Abstract
Poor immune reconstitution after haploidentical stem cell transplantation results in a high mortality from viral infections and relapse. One approach to overcome this problem is to selectively deplete the graft of alloreactive cells using an immunotoxin directed against the activation marker CD25. However, the degree of depletion of alloreactive cells is variable following stimulation with recipient peripheral blood mononuclear cells (PBMCs), and this can result in graft versus host disease (GVHD). We have refined this approach using recipient Epstein-Barr virus (EBV)–transformed lymphoblastoid cell lines (LCLs) as stimulators to activate donor alloreactive T cells. Our studies demonstrate that allodepletion with an anti-CD25 immunotoxin following stimulation with HLA-mismatched host LCLs more consistently depleted in vitro alloreactivity than stimulation with host PBMCs, as assessed in primary mixed lymphocyte reactions (MLRs). Allodepletion using this approach specifically abrogates cytotoxic T-cell responses against host LCLs. In interferon-γ (IFN-γ) enzyme-linked immunospot (ELISPOT) assays, antiviral responses to adenovirus and cytomegalovirus (CMV) were preserved following allodepletion. Likewise, using HLA-A2–pp65 tetramers, we have shown that the frequency of CMV-specific T cells is unaffected by allodepletion. Moreover, the donor anti-EBV response is partially retained by recognition of EBV antigens through the nonshared haplotype. Finally, we studied whether allodepletion affects the response to candidate tumor antigens in myeloid malignancies. Using HLA-A2–PR1 tetramer analysis, we found that the frequency of T cells recognizing the PR1 epitope of proteinase 3 was not significantly different in allodepleted and unmanipulated PBMCs from patients with chronic myeloid leukemia (CML) undergoing transplantation. Based on these data, we have embarked on a phase 1 clinical trial of addback of allo-LCL–depleted donor T cells in the haplo-identical setting.
Introduction
The lack of fully HLA-matched donors is a major limitation to the applicability of hematopoietic stem cell transplantation (HSCT). However, almost all patients have potential donors who are HLA haplo-identical. Recent advances in our ability to mobilize and select HSCs have made HSCT from haplo-identical donors feasible, both in terms of reliable engraftment and acceptable rates of graft versus host disease (GVHD).1,2
Because of the high frequency of alloreactive T cells in donor peripheral blood, rigorous T-cell depletion is necessary to prevent GVHD in the haplo-identical setting. Together with in vivo immunosuppression and the HLA disparity between host and donor, this results in profound posttransplantation immunodeficiency.3,4 This is the major barrier to the broader application of haplo-identical HSCT and results in high morbidity/mortality from viral infections due to the loss of antiviral immunity and high relapse rates due to the loss of the graft versus leukemia (GVL) response.1,2 Simple T-cell addback is unlikely to be effective in preventing these problems because the frequency of alloreactive T cells is much higher than that of either virus-specific T cells or T cells directed against hemopoietic-specific targets on leukemic cells in the early post-SCT period. Two major approaches have evolved to circumvent this. Induction of anergy in donor alloreactive T cells can be achieved by coculture with host peripheral blood mononuclear cells (PBMCs) in the presence of costimulatory blockade.5,6 However, anergized cells may have an inhibitory effect on bystander T cells, and anergy may be overcome in the context of high levels of interleukin-2 (IL-2). An alternative approach is to deplete the graft specifically of alloreactive T cells responsible for GVHD by deleting T cells that are activated in response to recipient antigen-presenting cells. This approach has the advantages that alloreactive T cells are permanently removed and cannot influence the function of the remaining T cells. Activated T cells express a number of markers including CD25 (IL-2 receptor α), CD69, CD71, CD147, and HLA-DR. Van Dijk et al have demonstrated that depletion of allo-antigen–activated T cells using a cocktail of anti-CD25, -CD69, -CD71, and –HLA-DR microbeads with the magnetic-activated cell separation (MACS) system (Miltenyi Biotec, Auburn, CA) resulted in a 10-fold reduction in allo-antigen–specific helper T-lymphocyte precursor (HTLp) frequency without significant reduction in third party–reactive HTLp.7 The group from Hopital Necker, Paris, has performed similar studies using an immunotoxin (RFT5-SMPT-dgA) recognizing CD25. It has been demonstrated that this anti-CD25 immunotoxin can deplete alloreactive T cells after incubation with haplo-identical PBMCs ex vivo, while retaining proliferative responses to CMV antigens and candida8 and cytotoxic T-lymphocyte precursor (CTLp) frequencies against cytomegalovirus/Epstein-Barr virus (CMV/EBV)–infected targets.9 More recently, the same group has performed a phase 1/2 clinical study of addback of allodepleted donor T cells in 15 patients undergoing HLA-mismatched stem cell transplantation.10,11
One major limitation of applying this approach to patients with leukemia and bone marrow failure concerns the source of recipient stimulator cells. For patients who are aplastic either due to disease or to chemotherapy it may be difficult to obtain sufficient PBMCs for this approach. Further, crude PBMC preparations are relatively ineffective antigen-presenting cells. In the clinical study of Andre-Schmutz et al,11 the presence of a residual proliferative response to recipient PBMCs was strongly associated with the subsequent development of GVHD. Moreover, in leukemic patients, PBMCs may be contaminated with leukemic blasts or dendritic cells that have processed leukemic antigens, so that allodepletion results in the loss of antileukemic activity. Dendritic cells, likewise, are difficult to prepare in adequate numbers for clinical protocols and require the use of expensive cytokines. We have used recipient EBV-transformed lymphoblastoid cell lines (LCLs) as stimulators to activate alloreactive T cells. LCLs are excellent antigen-presenting cells and therefore are likely to be more effective than bulk PBMCs in activating alloreactive T cells. Additionally, LCLs are relatively cheap to prepare, are easily expanded to large numbers, and have a standard phenotype, with less variability in expression of immunostimulatory molecules than PBMCs. Finally, unlike crude PBMCs, LCLs do not express myeloid or tumor antigens that may serve as targets for the GVL effect, so that donor T cells allodepleted after stimulation with recipient LCLs would be predicted to retain their antileukemic effect, particularly in myeloid malignancies. We have developed a protocol for allodepletion utilizing an anti-CD25 immunotoxin after stimulation of donor cells with HLA-mismatched LCLs and have evaluated residual alloreactivity and antiviral and antitumor activity.
Materials and methods
Isolation of PBMCs and generation of LCLs
PBMCs were isolated from normal donor peripheral blood by Lymphoprep (Nycomed, Oslo, Norway) density gradient centrifugation. For LCL generation, 5 × 106 PBMCs were infected with concentrated supernatant from the B95-8 EBV-producer cell line, as previously reported.12 LCLs were cultured in RF10 medium consisting of RPMI 1640 (Biowhittaker, Walkersville, MD) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT).
Immunotoxin
RFT5-SMPT-dgA is an immunotoxin generated by cross-linking a murine anti-CD25 monoclonal antibody (MoAb) (immunoglobulin G1 [IgG1]) with a chemically deglycosylated ricin α chain (dgA) using an N-succinimidyloxycarbonyl-α-methyl-2 pyridyldithiol toluene (SMPT) linker according to published methods.13 Clinical grade immunotoxin was made available to us through collaboration with Dr Ellen Vitetta (University of Texas Southwestern Medical Center).
Generation of allodepleted donor T cells
Normal donor PBMCs and HLA-mismatched or haplo-identical irradiated (70 Gy) recipient LCLs were each diluted to 2 × 106/mL in AIM V serum-free medium (Invitrogen, Carlsbad, CA). Donor PBMCs were then cocultured with or without irradiated recipient LCLs at a responder-to-stimulator ratio of 40:1 in T-75 or T-175 flasks for 3 days. After 72 hours cocultures were harvested and resuspended at 107/mL in immunodepletion medium consisting of AIM V supplemented with 20 mM ammonium chloride (Sigma, St Louis, MO) to improve the bioactivity of the immunotoxin with pH adjusted to 7.75 using Na HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (Sigma). Donor PBMCs alone and donor PBMCs plus recipient LCL cocultures were each split into 2 equal aliquots and 0.22 μm filtered RFT5-SMPT-dgA anti-CD25 immunotoxin added to 1 aliquot at a final concentration of 3 μg/mL. Cocultures in the presence and absence of immunotoxin were incubated overnight at 37°C. The next morning cocultures were washed twice and then resuspended at 2 × 106/mL in AIM V; 100 μL(2 × 105 cells) from each day 4 coculture was sampled in triplicate for primary mixed lymphocyte reactions (MLRs) and 300 μL for fluorescence-activated cell sorter (FACS) analysis. The remaining cells were rested in 24-well plates at 2 × 106 per well for 3 days prior to secondary stimulation, secondary MLRs, culture of CTLs, and enzyme-linked immunospot (ELISPOT) assays.
Flow cytometry and tetramer analysis
A total of 2 × 105 cultured cells were washed, stained for 30 minutes at 4°C with antibody, and analyzed by flow cytometry (FACSCalibur and CellQuest software; Becton Dickinson, San Jose, CA). A total of 10 000 events were analyzed. Cells were stained with fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, or peridinin chlorophyll protein (PerCP)–conjugated monoclonal antibodies to CD3 (clone UCHT1), CD4 (L200), CD8 (RPA-T8), CD25 (M-A251), CD40 (5C3), CD54 (HA-58), CD80 (L307.4), CD86 (FUN-1), HLA-ABC (G46-2.6), and HLA-DR (L243) (BD Biosciences, San Jose, CA). For tetramer analysis, 106 PBMCs or allodepleted T cells from donors known to have tetramer-positive populations were costained with CD8 FITC, CD3 PerCP, and either isotype PE control antibody or PE-conjugated HLA-A2–CMV pp65 (NLVPMVATV) or HLA-A2–LMP2 (CLGGLLTMV), titrated for optimal specific staining. For the HLA-A2–PR1 (VLQELNVTV) tetramer, which stains with lower fluorescence intensity, CD8 FITC clone 3B5 (Caltag, Burlingame, CA) and a “dump” channel consisting of CD4/CD14/CD16 and CD19 PerCP were used to reduce background. The percentage of tetramer-positive cells in the CD3+/CD8+ lymphocyte gate was expressed as a proportion of the CD8+ cells.
Proliferation and cytotoxicity assays
For proliferation assays, 2 × 105 PBMCs cultured with 5000 γ-irradiated (70 Gy) LCLs in 200 μL AIM V medium or 2 × 105 day 4 cocultures were plated in triplicate U-bottom 96-well plates (Nunc, Rochester, NY). After 5 days, cultures were pulsed with 0.037 MBq (1 μCi) 3H-thymidine per well (Amersham Biosciences, Piscataway, NJ) and harvested onto glass fiber strips 18 hours later using a Brandel PHD cell harvester. 3H-thymidine uptake was measured using a Matrix B liquid scintillation counter (Canberra Packard, Meriden, CT) and specific proliferation calculated by subtracting the mean counts per minute of triplicate responder alone and stimulator alone from mean counts per minute of test cultures. The cytotoxic activity of CTL lines was evaluated in a standard 6-hour 51Cr release assay, as previously described.14 Briefly, 3 × 106 target LCLs were labeled with 3700 MBq (100 mCi) 51Cr (Amersham Biosciences) for 2 hours, washed 3 times, and diluted to 5 × 104/mL (K562) in RF10 medium; 100 μL target cells were added to serial dilutions of effector cells in triplicate round-bottom, 96-well plates to obtain a total volume of 200 μL per well, giving varying effector-target (E/T) ratios. In some experiments a 20-fold excess of cold HSB-2 cells was added to cultures to assess specificity. HSB-2 cells were used because LCL targets are more sensitive to lymphokine-activate killer (LAK)– than natural killer (NK)–mediated lysis, and this cell line has previously been shown to be an excellent target for LAK cells.15 Assay plates were incubated for 6 hours at 37°C, 5% CO2, and 100 μL supernatant was harvested and counted using a Packard Cobra Gamma Counter (Packard Instrument Company, Downer's Grove, IL). The percentage of specific lysis was calculated as follows: ([cpm released experimental – cpm spontaneous]/[cpm total lysis – cpm spontaneous]) × 100%.
Optimization of allodepletion following stimulation with recipient LCLs
As a prelude to subsequent clinical studies we optimized a variety of parameters in our allodepletion protocol using recipient LCLs as stimulators. A donor PBMC/recipient LCL ratio of 40:1 was found to result in the lowest percentage of residual CD3+/CD25+ and residual proliferation. An immunotoxin concentration of 3 μg/mL was found to give effective depletion of secondary proliferative responses to host without significant diminution of responses to third party, and this concentration was therefore used in further studies. Based on sequential FACS analysis showing peak levels of CD25 expression at 3 to 4 days of coculture, allodepletion was performed at this time. To adapt our allodepletion protocol for a clinical trial, we scaled up our experiments using serum-free medium and clinical grade reagents. The residual percentage CD3+/CD25+ cells and residual proliferation following allodepletion in serum-free AIM V medium was equivalent to that in medium containing fetal calf serum. Assuming a 70 kg recipient, multiple doses of 105/kg or 106/kg allodepleted donor T cells could routinely be generated from 150 mL or 500 mL donor peripheral blood.
Generation of EBV-specific and alloreactive CTLs
Polyclonal EBV-specific and alloreactive T-cell lines were generated as previously reported.16 Briefly, allodepleted donor T-cell cultures (2 × 106 per well of a 24-well plate) were stimulated with 40 Gy–irradiated LCLs from the initial stimulator, an HLA-mismatched third-party donor (alloreactive CTL cultures), or autologous donor LCLs at a responder-stimulator ratio of 4:1. After 9 days, cultures were restimulated with irradiated LCLs (at 4:1 R/S ratio) and after 24 and 96 hours supplemented with 20 U/mL recombinant human interleukin-2 (rhIL-2; Proleukin, Chiron, Emeryville, CA). Cultures were restimulated in a similar fashion weekly and cytotoxicity assayed 5 to 6 days following the third round of stimulation.
ELISPOT assay
ELISPOT assays were used to determine the frequency of adenovirus-, CMV-, and EBV-specific T precursors in allodepleted donor T-cell cultures producing interferon-γ (IFN-γ) in response to stimulation with autologous PBMCs transduced with vaccinia or adenoviral vectors carrying green fluorescent protein (Vacc-GFP, Ad5f35-GFP) or CMV pp65 transgenes (Vacc-pp65, Ad5f35-pp65-GFP) or autologous LCLs, as reported previously with slight modifications.17 Briefly, to generate pp65-expressing stimulator cells, 106 thawed autologous PBMCs were resuspended in 200 μL AIM V and transduced with either Ad5f35-pp65-GFP or an identical control vector lacking the pp65 transgene (Ad5f35-GFP) at a multiplicity of infection (MOI) of 100 for 2 hours, washed twice, resuspended at 106/mL in AIM V, and irradiated (30 Gy). For assessment of response to EBV, irradiated (70 Gy) autologous LCLs diluted to 106/mL in AIM V were used as stimulators. MAHAS4510 plates (Millipore, Billerica, MA) were coated with anti–IFN-γ catcher MAB91 DIK (Mabtech, Cincinnati, OH) overnight and blocked with RF10 medium for 1 hour at 37°C. Serial dilutions starting at 2 × 105 allodepleted donor T cells or thawed donor PBMCs per well were plated in the presence of 105 stimulators in duplicate or triplicate wells for 18 to 24 hours at 37°C. Controls consisting of 2 × 105 responder alone, 105 stimulator alone, and 2 × 105 responder plus 105 untransduced autologous PBMCs cells were also plated. Plates were washed the next day and then incubated for 2 hours at 37°C with biotin–anti-IFN-γ detection antibody 7-B6-1 (Mabtech). Avidin-peroxidase complex (Vector Laboratories, Burlingame, CA) was added for 1 hour at room temperature and spots developed with 3-amino-9-ethylcarbazole (AEC, Sigma) substrate mix. The numbers of spots were counted (Zellnet Consulting, New York, NY), the means of duplicate/triplicate wells calculated and expressed spot-forming cells per 106 cells. The mean number of specific spot-forming cells was calculated by subtracting the mean number of spots produced by responder alone and stimulator-alone wells from the mean number of spots in test wells for each dilution. Linear regression analysis was then used to determine the number of specific spot-forming cells per 106 cells.
Viral vectors
Vacc-pp65 was a gift from Dr Stan Riddell (Fred Hutchinson Cancer Research Center, Seattle, WA). Vacc-GFP was constructed in our laboratory using published protocols.18 A genomic copy of pp65 cloned into pEGFP-C1 (Clontech, Palo Alto, CA) was a gift from Dr William J. Britt (Birmingham, AL). To construct Ad5f35-pp65-GFP, the GFPpp65 fusion gene was first subcloned into pShuttle vector (Adeno-X Expression System, Clontech) and then into pAd5f35 backbone (Ad5 vector backbone modified with Ad35 fiber protein).19 pAd5f35GFPpp65 was then transfected into 293 cells (American Type Culture Collection [ATCC], Manassas, VA) for homologous recombination.20 Green plaques were purified thrice before large-scale production. pp65 expression was verified by Western blotting using an anti-pp65 (ViroStat, Portland, ME) antibody. The GFPpp65 fusion protein was approximately 90 kDa in size. The titer of Ad5f35-pp65-GFP vector was 1 × 1012 viral particles per milliliter and 1 × 1010 plaque-forming units per milliliter (pfu/mL), as determined on 293 cells.
Statistical analysis
A paired, 2-tailed Student t test (95% confidence interval) was used to determine the statistical significance of differences between samples. All data are represented as mean ± 1 standard deviation (SD).
Results
Comparison of allodepletion following stimulation with HLA-mismatched PBMCs and LCLs
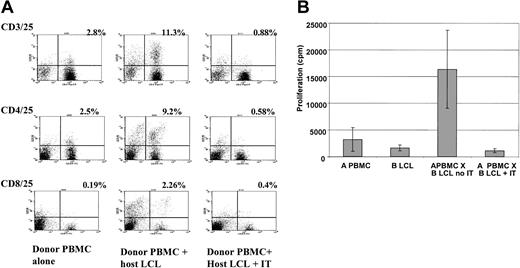
We initially compared the expression of a variety of immunostimulatory molecules on PBMCs and LCLs from 5 individuals using FACS analysis. Table 1 shows that although the expression of HLA class 1 was similar in PBMCs and LCLs, the latter expressed much higher and less variable levels of HLA class 2, the costimulatory molecules CD40, CD80, and CD86 and the adhesion molecule CD54. We then investigated the capacity of recipient LCLs to activate alloreactive T cells from HLA-mismatched donors. As shown in Figure 1A, coculture with recipient LCLs results in a marked increase in expression of CD25 in both CD4+ and CD8+ T cells. Overnight treatment of such cocultures with the anti-CD25 immunotoxin very effectively depletes CD3+/CD25+ in both CD4+ and CD8+ subsets. As seen in Figure 1B, in 5 different haplo-identical patient-donor pairs, allodepletion with the anti-CD25 immunotoxin after stimulation with recipient LCLs resulted in an average 15-fold decrease in proliferation in primary mixed lymphocyte reactions (MLRs) compared with untreated cocultures. To compensate for the effect of overnight culture in the immunodepletion medium, we included controls that were mock-treated with immunodepletion medium but in the absence of immunotoxin. Because the absolute counts per minute in primary MLRs is highly variable depending on the degree and nature of the HLA mismatch, we standardized these results by calculating the residual proliferation according to the following formula: cpm (donor PBMCs + host LCLs + immunotoxin) – cpm (donor PBMCs alone + immunotoxin) ÷ cpm (donor PBMCs + host LCLs; no immunotoxin) – cpm (donor PBMCs alone; no immunotoxin).
LCLs express higher levels of immunostimulatory molecules than PBMCs
Marker . | PBMCs, % positive . | PBMCs, MFI . | LCLs, % positive . | LCLs, MFI . |
|---|---|---|---|---|
| HLA class 1 | 96.6 ± 4.2 | 185.5 ± 159.5 | 97.4 ± 2.8 | 334.4 ± 146.3 |
| HLA-DR | 31.5 ± 4.0 | 181.4 ± 127.4 | 99.7 ± 0.2 | 1904.8 ± 545 |
| CD40 | 3.9 ± 5.0 | 3.3 ± 1.4 | 42.4 ± 8.0 | 36 ± 18.6 |
| CD54 | 25.4 ± 12.3 | 28.8 ± 28.5 | 95.4 ± 2.3 | 393.6 ± 203.7 |
| CD80 | 1.0 ± 0.8 | 2.2 ± 0.5 | 95.1 ± 3.6 | 206.8 ± 135 |
| CD86 | 10.4 ± 4.0 | 8.3 ± 2.2 | 81.3 ± 5.5 | 184.1 ± 132 |
Marker . | PBMCs, % positive . | PBMCs, MFI . | LCLs, % positive . | LCLs, MFI . |
|---|---|---|---|---|
| HLA class 1 | 96.6 ± 4.2 | 185.5 ± 159.5 | 97.4 ± 2.8 | 334.4 ± 146.3 |
| HLA-DR | 31.5 ± 4.0 | 181.4 ± 127.4 | 99.7 ± 0.2 | 1904.8 ± 545 |
| CD40 | 3.9 ± 5.0 | 3.3 ± 1.4 | 42.4 ± 8.0 | 36 ± 18.6 |
| CD54 | 25.4 ± 12.3 | 28.8 ± 28.5 | 95.4 ± 2.3 | 393.6 ± 203.7 |
| CD80 | 1.0 ± 0.8 | 2.2 ± 0.5 | 95.1 ± 3.6 | 206.8 ± 135 |
| CD86 | 10.4 ± 4.0 | 8.3 ± 2.2 | 81.3 ± 5.5 | 184.1 ± 132 |
Data are the results of FACS analysis of PBMCs or LCLs from the same individuals. The results are the means ± SDs of 5 healthy donors.
MFI indicates mean fluorescence intensity.
Depletion of alloreactive T cells by anti-CD25 immunotoxin. (A) FACS analysis showing increased expression of CD25 (y axis) on CD3/4/8+ T cells after activation with HLA-mismatched LCLs and effective depletion of CD3+/CD25+, CD4+/CD25+, and CD8+/CD25+ cells following treatment with anti-CD25 immunotoxin. The figure shows a representative FACS analysis from 6 different donor-recipient pairs. The percentage of double-positive cells is indicated. (B) Primary mixed lymphocyte reaction showing a mean 15-fold decrease in proliferation in response to HLA-mismatched LCL stimulators after treatment with anti-CD25 immunotoxin (IT). Results are the mean ± SD of 5 haplo-identical donor-patient pairs each assayed in triplicate.
Depletion of alloreactive T cells by anti-CD25 immunotoxin. (A) FACS analysis showing increased expression of CD25 (y axis) on CD3/4/8+ T cells after activation with HLA-mismatched LCLs and effective depletion of CD3+/CD25+, CD4+/CD25+, and CD8+/CD25+ cells following treatment with anti-CD25 immunotoxin. The figure shows a representative FACS analysis from 6 different donor-recipient pairs. The percentage of double-positive cells is indicated. (B) Primary mixed lymphocyte reaction showing a mean 15-fold decrease in proliferation in response to HLA-mismatched LCL stimulators after treatment with anti-CD25 immunotoxin (IT). Results are the mean ± SD of 5 haplo-identical donor-patient pairs each assayed in triplicate.
In 5 independent experiments with haplo-identical patientdonor pairs, the residual proliferation after stimulation with recipent LCLs followed by treatment with anti-CD25 immunotoxin was less than 0.5%.
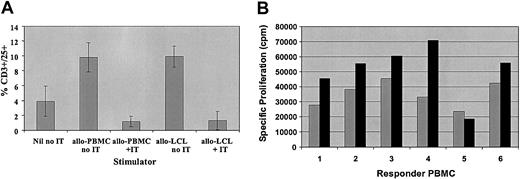
Based on these results, we compared T-cell activation and the efficacy of depletion of alloreactive donor T cells following stimulation with HLA-mismatched recipient PBMCs or LCLs. Normal donor PBMCs were stimulated for 4 days with irradiated HLA-mismatched PBMCs at a ratio of 1:1 or LCLs from the same recipients at a ratio of 40:1. As illustrated in Figure 2A, in 5 donor-patient pairs, the percentage of activated CD3+/CD25+ cells was similar after stimulation with PBMCs or LCLs, although the mean fluorescence intensity for CD25 was generally higher after stimulation with LCLs. Likewise, after depletion of alloreactive cells with the CD25 immunotoxin the percentage of residual CD3+/CD25+ cells was equivalent after stimulation with PBMCs or LCLs. However, as seen in Figure 2B, the specific proliferation of PBMCs from 6 different donors stimulated with PBMCs or LCLs from the same HLA-mismatched recipients was significantly higher after stimulation with LCLs, despite the much lower number of stimulators used (P < .05). More importantly, the residual proliferation after treatment of these cocultures with anti-CD25 immunotoxin was more variable after stimulation with HLA-mismatched recipient PBMCs (mean, 8.5% ± 11.3%). In contrast, the residual proliferation after stimulation with LCLs from the same recipients was lower and consistently less than 5% (mean, 0.8% ± 1.8%) and was statistically significantly lower than after stimulation with PBMCs from the same donors (P < .05) (Table 2). Thus, allodepletion with anti-CD25 immunotoxin following stimulation with host LCLs appears more consistently effective in depleting in vitro alloreactivity than after stimulation with host PBMCs.
Comparison of activation and allodepletion following stimulation with HLA-mismatched PBMCs or LCLs. (A) FACS analysis showing percent CD3+/CD25+ cells after stimulation with HLA-mismatched PBMCs or LCLs in the presence or absence of immunotoxin. Results are the mean ± SD of 5 HLA-mismatched donor-patient pairs. (B) Primary mixed lymphocyte reactions showing proliferation of 2 × 105 donor PBMCs by HLA-mismatched PBMCs (R/S 1:1; ▦) or LCLs (R/S 40:1; ▪) from the same recipient. The results are the mean specific proliferation of triplicate wells for 6 HLA-mismatched donor-recipient pairs.
Comparison of activation and allodepletion following stimulation with HLA-mismatched PBMCs or LCLs. (A) FACS analysis showing percent CD3+/CD25+ cells after stimulation with HLA-mismatched PBMCs or LCLs in the presence or absence of immunotoxin. Results are the mean ± SD of 5 HLA-mismatched donor-patient pairs. (B) Primary mixed lymphocyte reactions showing proliferation of 2 × 105 donor PBMCs by HLA-mismatched PBMCs (R/S 1:1; ▦) or LCLs (R/S 40:1; ▪) from the same recipient. The results are the mean specific proliferation of triplicate wells for 6 HLA-mismatched donor-recipient pairs.
Residual proliferation in 7 donor-patient pairs
. | Responder . | . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stimulator . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | Mean ± SD . | ||||||
| Allo-PBMCs | 0 | 14.8 | 0 | 27.4 | 0 | 17.4 | 0 | 8.5 ± 11.3 | ||||||
| Allo-LCLs | 4.8 | 0 | 0.7 | 0 | 0 | 0 | 0 | 0.8 ± 1.8 | ||||||
. | Responder . | . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stimulator . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | Mean ± SD . | ||||||
| Allo-PBMCs | 0 | 14.8 | 0 | 27.4 | 0 | 17.4 | 0 | 8.5 ± 11.3 | ||||||
| Allo-LCLs | 4.8 | 0 | 0.7 | 0 | 0 | 0 | 0 | 0.8 ± 1.8 | ||||||
Residual proliferation in 7 donor-patient pairs after allodepletion with anti-CD25 immunotoxin following stimulation of donor PBMCs with PBMCs (R/S 1:1) or LCLs (R/S 40:1) from the same HLA-mismatched recipient. Residual proliferation was calculated using the formula in “Results” and was significantly higher after stimulation with PBMCs (P < .05).
Allodepletion specifically abrogates the ability to generate alloreactive CTLs
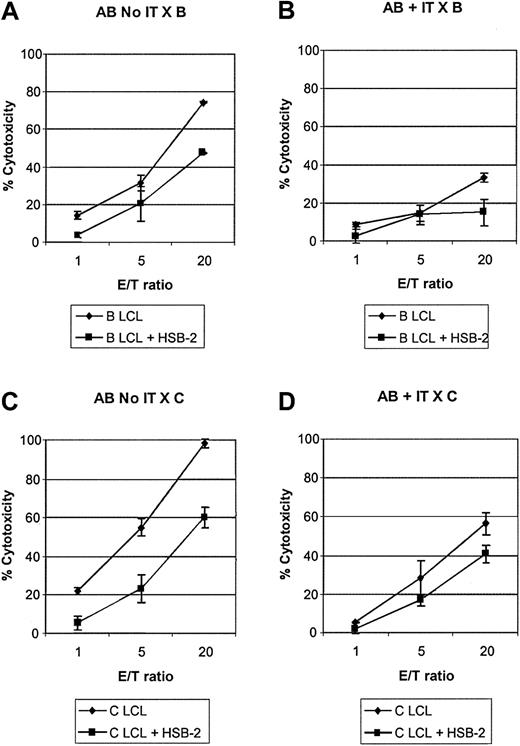
To determine the effect of allodepletion with anti-CD25 immunotoxin on alloreactive and bystander cytotoxic T-cell precursors, we restimulated allodepleted donor T cells from 3 different donors with either LCLs from the initial recipient or from an HLA-mismatched third party and then performed cytotoxicity assays. Cold-target inhibition with a 20-fold excess of unlabeled HSB-2 cells was used to assess specificity. The results of a representative experiment are shown in Figure 3. As seen in Figure 3A, in the absence of immunotoxin treatment, donor cells restimulated with LCLs from the initial recipient efficiently lysed recipient LCLs and most of the observed cytotoxicity was specific, because it was largely preserved in the presence of cold HSB-2 targets. In contrast, allodepleted donor T cells showed much lower cytolytic activity against the recipient, and much of the residual cytotoxicity seen at high E/T ratios was nonspecific, because it was lost in the presence of cold HSB-2 targets (Figure 3B). When donor T cells exposed to recipient LCLs were restimulated with third-party LCLs (Figure 3C-D), the cytolytic activity against third-party LCLs was somewhat reduced with immunotoxin treatment, but allodepleted donor T cells nonetheless showed good killing of third-party targets, which appeared specific, in that it was largely preserved in the presence of cold target inhibitors. In 3 different HLA-mismatched donor recipient pairs, at an E/T ratio of 20:1, the mean specific cytotoxicity of cocultures against recipient LCLs in the presence of excess cold HSB-2 was reduced from 46.3% ± 1.8% in the absence of immunotoxin to 13.6% ± 1.8% in its presence. In contrast, the cytotoxicity against third-party LCLs was largely preserved (68.3%± 11.5% versus 44.9%± 5.9%).
Allodepletion specifically abrogates the generation of alloreactive CTLs. Cytotoxicity assay showing lysis of LCL targets by cocultures of donor A PBMCs with recipient B LCLs in the presence or absence of anti-CD25 immunotoxin after secondary stimulation with B LCLs (A-B) or with third-party LCLs (C-D). In each case cytotoxicity was also assayed in the presence of a 20-fold excess of cold HSB-2 targets to assess specificity. Results are the mean specific cytotoxicity ± SD of triplicate wells in an assay representative of 3 experiments with different patient-donor pairs.
Allodepletion specifically abrogates the generation of alloreactive CTLs. Cytotoxicity assay showing lysis of LCL targets by cocultures of donor A PBMCs with recipient B LCLs in the presence or absence of anti-CD25 immunotoxin after secondary stimulation with B LCLs (A-B) or with third-party LCLs (C-D). In each case cytotoxicity was also assayed in the presence of a 20-fold excess of cold HSB-2 targets to assess specificity. Results are the mean specific cytotoxicity ± SD of triplicate wells in an assay representative of 3 experiments with different patient-donor pairs.
Antiviral T-cell responses are preserved following allodepletion
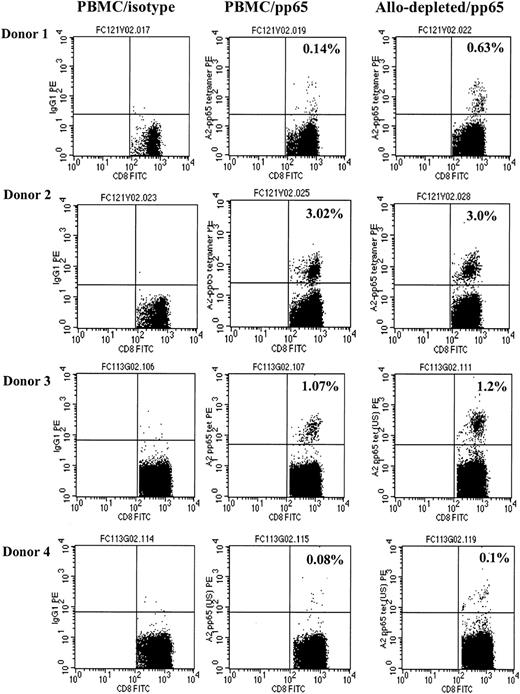
To determine the specificity of allodepletion, we studied whether antiviral T-cell responses were retained following immunotoxin treatment. PBMCs from 4 HLA-A2–positive donors known to have significant populations of CMV-specific CD8+ cells detectable by HLA-A2–pp65 peptide tetramers were cocultured with HLA-mismatched (HLA-A2–negative) LCLs for 3 days and then treated overnight with anti-CD25 immunotoxin. As shown in Figure 4, there was no significant difference in the frequency of HLA-A2-pp65–specific CD8+ T cells in allodepleted donor T-cell cultures and unmanipulated PBMCs. These results suggested that virus-specific T cells are retained following allodepletion. To study the functionality of antiviral T cells, we then performed ELISPOT analyses to determine the frequency of T cells secreting IFN-γ in response to adenoviral antigens and CMV pp65. Unmanipulated or allodepleted donor T cells were stimulated with irradiated autologous PBMCs transduced with vaccinia vectors carrying GFP (Vacc-GFP)orCMVpp65 (Vacc-pp65) transgenes or with adenoviral vector carrying the GFP transgene (Ad5f35-GFP) or GFP and CMVpp65 (Ad5f35-pp65-GFP). As can be seen in Figure 5, in 5 different CMV-seropositive donors, there was no statistically significant difference in frequency of IFN-γ–secreting cells after stimulation with Ad5f35-GFP– (Figure 5A, P = .22) or Vaccpp65– (Figure 5B, P = .95) transduced PBMCs in allodepleted T-cell cocultures and unmanipulated PBMCs, implying that allodepletion does not affect the function of adenovirus- or CMV-specific T cells. IFN-γ responses to PBMCs transduced with the control vector Vacc-GFP were always less than 30 spot-forming cells per 106 cells, demonstrating the specificity of these responses. Likewise, when responder cells were stimulated with antigens from both adenovirus and CMV, using the Ad5f35pp65GFP vector, the frequency of IFN-γ–secreting cells was similar in allodepleted donor T cells and unmanipulated PBMCs (Figure 5C, P = .37). In some donors the response to Ad5f35-pp65-GFP was greater than the sum of the responses to Ad5f35-GFP and to Vacc-pp65: This may reflect the known inhibitory effect of vaccinia on IFN-γ secretion by T cells.
CMV-specific CD8+ T cells are not deleted by allodepletion. The figure shows FACS analysis following staining of either unmanipulated PBMCs (right and center columns) or allodepleted cells (left column) from 4 HLA-A2–positive, CMV-seropositive donors with IgG PE (left column) or an HLA-A2–CMV pp65 tetramer (center and right columns). The percentages of tetramer-positive cells as a proportion of CD8+ cells with isotype subtracted are shown.
CMV-specific CD8+ T cells are not deleted by allodepletion. The figure shows FACS analysis following staining of either unmanipulated PBMCs (right and center columns) or allodepleted cells (left column) from 4 HLA-A2–positive, CMV-seropositive donors with IgG PE (left column) or an HLA-A2–CMV pp65 tetramer (center and right columns). The percentages of tetramer-positive cells as a proportion of CD8+ cells with isotype subtracted are shown.
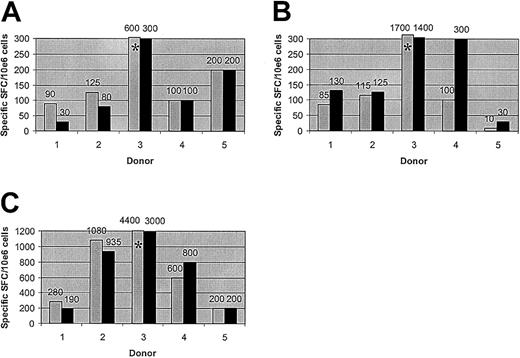
T-cell responses to adenoviral and CMV antigens are preserved after allodepletion. The figure shows the frequency of cells secreting IFN-γ as determined by ELISPOT assays. Unmanipulated PBMCs from 5 different seropositive donors (▦) or donor PBMCs allodepleted after stimulation with HLA-mismatched LCLs (▪) were stimulated with irradiated autologous PBMCs transduced with an adenoviral vector carrying the GFP gene (A), vaccinia vectors carrying the GFP gene with (B) or without (not shown) the CMV pp65 gene, or an adenoviral vector carrying the CMV pp65 and GFP genes (C). Results are shown numerically above columns. *The result falls above the axis limit. Results are the mean number of specific spot-forming cells calculated by linear regression of duplicate wells assayed at 3 dilutions.
T-cell responses to adenoviral and CMV antigens are preserved after allodepletion. The figure shows the frequency of cells secreting IFN-γ as determined by ELISPOT assays. Unmanipulated PBMCs from 5 different seropositive donors (▦) or donor PBMCs allodepleted after stimulation with HLA-mismatched LCLs (▪) were stimulated with irradiated autologous PBMCs transduced with an adenoviral vector carrying the GFP gene (A), vaccinia vectors carrying the GFP gene with (B) or without (not shown) the CMV pp65 gene, or an adenoviral vector carrying the CMV pp65 and GFP genes (C). Results are shown numerically above columns. *The result falls above the axis limit. Results are the mean number of specific spot-forming cells calculated by linear regression of duplicate wells assayed at 3 dilutions.
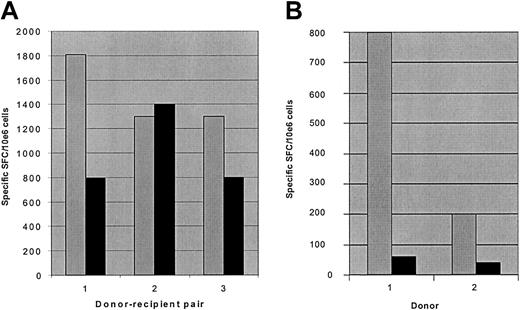
One potential concern about the use of donor T cells allodepleted after stimulation with recipient LCLs is that, in the haplo-identical setting, T-cell responses to EBV would be abrogated, thereby increasing the risk of lymphoproliferative disease after transplantation. We reasoned that because T cells recognizing EBV antigens in the context of HLA molecules from the nonshared donor haplotype should not be activated in response to recipient LCLs, some anti-EBV activity should be retained following allodepletion. To investigate this, we stimulated PBMCs from EBV-seropositive donors with haplo-identical recipient LCLs for 3 days followed by allodepletion with anti-CD25 immunotoxin. We then used ELISPOT assays to determine the frequency of IFN-γ– secreting cells after secondary stimulation with autologous donor LCLs to assess anti-EBV responses. As shown in Figure 6A, the frequency of cells secreting IFN-γ after stimulation with autologous LCLs in allodepleted donor T-cell cultures was partially retained in all 3 haplo-identical donor-patient pairs, demonstrating that significant anti-EBV responses persist following allodepletion. In contrast, control cultures depleted with immunotoxin after initial stimulation with autologous LCLs showed a much more marked loss of T cells secreting IFN-γ in response to EBV antigens than observed in the haplo-identical setting (Figure 6B). These results suggest that the residual responses to EBV following allodepletion are likely to be mediated through recognition of EBV antigens in the context of the nonshared haplotype.
T-cell responses to EBV are partially preserved following allodepletion with haplo-identical LCLs. The figure shows the frequency of cells secreting IFN-γ as determined by ELISPOT assays. (A) Unmanipulated donor PBMCs (▦) or donor PBMCs allodepleted after stimulation with recipient LCLs from 3 different haplo-identical donor-recipient pairs (▪) were stimulated with irradiated autologous donor LCLs. (B) Unmanipulated donor PBMCs (▦) or donor PBMCs depleted with immunotoxin after stimulation with autologous LCLs (▪) were restimulated with irradiated autologous LCLs. Results are the mean number of specific spot-forming cells calculated by linear regression of duplicate wells assayed at 3 dilutions.
T-cell responses to EBV are partially preserved following allodepletion with haplo-identical LCLs. The figure shows the frequency of cells secreting IFN-γ as determined by ELISPOT assays. (A) Unmanipulated donor PBMCs (▦) or donor PBMCs allodepleted after stimulation with recipient LCLs from 3 different haplo-identical donor-recipient pairs (▪) were stimulated with irradiated autologous donor LCLs. (B) Unmanipulated donor PBMCs (▦) or donor PBMCs depleted with immunotoxin after stimulation with autologous LCLs (▪) were restimulated with irradiated autologous LCLs. Results are the mean number of specific spot-forming cells calculated by linear regression of duplicate wells assayed at 3 dilutions.
T cells recognizing myeloid tumor antigens are retained following allodepletion
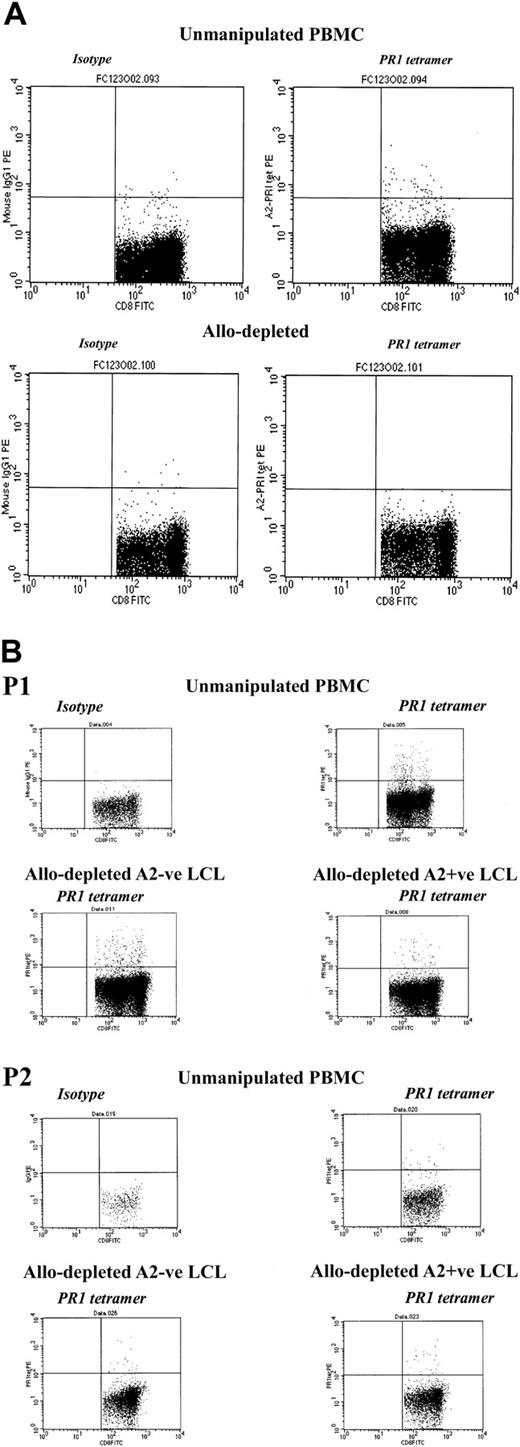
We then investigated whether allodepletion after stimulation with recipient LCLs affected the response to candidate tumor antigens in myeloid malignancies. As our model, we studied T cells recognizing the PR1 epitope from proteinase 3, which have been shown to have potent cytolytic activity against primary leukemic cells in chronic myeloid leukemia (CML) and acute myeloid leukemia (AML) and to preferentially inhibit growth of leukemic granulocytemacrophage colony-forming units (CFU-GMs) from patients with CML.21,22 Using HLA-A2–PR1 tetramer analysis in 3 patients with CML undergoing transplantation and known to have detectable circulating PR1-specific CTLs, we compared the frequency of T cells recognizing the PR1 epitope in unmanipulated PBMCs and after stimulation with HLA-mismatched stimulators and allodepletion with anti-CD25 immunotoxin. As shown in Figure 7A, after stimulation with allogeneic, mismatched HLA-A2–positive CML PBMCs, allodepletion resulted in the complete loss of PR1-specific T cells. By contrast, when LCLs were used as stimulators, PR1-specific CD8+ cells were largely preserved following allodepletion, regardless of whether this was done following stimulation with HLA-A2–positive or HLA-A2–negative LCLs (Figure 7B).
CD8+ T cells specific for the myeloid tumor epitope PR1 are retained after allodepletion after stimulation with mismatched LCLs but not CML PBMCs. (A) FACS analysis following staining with HLA-A2–PR1 tetramer of unmanipulated PBMCs (top row) or allodepleted PBMCs (bottom row) from a patient with CML. In each case isotype controls are shown on the left and tetramer-stained cells on the right. Allodepletion was performed after stimulation with allogeneic HLA-A2–positive PBMCs from a mismatched donor with CML. (B) FACS analysis following staining with HLA-A2–PR1 tetramer of unmanipulated PBMCs or allodepleted T cells from 2 HLA-A2–positive patients with CML. Allodepletion was performed after stimulation with either HLA-A2–positive or –negative LCLs. The percentages of tetramer-positive cells as a proportion of CD8+ cells (isotype subtracted) are shown.
CD8+ T cells specific for the myeloid tumor epitope PR1 are retained after allodepletion after stimulation with mismatched LCLs but not CML PBMCs. (A) FACS analysis following staining with HLA-A2–PR1 tetramer of unmanipulated PBMCs (top row) or allodepleted PBMCs (bottom row) from a patient with CML. In each case isotype controls are shown on the left and tetramer-stained cells on the right. Allodepletion was performed after stimulation with allogeneic HLA-A2–positive PBMCs from a mismatched donor with CML. (B) FACS analysis following staining with HLA-A2–PR1 tetramer of unmanipulated PBMCs or allodepleted T cells from 2 HLA-A2–positive patients with CML. Allodepletion was performed after stimulation with either HLA-A2–positive or –negative LCLs. The percentages of tetramer-positive cells as a proportion of CD8+ cells (isotype subtracted) are shown.
Discussion
Previous studies have demonstrated that after activation with recipient PBMCs, alloreactive donor T cells can be effectively depleted by targeting activation markers such as CD25 and CD69.8,9,23-25 We have attempted to refine this approach to make it more reproducible in a clinical setting, particularly for patients with malignant or aplastic disorders.
A major limitation of current approaches to allodepletion is the source of recipient stimulator cells. In patients who are aplastic either due to disease or to therapy it may be difficult to obtain adequate numbers of recipient PBMCs or dendritic cells to stimulate donor T cells prior to allodepletion. In contrast, even in these patients LCLs are readily generated and expanded to large numbers. Even when sufficient PBMCs are available, these cells are inefficient at antigen presentation and have a variable phenotype, resulting in inconsistent allo-activation and hence allodepletion. Indeed, in the only clinical study of addback of allodepleted donor T cells to date,11 the 4 patients who developed GVHD all had significant residual proliferation to recipient stimulators in primary mixed lymphocyte reactions, demonstrating ineffective allodepletion. GVHD was significantly correlated with this residual proliferation. In the current study we have shown that LCLs activate alloreactive T cells from HLA-mismatched donors to proliferate more effectively than PBMCs, even at much lower responderstimulator ratios. Further, we have demonstrated that allodepletion with an anti-CD25 immunotoxin following stimulation with HLA-mismatched LCLs is more consistently effective at removing alloreactive cells, as assessed by the residual proliferation, than following stimulation with PBMCs. This may reflect the heterogeneity of crude PBMC preparations as antigen-presenting cells compared with LCLs, which have a more standard phenotype and more uniformly express high levels of immunostimulatory molecules. If adoptive immunotherapy with allodepleted donor T cells is to be useful in restoring antiviral and antileukemic T-cell responses clinically, it is critical to demonstrate the specificity of depletion. T-cell receptors on alloreactive T cells may recognize limited sets of cross-reactive peptides presented by foreign major histocompatibility complex (MHC) molecules26 and, conversely, T cells specific for environmental antigens may cross-react with foreign MHC.27-29 Potentially then, allodepletion could be associated with a reduction in T cells specific for viral antigens, limiting the clinical usefulness of this approach. Likewise, the CD25 immunotoxin could bind to bystander T cells nonspecifically activated by paracrine secretion of cytokines, and its ricin moiety could exert negative effects on bystander T cells. Previous in vitro studies on allodepletion have not adequately addressed the issue of whether antiviral responses are preserved following allodepletion. In general they have relied on demonstration of preserved proliferative or CTLp responses to third party as surrogate markers for antiviral T-cell responses in allodepleted donor T cells.23,25 In our hands, however, secondary proliferative responses to third party are highly dependent on the timing of secondary stimulation in relation to immunodepletion. Similarly, the preservation of proliferative responses to candidin and CMV lysates after allodepletion shown by Valteau-Couanet et al8 may not truly reflect preservation of donor T-cell responses to infectious agents because of the supraphysiological levels of antigen used in these assays.
We have used ELISPOT and tetramer assays to compare the frequency of antiviral T cells before and after allodepletion: These assays have the advantage that they enable direct quantification of the frequency of viral-specific T cells. Using these assays, we have clearly shown that the frequency of T cells recognizing CMV and adenoviral antigens is unaffected by allodepletion with our protocol. Thus, adoptive immunotherapy with allodepleted donor T cells should result in improved T-cell responses to these viruses. Likewise, we have shown that the donor anti-EBV response is partially retained following allodepletion with recipient LCL stimulators, through recognition of EBV antigens on the nonshared haplotype, so that addback of allodepleted donor T cells after haplo-identical SCT would be predicted to confer some degree of immunity against EBV.
Given the high relapse rates after haplo-identical SCT,1,2 the issue of whether antileukemic responses are preserved following allodepletion is also critical. If allodepletion is performed after stimulation with host PBMCs, it would be predicted that T-cell responses to myeloid antigens overexpressed in AML and CML, such as proteinase 3 and WT-1, which may be important targets for the GVL effect,21,22,30,31 would be lost. Our data suggest that this may indeed be the case. Similarly, recipient PBMCs are frequently contaminated with leukemic cells, so that the response to leukemia-specific antigens may be lost. In contrast, using our approach with recipient LCLs as stimulators, these responses should be preserved. The studies of Montagna et al9 and Mavroudis et al32 suggest that CTLp and HTLp frequencies against leukemic cells may be maintained after allodepletion. These data, however, are complicated by the indirect nature of the CTLp and HTLp assays, which involve in vitro restimulation, as well as by the wide confidence intervals for these assays, which may obscure significant changes. We have instead directly studied the T-cell response to a defined candidate tumor antigen, the PR1 epitope of proteinase 3. PR1-specific CTLs lyse primary leukemic cells from patients with AML,21 preferentially inhibit leukemic CFU-GM colony formation,22 and have been correlated with clinical responses to IFN-α and allogeneic HSCT in patients with CML.33 We have demonstrated that, after stimulation with HLA-mismatched LCLs, T-cell responses to PR1 are preserved following allodepletion.
Donor NK cell alloreactivity, mediated through absent expression of the killer cell inhibitory receptor (KIR) ligand on recipient cells, may play a major role in the GVL response in haplo-identical SCT, particularly in AML.34 The percentage of NK cells and cytotoxicity against K562 targets is unaffected by allodepletion using our protocol (data not shown). Alloreactive NK cells express CD25 upon activation,35 and these CD56+/CD25+ cells are depleted by anti-CD25 immunotoxin (data not shown), so that adoptive immunotherapy with allodepleted donor cells would not be predicted to enhance NK-mediated GVL reactions. However, because the recovery of donor NK cells is rapid following haplo-identical SCT,4 this should not adversely impact on the efficacy of such a strategy.
The pace of immune reconstitution has been directly correlated with the number of T cells in the infused graft.36 Likewise, it is known that doses of unmanipulated donor T cells as low as 3 × 104/kg can be associated with severe GVHD in the haploidentical setting. We can routinely generate multiple doses of 106 allodepleted donor T cells per kilogram from 500 mL donor peripheral blood. It remains unclear, however, how many allodepleted donor T cells can safely be given in the haploidentical setting and whether infusion of this number would be sufficient to confer useful antiviral and antileukemic activity. To address this question we have initiated a phase 1 clinical study of addback of escalating doses of allodepleted donor T cells after haplo-identical SCT.
Prepublished online as Blood First Edition Paper, May 22, 2003; DOI 10.1182/blood-2002-11-3516.
Supported by National Institutes of Health grant R21CA093069 (M.K.B.) and by a Doris Duke Distinguished Clinical Scientist Award (H.E.H.). P.J.A. was a recipient of a Clinician-Scientist fellowship from the Medical Research Council of the United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We gratefully acknowledge Dr Ellen Vitetta (University of Texas Southwestern Medical Center, Dallas) for the gift of RFT5-SMPT-dgA immunotoxin.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal