Abstract
Expression of one or more natural killer (NK) receptors on T cells may correlate with effector function. This study investigated the frequency of neonatal NK receptor–positive (NKR+) T cells and their expansionary properties with interleukin-2 (IL-2), IL-7, or IL-15. While cord blood contains significantly decreased frequencies of NKR+ T cells compared with adult blood, newborn CD56+CD3+ cells could be expanded 200-fold during culture with IL-15. By depleting CD56+ cells, we were able to determine that this expansion was due to a subpopulation of T cells acquiring CD56 expression. Moreover, CD56 acquisition was associated with a distinct CD8+CD25+ interferon γ–positive (IFN-γ+) phenotype. This property could therefore be exploited during bone marrow reconstitution and may partially account for the resilience of the newborn to infection.
Introduction
The low frequency and severity of graft-versus-host disease (GVHD) following cord blood transplantation is thought to be largely due to the “naivety” of cells present in cord blood.1,2 Compared with adult T cells, neonatal T cells secrete increased levels of interleukin-10 (IL-10) following stimulation,3 but reduced levels of many other cytokines, including IL-2, IL-4, IL-8, interferon γ (IFN-γ), transforming growth factor β (TGF-β), and tumor necrosis factor α (TNF-α).4-7 In addition to classical T cells, natural killer (NK) and NKT cells may also be partially responsible for the low incidence of GVHD and may play an important role in protecting the newborn against infection. Interestingly, NK cells are one of the first populations to recover following cord blood transplantation,8 and while host NK cells mediate graft rejection,9 donor NK cell alloreactivity decreases leukemia relapse and protects patients against GVHD.10 In contrast to NK cells, little attention has been placed on the possible protective roles of NKT cells. NKT cells are distinguished by a highly conserved T-cell receptor (TCR) repertoire, composed of an invariant TCR α-chain (Vα24-JαQ) and a biased set of TCR β-chains (Vβ11), although most also express the NK cell marker CD56.11 Since NKT cells comprise less than 0.1% lymphocytes in adult and cord blood,12 there is likely to be a wider population of CD56+ T cells that display characteristics of NKT cells but do not express an invariant TCR. These cells may be of particular importance since effector function of CD8+ cells correlates with CD56 expression.13 The aims of this study were to investigate whether NK receptor–positive (NKR+) T cells are present in, or may be expandable from, cord blood, and whether such cells may provide the newborn with a phenotypically distinct cytotoxic population.
Study design
Sample preparation
Cord blood and peripheral blood samples were obtained from healthy subjects. Mononuclear cells were isolated by density centrifugation (Lymphoprep; Nycomed, Oslo, Norway). Informed consent was obtained in all cases and the project received local ethical committee approval.
Flow cytometry
Cord blood mononuclear cells (CBMCs) or peripheral blood mononuclear cells (PBMCs) were stained with peridinin chlorophyll protein (PerCP)–labeled anti-CD3, anti-CD56 (fluorescein isothiocyanate [FITC]– or phycoerythrin [PE]–labeled), and one of the following antibodies: FITC-labeled anti-CD45RA, anti-CD25, anti-NKB1, anti-CD57, anti-TCRVα24, anti-CD158a; PE-labeled anti-CD8, anti-CD45RO, anti-CD62L, anti-CD16, anti-CD161, anti-CD94, anti-TCRγδ, or anti-CD158b. Levels of perforin, IFN-γ, and IL-2 were assessed by intracellular staining.14,15 CBMCs/PBMCs were also stained with isotype-matched controls.
Phenotypic analysis of lymphocyte subsets (gated using forward- and side-scatter parameters) was performed by 3-color monoclonal antibody staining and flow cytometry.
Culture of CD56– CBMCs
CBMCs were depleted of CD56+ cells using anti-CD56 microbeads (Miltenyi Biotec, Bisley, United Kingdom). Depleted CBMCs (< 1% CD56+) were resuspended to 1 × 106/mL in complete RPMI medium (RPMI 1640 containing 25 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 2 mM l-glutamine, antibiotics, 10% fetal calf serum), cultured with 50 U/mL IL-2, 10 ng/mL IL-7, or 10 ng/mL IL-15 (R&D Systems, Oxon, United Kingdom), and restimulated every 3 to 4 days. Cells were harvested on days 7, 14, 21, and 28, and analyzed by flow cytometry.
Analysis of cell division
Quantification of dividing cells in response to IL-15 was achieved by labeling with carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Leiden, Holland) prior to culture.16
Statistical analysis
Statistical analysis was performed using the Mann-Whitney test; P < .05 was considered significant.
Results and discussion
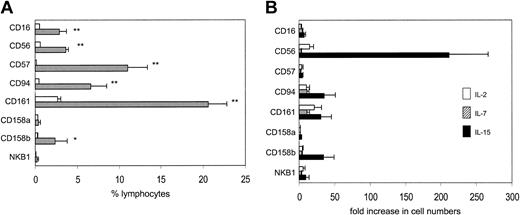
TCR-restricted NKT cells are present in comparable frequencies in adult and cord blood.12 However, to investigate the overlap between NKT and NKR+ T cells, we determined the frequency of lymphocytes that coexpress CD3 with 1 of 8 NK (CD16, CD56, CD57, CD94, CD161) or killer cell immunoglobulin-like receptor (KIR) (CD158a, CD158b, NKB1) molecules. All NKR+ T-cell subsets were decreased in cord blood compared with adult blood (Figure 1A), and all were predominantly TCRVα24–, suggesting that NKR+ T cells are much more heterogeneous than NKT cells.
Cord blood contains a low frequency of NK receptor–positive T cells, which can be expanded with common γ-chain cytokines. (A) Cord blood (□) and adult mononuclear cells (▧) were stained for CD3 and 1 of 8 NK/KIR molecules. (B) CBMCs were cultured with IL-2, IL-7, or IL-15, harvested on day 14, and stained for CD3 and 1 of 8 NK/KIR molecules. Cell expansions are expressed as fold increase in the number of cells present after culture, compared with the numbers present before culture. Results are means (± SEMs) of 5 to 9 samples. *P < .01; **P < .005.
Cord blood contains a low frequency of NK receptor–positive T cells, which can be expanded with common γ-chain cytokines. (A) Cord blood (□) and adult mononuclear cells (▧) were stained for CD3 and 1 of 8 NK/KIR molecules. (B) CBMCs were cultured with IL-2, IL-7, or IL-15, harvested on day 14, and stained for CD3 and 1 of 8 NK/KIR molecules. Cell expansions are expressed as fold increase in the number of cells present after culture, compared with the numbers present before culture. Results are means (± SEMs) of 5 to 9 samples. *P < .01; **P < .005.
As neonatal CD4+CD45RA+ T cells proliferate to common γ-chain cytokines,14,17 we examined whether neonatal NKR+ T cells could be expanded with IL-2, IL-7, or IL-15. Following 14 days in culture, most NKR+ T-cell subsets showed marginal expansion, but by far the greatest expansion was seen in the CD56+ T-cell population with IL-15 (>200-fold, versus 15-fold with IL-2; Figure 1B). Adult CD56+ T cells could also be expanded with IL-2 and IL-15, although the level of expansion was lower than that for neonatal CD56+ T cells (3-fold and 19-fold, respectively; not shown). These results are consistent with those of a recently published tumor model in which IL-15 induced significantly greater expansion and survival of human CD8 effector T cells, compared with IL-2.18
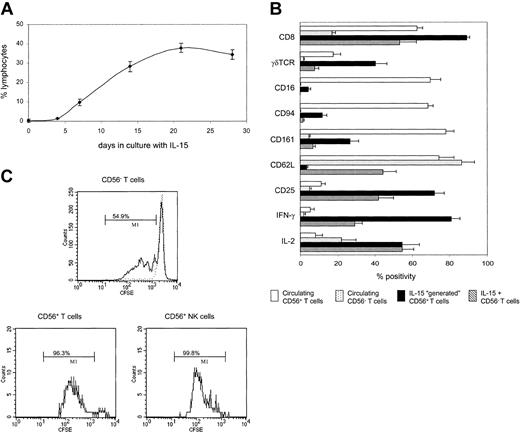
To determine if the CD56+ T cells were derived from simple expansion or from induction of CD56 expression, we depleted CD56+ cells from CBMCs prior to culture. Figure 2A shows that IL-15 can drive the “generation” of CD56+ T cells from a CD3 population that was previously CD56–. This process was rapid and long lasting, so that after 14 days of incubation, one third of all viable lymphocytes expressed the CD56+CD3+ phenotype. Lower numbers of CD56+CD3– NK cells (10.4% lymphocytes) were also generated in this system.
IL-15 drives the generation of neonatal CD56+ T cells from CD56– populations. (A) CD56-depleted populations were cultured with IL-15, harvested on days 4, 7, 14, 21, and 28, and labeled with CD3 and CD56. Results show the generation of CD56+ T cells as a percentage of total lymphocytes. (B) CD56-depleted cells were cultured with IL-15 for 14 days and labeled with CD3, CD56, and 1 of 17 other antibodies. By gating dotplots of CD3 versus CD56, 3 distinct subpopulations could be analyzed: CD56+CD3+ T cells, CD56+CD3– NK cells, and CD56–CD3+ T cells. The cell surface and intracellular cytokine phenotype of generated CD56+ T cells was compared with that of IL-15–expanded CD56– T cells and circulating T cells (CD56+ and CD56–). Data, shown for 9 antibodies, are expressed as percent positivity within each cell population. (C) CD56-depleted cells were labeled with CFSE prior to culture with IL-15 for 7 days. CFSE-labeled cells were also cultured with low levels of IL-4 (dotted line) in order to maintain a viable but nondividing control population. Cells were stained for CD3 and CD56, and cell division within the 3 cell populations was determined by flow cytometry as in panel B. Bars indicate the percentage of cells that have undergone at least one division. Results are means (± SEMs) of 5 to 9 samples (A-B) or representative of 6 samples (C).
IL-15 drives the generation of neonatal CD56+ T cells from CD56– populations. (A) CD56-depleted populations were cultured with IL-15, harvested on days 4, 7, 14, 21, and 28, and labeled with CD3 and CD56. Results show the generation of CD56+ T cells as a percentage of total lymphocytes. (B) CD56-depleted cells were cultured with IL-15 for 14 days and labeled with CD3, CD56, and 1 of 17 other antibodies. By gating dotplots of CD3 versus CD56, 3 distinct subpopulations could be analyzed: CD56+CD3+ T cells, CD56+CD3– NK cells, and CD56–CD3+ T cells. The cell surface and intracellular cytokine phenotype of generated CD56+ T cells was compared with that of IL-15–expanded CD56– T cells and circulating T cells (CD56+ and CD56–). Data, shown for 9 antibodies, are expressed as percent positivity within each cell population. (C) CD56-depleted cells were labeled with CFSE prior to culture with IL-15 for 7 days. CFSE-labeled cells were also cultured with low levels of IL-4 (dotted line) in order to maintain a viable but nondividing control population. Cells were stained for CD3 and CD56, and cell division within the 3 cell populations was determined by flow cytometry as in panel B. Bars indicate the percentage of cells that have undergone at least one division. Results are means (± SEMs) of 5 to 9 samples (A-B) or representative of 6 samples (C).
To investigate whether the generated CD56+ T cells showed characteristics of T cells or NK cells, CD56-depleted CBMCs were incubated with IL-15, harvested after 14 days, and stained for CD3, CD56, and a number of other cell surface and intracellular molecules. NK cells exhibit differential cytotoxic capabilities, with CD56dim cells containing 10-fold more perforin and granzyme A than CD56bright cells.15 The CD56+ T cells generated during our cultures were largely restricted to the CD56dim phenotype.
IL-15 is known to expand both CD8+ T cells19 and γδTCR+ T cells.20 Indeed, 89% of the generated CD56+ T cells were CD8+, and 40% expressed a γδTCR (Figure 2B). Most of these generated cells were also CD25+ and were capable of producing IFN-γ. However CD8, CD25, γδTCR, and IFN-γ were all expressed more frequently by CD56+ T cells, compared with those CD3 cells that did not acquire CD56, as was the case with perforin (45% versus 1.4%, respectively; not shown). Furthermore, while distribution of CD45RA and CD45RO was similar on CD56+ compared with CD56– T cells (data not shown), the CD56+ T-cell population showed a down-regulation of the lymph node homing receptor, L-selectin (CD62L). Loss of CD62L has been associated with a switch from naive to memory phenotype, and neonatal TCR-restricted NKT cells express low levels of CD62L, in addition to high levels of CD25.21 In contrast, the CD56+ T cells generated in culture were predominantly TCRVα24– (data not shown). Taken together, these data suggest that CD56+ T cells generated with IL-15 are highly activated effector cells.
The phenotype of IL-15–generated CD56+ T cells also contrasted sharply with that of circulating CD56+ T cells, particularly in respect to coexpression of other NK cell molecules. While most of circulating CD56+ T cells expressed CD16, CD94, and CD161, generated CD56+ T cells expressed these markers less frequently (Figure 2B), reflecting their T-cell lineage.
Since NK cells proliferate in response to IL-15,22 we then looked at whether CD56+ T cells show a similar kinetics of response. By labeling CD56-depleted CBMCs with CFSE prior to culture, we were able to determine the level of cell division within each population. After 7 days in culture, almost all NK and CD56+ T cells had undergone at least one division, compared with only 54% to 68% of CD56– T cells (Figure 2C). Hence CD56 expression is also associated with increased ability to proliferate in response to IL-15.
In conclusion, we have shown that cord blood contains significantly decreased frequencies of NKR+ T cells compared with adult blood, and that only the CD56+CD3+ subset is susceptible to major expansion with IL-15. We have further shown that rather than being a receptor linked to lineage, CD56 may be induced on cells that were previously CD56–,in an antigen-independent manner. Acquisition of CD56 on T cells was associated with a unique phenotype that was T cell–like in surface marker expression, but NK cell–like in both cytokine production and in proliferative response to IL-15. Recently, NKR+ T cells isolated from tumor-bearing mice have been shown to exhibit potent NK-like cytotoxic activity against a range of tumor targets.23 While there is no murine homolog of CD56, these cytotoxic cells displayed similar characteristics to the CD56+ T cells generated in the study presented here. Moreover, in separate studies of murine lymphoma, cotransfer of allogeneic stem cells with expanded CD8+ NKR+ T cells resulted in substantial antitumor activity but minimal GVHD.24 The ability of newborn T cells to acquire CD56 may therefore not only provide the neonate with a cytotoxic cell population that can be rapidly expanded and mobilized following infection, but also may be of therapeutic potential in the treatment of hematopoietic malignancy.
Prepublished online as Blood First Edition Paper, May 22, 2003; DOI 10.1182/blood-2003-01-0232.
Supported by Conway Institute of Biomolecular and Biomedical Research, University College Dublin, Ireland.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are extremely grateful to Julie Grantham and the nursing staff of the Coombe Women's Hospital (Dublin) for collecting cord blood samples. We would also like to thank Dr Cliona O'Farrelly (St Vincent's Hospital, Dublin), Dr Derek Doherty (National University of Maynooth, Co Kildare), and Dr Stephen Todryk (Trinity College, Dublin) for useful discussion.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal