 4A). However, the observation that the combination of tg erythrocytes with wt plasma was slightly less viscous than wt erythrocytes in tg plasma (significant at shear rates 5 s–1 and 11 s–1) implied that the tg erythrocytes are responsible for the reduced viscosity. This interpretation is supported by the fact that plasma viscosity did not differ between tg and wt mice (Figure 4B). In agreement with this, analysis of serum and plasma by SDS-PAGE revealed no differences in the protein patterns (Figure 4C).
Blood and plasma viscosity of wt and tg mice. (A) Blood viscosity from wt (109 ± 6 days old) or tg (111 ± 9 days old) mice was measured at predefined shear rates ranging from 2 s–1 to 450 s–1. Transgenic blood (▦; hematocrit value, 0.88) was about 4 times as viscous as normal wt blood (□; hematocrit value, 0.43) but only half as viscous as hemoconcentrated wt blood reaching an hematocrit value of 0.89 (▪). Due to the extreme viscosity of the concentrated wt blood its viscosity at the highest shear rate could not be determined with our viscosimeter (missing black column at 450 s–1). The viscosity of reconstituted blood consisting of wt erythrocytes in tg plasma (▧) or tg erythrocytes in wt plasma ( ), both adjusted to an hematocrit value of 0.44, did not differ from that of normal wt blood. Although in tendency the viscosity of tg erythrocytes in wt plasma was slightly lower than wt erythrocytes in tg plasma, this observation was significant only at 5 s–1 and 11 s–1. *P < .05, **P < .01 compared with wt blood or as indicated. (B) Plasma viscosity (wt, □; tg, ▦) was measured at predefined shear rates ranging from 45 s–1 to 450 s–1. (C) SDS-PAGE of plasma and serum of wt and tg mice. Neither plasma viscosity nor plasma or serum protein pattern and concentrations differed significantly between the mouse groups.
), both adjusted to an hematocrit value of 0.44, did not differ from that of normal wt blood. Although in tendency the viscosity of tg erythrocytes in wt plasma was slightly lower than wt erythrocytes in tg plasma, this observation was significant only at 5 s–1 and 11 s–1. *P < .05, **P < .01 compared with wt blood or as indicated. (B) Plasma viscosity (wt, □; tg, ▦) was measured at predefined shear rates ranging from 45 s–1 to 450 s–1. (C) SDS-PAGE of plasma and serum of wt and tg mice. Neither plasma viscosity nor plasma or serum protein pattern and concentrations differed significantly between the mouse groups.
Blood and plasma viscosity of wt and tg mice. (A) Blood viscosity from wt (109 ± 6 days old) or tg (111 ± 9 days old) mice was measured at predefined shear rates ranging from 2 s–1 to 450 s–1. Transgenic blood (▦; hematocrit value, 0.88) was about 4 times as viscous as normal wt blood (□; hematocrit value, 0.43) but only half as viscous as hemoconcentrated wt blood reaching an hematocrit value of 0.89 (▪). Due to the extreme viscosity of the concentrated wt blood its viscosity at the highest shear rate could not be determined with our viscosimeter (missing black column at 450 s–1). The viscosity of reconstituted blood consisting of wt erythrocytes in tg plasma (▧) or tg erythrocytes in wt plasma ( ), both adjusted to an hematocrit value of 0.44, did not differ from that of normal wt blood. Although in tendency the viscosity of tg erythrocytes in wt plasma was slightly lower than wt erythrocytes in tg plasma, this observation was significant only at 5 s–1 and 11 s–1. *P < .05, **P < .01 compared with wt blood or as indicated. (B) Plasma viscosity (wt, □; tg, ▦) was measured at predefined shear rates ranging from 45 s–1 to 450 s–1. (C) SDS-PAGE of plasma and serum of wt and tg mice. Neither plasma viscosity nor plasma or serum protein pattern and concentrations differed significantly between the mouse groups.
), both adjusted to an hematocrit value of 0.44, did not differ from that of normal wt blood. Although in tendency the viscosity of tg erythrocytes in wt plasma was slightly lower than wt erythrocytes in tg plasma, this observation was significant only at 5 s–1 and 11 s–1. *P < .05, **P < .01 compared with wt blood or as indicated. (B) Plasma viscosity (wt, □; tg, ▦) was measured at predefined shear rates ranging from 45 s–1 to 450 s–1. (C) SDS-PAGE of plasma and serum of wt and tg mice. Neither plasma viscosity nor plasma or serum protein pattern and concentrations differed significantly between the mouse groups.
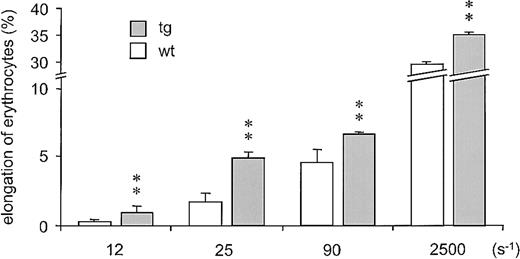
Considering that the internal fluid viscosity of erythrocytes increases with the MCHC,32 we measured this parameter in tg mice. Although the total hemoglobin concentration was increased in the tg blood (wt: 14 ± 1 g/dL vs tg6: 26 ± 1 g/dL; P < .01), the MCHC did not differ significantly between both mouse lines (wt: 33 ± 2 g/dL vs tg6: 29.9 ± 3 g/dL). Furthermore, the doubled percentage of reticulocytes (wt: 1.7 ± 0.6% vs tg6: 3.4 ± 1.6%; P < .01) is indicative for an elevated number of young and thus well-deformable erythrocytes.33 This notion is supported by the increased MCV we observed in tg erythrocytes (wt: 44.8 ± 1.8 fL vs tg6: 57.4 ± 5.1 fL; P < .05); elevated MCV is a typical side effect of massively stimulated erythropoiesis34 also known to enhance the flexibility of erythrocytes.35 To directly and unequivocally prove that tg erythrocytes are more deformable compared with wt erythrocytes, thereby reducing whole-blood viscosity, we measured their elongation by ektacytometry. As shown in Figure 5, tg erythrocytes showed a 1.5- to 3-fold higher elongation at shear rates within the physiologic range and an about 20% higher elongation at the maximal shear forces that can be applied without damaging the cells.
Ektacytometry of tg and wt erythrocytes. Tg erythrocytes (▪) were up to 3 times more elongable than wt erythrocytes (□) at shear rates that correspond to those found in the vasculature in vivo (about 11.5 s–1 to 115 s–1).44 Moreover, enhanced elongation of tg erythrocytes was also observed at the maximal experimental shear rate (2500 s–1) that can be applied without damaging the red cells. ** = P < .01 versus wt.
Ektacytometry of tg and wt erythrocytes. Tg erythrocytes (▪) were up to 3 times more elongable than wt erythrocytes (□) at shear rates that correspond to those found in the vasculature in vivo (about 11.5 s–1 to 115 s–1).44 Moreover, enhanced elongation of tg erythrocytes was also observed at the maximal experimental shear rate (2500 s–1) that can be applied without damaging the red cells. ** = P < .01 versus wt.
Discussion
Our results demonstrate that total blood volume of tg mice overexpressing Epo is dramatically elevated and may attain one-quarter of the body weight as a consequence of increased erythrocyte counts without alteration of the plasma volume. The elevation of blood volume might explain the increased central venous pressure in tg mice that culminates in congestive heart failure and increased systolic diameters described earlier.10 Interestingly, erythrocytotic mice did not develop hypertension nor was there a change in heart rate or cardiac output. As shown previously, one adaptive mechanism involves increased activity of endothelial nitric oxide synthase (eNOS or NOS III). Enhanced NO synthesis results in generalized peripheral vasodilation9 despite a concomitant increase of the potent vasoconstrictor endothelin-1.22 Another unexpected adaptive mechanism to this excessive erythrocytosis is the moderate increase of blood viscosity that is most likely due to an increased flexibility of the tg erythrocytes.
We demonstrated that enhanced erythropoiesis occurs in the tg spleen that attains up to a 5-fold increase in weight. Massive splenic erythropoiesis in tg mice was visualized by using the erythropoiesis-associated antibody ER-HR3.30 As expected, only little ER-HR3 staining was found in wt spleens, indicating that there is virtually no erythropoiesis in the spleen of wt mice and suggesting that splenectomy of wt mice will not alter the hematocrit level. Note that there are no reports of reduced erythropoiesis in human patients who have had splenectomies. In contrast, extramedullary erythropoiesis and splenomegaly are classic complications in human patients suffering from polycythemia vera, a human disease that, in contrast to our tg mouse model, is accompanied by normal or even reduced Epo plasma levels.36 Splenectomy in some polycythemia vera patients has gone from being the least favorable alternative to the best therapeutic intervention since the postoperative complications have been minimized and no negative impact on survival has been observed.37,38 Similar to these observations in patients, we confirmed the functional relevance of the extramedullary erythropoiesis in our Epo-overexpressing tg mice by splenectomy that resulted in an about 30% reduction of the hematocrit level. At present, however, we cannot exclude the possibility that the hematocrit level of splenectomized tg mice will increase again over time, since the bone marrow may have the potential for compensation.
The regulation of blood viscosity represents an important adaptive mechanism to the excessive erythrocytosis observed in our tg mice. Whereas the hemoconcentrated whole blood from wt control mice increased its viscosity 8-fold, the increase in viscosity of tg blood was only 4-fold. What are the corresponding adaptive mechanisms? The hematocrit value is a main factor of viscosity that influences the resistance to flow as well as the circulatory transport. As a non-Newtonian fluid, blood decreases its viscosity with increasing shear stress. This phenomenon is due primarily to the remarkably high deformability of red blood cells.39 Indeed, chemically hardened erythrocytes led to increased flow resistance.40,41 Juvenile erythrocytes have been reported to be larger and more deformable than older ones.39 The increased MCV and the doubled number of reticulocytes suggests that the raised blood viscosity in our erythrocytotic mice was modulated by an increased proportion of juvenile flexible erythrocytes. We proved our hypothesis directly by measurement of the erythrocyte elongation using ektacytometry, a method allowing simple and rapid measurements of red cell deformability in small aliquots of blood.42,43 Using this technique, we demonstrated that tg erythrocytes were 1.5- to 3-fold more elongable compared with wt erythrocytes predominantly at physiologic shear rates found in the vasculature in vivo.44
Interestingly, gene-therapeutically induced but hypoxia-independent Epo expression in adult mice led to similar hematocrit values as those in our tg mice, but ended lethal.45 This fatal effect that is also sometimes observed in patients suffering from polycythemia might be due to the lack of time to establish the additional adaptive mechanisms, such as increased NO levels, as observed in our tg mice that cope with excessive erythrocytosis for a lifetime.9 Since NO has been shown to reduce erythrocyte aggregation,46 it is tempting to speculate that this mechanism occurs in our erythrocytotic tg mice as well. Furthermore, circulating NO has been reported to increase deformability of erythrocytes.46-48 The findings of Binley et al45 and the observations in patients with polycythemia vera,49 together with the results of our present and previous9 studies suggest that the adaptation to extreme hematocrit values requires both increased erythrocyte flexibility and increased plasma NO levels.
Erythrocyte deformability is not only necessary to enable the passage of red blood cells through the microcirculation but also enhances oxygen release from red blood cells.50 Some time ago it could be demonstrated that for the same level of increased hematocrit the oxygen delivery to different tissues varies markedly.44 To test oxygen delivery in our tg mice, we performed preliminary Western blot analysis of HIF-1α. The oxygen-dependent subunit of HIF-1 represents the master regulator of oxygen homeostasis (reviewed in Semenza,19 Wenger,20 and Hofer et al51 ). Normoxic and hypoxic tg mice showed reduced levels of HIF-1α in kidney and liver, suggesting enhanced oxygen delivery to these tissues (Ursula Jewell and M. G., unpublished observations, June 2002). These findings indicate that oxygen delivery to the tissue in our tg mice is not impaired despite excessive erythrocytosis and increased blood viscosity, different from previously reported hemodilution/hemoconcentration experiments.44
Another important determinant of whole-blood viscosity is the plasma viscosity that is a function of the concentration of plasma proteins, especially fibrinogen, and some globulin fractions.52 Indeed, in some polycythemia vera cases, hyperviscous plasma and increased plasma fibrinogen levels have been described.53,54 The plasma viscosity values in our tg mice did not differ from those obtained in the wt control plasma. This finding does not exclude that the serum or plasma protein composition might be altered. However, analysis of serum and plasma by electrophoresis revealed no differences in protein pattern and concentration between both lines. Thus, plasma viscosity or a change of the serum/plasma protein pattern most likely is not involved in adaptive mechanisms in our tg mice. This interpretation is in line with the viscosity measurements of reconstituted blood consisting of wt erythrocytes in tg plasma and tg erythrocytes in wt plasma at an hematocrit value of 0.44 that revealed no differences compared with control wt blood. At most, these measurements show again that the tg erythrocytes are more flexible since tg erythrocytes in wt plasma were less viscous than wt erythrocytes in tg plasma.
In contrast to two-thirds of polycythemia vera patients who showed elevated platelet counts,55 our mice were thombocytopenic, with a 60% reduction of blood platelets per unit of whole blood. As described recently,8,56 however, one must remember that platelet counts are presented as a number per unit of volume of whole blood despite the fact that platelets are distributed only in the plasma. Taking this into account, the concentration of platelets per unit of plasma did not differ between tg and wt animals.56 Thus, tg blood contains fewer platelets per erythrocyte, which implies that contact between red blood cells and platelets is reduced.
Several previous studies suggest that Epo raises blood pressure directly, independent of the increase of the hematocrit level.57-59 In contrast to these observations, our mice did not show increased blood pressure despite extremely increased Epo levels (and extreme hematocrit values).9,10 This might be at least partly due to the vasodilatory effect of the increased plasma NO levels in our tg mice.9 However, putative additional adaptive mechanisms in these mice await elucidation.
In summary, by means of our tg mouse model, we provide convincing evidence that several adaptive mechanisms exist to control blood viscosity in chronic Epo-induced excessive erythrocytosis. These include, next to the previously shown increased plasma NO levels,9 an increased red cell flexibility resulting in a moderately increased whole-blood viscosity. It would be of utmost interest to determine whether humans with a high hematocrit level (eg, high altitude dwellers and polycythemia vera and other erythrocytotic patients) show similar long-term adaptation of blood viscosity.
Prepublished online as Blood First Edition Paper, May 15, 2003; DOI 10.1182/blood-2003-01-0283.
Supported by grants from the Roche Research Foundation, the EMDO Stiftung, the Stiftung für wissenschaftliche Forschung an der Universität Zürich, the Swiss National Science Foundation (3100A0-100214; M.G.), by the Forschungsförderungsprogramm of the University of Heidelberg (J.V.), and by the Deutsche Forschungsgemeinschaft (HE 3471/1-1; K.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank S. Keller, M. Harlacher, F. Stadler, B. de los Angeles, and R. Degenhardt for support and C. Bauer, F. Ruschitzka, J. Fandrey, K. Wagner, and T. Fischer for fruitful discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal