Abstract
Dendritic cells (DCs) have been thought to follow a life history, typified by Langerhans cells (LCs), with 2 major developmental stages: an immature stage that captures antigens in the periphery and a mature stage that presents those antigens in the lymphoid organs. However, a systematic assessment of the maturity of lymphoid organ DCs has been lacking. We have analyzed the maturity of the DC types found in the steady state in the spleen, lymph nodes (LNs), and thymus. The DCs that migrate into the iliac, mesenteric, mediastinal, or subcutaneous LNs from peripheral tissues were mature and therefore could not process and present newly encountered antigens. However, all the other DC types were phenotypically and functionally immature: they expressed low levels of surface major histocompatibility complex class II (MHC II) and CD86, accumulated MHC II in their endosomes, and could present newly encountered antigens. These immature DCs could be induced to mature by culture in vitro or by inoculation of inflammatory stimuli in vivo. Therefore, the lymphoid organs contain a large cohort of immature DCs, most likely for the maintenance of peripheral tolerance, which can respond to infections reaching those organs and mature in situ.
Introduction
Dendritic cells (DCs) are highly specialized antigen-presenting cells that undergo complex developmental changes. The term “maturation” was originally coined to describe the phenotypic and functional changes undergone by Langerhans cells (LCs; a DC type) extracted from the skin during culture in vitro.1 During maturation, the LCs changed their surface phenotype, their capacity to stimulate naive T cells, and their capacity to process and present antigens. From this, 2 developmental stages, “immature” and “mature,” were defined and later reproduced in cultures of DCs grown in vitro from bone marrow, spleen, or blood precursors.2-6 The immature DCs are very efficient at capturing antigens, but they are inefficient at presenting those antigens on their major histocompatibility complex class II (MHC II) molecules and are poor T-cell stimulators. Inflammatory stimuli activate the immature DCs to become “mature.” Mature DCs display several distinctive features: (1) they gain the capacity to activate naive T cells owing to their high expression levels of costimulatory molecules such as CD40, CD80, and CD867 ; (2) they are very efficient at presenting the antigens they captured at the time of receiving the maturation stimulus; and (3) they cannot process and present newly encountered antigens.
In vivo, immature and mature DCs have been generally thought to occur in distinct anatomic locations: immature DCs capture antigens in peripheral tissues, while mature DCs present those antigens in the draining lymphoid organs. Indeed, studies tracking the phenotype of DCs that captured antigens in the skin, the lung, or the gut and then migrated to their corresponding draining lymph nodes (LNs) support this “LC paradigm.”8-12 However, it is not clear if this scheme applies to all the DCs present in lymphoid organs in the steady state.13 For instance, splenic DCs show considerable phagocytic activity14,15 and express low levels of costimulatory molecules,16,17 2 hallmarks of immaturity. An additional complication is the phenotypic heterogeneity among lymphoid organ DC populations.18 It is possible that some discrete populations follow the LC paradigm, while other populations behave differently.
Characterizing the maturational state of the lymphoid organ DCs is important because several studies indicate that immature DCs are involved in maintaining tolerance. Since the presentation of foreign antigens is always accompanied by that of self components, mature DCs could activate autoreactive T cells circulating through the secondary lymphoid organs. To prevent this, it has been proposed that immature DCs are responsible for the induction of tolerogenic mechanisms19-22 (reviewed in Steinman and Nussenzweig23 ). However, this would require the presence of immature DCs in the lymphoid organs. Therefore several questions remain. Are there immature DCs in the lymphoid organs in the steady state? How abundant are they? Do all the DC types follow the life history typified by LCs?
Here we present a comprehensive phenotypic and functional comparison of the maturational state of the different DC types found in the LNs, spleen, and thymus of conventional laboratory mice. The only DC types that had a mature phenotype were found in the LNs, where they accounted for approximately 50% of the total DCs. These mature DCs expressed the surface markers characteristic of DCs that traffic from the skin, the respiratory epithelium, the gut, and probably other mucosal surfaces, into their corresponding draining LNs. The other LN DC types, and all the splenic and thymic DCs, were immature. These immature lymphoid organ DCs could be induced to mature by in vitro culture, or in vivo, by injecting mice with inflammatory compounds such as lipopolysaccharide (LPS) or CpG oligonucleotides. Therefore, most lymphoid organ DC types do not follow the life cycle typified by LCs. They are present in the lymphoid organs in an immature state, perhaps dedicated to maintaining peripheral tolerance in the steady state. However, they retain the potential to respond to inflammatory signals and mature in situ, a capacity that may enable them to respond to infections reaching the lymphoid organs.
Materials and methods
Mice and injections of LPS in vivo
The mice used were 6- to 8-week-old C57Bl/6 and ovalbumin (OVA)– specific, MHC II–restricted, T-cell–receptor transgenic mice (OT-II mice).24 Where indicated, mice were injected in the tail vein with 3 μg lipopolysaccharide (LPS; Sigma, St Louis, MO) or 50 nmol synthetic phosphorothioated CpG166825 (GeneWorks, Adelaide, Australia) dissolved in phosphate-buffered saline (PBS), and killed 12 hours later. The animals were bred and treated at The Walter and Eliza Hall Institute animal breeding facility according to institutional guidelines.
Antibodies and flow cytometry
The antibodies used for preparative or analytic fluorescence-activated cell sorting (FACS) were hamster monoclonal antibody (mAb) N418 (anti-CD11c), rat mAb YTS (anti-CD8), rat mAb GK1.5 (anti-CD4), hamster mAb NLDC-145 (anti-CD205), rat mAb M5/114 (anti-MHC II), and rat mAb GL1 (anti-CD86). All the antibodies were purified and conjugated to fluorochromes (fluorescein isothiocyanate [FITC], phycoerythrin [PE], Texas Red, or Cy5) in our laboratory. Samples were analyzed using a FACS II, FACStarPlus, FACScan, or LSR instrument (Becton Dickinson, San Jose, CA). Preparative FACS was performed using a Mo-Flo (Cytomation, Fort Collins, CO) or a Diva (Becton Dickinson) high-speed sorter.
Dendritic cell preparation
Spleen, subcutaneous LNs, mesenteric LNs, and thymus DCs were purified as described.8,26 DCs from the iliac LNs and the mediastinal LNs were purified following the same protocol but omitting the cell density purification step. B220+ cells, including plasmacytoid DCs, were excluded during the magnetic bead–depletion step. The whole process of DC purification and FACS sorting was carried out on ice except for the initial 20-minute digestion of the lymphoid organs with collagenase/DNase, which was done at room temperature. Culture of the lymphoid organ DCs was performed in RPMI 1640 supplemented with 10% fetal calf serum (FCS) and 200 units/mL granulocyte-macrophage colony-stimulating factor (PeproTech, Rocky Hill, NJ). DCs derived from bone marrow precursors (BMDCs) were generated exactly as described5 and stimulated by addition of 1 μg/mL LPS for 20 hours.
Induction of OT-II proliferation in vitro
DCs were incubated with the indicated concentration of OVA (Sigma) or synthetic OVA323-339 peptide (Auspep, Melbourne, Australia) for 45 minutes at 37°C in culture medium, washed 3 times, and then plated in round-bottom 96-well plates at 5 × 103 cells/well. The naive OT-II cells were purified from transgenic mice and labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) as described.27 The CFSE-labeled OT-II cells were added to the DCs at 5 × 104 cells/well. The plates were incubated for 2.5 days and then the cells were harvested and resuspended in 200 μL balanced salt solution (BSS)/EDTA (ethylenediaminetetraacetic acid)/3% FCS containing 2.5 × 104 Callibrite beads (Becton Dickinson). Samples were analyzed by FACS until 1 × 104 beads were collected. The number of dividing (propidium iodide [PI–], CFSElow) OT-II cells was then determined (Figure 3A). For the experiments using fixed DCs, fixation was performed at room temperature by incubation for 10 minutes in 4% paraformaldehyde (PFA) in PBS followed by 3 washes. The fixed DCs were incubated with 5 × 104 CFSE-labeled OT-II cells in the presence of the indicated concentration of synthetic OVA323-339 peptide. OT-II proliferation was assessed 2.5 days later.
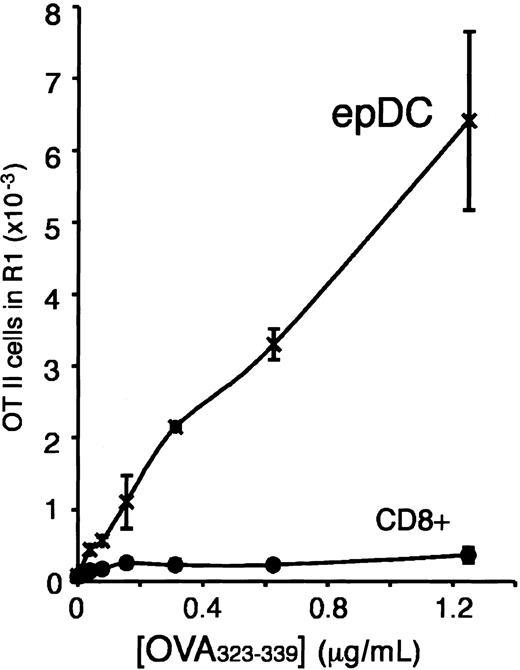
Antigen presentation and T-cell stimulation by the lymphoid organ DCs. (A) FACS analysis of proliferation of CFSE-labeled OT-II cells. DCs were incubated without (top) or with (center) OVA for 45 minutes, washed, and then incubated with CFSE-labeled OT-II cells for 2.5 days. The number of live (PI–), proliferating OT-II cells (region R1) was then determined by FACS analysis. The histogram at the the bottom shows the number of dividing OT-II cells obtained in response to DCs that had been incubated with the indicated concentrations of OVA. (B) Induction of OT-II proliferation by the spleen, thymus, and subcutaneous LN DC populations. DCs were incubated for 45 minutes with the indicated concentrations of OVA protein (left) or OVA323-339 peptide (right), washed, and then incubated with CFSE-labeled OT-II T cells for 2.5 days. The amount of proliferating OT-II cells was determined as in panel A. The data shown are the average of duplicate determinations. The results in each panel were obtained in a single experiment in which all the DC populations described in Figure 1A were purified and analyzed in parallel. The number of thymic DCs (TDC) in this particular experiment allowed us to analyze only one concentration of OVA. Multiple experiments have been performed comparing only the populations from a single organ or 2 organs, and the results were consistent with the data shown. (C) Presentation of OVA and induction of OT-II proliferation by CD8+CD205+ DCs and epDCs purified from mesenteric LNs. The experiment was performed as described in panel B, left panel. (D) Antigen presentation of hen egg lysozyme (HEL) to the hybridoma BO4H9.1. The indicated number of the splenic and subcutaneous LN DC populations was incubated for 18 hours with the BO4H9.1 T-cell hybridoma in the presence of 0.1 mM HEL. The amount of IL-2 released was then determined using a standard CTLL2 proliferation assay. The data shown were obtained in triplicate and are representative of 2 experiments.
Antigen presentation and T-cell stimulation by the lymphoid organ DCs. (A) FACS analysis of proliferation of CFSE-labeled OT-II cells. DCs were incubated without (top) or with (center) OVA for 45 minutes, washed, and then incubated with CFSE-labeled OT-II cells for 2.5 days. The number of live (PI–), proliferating OT-II cells (region R1) was then determined by FACS analysis. The histogram at the the bottom shows the number of dividing OT-II cells obtained in response to DCs that had been incubated with the indicated concentrations of OVA. (B) Induction of OT-II proliferation by the spleen, thymus, and subcutaneous LN DC populations. DCs were incubated for 45 minutes with the indicated concentrations of OVA protein (left) or OVA323-339 peptide (right), washed, and then incubated with CFSE-labeled OT-II T cells for 2.5 days. The amount of proliferating OT-II cells was determined as in panel A. The data shown are the average of duplicate determinations. The results in each panel were obtained in a single experiment in which all the DC populations described in Figure 1A were purified and analyzed in parallel. The number of thymic DCs (TDC) in this particular experiment allowed us to analyze only one concentration of OVA. Multiple experiments have been performed comparing only the populations from a single organ or 2 organs, and the results were consistent with the data shown. (C) Presentation of OVA and induction of OT-II proliferation by CD8+CD205+ DCs and epDCs purified from mesenteric LNs. The experiment was performed as described in panel B, left panel. (D) Antigen presentation of hen egg lysozyme (HEL) to the hybridoma BO4H9.1. The indicated number of the splenic and subcutaneous LN DC populations was incubated for 18 hours with the BO4H9.1 T-cell hybridoma in the presence of 0.1 mM HEL. The amount of IL-2 released was then determined using a standard CTLL2 proliferation assay. The data shown were obtained in triplicate and are representative of 2 experiments.
Antigen-presentation assay to T-cell hybridomas
Purified DCs were incubated for 20 hours in 96-well plates with 5 × 104 BO4H9.1 (anti–I-Ab-HEL74-88)28 or 1 × 105 OT-II (anti–I-Ab-OVA323-339) hybridoma T cells in the presence of 0.1 mM hen egg lysozyme (HEL) or OVA (Sigma), respectively. Interleukin-2 (IL-2) secretion was measured by a standard CTLL-2 proliferation assay or by enzyme-linked immunosorbent assay (ELISA).
Immunofluorescence confocal microscopy (ICM)
Cells were attached to glass coverslips, fixed, permeabilized, and stained for ICM as described.5 Glass coverslips were coated with hamster anti–MHC II mAb N22 by placing a drop of a 10 μg/mL solution of the purified Ab on top of the coverslip for one hour at 37°C and then they were washed. Cells were incubated in BSS/EDTA/3% FCS on the coverslips for 30 minutes; the coverslips were then covered with 1 mL RPMI 1640 (10% FCS) and the incubation continued for another 30 minutes. Analysis of DCs attached to polylysine-coated coverslips yielded similar results to those shown, but in this case the resolution of the intracellular compartments was much poorer. Following attachment, cells were washed, fixed for 10 minutes in 4% PFA dissolved in PBS, and washed. Fixed cells were then permeabilized by incubation for 15 minutes in PBS containing 10% normal goat serum and 0.05% saponin (Sigma). The coverslips were then incubated for 30 minutes with rat anti-Lamp1 mAb 1D4B (Becton Dickinson) and biotinylated N22 diluted in permeabilization buffer, washed 3 times, and then incubated for 30 minutes in permeabilization buffer containing the secondary reagents. These were streptavidin conjugated to FITC or Cy5 (Amersham, Castle Hill, Australia) and goat antirat serum conjugated to FITC (Caltag, Burlingame, CA) or donkey antirat serum conjugated to Texas Red (Jackson Immunoresearch, West Grove, PA). Where indicated, DAPI (4′,6-diamidino-2-phenyindole; Sigma) was also included in the second incubation to label the nuclei. The coverslips were then mounted on microscopy slides using Fluorescent Mounting Medium (Dako, Carpinteria, CA) containing 100 μg/mL DABCO (1,4-diazabicyclooctane; Sigma). The pattern of staining obtained with an anti–I-Abα rabbit serum was similar to that obtained with biotinylated mAb N22. No intracellular staining was observed in the absence of saponin, and no staining occurred if the primary antibodies were omitted (data not shown). ICM analysis was performed with a Leitz Fluovert FU or a Leica STS SP2 confocal microscopes (Leica Microsystems, Wetzlar, Germany).
Results
Phenotype of freshly isolated spleen, lymph node, and thymus DCs
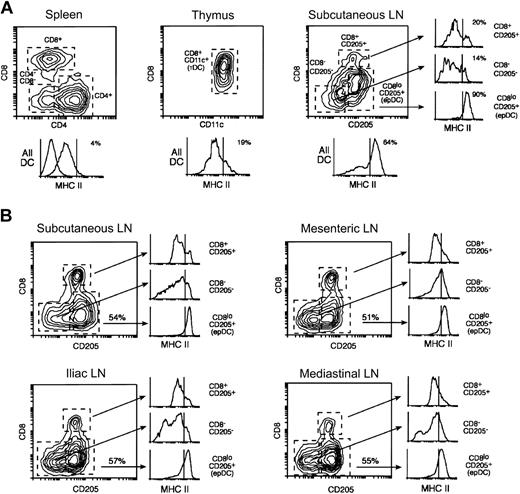
The DCs of mouse spleens can be subdivided into 3 populations based on their expression of CD4 and CD8: CD4+CD8– DCs (“CD4+ DCs” hereafter), CD4–CD8+ DCs (“CD8+ DCs”) and CD4–CD8– DCs (“CD4–CD8– DCs”) (Figure 1A).26 The CD8+ DCs also express CD205, which is absent in the other 2 populations. The DCs found in the mouse thymus are more homogeneous and all express CD8 (Figure 1A).26 Subcutaneous LNs contain, in addition to the 3 populations found in the spleen, another DC group that is CD8loCD205+ (Figure 1A). The CD8loCD205hi DC group originates from the skin and comprises both langerin+ (an LC marker) and langerin– DCs.8,29,30 This DC group most likely corresponds to 2 different populations, dermal-derived DCs and epidermal-derived LCs that have migrated from the skin to the draining LNs and acquired a mature phenotype (this DC will be termed “epithelium-derived DC [epDC]” hereafter). Splenic and thymic DCs expressed moderate levels of MHC II and very low levels of CD86 (Figures 1A, 2A; and data not shown). In contrast, the FACS profile of MHC II expression in the DCs from the subcutaneous LNs was more heterogeneous, with 50% to 65% of the DCs expressing high MHC II levels (Figure 1A). This was due to the presence in the subcutaneous LNs of the one DC type that was absent in the spleen and the thymus, namely the epDCs. Indeed, the epDCs expressed homogeneously high levels of MHC II and CD86, whereas the other subcutaneous LN DC populations expressed low levels, similar to those of their splenic and thymic counterparts (Figure 1A and data not shown).8
Dendritic cell populations of the spleen, the lymph nodes, and the thymus. (A) DCs were purified in parallel from spleens, thymi, and subcutaneous LNs and analyzed by FACS using antibodies for CD11c, MHC II, and the following combinations depending on the particular lymphoid organ: CD8 and CD4 (spleen), CD8 and CD205 (LN), or only CD8 (thymus). The plots shown were obtained by gating on the CD11c+ live cells (PI–) in each preparation. Mouse spleens contain 3 populations of CD11c+ DCs that can be distinguished by their expression of CD4 and CD8. Subcutaneous LNs contain similar populations (the CD4+ and CD4–CD8– DCs are both CD205–) and, in addition, epDCs. The thymus DCs have a more homogeneous phenotype. The histograms at the bottom of each plot show the expression of MHC II in all the DCs (the numbers indicate the percentages of MHC IIhi DCs). The dashed line shows the fluorescent level of unstained cells. The 3 smaller histograms at the right side show the expression of MHC II in each of the subcutaneous LN DC populations. Note that the occurrence of MHC IIhigh DCs in the subcutaneous LNs can be attributed almost entirely to the presence of epDCs. The 3 splenic DC populations expressed comparable levels of MHC II. All the results were obtained in parallel using the same MHC II antibody and are representative of multiple experiments. (B) The DCs from the subcutaneous LNs, mesenteric LNs, iliac LNs, and mediastinal LNs were purified and analyzed in parallel as in (A). The number in the plots indicates the percentage of epDCs in each LN. In all cases, the CD205–CD8– and the CD205+CD8+ DCs expressed low levels of MHC II, whereas the epDCs accounted for most of the MHC IIhigh DCs. Note that overlapping populations obscure the distinction between MHC IIlow and MHC IIhigh DCs in the gated histograms. The results shown are representative of at least 2 experiments.
Dendritic cell populations of the spleen, the lymph nodes, and the thymus. (A) DCs were purified in parallel from spleens, thymi, and subcutaneous LNs and analyzed by FACS using antibodies for CD11c, MHC II, and the following combinations depending on the particular lymphoid organ: CD8 and CD4 (spleen), CD8 and CD205 (LN), or only CD8 (thymus). The plots shown were obtained by gating on the CD11c+ live cells (PI–) in each preparation. Mouse spleens contain 3 populations of CD11c+ DCs that can be distinguished by their expression of CD4 and CD8. Subcutaneous LNs contain similar populations (the CD4+ and CD4–CD8– DCs are both CD205–) and, in addition, epDCs. The thymus DCs have a more homogeneous phenotype. The histograms at the bottom of each plot show the expression of MHC II in all the DCs (the numbers indicate the percentages of MHC IIhi DCs). The dashed line shows the fluorescent level of unstained cells. The 3 smaller histograms at the right side show the expression of MHC II in each of the subcutaneous LN DC populations. Note that the occurrence of MHC IIhigh DCs in the subcutaneous LNs can be attributed almost entirely to the presence of epDCs. The 3 splenic DC populations expressed comparable levels of MHC II. All the results were obtained in parallel using the same MHC II antibody and are representative of multiple experiments. (B) The DCs from the subcutaneous LNs, mesenteric LNs, iliac LNs, and mediastinal LNs were purified and analyzed in parallel as in (A). The number in the plots indicates the percentage of epDCs in each LN. In all cases, the CD205–CD8– and the CD205+CD8+ DCs expressed low levels of MHC II, whereas the epDCs accounted for most of the MHC IIhigh DCs. Note that overlapping populations obscure the distinction between MHC IIlow and MHC IIhigh DCs in the gated histograms. The results shown are representative of at least 2 experiments.
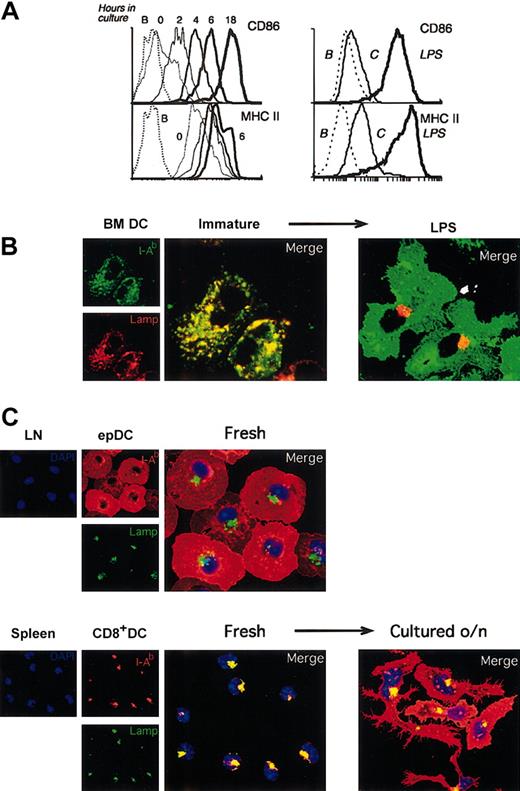
Immature lymphoid organ DCs acquire a mature phenotype during culture in vitro. (A) Left: FACS analysis of CD86 and MHC II expression in splenic DCs freshly isolated (0 time point, thinnest continuous line) or after incubation for the times indicated. The results were similar for each individual splenic DC population. The FACS profiles of MHC II expression at 6 and 18 hours were comparable, so the latter has been removed for clarity. The dashed line corresponds to unlabeled cells. The result shown is representative of multiple experiments. Right: FACS analysis of the expression of MHC II and CD86 in BMDCs after incubation without (thin line) or with (thick line) LPS for 20 hours. The dashed line corresponds to unlabeled cells. The result shown is representative of multiple experiments. (B) Immunofluorescence confocal microscopy (ICM) analysis of MHC II (green) and Lamp 1 (red) localization in BMDCs incubated for 20 hours in the absence (immature, left panels) or the presence (right) of 1 μg/mL LPS. (C) Subcutaneous LN epDCs and splenic CD8+ DCs were purified and analyzed by ICM immediately after isolation (“Fresh”) or after culture for 18 hours in vitro (“Cultured o/n”). Cells were stained with antibodies for MHC II (I-Ab, red), the late endosomal/lysosomal marker Lamp1 (green), and the nuclear dye DAPI (blue).
Immature lymphoid organ DCs acquire a mature phenotype during culture in vitro. (A) Left: FACS analysis of CD86 and MHC II expression in splenic DCs freshly isolated (0 time point, thinnest continuous line) or after incubation for the times indicated. The results were similar for each individual splenic DC population. The FACS profiles of MHC II expression at 6 and 18 hours were comparable, so the latter has been removed for clarity. The dashed line corresponds to unlabeled cells. The result shown is representative of multiple experiments. Right: FACS analysis of the expression of MHC II and CD86 in BMDCs after incubation without (thin line) or with (thick line) LPS for 20 hours. The dashed line corresponds to unlabeled cells. The result shown is representative of multiple experiments. (B) Immunofluorescence confocal microscopy (ICM) analysis of MHC II (green) and Lamp 1 (red) localization in BMDCs incubated for 20 hours in the absence (immature, left panels) or the presence (right) of 1 μg/mL LPS. (C) Subcutaneous LN epDCs and splenic CD8+ DCs were purified and analyzed by ICM immediately after isolation (“Fresh”) or after culture for 18 hours in vitro (“Cultured o/n”). Cells were stained with antibodies for MHC II (I-Ab, red), the late endosomal/lysosomal marker Lamp1 (green), and the nuclear dye DAPI (blue).
We extended the analysis of lymphoid organ DCs to the mesenteric, iliac, and mediastinal LNs. While these LNs do not drain the skin, they are the final destination of DCs that have migrated from other epithelial surfaces such as the gut mucosa, the peritoneal cavity, and the respiratory epithelium. In all cases, approximately 50% of the total DCs were CD8loCD205hi DCs (Figure 1B). As in the subcutaneous LNs, this population accounted for all the DCs that expressed high levels of MHC II in the mesenteric, iliac, and mediastinal LNs (Figure 1B, note that overlapping populations confuse the distinction of MHC IIhi and MHC IIlo DCs in the histograms gated on individual populations). The origin of the CD8loCD205hiMHC IIhigh DCs of the subcutaneous LNs and the mediastinal LNs is the skin and the respiratory epithelium, respectively.8,11,29,30 By inference, the CD8loCD205hiMHC IIhigh DCs of the mesenteric LNs and the iliac LNs most likely correspond to DCs migrating from the gut and the peritoneum (or other visceral epithelia that drain to the iliac LNs), respectively. For consistency, we will term this population in all LNs as epDCs. This analysis showed that in the steady state most of the lymphoid organ DCs that had a mature phenotype were those that migrate from peripheral tissues (the epDCs); all the other DC subsets appeared immature.
Activation of the lymphoid organ DCs by culture in vitro
When the freshly isolated spleen DCs were cultured in vitro their phenotype changed dramatically: the mean linear fluorescence value of their CD86 expression increased 3-fold in 2 hours, 32-fold in 6 hours, and 100-fold in 18 hours; that of MHC II increased by a factor of 1.8, 4, and 4.4 at the same time points (Figure 2A).17 Similar changes were observed in the thymic DCs and the CD8–CD205– and CD8hiCD205hi LN DCs cultured in vitro, whereas the already mature epDCs only slightly increased their levels of CD86 and MHC II (data not shown).8 These changes resembled those undergone by bone marrow–derived DCs (BM-DCs), a commonly used model of reference for studies of DC maturation, upon stimulation with lipopolysaccharide (LPS) (Figure 2A). These results supported the notion that all the lymphoid organ DCs except the LN epDCs were immature and could be induced to mature by culture in vitro, so that they acquired the phenotypic markers of the epDCs that had matured in vivo.
Subcellular distribution of MHC II in lymphoid organ DCs
The increase in MHC II expression that accompanies BMDC maturation is linked to a dramatic change in its subcellular distribution. In the immature BMDCs most of the MHC II molecules are contained in late endosomal/lysosomal Lamp+ compartments, whereas in their mature counterparts the MHC II molecules accumulate on the cell surface and the Lamp+ vesicles cluster in a perinuclear region (Figure 2B).3,5 This difference in the subcellular distribution of MHC II is a characteristic marker of the maturational state of DCs, common to other DC types such as DCs grown in vitro from spleen precursors (D1DCs), LCs, and human monocyte–derived DCs.3-5,31 We used this criterion to further assess the maturational state of the lymphoid organ DC populations. In agreement with the FACS data, the localization of MHC II in freshly isolated subcutaneous LN epDCs was consistent with an already mature phenotype (Figure 2C). In stark contrast, the other freshly isolated lymphoid organ DCs showed the distinctive subcellular distribution of MHC II associated with an immature phenotype, but this distribution changed to that of mature DCs after culture in vitro (illustrated for CD8+ spleen DCs in Figure 2C, and similar for the other populations, not shown).
Thus, the spleen and thymic DCs and the non-epDCs from the LNs had the phenotypic characteristics of immature DCs. Incubation in vitro activated all these DC populations so that they acquired a mature phenotype similar to that of the epDCs that had matured in vivo. To determine whether these DCs were also functionally immature, we assessed the features that define DC maturation, namely the change in the capacity to process and present newly encountered antigens and the change in the capacity to induce naive T-cell proliferation.
All the lymphoid organ DC types except the epDCs can process and present newly encountered antigens
We assessed the capacity of the spleen, LN, and thymus DC populations to process and present newly encountered antigens using an in vitro assay of stimulation of naive T cells. The DCs were first incubated in vitro with ovalbumin (OVA) for 45 minutes and then washed, simulating a brief encounter with a foreign antigen in the presence of an activatory stimulus (in this case, incubation in vitro). The DCs were then incubated with CFSE-labeled naive CD4+ T cells (OT-II) specific for I-Ab + OVA323-339.24 After 2.5 days, the proliferation of the OT-II cells was determined by FACS analysis. The naive OT-II cells proliferated only in the presence of DCs and in an antigen-dose–dependent manner (Figure 3A).
We used this experimental system to assess, in parallel, the capacity of the splenic, thymic, and subcutaneous LN DC populations to present newly encountered antigens. According to the original definition of maturity, an immature DC that encounters an antigen in the presence of an activatory signal will (1) take up, process, and present the antigen; and (2) acquire the capacity to stimulate naive T cells as it undergoes maturation. In contrast, a mature DC should not be capable of processing and presenting newly encountered antigens. Indeed, the subcutaneous LN epDCs behaved, in this test, as mature DCs and were unable to present the OVA antigen to naive T cells (Figure 3B). In contrast, the non-epDC types of the LN, and all the splenic and thymic DCs, presented the OVA antigen and induced OT-II proliferation (Figure 3B). The failure of the epDCs to stimulate the OT-II cells could be overcome by using a synthetic OVA323-339 peptide antigen instead of OVA protein (Figure 3B). Therefore, the epDCs could stimulate naive T cells, but they were unable to process and present newly encountered antigens, as would be expected of mature DCs. Similar results were obtained in a separate experiment that compared the DC populations of the mesenteric LN (Figure 3C). This result supports our interpretation that the epDCs of the mesenteric LN are the gut equivalent of the skin-derived epDCs of the subcutaneous LN.
The results obtained with the naive OT-II cells were not due to a peculiarity of the OVA antigen or the assay used, as similar conclusions were obtained in assays of antigen presentation to the hen egg lysozyme (HEL)–restricted T-cell hybridoma BO4H9.1 (Figure 3D) and to an OT-II hybridoma (not shown). Since the production of IL-2 by the T-cell hybridomas depends only on antigen presentation and not costimulation, these results confirmed that the difference between the epDCs and the other DC types was at the level of antigen processing and presentation. A previous report concluded that mature LCs were still capable of capturing, processing, and presenting newly encountered antigens.32 However, that study analyzed only the antigen-presenting capacity of CD11c+CD40hi cells from the LN, and did not perform parallel comparisons with the LN DC populations that have an immature phenotype. It is possible that the antigen processing and presenting capacity of mature LCs reported by Ruedl et al32 was equivalent to the residual capacity that we observed in our epDCs (Figure 3B,D), which is nevertheless very low when compared with the other DC populations.
Stimulatory capacity of the lymphoid organ DC populations after fixation
The ability of the immature DC populations to induce OT-II proliferation may seem paradoxical, given their low level of expression of CD86, but it is important to note that these DCs up-regulated their expression of both MHC II and CD86 very rapidly as they matured during culture in vitro (Figure 2A). If this process of spontaneous maturation was impaired, it would be expected that the immature lymphoid organ DC types would be poor T-cell stimulators when compared with the already mature epDCs. To test this, immature CD8+ DCs from the spleen and mature epDCs from the subcutaneous LNs were purified, fixed with paraformaldehyde (PFA), and then incubated with naive OT-II cells in the presence of OVA peptide (OVA protein could not be used because fixed cells cannot process antigens). Only the epDCs were capable of inducing significant proliferative responses (Figure 4). These results support our conclusion that the non-epDCs were immature; although they do not discard that, in vivo, these DCs may be able to induce some type of response in naive T cells, perhaps leading to the elimination of autoreactive T cells or the induction of suppressive or regulatory T cells.20,23,33,34
Activation of naive antigen-specific T cells by fixed DCs. Purified splenic CD8+ DCs and epDCs were fixed in PFA immediately after purification, washed, and then incubated for 3 days with CFSE-labeled OT-II cells in the presence of the indicated concentration of OVA323-339 peptide. The result is representative of 2 experiments.
Activation of naive antigen-specific T cells by fixed DCs. Purified splenic CD8+ DCs and epDCs were fixed in PFA immediately after purification, washed, and then incubated for 3 days with CFSE-labeled OT-II cells in the presence of the indicated concentration of OVA323-339 peptide. The result is representative of 2 experiments.
The immature lymphoid organ DCs become functionally mature after stimulation in vitro and in vivo
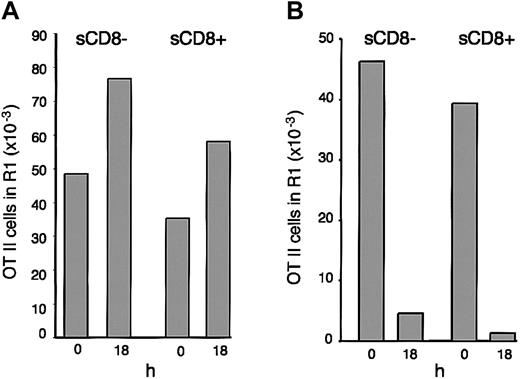
During in vitro culture, the spleen DCs acquired the phenotypic features of mature DCs: increased surface expression of MHC II and CD86 and redistribution of their MHC II molecules from endosomal compartments to the plasma membrane (Figure 2). Were the antigen-presentation properties of the cultured DCs consistent with a mature phenotype? To address this question, we first determined whether the immature DCs that endocytosed OVA early during activation maintained their capacity to present the antigen for a long time, a hallmark of maturation. Spleen CD8– and CD8+ DCs were incubated with OVA for 45 minutes, washed, and then cultured for 18 hours. The cultured DCs were then washed and incubated with CFSE-labeled OT-II cells. Their stimulatory capacity was higher than that of their counterparts that were incubated with the OT-II cells immediately after capturing the OVA antigen (Figure 5A). The enhanced stimulatory capacity of the DCs that were cultured for 18 hours probably reflected that the OT-II cells encountered fully mature DCs from the start of the assay rather than freshly isolated DCs that had just begun to mature. Since the DCs that had been cultured for 18 hours were washed before incubation with the OT-II cells, this result also indicated that OT-II stimulation did not depend on soluble factors released by the DCs during maturation. We next tested if the spleen DCs could process and present OVA after first being matured in vitro; this was not the case (Figure 5B). Since, in contrast, the DCs that had been cultured for 18 hours after receiving their OVA pulse were highly stimulatory (Figure 5A), the only explanation for this result is that once the spleen DCs had reached the mature stage they could not process and present newly encountered antigens, thus resembling the epDCs that had matured in vivo.
Immature splenic DCs become functionally mature during culture in vitro. (A) Purified CD8– and CD8+ spleen DCs were incubated with OVA for 45 minutes, washed, and then incubated with CFSE-labeled OT-II cells immediately (0) or after 18 hours of culture. OT-II proliferation was assessed 3 days later. The results are representative of 4 experiments. (B) Spleen CD8– and CD8+ DCs were incubated for 45 minutes with OVA immediately after purification (0) or after culture in vitro for 18 hours, washed, and then cultured for 3 days with CFSE-labeled OT-II cells. The results are representative of 3 experiments.
Immature splenic DCs become functionally mature during culture in vitro. (A) Purified CD8– and CD8+ spleen DCs were incubated with OVA for 45 minutes, washed, and then incubated with CFSE-labeled OT-II cells immediately (0) or after 18 hours of culture. OT-II proliferation was assessed 3 days later. The results are representative of 4 experiments. (B) Spleen CD8– and CD8+ DCs were incubated for 45 minutes with OVA immediately after purification (0) or after culture in vitro for 18 hours, washed, and then cultured for 3 days with CFSE-labeled OT-II cells. The results are representative of 3 experiments.
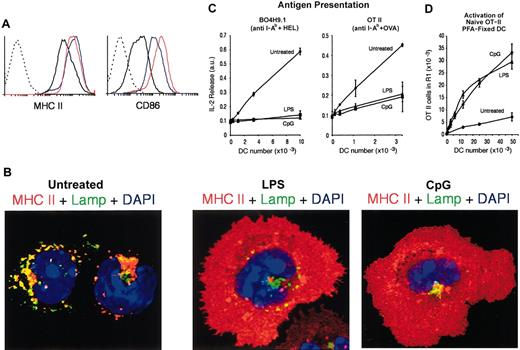
Finally, we assessed how the DC populations that showed an immature phenotype in the steady state responded to inflammatory stimuli in vivo. Mice were injected intravenously with LPS or CpG, and their splenic DCs were analyzed 12 hours later. Neither treatment altered significantly the total numbers or relative ratios of the 3 splenic DC populations at this time point (data not shown),14 but the treatments did provoke the up-regulation of surface MHC II and costimulatory molecules in the DCs (Figure 6A).14,35,36 ICM analysis revealed a mature morphology, and subcellular distribution of MHC II, in the DCs from mice inoculated with LPS or CpG (Figure 6B). These DCs were then tested for their capacity to present antigen to hybridoma T cells (Figure 6C) and for their capacity to stimulate naive OT II cells after fixation (Figure 6D). The DCs activated in vivo behaved as the epDCs: they down-regulated their capacity to present OVA or HEL and were capable of stimulating naive T cells. Therefore, the immature lymphoid organ DCs can acquire the phenotypic and functional characteristics of mature DCs in response to inflammatory stimuli in vivo.
Maturation of splenic DCs in vivo. (A) FACS analysis of MHC II (left) and CD86 (right) expression in freshly purified splenic DCs from untreated (black line), LPS-injected (red line), or CpG-injected (blue line) mice. The dashed line corresponds to unlabeled DCs. (B) ICM analysis of MHC II localization in freshly purified splenic DCs from untreated (left), LPS-injected (center), or CpG-injected (right) mice. (C) The indicated number of DCs freshly purified from untreated, LPS-, or CpG-treated mice was used in an assay of antigen presentation to the anti-HEL T-cell hybridoma BO4H9.1 (left) and the anti-OVA T-cell hybridoma OT II (right). (D) The indicated number of freshly purified and fixed DCs was assessed for its capacity to induce OT-II proliferation in the presence of the OVA323-339 peptide.
Maturation of splenic DCs in vivo. (A) FACS analysis of MHC II (left) and CD86 (right) expression in freshly purified splenic DCs from untreated (black line), LPS-injected (red line), or CpG-injected (blue line) mice. The dashed line corresponds to unlabeled DCs. (B) ICM analysis of MHC II localization in freshly purified splenic DCs from untreated (left), LPS-injected (center), or CpG-injected (right) mice. (C) The indicated number of DCs freshly purified from untreated, LPS-, or CpG-treated mice was used in an assay of antigen presentation to the anti-HEL T-cell hybridoma BO4H9.1 (left) and the anti-OVA T-cell hybridoma OT II (right). (D) The indicated number of freshly purified and fixed DCs was assessed for its capacity to induce OT-II proliferation in the presence of the OVA323-339 peptide.
Discussion
The process of DC maturation consists of a concerted series of developmental changes whereby the immature DCs gain the capacity to stimulate naive T cells and simultaneously lose their capacity to process and present newly encountered antigens, thus becoming mature DCs.1,6 The life history of DCs has been generally thought to follow the “LC paradigm,” according to which immature and mature DCs occur in peripheral tissues and lymphoid organs, respectively. If this were the case for all the DC types, the DCs found in the steady state in the lymphoid organs would be expected to have the antigen-presentation and T-cell stimulatory properties of mature DCs. However, a systematic analysis of the maturational state of lymphoid organ DCs has been lacking. We addressed this issue using the epDCs as a reference. Indeed, in the steady state the epDCs are known to capture antigens in the skin and respiratory tract and migrate to the subcutaneous and mediastinal LNs, respectively, acquiring a mature phenotype.8,11 Given the similarities in their surface markers, it is reasonable to infer that the epDCs of the mesenteric LN and the iliac LN follow a similar life history, so that they are the mature form of DCs that captured antigens in the gut and the peritoneal cavity, respectively. This allowed us to use the epDCs as a reference to address the maturational state of the other DC populations.
Our results show that virtually all the DCs contained in the spleen, and most of those in the thymus, of a conventional laboratory mouse are immature. It is highly unlikely that these DCs are undergoing maturation, considering the fast kinetics of this process (Figure 1C), since if many DCs were maturing a considerable number would have a mature phenotype in the steady state, and we observed very few. In fact, since the half-life of all the splenic DCs is less than 2 days,14,30 most of them probably die in an immature state. Even in the LNs, which drain tissues that are more prone to inflammatory stimuli, approximately 50% of the DCs had an immature phenotype, and virtually all the mature DCs corresponded to the epDC population (Figure 1A-B). These conclusions contrast with the generally assumed notion that the DCs present in lymphoid organs are mature because of their location and their ability to stimulate naive allogeneic and antigen-specific T cells. The problem with drawing conclusions about DCs' maturational state by measuring their capacity to stimulate T cells is that such experiments typically require culture for several days before T-cell proliferation is assessed. This would be sufficient time for immature DCs to become activated, since in just 6 hours their expression of MHC II and CD86 increased 4-fold and 30-fold, respectively (Figure 2A). If the lymphoid organ DCs were fixed to prevent maturation, only the epDCs were able to stimulate naive T cells (Figure 4).
It could be argued that the non-epDCs we describe as immature are simply at an intermediate stage of maturation. However, our results do not support a simple quantitative difference between the (immature) non-epDCs and the (mature) epDCs (or the non-epDCs after maturation in culture). For instance, the contrast in the capacity of these DC subsets to process and present newly encountered antigens (Figure 3) and to stimulate naive T cells when maturation is impaired (Figure 4) supports a qualitative rather than a quantitative distinction. This conclusion is supported by the analysis of other parameters commonly used to assess the maturational state of DCs, such as the phagocytic activity,14 the subcellular distribution of MHC II (Figure 2), the rate of MHC II synthesis, and the trafficking pathways followed by the MHC II molecules (N.S.W. and J.A.V., unpublished observations, June 2002). Indeed, based on these criteria, the freshly isolated non-epDCs resemble the immature stage of the DCs that have been used as reference models in studies of DC maturation, namely LCs extracted from the skin,1 BMDCs,3,5 D1DCs,4,5 and human monocyte–derived DCs.31 Conversely, the epDCs (or the non-epDCs after culture) resemble the mature stage of those same reference models. Our conclusions match the analysis of the surface phenotype of human tonsil and spleen DCs carried out by the laboratories of D. Hart and A. Hosmalin, respectively, which concluded that only 4% to 12% of the DCs present in these lymphoid organs in the steady state have a mature phenotype.37,38 Of course this does not imply that the immature DCs located in the lymphoid organs are identical to those in peripheral tissues. For instance the expression of molecules involved in tissue localization such as integrins, chemokines, or their receptors are probably different between peripheral and lymphoid organ immature DCs. This supports the hypothesis that the developmental programs for DC localization and DC maturation are controlled independently.39
Therefore, it appears that the only lymphoid organ DCs that follow the “LC paradigm” are the epDCs. The question remains: have the other DC types ever been in peripheral tissues? The current data support 2 nonexclusive possibilities.30 First, all the lymphoid organs may receive a constant input of DCs that migrate from peripheral tissues but do not mature.33,34,40 Second, the immature lymphoid organ DCs may develop in situ from earlier precursors and die without acquiring a mature phenotype. In the latter case, the endocytic activity of these “resident” DCs would still enable them to capture peripheral antigens reaching the lymphoid organs by themselves or brought by other incoming cells.29,33,34,41-43
What is the function of the immature lymphoid organ DCs? It is well established that thymic DCs are involved in the negative selection of autoreactive thymocytes.44 With regard to the secondary lymphoid organs, it has been hypothesized that immature DCs induce peripheral tolerance by presenting self-antigens but expressing low levels of costimulatory molecules.19,23 Indeed, a number of recent reports have shown that when antigens are targeted to DCs in the absence of inflammatory signals the DCs induce tolerance, whereas if the antigens are administered together with an inflammatory signal the DCs induce immunity.20-22 Our results show that the secondary lymphoid organs contain a large fraction (representing the vast majority in the spleen) of immature DCs whose function might be the maintenance of peripheral tolerance. We stress that we have measured the capacity of the lymphoid organ DCs to stimulate OT-II proliferation simply as a read-out of DC maturity. Our observation that fixed immature DCs do not stimulate naive OT II cells in vitro does not imply that these cells cannot induce tolerogenic mechanisms in vivo.
In retrospect, it appears appropriate that the environment encountered by naive T cells in lymphoid organs is strongly tolerogenic. The presentation of foreign antigens is restricted in space and time, but self-antigens are expressed constantly. Thus, in the steady state a potentially autoreactive T-cell circulating between lymphoid organs is very likely to encounter a DC presenting its autoantigen. Maintaining a large cohort of tolerogenic DCs would ensure that the T-cell repertoire is continuously purged of autoreactive T cells. This conclusion does not imply that the immature lymphoid organ DCs never mature; inoculation of LPS or CpG in vivo led to their functional maturation (Figure 6), and it is known that LPS promotes DCs to redistribute within the lymphoid organ and to secrete cytokines.14,35,36,45 Similarly, the number of mature human splenic DCs increases dramatically in individuals suffering bacterial infections or multiple trauma.38 The potential of lymphoid organ DCs to capture antigens and mature in situ might be important to trigger immune responses against blood-borne pathogens, particularly in the spleen, an organ ideally suited to sample the blood.46,47 Thus, the function of the immature lymphoid organ DCs may be to promote tolerance in the steady state while maintaining their capacity to respond to infections reaching those organs.
Prepublished online as Blood First Edition Paper, June 5, 2003; DOI 10.1182/blood-2003-02-0513.
Supported by the National Health and Medical Research Council (NHMRC) (K.S., W.R.H., and J.A.V.), the Cooperative Research Center for Vaccine Technology (N.S.W., C.M.S., W.R.H., and J.A.V.), the Human Frontiers Science Program Organization (J.A.V.), a Howard Hughes Medical Institute Fellowship (W.R.H.), a Welcome Trust Senior Overseas Fellowship (G.T.B.), and a Special Fellowship of the Leukemia and Lymphoma Society (J.A.V.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ana Maria Lennon-Dumenil, Hidde Ploegh, Frank Carbone, and Paul Gleeson for critically reading the manuscript and helpful suggestions. We are grateful to the personnel of the FACS sorting and animal breeding facilities at WEHI for their excellent technical assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal