Abstract
Deletions of the derivative chromosome 9 occur in a subset of patients with Philadelphia chromosome–positive chronic myeloid leukemia (CML) and are associated with a poor prognosis on standard drug therapy. However, it is currently unknown if the presence of deletions influences the response to imatinib, an Abl-specific tyrosine kinase inhibitor, that has recently shown excellent hematologic and cytogenetic responses in patients with CML. We, therefore, compared hematologic and cytogenetic responses with imatinib in 397 patients with CML, and survival and progression in 354 of these patients, according to deletion status and disease phase. We found no difference in survival between patients with and without deletions, contrasting with previous reports in cohorts with a lower proportion of patients treated with imatinib. However, the time to disease progression on imatinib treatment was significantly shorter for patients with deletions, both in chronic phase (P = .02) and advanced phases (P = .02). Moreover, both in chronic phase and more advanced phases of CML, hematologic and cytogenetic responses were uniformly lower in patients with deletions, with significant differences seen for hematologic response (P = .04), for major cytogenetic response (P = .008) in chronic phase, and for hematologic response in advanced phases (P = .007) of CML. This finding suggests that differences in survival may become apparent with longer follow-up.
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder that arises in the stem cell compartment.1-3 The molecular hallmark of the disease is the BCR-ABL gene rearrangement,4,5 which usually occurs as the result of a reciprocal translocation between chromosomes 9 and 22.6 The chimeric Bcr-Abl protein derived from this translocation has constitutive protein tyrosine kinase (PTK) activity that is essential for its transforming capacity7 and leads to the activation of multiple signaling pathways, with profound effects on cell cycle, adhesion, and apoptosis. Transgenic and retroviral murine models of CML have confirmed the requirement of BCR-ABL expression and PTK activity for both the initiation and maintenance of the leukemic phenotype,8,9 although not all models exactly reproduce the human disease.
CML is a biphasic disease, with an initial, mostly asymptomatic chronic phase (CP) that progresses through a rather ill-defined stage termed accelerated phase (AP) to the terminal blast crisis (BC).2 During CP, the myeloid compartment is expanded, but the cells retain their capacity to differentiate and function normally, and drug treatment is usually effective. By contrast, BC is characterized by a loss of differentiation capacity and refractoriness to therapy. The clinical course of CML is very heterogeneous, in contrast to the apparent molecular homogeneity of its chronic phase, with marked differences between individual patients both in laboratory and clinical characteristics at diagnosis, in the rate of progression to BC, and ultimately in survival. The nature of this heterogeneity and the molecular basis of the progression to BC remain poorly understood.
The presence of deletions adjacent to the translocation breakpoints on the derivative chromosome 9 has been described in between 10% and 15% of patients with CML.10-15 These deletions span the translocation breakpoint, often involve both chromosome 9 and 22 sequences, are large, sometimes measuring many megabases, and occur at the same time as the formation of the Philadelphia translocation.10,13,15 They are associated with an extremely poor prognosis, with those patients who carry them having shorter length of chronic phase, earlier disease transformation, and shorter survival.14,15 Also, in a direct comparison, deletion status was a far more powerful prognostic indicator than either the commonly used Sokal or Hasford scores.16,17
Conventional drug treatment for chronic-phase CML includes hydroxyurea and interferon-α with or without cytosine arabinoside. Although interferon-α prolongs survival and some patients achieve long-lasting remissions, conventional drug therapy is rarely if ever curative, although it offers good symptomatic control.18-20 By contrast, allogeneic stem cell transplantation is capable of eradicating the malignant clone and thus has curative potential, but it is associated with considerable treatment-associated mortality.21 None of the standard medical treatments appear to improve the outcome of patients with deletions of the derivative chromosome 9.14,15 Moreover, in one study there was also an increased rate of relapse in patients with deletions following allografting.14 Although the numbers of patients in this study were small (12 patients with deletions and 58 patients who lacked deletions), this finding suggests that allografting may be less effective in achieving disease eradication in patients with deletions of the derivative chromosome 9.
Recently, imatinib (formerly STI571; Novartis, Basel, Switzerland), a novel agent specifically designed to inhibit the PTK activity of Bcr-Abl has been tested in several large clinical trials. In a recent randomized study in newly diagnosed patients, complete hematologic response (CHR) (97% versus 69%, P < .001), major cytogenetic response (MCR) (87% versus 34%, P < .001), and complete cytogenetic response (CCR) (76% versus 14.5%, P < .001) with imatinib were all superior to a combination of interferon-α and low-dose cytarabine.22 In this study, freedom from progression to AP or BC was also significantly higher in patients treated with imatinib (97% versus 91%, P < .001). In patients in CP failing interferon-α, treatment with imatinib resulted in complete hematologic response in 95% of patients, major cytogenetic response in 60% of patients, and complete cytogenetic response in 41% of patients after 18 months' follow-up.23 In AP, following treatment with 600 mg imatinib, there was an overall hematologic response rate of 71% (37% complete responses), MCR of 28%, and CCR of 19%.24 The effect of imatinib was less impressive in patients with BC, with sustained (> 4 weeks) responses in approximately one third of the patients.25 In addition, there was a very high rate of relapse, particularly in the lymphoid phenotype.26
Because various and highly divergent treatment options are available for patients with CML, it is becoming ever more important to determine the effect of baseline characteristics on the efficacy of a given therapy. The effect of derivative chromosome 9 deletions on imatinib response is not known. We, therefore, determined the deletion status in a cohort of 397 patients treated with imatinib in various phases of CML and compared survival and disease progression, overall and following initiation of imatinib, as well as the rate of hematologic and cytogenetic responses according to deletion status. Our results indicate that, within the limits of a relatively short follow-up, treatment with imatinib abrogates the survival difference between patients with and without deletions. However, a significantly lower rate of both hematologic and cytogenetic response in chronic phase and hematologic response in more advanced phases, along with an increased rate of progression of disease following the initiation of imatinib therapy in patients with deletions, suggests that imatinib does not fully reverse the adverse prognostic risk associated with these deletions.
Patients, materials, and methods
Patient samples and clinical and laboratory data
Fixed cytogenetic preparations from cultured bone marrow samples and RNA (397 and 244 samples, respectively) from either blood or bone marrow were obtained from patients undergoing treatment with imatinib for BCR-ABL–positive CML. These patients were diagnosed between December 1969 and August 2001 and were consecutive patients commenced on imatinib therapy between August and August 2001 at the Hematology Departments of Addenbrooke's Hospital Cambridge, Cambridge, United Kingdom, the City Hospital Nottingham, Nottingham, United Kingdom, Royal Liverpool University Hospital, Liverpool, United Kingdom, the Royal Victoria Infirmary Newcastle, Newcastle, United Kingdom, the University Hospital of Poitiers, Poitiers, France, and the University Hospital, Leipzig, Germany. Except for those patients newly diagnosed, most patients had previously been treated with an interferon-α–containing regimen. The disease characteristics, at diagnosis and at the initiation of imatinib, of all patients included in the response and outcome analysis are shown in Tables 1 and 2. Risk categories were determined with the use of standard methods,16,17 and sufficient laboratory data to calculate the Sokal and Hasford scores were available for 194 and 191 patients, respectively. Survival outcome was available for 351 patients (54 with deletions and 297 without) from diagnosis and 354 patients (54 with deletions and 300 without) from initiation of imatinib. Data for disease progression on imatinib therapy were available for 328 patients (50 with deletions and 278 without). Hematologic response, MCR, and CCR were available for 337 (50 with deletions and 287 without), 383 (54 with deletions and 329 without), and 374 patients (55 with deletions and 319 without), respectively (Table 3). Both the samples and data were obtained in accordance with local institutional ethical protocols.
Comparison of patient characteristics at diagnosis
Patient characteristics . | Patients with deletions (n = 59) . | Patients without deletions (n = 338) . | P . |
|---|---|---|---|
| Sex, M/F | 27/32 | 183/155 | 18 |
| Median age, y (interquartile range) | 50 (38-56) | 52 (42-59) | 17 |
| Available Sokal score | 32 | 162 | |
| High (%) | 10 (31) | 62 (38) | 31 |
| Intermediate (%) | 9 (28) | 56 (34) | |
| Low (%) | 13 (41) | 44 (27) | |
| Available Hasford score | 32 | 159 | |
| High (%) | 12 (37) | 58 (36) | 20 |
| Intermediate (%) | 14 (44) | 64 (40) | |
| Low (%) | 6 (19) | 37 (24) |
Patient characteristics . | Patients with deletions (n = 59) . | Patients without deletions (n = 338) . | P . |
|---|---|---|---|
| Sex, M/F | 27/32 | 183/155 | 18 |
| Median age, y (interquartile range) | 50 (38-56) | 52 (42-59) | 17 |
| Available Sokal score | 32 | 162 | |
| High (%) | 10 (31) | 62 (38) | 31 |
| Intermediate (%) | 9 (28) | 56 (34) | |
| Low (%) | 13 (41) | 44 (27) | |
| Available Hasford score | 32 | 159 | |
| High (%) | 12 (37) | 58 (36) | 20 |
| Intermediate (%) | 14 (44) | 64 (40) | |
| Low (%) | 6 (19) | 37 (24) |
For those patients in whom diagnostic information was available, there were no significant differences between patients with and without deletions (P as shown)
Comparison of patient characteristics at initiation of imatinib
Patient characteristics . | Patients with deletions, n = 59 (%) . | Patients without deletions, n = 338 (%) . | P . |
|---|---|---|---|
| Disease phase | |||
| BC | 11 (18) | 57 (18) | .3 |
| AP | 4 (7) | 50 (15) | |
| CP1, IFN failure | 33 (57) | 191 (55) | |
| CP1, newly diagnosed | 11 (18) | 40 (12) | |
| Available IFN response | 41 | 210 | |
| Newly diagnosed | 8 (20) | 34 (16) | .09 |
| Hematologic resistance | 2 (5) | 15 (7) | |
| Hematologic relapse | 6 (15) | 9 (4) | |
| Cytogenetic resistance | 9 (21) | 80 (38) | |
| Cytogenetic relapse | 12 (29) | 46 (22) | |
| IFN intolerant | 4 (10) | 26 (13) | |
| Weight, available | 24 | 122 | |
| Less than 70 kg | 7 (29) | 39 (32) | .78 |
| More than 70 kg | 17 (71) | 83 (68) | |
| Splenomegaly | 17/42 (40) | 110/211 (52) | .17 |
| Platelet count, available | 38 | 172 | |
| Less than 450 × 109/L | 32 (85) | 119 (69) | .16 |
| 450-699 × 109/L | 2 (5) | 32 (19) | |
| More than 700 × 109/L | 4 (10) | 21 (12) | |
| Hb, available | 39 | 199 | |
| Less than 12 g/dL | 20 (51) | 97 (49) | .88 |
| More than 12 g/dL | 19 (49) | 102 (51) | |
| WCC, available | 44 | 218 | |
| Less than 10 × 109/L | 21 (48) | 97 (44) | .37 |
| 10-49.9 × 109/L | 21 (48) | 95 (43) | |
| More than 50 × 109/L | 2 (4) | 26 (13) | |
| PB blasts, available | 34 | 155 | |
| 0 | 29 (85) | 106 (68) | .07 |
| 0-2 | 0 | 15 (9) | |
| More than 3 | 5 (15) | 34 (23) | |
| BM blasts, available | 34 | 153 | |
| Less than 5 | 29 (85) | 105 (68) | .12 |
| More than 5 | 5 (15) | 48 (32) | |
| Ph positive metaphases, available | 51 | 291 | |
| Less than 90 | 8 (16) | 53 (18) | .66 |
| More than 90 | 43 (84) | 238 (82) | |
| Additional cytogenetic abnormalities | 15/59 (25) | 73/331 (22) | .66 |
Patient characteristics . | Patients with deletions, n = 59 (%) . | Patients without deletions, n = 338 (%) . | P . |
|---|---|---|---|
| Disease phase | |||
| BC | 11 (18) | 57 (18) | .3 |
| AP | 4 (7) | 50 (15) | |
| CP1, IFN failure | 33 (57) | 191 (55) | |
| CP1, newly diagnosed | 11 (18) | 40 (12) | |
| Available IFN response | 41 | 210 | |
| Newly diagnosed | 8 (20) | 34 (16) | .09 |
| Hematologic resistance | 2 (5) | 15 (7) | |
| Hematologic relapse | 6 (15) | 9 (4) | |
| Cytogenetic resistance | 9 (21) | 80 (38) | |
| Cytogenetic relapse | 12 (29) | 46 (22) | |
| IFN intolerant | 4 (10) | 26 (13) | |
| Weight, available | 24 | 122 | |
| Less than 70 kg | 7 (29) | 39 (32) | .78 |
| More than 70 kg | 17 (71) | 83 (68) | |
| Splenomegaly | 17/42 (40) | 110/211 (52) | .17 |
| Platelet count, available | 38 | 172 | |
| Less than 450 × 109/L | 32 (85) | 119 (69) | .16 |
| 450-699 × 109/L | 2 (5) | 32 (19) | |
| More than 700 × 109/L | 4 (10) | 21 (12) | |
| Hb, available | 39 | 199 | |
| Less than 12 g/dL | 20 (51) | 97 (49) | .88 |
| More than 12 g/dL | 19 (49) | 102 (51) | |
| WCC, available | 44 | 218 | |
| Less than 10 × 109/L | 21 (48) | 97 (44) | .37 |
| 10-49.9 × 109/L | 21 (48) | 95 (43) | |
| More than 50 × 109/L | 2 (4) | 26 (13) | |
| PB blasts, available | 34 | 155 | |
| 0 | 29 (85) | 106 (68) | .07 |
| 0-2 | 0 | 15 (9) | |
| More than 3 | 5 (15) | 34 (23) | |
| BM blasts, available | 34 | 153 | |
| Less than 5 | 29 (85) | 105 (68) | .12 |
| More than 5 | 5 (15) | 48 (32) | |
| Ph positive metaphases, available | 51 | 291 | |
| Less than 90 | 8 (16) | 53 (18) | .66 |
| More than 90 | 43 (84) | 238 (82) | |
| Additional cytogenetic abnormalities | 15/59 (25) | 73/331 (22) | .66 |
For those patients in whom information was available at the initiation of imatinib, there were no significant differences between patients with and without deletions (P as shown). IFN indicates interferon-α; PB, peripheral blood; BM, bone marrow; WCC, white cell count; Hb, hemoglobin.
Comparison of hematologic and cytogenetic response rates by deletion status and disease phase
Further phase/response . | Patients with deletions, n (%) . | Patients without deletions, n (%) . | P . |
|---|---|---|---|
| CP1, all patients | 44 | 231 | |
| CHR | 33/37 (89) | 178/184 (97) | .04 |
| MCR | 23/42 (55) | 173/231 (75) | .008 |
| CCR | 16/44 (36) | 115/227 (50) | .08 |
| CP1, newly diagnosed | 11 | 40 | |
| CHR | 8/9 (88) | 37/40 (92) | .56* |
| MCR | 8/11 (73) | 35/40 (87) | .23* |
| CCR | 8/11 (73) | 29/38 (76) | .54* |
| CP1, interferon-α failure | 33 | 191 | |
| CHR | 25/28 (89) | 141/144 (98) | .02 |
| MCR | 15/31 (48) | 138/191 (72) | .008 |
| CCR | 8/33 (24) | 86/189 (45) | .02 |
| Advanced phase | 13 | 103 | |
| HR | 6/13 (46) | 85/103 (83) | .007 |
| MCR | 1/12 (8) | 27/98 (27) | .13 |
| CCR | 0/11 | 11/92 (12) | .27 |
Further phase/response . | Patients with deletions, n (%) . | Patients without deletions, n (%) . | P . |
|---|---|---|---|
| CP1, all patients | 44 | 231 | |
| CHR | 33/37 (89) | 178/184 (97) | .04 |
| MCR | 23/42 (55) | 173/231 (75) | .008 |
| CCR | 16/44 (36) | 115/227 (50) | .08 |
| CP1, newly diagnosed | 11 | 40 | |
| CHR | 8/9 (88) | 37/40 (92) | .56* |
| MCR | 8/11 (73) | 35/40 (87) | .23* |
| CCR | 8/11 (73) | 29/38 (76) | .54* |
| CP1, interferon-α failure | 33 | 191 | |
| CHR | 25/28 (89) | 141/144 (98) | .02 |
| MCR | 15/31 (48) | 138/191 (72) | .008 |
| CCR | 8/33 (24) | 86/189 (45) | .02 |
| Advanced phase | 13 | 103 | |
| HR | 6/13 (46) | 85/103 (83) | .007 |
| MCR | 1/12 (8) | 27/98 (27) | .13 |
| CCR | 0/11 | 11/92 (12) | .27 |
The rates of CHR, MCR, and CCR achieved in patients according to deletion status and disease phase are shown when available. These rates are compared for deletion status by the χ2 test with the P values shown.
P determined by Fisher exact test.
Definition of disease phase, disease progression, and response criteria
The diagnostic criteria for disease phase were the same as used in 3 recently published clinical trials.24,25,27 AP was defined as the presence of at least 1 of the following criteria: (1) blasts in the bone marrow (BM) and/or peripheral blood (PB) 15% to 30%, (2) blasts and promyelocytes in PB or BM more than 30%, and (3) basophils in PB more than 20% and platelets less than 100 × 109/L (unrelated to therapy). BC was diagnosed if more than 30% of blasts were present in BM or PB. Fifty-one patients were available for analysis in whom imatinib therapy was initiated in CP phase within 6 months of diagnosis, 224 patients for interferon failure in CP, 48 patients were treated following progression to AP, and 68 patients following progression to BC. Patients with BC limited to an extramedullary site were not included in the response analysis but were included in the survival and progression analysis. Disease progression was defined as progression from the stage of disease at initiation of imatinib to a further disease phase, as defined by the criteria given earlier, or patient death if this was related to CML. Karyotypic evolution alone was not considered as indicative of disease progression.
The definition of hematologic responses differed according to the phase of disease. For all patients, CHR required a normal differential and full blood count, with no signs of extramedullary disease. For patients in AP, the responses were either CHR or CHR without peripheral blood recovery, which required a normal differential without the full blood count returning to normal. In BC the responses were CHR, “marrow” response (< 5% marrow blasts, no blasts in the peripheral blood, but incomplete peripheral blood recovery),or return to CP, as previously defined.25 Patients included in the analysis of hematologic response required a minimum follow-up of 3 months unless the response occurred prior to this time. Transient responses lasting less than 4 weeks were not included. An MCR was defined as Philadelphia chromosome–positive (Ph+) metaphases less than 35% of the total on conventional cytogenetic banding analysis, and patients required a minimum of 6 months of follow-up prior to inclusion in the analysis unless the MCR occurred prior to this time. CCR was defined as the absence of Ph+ metaphases on cytogenetic analysis and required a minimum of 12 months' follow-up, unless occurring earlier.
Detection of derivative chromosome 9 deletions
We and others have recently shown that the expression of the reciprocal transcript ABL-BCR is abrogated in patients who carry deletions.28,29 Thus, screening for the presence of ABL-BCR transcripts by reverse transcription– polymerase chain reaction (RT-PCR) provides a reliable method of identifying patients without deletions (negative predictive value, 100%; 95% confidence interval [CI], 93%-100%).28
The 244 patients with available RNA were initially screened for expression of ABL-BCR transcripts by RT-PCR as previously described.28 The ABL-BCR expression and survival of 160 of these patients have been previously reported, with the deletion status of 149 also reported.28 Those patients who did not express ABL-BCR (89 patients) and the remaining 153 patients with metaphases but no RNA available were further analyzed for deletions of the derivative chromosome 9 by fluorescence in-situ hybridization (FISH). In 189 patients the probe system used was the Vysis dual-color dual-fusion FISH probe (Vysis, Richmond, United Kingdom) which screens for the deletion of both chromosome 9 and 22 sequences from the derivative chromosome 9.28 In the remaining 53 patients the probe system used was the Vysis ES (extra signal) probe (Vysis). This probe system screens only for deletions of sequences from the derivative chromosome 9. Both probe systems were used following the manufacturer's instructions. FISH detection of deletions was performed and interpreted as previously described.15
Cytogenetic analysis of response and additional chromosomal abnormalities
Cytogenetic analysis was available for 378 patients at the initiation of imatinib therapy and was repeated every 3 months on all patients to assess cytogenetic response to imatinib. A minimum of 20 metaphases was analyzed using conventional G-banding methods.30 When insufficient numbers of metaphases were available, a minimum of 100 interphases were analyzed using FISH probes.
Statistical methods
The characteristics for patients with and without deletions at diagnostic and at initiation of imatinib are shown in Tables 1 and 2 and were compared by the chi-square test and Fisher exact tests for categorical variables and by the Mann-Whitney U test for continuous variables. Diagnostic Sokal and Hasford risk categories, when available, were compared between these groups by chi-square test, as was disease phase and the presence of additional cytogenetic abnormalities at the time of imatinib initiation. Disease duration prior to initiation of imatinib was also compared in patients with and without deletions by Mann-Whitney U test. Overall survival was calculated from the month of diagnosis until the month of death. Survival time following initiation of imatinib was calculated from the month of commencing imatinib treatment until the month of death. The rate of progression following initiation of imatinib was calculated from the month of commencing imatinib treatment until the month of progression as defined earlier. The estimated median survival and time to disease progression were calculated with the Kaplan-Meier estimator, and the significance of any difference between patients with and without deletions was assessed with the log-rank test. The chi-square test and Fisher exact tests were used to compare the overall proportions of patients with and without deletions achieving hematologic and cytogenetic responses. The same response comparisons were then made for patients with and without deletions according to disease phase.
Results
Deletion status and disease characteristics of the patient population
The presence of chromosome 9 deletions was determined in 397 patients treated with imatinib, either by ABL-BCR transcript analysis or by FISH. Fifty-nine of the 391 patients analyzed were found to have deletions of the derivative chromosome 9, an incidence (15%) in keeping with previous reports.10-12,14,15 Twenty-eight patients had a deletion of both chromosome 9 and 22 sequences, 12 patients a deletion of only chromosome 9 sequences, and 5 patients a deletion of only chromosome 22 sequences. Because of the analysis of some patients with the ES probe system, the deletion status of chromosome 22 sequences in 14 patients known to be have a deletion of chromosome 9 was not determined.
Baseline characteristics and disease phase according to deletion status
A number of disease characteristics relating to age, sex, and Sokal and Hasford prognostic score were compared for patients with and without deletions at initial diagnosis (Table 1). No significant differences between the 2 groups were observed. Moreover, there were no differences at the initiation of imatinib therapy between the 2 groups in regard to disease state (CP, AP, BC), type of blast crisis (myeloid versus lymphoid and medullary versus extramedullary), presence of cytogenetic abnormalities in addition to the Ph chromosome, or in a number of other characteristics shown to have prognostic significance for patients treated with imatinib in chronic and accelerated phases of CML23-25 (Table 2).
Response to imatinib by deletion status and disease phase
Deletion status and imatinib response in chronic phase. The rates of CHR, MCR, and CCR were compared between patients in first CP (CP1) with deletions and those without (Table 3). The rates of CHR, MCR, and CCR were uniformly lower in patients with deletions, with a significant difference seen for CHR and MCR (89% versus 97%, patients with a deletion versus those without P = .04 and 55% versus 75%, P = .008, respectively). The CP1 cohort was, therefore, broken down into newly diagnosed patients and the patients failing interferon. In patients who had failed interferon, there was a significant difference between patients with and without deletions for CHR, MCR, and CCR (89% versus 98%, patients with a deletion versus those without, P = .02, 48% versus 72%, P = .008, and 24% versus 45%, P = .02, respectively). By contrast, in newly diagnosed patients, although the group size was small (n = 51), there was no difference in CHR, MCR, or CCR, although again the response rates were uniformly lower in patients with deletions.
Deletion status and imatinib response in advanced phase. Patients in BC or AP were grouped together for analysis of hematologic response (which included the categories of hematologic response described in “Patients, materials, and methods”), MCR, and CCR according to deletion status. A significant difference in hematologic response was seen between patients who carried deletions and those who did not (46% versus 83%, patients with a deletion versus those without, P = .007). No significant difference between cytogenetic response rates was observed in patients with or without deletions. However, as in the case of CP, response rates were lower in patients with deletions.
Survival from diagnosis by deletion status in all patients
To determine the effect of imatinib on the poor prognosis associated with deletions, we compared the overall survival and survival following the initiation of imatinib therapy between patients with deletions and those without. The duration of disease prior to imatinib therapy did not differ between the 2 groups of patients (29 months [interquartile range (IQ), 11-59] versus 30 months [IQ, 11-57], for patients with and without deletions, respectively, P = .71 by Mann-Whitney U test). The median overall follow-up for both groups was also similar (48 months [IQ 30-77] versus 49 months [IQ 28-78], for patients with and without deletions, respectively, P = .95 by Mann-Whitney U test).
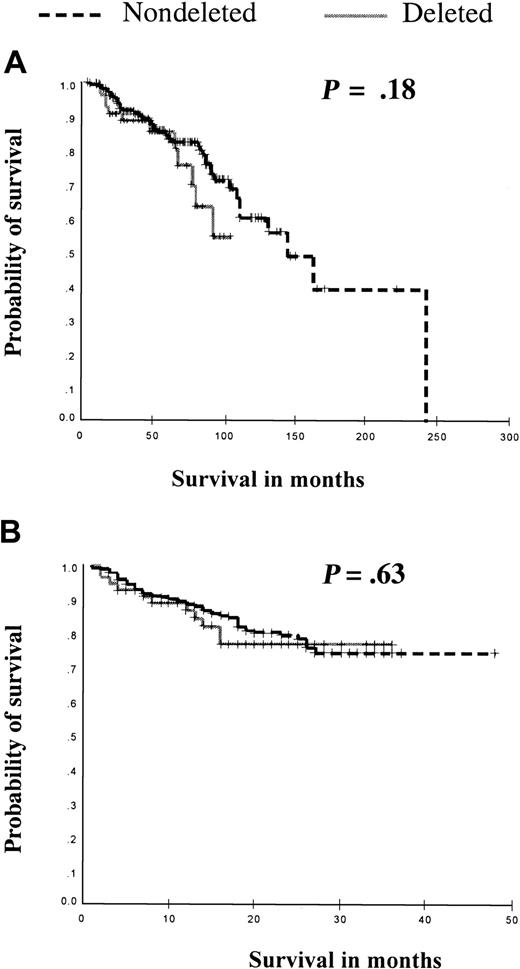
For the whole cohort, the estimated median survival from diagnosis for patients without deletions was 144 months (95% CI, 111-176), whereas the median survival for patients with a deletion could not be estimated as more than 50% of patients were still alive at the time of maximal follow-up. However, there was no significant difference by log-rank analysis (P = .18) (Figure 1). We next compared the survival of all patients with and without deletions following the initiation of imatinib therapy. The median follow-up was similar for both groups (17 months [IQ range, 12-24 months] versus 18 months [IQ range, 12-25 months], for patients with and without deletions, respectively, P = .47 by Mann-Whitney U test). Because of the short follow-up, estimated median survival could not be calculated for either patients with or without deletions, as more than 50% of patients were still alive in both groups. However, there was no significant difference by log-rank analysis (P = .63) (Figure 1).
Kaplan-Meier analysis of overall survival and survival following initiation of imatinib in all patients according to deletion status. (A) Overall survival is compared between those patients with (n = 54) and those without deletions (n = 297). (B) Comparison of survival between patients with (n = 54) and without (n = 300) deletions, following the initiation of treatment with imatinib. No significant differences were seen as compared by log-rank analysis with the P values as compared by log-rank analysis shown.
Kaplan-Meier analysis of overall survival and survival following initiation of imatinib in all patients according to deletion status. (A) Overall survival is compared between those patients with (n = 54) and those without deletions (n = 297). (B) Comparison of survival between patients with (n = 54) and without (n = 300) deletions, following the initiation of treatment with imatinib. No significant differences were seen as compared by log-rank analysis with the P values as compared by log-rank analysis shown.
Survival and time to disease progression following initiation of imatinib
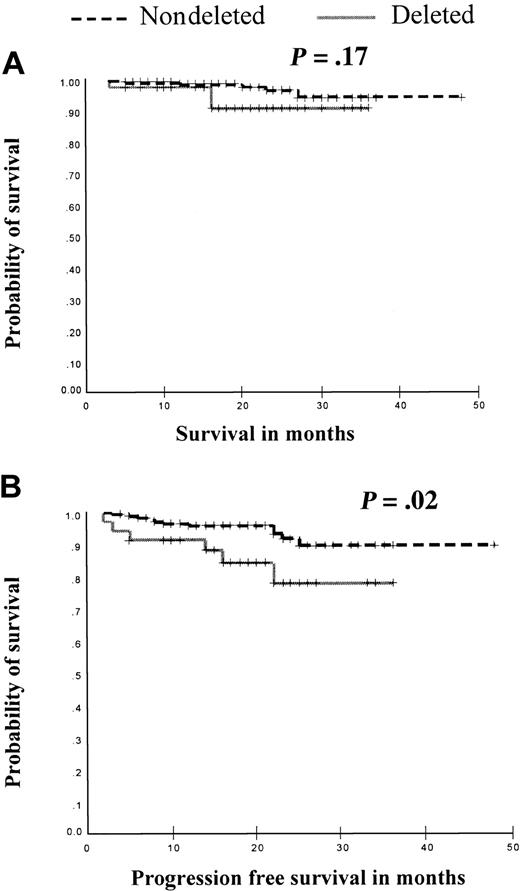
Patients in chronic phase. We next looked at the survival and time to disease progression following the initiation of imatinib of patients in CP (either newly diagnosed or after failing interferon-α) according to deletion status. The median follow-up was similar for both groups (19 months [IQ range, 14-24 months] versus 19 months [IQ range, 15-26 months], for patients with and without deletions, respectively, P = .91 by Mann-Whitney U test). Of the 231 patients in this analysis only 9 patients died; therefore, no survival difference could be determined (P = .17, Figure 2). However, although only 17 of the 207 patients for whom data were available progressed, this represented 6 of 35 (17%) of these patients with deletions but only 11 of 172 (6%) of patients without deletions. Although the estimated median time to progression could not be calculated in either group, there was a significant difference in the rate of progression between the 2 groups with patients with deletions progressing more rapidly (P = .02, Figure 2). This finding suggests that patients in chronic phase with derivative chromosome 9 deletions continue to progress to advanced phase more rapidly than those patients without deletions even when treated with imatinib.
Kaplan-Meier analysis of rate of survival and disease progression in patients in chronic phase following imatinib therapy according to deletion status. (A) Survival from initiation of imatinib was compared in 231 patients (39 with deletions and 192 patients without deletions). No survival differences were seen (P = .17). (B) Progression-free survival was compared in 207 patients (35 with deletions and 172 patients without deletions). Patients with deletions had a significantly shorter time to disease progression (P = .02).
Kaplan-Meier analysis of rate of survival and disease progression in patients in chronic phase following imatinib therapy according to deletion status. (A) Survival from initiation of imatinib was compared in 231 patients (39 with deletions and 192 patients without deletions). No survival differences were seen (P = .17). (B) Progression-free survival was compared in 207 patients (35 with deletions and 172 patients without deletions). Patients with deletions had a significantly shorter time to disease progression (P = .02).
Patients in advanced phase. The same comparisons were then made for patients in the advanced phases of CML. The median follow-up was similar for both groups by Fisher exact test (11 months [IQ range, 5-16 months] versus 13 months [IQ range, 6-18 months], for patients with and without deletions, respectively, P = .07 by Mann-Whitney U test). No survival difference was observed between patients with deletions and those without (median survival, 13 months [95% CI, 11-15] versus 19 months [95% CI, 13-25], for patients with and without deletions, respectively, P = .14, Figure 3). However, when time to disease progression following imatinib therapy was analyzed, there was a significant difference between patients in advanced phase according to deletion status (median time to progression, 8 months [95% CI, 3-13] versus 16 months [95% CI, 13-19], for patients with and without deletions, respectively, P = .02, Figure 3). This finding suggests that the presence of a deletion of the derivative chromosome 9 continues to confer a poor prognosis on patients even after disease progression beyond first CP.
Kaplan-Meier analysis of survival and rate of disease progression in patients in advanced phases following imatinib therapy according to deletion status. (A) Survival from initiation of imatinib was compared in 122 patients (15 with deletions and 107 patients without deletions). No survival differences were seen (P = .14). (B) Progression-free survival was compared in 121 patients (15 with deletions and 106 patients without deletions). Patients with deletions had a significantly shorter time to disease progression (P = .02).
Kaplan-Meier analysis of survival and rate of disease progression in patients in advanced phases following imatinib therapy according to deletion status. (A) Survival from initiation of imatinib was compared in 122 patients (15 with deletions and 107 patients without deletions). No survival differences were seen (P = .14). (B) Progression-free survival was compared in 121 patients (15 with deletions and 106 patients without deletions). Patients with deletions had a significantly shorter time to disease progression (P = .02).
Discussion
Deletions of the derivative chromosome 9 in patients with CML are associated with a poor prognosis. These patients respond less well to conventional drug therapy and have a shorter duration of chronic phase and survival than patients without deletions. We have, therefore, analyzed the hematologic and cytogenetic responses as well as survival and time to disease progression in patients treated with imatinib according to the presence or absence of deletions of the derivative chromosome 9. Those patients with available RNA were initially screened for ABL-BCR expression, followed by the detection of deletion status by FISH in patients who lacked expression of ABL-BCR or for whom only metaphase preparations were available. Two separate, commercially available, FISH probe systems were used, according to the practice of the host clinical institution. These systems are equivalent, except in their ability to detect deletions of chromosome 22 sequences in addition to deletions of sequences from the derivative chromosome 9.31 In this regard, deletions solely of chromosome 22 sequences may have been missed in the 53 patients analyzed with the ES probe system. However, in the analysis of the remaining patients with the dual-color, dual-fusion system such deletions were infrequent (5 of 189, 2.7%) and, therefore, unlikely to influence the results presented.
We did not observe significant differences between patients with and without deletions in regard to demographics, risk factors, and certain disease characteristics (Tables 1, 2), including time from diagnosis. The latter is somewhat surprising, given the more rapid disease progression seen in patients with deletions,14 which implies that time from diagnosis should be shorter in this group of patients. One possible explanation is that the patients without deletions in our cohort constitute a high-risk group that was selected by the entry criteria of the studies.23-25 In fact, only 66 of 338 (20%) patients without deletion were in CP1 and either newly diagnosed or intolerant to interferon-α and were, thus, not selected on the basis of progression beyond CP or resistance to interferon.
Overall, hematologic and cytogenetic response rates in our cohort accord with recently published results for imatinib treatment of CML patients in the chronic, accelerated and blastic phase of their disease.22-26 We demonstrate that, generally, response rates to imatinib are comparable in most groups of patients with CML with and without a deletion of the derivative chromosome 9, in all disease phases (Table 2). However, the response rates were uniformly lower in patients with deletions and there was a significantly lower rate of hematologic response in both chronic phase and advanced phases of CML, and of MCR in chronic phase CML. A failure to achieve MCR is associated with an increased risk of disease progression on imatinib.32,33 Although the deletion status of the cohorts in which this progression is described is not known, the data are compatible with the reduced rate of MCR and increased risk of disease progression that we observed in our patients, both in CP and advanced phase CML.
Analysis of overall survival failed to show a significant difference between patients with and without deletions, both in regard to survival from diagnosis and from the start of imatinib therapy. Regarding survival from diagnosis, this observation is in contrast to our previous reports of patients diagnosed over a similar time period, in which, with comparable follow-up, the survival of patients with deletions was significantly shorter compared with patients without deletions, (P = .0001 in the first cohort followed for 34 months,15 and P = .03 in a second cohort followed for 39 months,28 Figure 4). The most likely variable to explain the decrease in survival difference between patients with and without deletion is the number of patients treated with imatinib. In the initial cohort no patient received imatinib therapy, whereas around 80% of patients in the second cohort had received the drug. Thus, imatinib renders at least the short-term prognosis of patients with deletions equal to the prognosis of patients without deletion. This finding is indirect evidence that imatinib improves the survival of patients with deletions relative to other treatment modalities. However, because of the cohort size and the short median follow-up, the power of our study to detect a survival difference making conservative assumptions (a median survival of 7 years in the nondeleted group and 5 years in the deleted group, and a ratio of nondeleted-to-deleted patients of 6:1) was only 0.14, that is, a 14% chance of being able to show a difference in survival, assuming there is a true difference at 2 years.34 Therefore, given the higher rate of disease progression in patients with deletions, it is likely that a difference in survival from patients without deletion may become apparent with longer follow-up. To resolve these issues definitively, a direct comparison between patients with deletions treated up-front with interferon-α versus imatinib and between patients with and without deletions treated up-front with imatinib will be required. An effort to collect these data retrospectively from an ongoing phase 3 protocol22 is currently being made.
The survival difference between patients with and without deletions narrows with the introduction of imatinib therapy. Kaplan-Meier analysis is shown for survival differences according to deletion status in 2 previously reported cohorts (A)15 and (B)28 and the cohort in this study to the same scale. No formal comparisons could be made between the 3 groups, but within each group the patients with and without deletions had similar disease characteristics. The median follow-up for each group is comparable. Note that the highly significant difference in survival (analyzed by log-rank test) gradually disappears with the inclusion of more patients treated with imatinib.
The survival difference between patients with and without deletions narrows with the introduction of imatinib therapy. Kaplan-Meier analysis is shown for survival differences according to deletion status in 2 previously reported cohorts (A)15 and (B)28 and the cohort in this study to the same scale. No formal comparisons could be made between the 3 groups, but within each group the patients with and without deletions had similar disease characteristics. The median follow-up for each group is comparable. Note that the highly significant difference in survival (analyzed by log-rank test) gradually disappears with the inclusion of more patients treated with imatinib.
The differences in hematologic and cytogenetic response rates according to deletion status seen in newly diagnosed patients compared with those in chronic phase who have failed interferon-α are intriguing and may have 3 plausible explanations. The first and very important possibility is that imatinib may completely abrogate the poor prognosis associated with derivative chromosome 9 deletions if given early enough in the course of CML. The second possibility is that the size of the cohort of newly diagnosed patients is simply too small to show any differences. The presumed molecular mechanism underlying the deletions is loss of a tumor suppressor gene,31 and, because of the heterogeneity of the size and type (chromosome 9 and/or 22 sequence loss) of deletions, it is likely that only a subgroup of patients with deletions actually carry this molecular lesion. However, the third explanation is that the molecular lesion associated with deletion of derivative chromosome 9 sequences may require time to irrevocably drive disease progression, irrespective of imatinib treatment, and however early it is started. These explanations would accommodate both a classical tumor suppressor model, in which the lag-time may be the inactivation of the remaining allele and haploinsufficient models in which a period of haploinsufficiency may be required to drive disease progression beyond any possible imatinib rescue. All of these possibilities further emphasize the need for a direct comparison, in large numbers of patients with adequate follow-up, between patients with and without deletions treated up front with imatinib.
Do the overall results suggest anything about the potential molecular lesion associated with deletions of the derivative chromosome 9? The improvement in survival seen with imatinib, which contrasts with such patients treated with interferon-α (Figure 4), indicates that the rapid progression of CML in patients with deletions requires continued Bcr-Abl PTK activity. However, the significantly lower rate of cytogenetic and hematologic responses and higher rate of progression of patients in chronic phase argue that inhibition of Bcr-Abl alone may not completely correct the phenotype of patients with deletions. Taken together, this suggests that the molecular aberration present in patients with derivative chromosome 9 deletions cooperates with, but is not wholly dependent on, Bcr-Abl to produce the poor prognosis in these patients. The longer follow-up of newly diagnosed patients with deletions treated with imatinib will confirm or deny this hypothesis.
Our results indicate that treatment with imatinib improves the prognosis of patients with CML who carry deletions of the derivative chromosome 9. However, significantly lower hematologic and cytogenetic response rates in chronic phase and hematologic response rates in more advanced phases along with a more rapid disease progression in both phases of CML suggest that imatinib does not completely reverse their poor prognosis and that survival differences may eventually be seen. Longer follow-up and the study of more patients treated up front are required to define the effect of chromosome 9 deletions on the prognosis of patients with CML treated with imatinib.
Prepublished online as Blood First Edition Paper, May 15, 2003; DOI 10.1182/blood-2002-09-2763.
Supported by the Leukemia Research Fund (LRF; United Kingdom) and the Kay Kendall Leukemia Fund (B.J.P.H., A.G.R., E.P.N., and A.R.G.). B.J.P.H. was previously funded by a Medical Research Council (United Kingdom) Clinical Training Fellowship and is currently an LRF (United Kingdom) Senior Clinical Fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Steven O'Brien and Anne Lennard for patient samples and helpful discussion; Kath Andrews, Ian Carter, Kate Martin, Francoise Brizard, and Laurence Daheron for assistance with samples; Peter Campbell for statistical discussions; Christel Mueller for technical excellence; Sandra Otto for data management; and Haifa-Kathrin Al-Ali and Phyllis Paterson for dedicated patient care.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal