Abstract
The prognosis for patients with B-cell chronic lymphocytic leukemia (B-CLL) is generally less favorable for those expressing CD38. Our working hypothesis is that CD38 is not merely a marker in B-CLL, but that it plays a receptor role with pathogenetic potential ruling the proliferation of the malignant clone. CD38 levels were generally low in the patients examined and monoclonal antibody (mAb) ligation was inefficient in signaling. Other cellular models indicated that molecular density and surface organization are critical for CD38 functionality. Interleukin 2 (IL-2) induced a marked up-modulation and surface rearrangement of CD38 in all the patients studied. On reaching a specific expression threshold, CD38 becomes an efficient receptor in purified B-CLL cells. Indeed, mAb ligation is followed by Ca2+ fluxes and by a markedly increased proliferation. The unsuitability of CD38 to perform as a receptor is obviated through close interaction with the B-cell–receptor (BCR) complex and CD19. On mAb binding, CD38 translocates to the membrane lipid microdomains, as shown by a colocalization with the GM1 ganglioside and with CD81, a raft-resident protein. Finally, CD38 signaling in IL-2–treated B-CLL cells prolonged survival and induced the appearance of plasmablasts, providing a pathogenetic hypothesis for the occurrence of Richter syndrome.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is characterized by the progressive accumulation of a neoplastic clone consisting entirely of CD5/CD19/CD23/surface immunoglobulin (Ig+) cells.1,2 Despite this homogeneity in phenotype, B-CLL can follow either an indolent or a progressive course.3,4 The search for prognostic factors to determine the course of the disease led to the finding that benign cases of B-CLL present somatic mutations in Ig variable (V) region genes, whereas malignant cases do not.5 The initial recommendation was to use CD38 expression by B-CLL cells as a surrogate for IgV gene rearrangement.6 Notwithstanding continuing controversy in this matter, CD38 expression is undisputedly a reliable prognostic marker in B-CLL,7-9 even though it does not always correlate to the rearrangement of the IgV genes.10 Independent groups have demonstrated that patients presenting with a CD38+ clone are characterized by an unfavorable clinical course with a more advanced stage of disease, poor responsiveness to chemotherapy, a shorter lapse in time before initial treatment is required, and a shorter survival rate. Moreover, the levels of CD38 expressed by peripheral blood lymphocytes are reported as predicting the progression rate following first-line therapy with high-dose chlorambucil.11
Human CD38 is the prototype member of a family of proteins that share a long evolutionary history as well as common functional traits.12 CD38 evolved from a soluble enzyme regulating calcium (Ca2+) homeostasis in mollusks to a complex cell surface glycoprotein in mammals, which has retained its peculiar catalytic activities as an ectoenzyme while acquiring new receptor functions.13 CD38 expression is tightly regulated during B-cell ontogeny and is present at high levels in the bone marrow (BM) precursors, is down-regulated in resting normal B cells, and then is re-expressed in terminally differentiated plasma cells.14 The dynamic behavior played by CD38 in the B-cell compartment is accompanied by drastic modifications in its functional properties; whereas it blocks B lymphopoiesis in BM,15 it rescues germinal center B cells from apoptosis.16 An explanation for this apparently contradictory behavior is likely to be found through investigation of the role of the microenvironment in providing soluble or cell-bound ligands for CD38. CD31/platelet-endothelial cell adhesion molecule 1 (PECAM-1) is thus far the only reported cell surface–bound ligand for CD38 and it has been shown that CD31/CD38 interactions control an active signaling pathway in circulating and residential lymphocytes.17,18 Further, recent data indicate that the qualitative and quantitative composition of cell surface receptors can influence both the signaling potential of CD38 and the nature of the messages delivered. Indeed, it has conclusively been established that CD38 works in physical and functional associations with specialized signaling molecules in human T and natural killer (NK) cells, such as the T-cell–receptor complex and CD16, respectively.19,20 Comparative analysis of circulating versus residential T lymphocytes showed that CD38 modifies selection of the signaling partner according to the operational environment,21 suggesting that CD38 signaling proceeds through distinct pathways, even within the same cell lineage. Research into the modalities of CD38-mediated signaling in B cells has been addressed in human22 and murine23 systems, where the molecule associates with the B-cell–receptor (BCR) complex.
This information serves as the background necessary for investigating whether this complex surface receptor molecule simply acts as a marker of other intracellular or extracellular events or whether it is directly involved in determining the prognosis of patients with B-CLL.
Patients, materials, and methods
Cells
The sample analyzed included 19 men and 14 women, with B-CLL, ranging in age from 49 to 78 years, either at diagnosis or who had received no treatment over the prior 6 months. B-CLL was diagnosed according to standard clinical and laboratory criteria. B cells were purified from the peripheral blood by Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient centrifugation followed by negative selection using a mixture of anti-CD3, anti-CD16, and anti-CD14 monoclonal antibodies (mAbs) and separation by immunomagnetic beads (Dynal, Oslo, Norway), as described.21 The studies were approved by the review board of the University of Torino Medical School, and informed consent from each patient was obtained according to the Declaration of Helsinki.
Flow cytometric analysis performed on purified B-CLL cells showed that these cells are more than 95% CD19+ and CD5+.
Antibodies
The anti-CD38 mAb IB4 was selected because of its agonistic properties and used whole or as F(ab′)2. Other reagents used were SUN-4B7 (nonagonistic anti-CD38), O1.65 (anti–HLA class I), Ab2.9 (anti–HLA class II), CB19 (anti-CD19), CB20 (anti-CD20), CB01 (anti-CD5), Moon-1 (anti-CD31), and JAS (anti–HIV-1 gp120), the latter used as irrelevant control. Rabbit antihuman IgM and IgD polyclonal antibodies as well as anti–IgM-fluorescein isothiocyanate (FITC) and anti–IgD-FITC were from Southern Biotechnology (Birmingham, AL). I.33.22 mAb (anti-CD81) was the kind gift of Dr R. Vilella (Hospital Clinic, Barcelona, Spain). Directly labeled reagents included anti–CD5-FITC, CD19-FITC, CD20-FITC, CD38-FITC, CD79α-FITC, CD79β-FITC, CD138-phycoerythrin (PE; all from Caltag Laboratories, Burlingame, CA), and anti–CD23-PE (BD Biosciences, Milan, Italy). An F(ab′)2 rabbit antimouse Ig (RαMIg) was locally produced and used as a cross-linking agent. FITC-conjugated F(ab′)2 goat antimouse Ig (GαMig; Caltag) was used in indirect immunofluorescence (IIF) studies. Tetrarhodamine isothiocyanate (TRITC)–conjugated F(ab′)2 GαMIg was from Dako (Glostrup, Denmark). FITC-labeled cholera toxin (CTX) was from Sigma (Milan, Italy).
Stained cells were analyzed with a FACSort flow cytometer (BD Biosciences), acquiring at least 5000 events per sample. Data were analyzed using the CellQuest software (BD Biosciences) and were expressed as histograms of the fluorescence intensity versus cell number or as mean fluorescence intensity (MFI).
Cell cultures
Purified B cells were cultured at a concentration of 1 × 106/mL in RPMI 1640 medium (Sigma) with 10% heat-inactivated fetal calf serum (FCS; Seromed, Berlin, Germany), 50 μg/mL gentamicin, 100 U/mL penicillin, 100 μg/mL streptomycin (all from Sigma). Cells were exposed to the IB4 mAb, or to the irrelevant JAS mAb (both at 10 μg/mL). Other agents used alone or in the indicated combinations included recombinant interleukin 2 (rIL-2; 100 IU/mL, unless specified), rIL-4 (100 IU/mL), rIL-6 (70 IU/mL), rIL-21 (10 ng/mL), and granulocyte-macrophage colony-stimulating factor (GM-CSF; 1000 IU/mL). Treatment was replaced every 5 days in long-term cultures.
Cell morphology was studied on cytospin preparations of the selected cultures stained with Giemsa. Samples were analyzed by light microscopy and images collected using a C-VIEW-12-BUND camera fitted to an Olympus 1 × 70 microscope (Milan, Italy), with the images being collected using the ANALYSIS software (Olympus), which was also used to measure cell size.
Ca2+ mobilization
Intracellular Ca2+ concentrations were measured by flow cytometry after loading the cells with Fluo 3-am (Molecular Probes, Leiden, The Netherlands), a Ca2+-sensitive fluorescent dye. Resting or IL-2–treated (100 U/mL, 72 hours) B-CLL cells were washed twice in RPMI 1640 medium plus 5% FCS and incubated (106/mL, 1 hour, 37°C) with 5 μM Fluo 3-am. Cells were then washed, incubated (10 minutes, room temperature) with the indicated mAb (10 μg/mL), and washed again. Cross-linking RαMIg (20 μg/mL) was added 10 seconds after starting a continuous FACSort analysis at 37°C. Changes in Ca2+ concentrations were monitored by plotting the shift in the Fluo 3-am fluorescence over a 540-second time period and presented as changes in Fluo 3-am intensity over time. The irrelevant isotype-matched JAS mAb was included as the control; the efficient loading of the cells was checked by the addition of the A23187 ionophore (Sigma).
Apoptosis determination
The rate of apoptosis was determined by double staining with annexin V–FITC and propidium iodide (PI). Briefly, 2 × 105 cells were washed in phosphate-buffered saline (PBS) and resuspended in 100 μL annexin V binding buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4, 2.5 mM CaCl2, 140 mM NaCl) containing 0.2 μL (1 μg/mL) annexin V–FITC. After incubation (15 minutes in the dark, room temperature), cells were diluted with 100 μL annexin V binding buffer containing 1 μg/mL PI and analyzed with a FACSort using the CellQuest software.
Cell proliferation and survival
Purified B-CLL cells (2 × 105/well) were plated into 96-well plates and exposed to different signals for 96 hours. To each well was added 1 μCi (0.037 MBq) 3H-thymidine (thy; NEN, Cologno Monzese, Italy); wells were incubated for 8 hours and the incorporated radioactivity measured in a β-counter (NEN). Cell survival in long-term cultures was assessed daily by microscope examination. At the indicated time points, cells were collected from culture and viability was assessed after the trypan blue exclusion test.
Cocapping experiments
B-CLL cells (0.5 × 106) were incubated with the anti-CD38 mAb (30 minutes on ice), washed, and reacted with TRITC-labeled GαMIg (20 minutes on ice). Samples were then moved to 37°C (40 minutes) to induce capping before adding ice-cold PBS plus 0.5% bovine serum albumin (BSA) and 0.1% NaN3. Counterstaining was performed with direct FITC-labeled mAbs. After washing, cells were fixed (4% paraformaldehyde in PBS with 2% sucrose, pH 7.6), settled on coverslips coated with poly-l-lysine, analyzed with a C-VIEW-12-BUND camera fitted to an Olympus 1 × 70 microscope and the images collected using the ANALYSIS software.
Results
CD38 expression in the B-CLL study group
The present study included 33 patients with an established diagnosis of B-CLL, who were untreated or who had received the last course of chemotherapy more than 6 months previously. The surface expression levels of CD38 were studied in purified B-CLL cells by means of an indirect technique. A leukemic population was arbitrarily scored as CD38+ when more than 20% of the cells expressed the molecule. Based on this cut-off value, 18 patients were defined as CD38+ and 15 as CD38–, respectively. The MFI values of the CD38+ subgroup demonstrated that the epitope density of CD38 was generally low. Table 1 shows the characteristics of the sample population and the percentage and MFI values for CD38 in basal conditions and on IL-2 exposure (vide infra).
CD38 expression in basal conditions and on IL-2 treatment in the B-CLL sample analyzed
. | . | . | Basal CD38 . | . | CD38/IL-2 . | . | ||
|---|---|---|---|---|---|---|---|---|
| Patient no. . | Age, y . | Sex . | % . | MFI . | % . | MFI . | ||
| 1 | 65 | M | 71 | 13 | ND | ND | ||
| 2 | 63 | F | 8 | 6 | ND | ND | ||
| 3 | 52 | F | 89 | 51 | 98 | 155 | ||
| 4 | 55 | M | 55 | 24 | 93 | 123 | ||
| 5 | 68 | M | 58 | 36 | 70 | 254 | ||
| 6 | 63 | M | 4 | 12 | 33 | 48 | ||
| 7 | 69 | M | 1 | 13 | ND | ND | ||
| 8 | 76 | M | 62 | 36 | 88 | 260 | ||
| 9 | 66 | F | 7 | 16 | 77 | 209 | ||
| 10 | 49 | M | 14 | 14 | 6 | 7 | ||
| 11 | 74 | M | 76 | 35 | 94 | 73 | ||
| 12 | 64 | M | 82 | 40 | 98 | 128 | ||
| 13 | 81 | F | 1 | 12 | 13 | 60 | ||
| 14 | 74 | F | 70 | 33 | 88 | 261 | ||
| 15 | 54 | F | 34 | 10 | 59 | 27 | ||
| 16 | 61 | M | 2 | 4 | 8 | 11 | ||
| 17 | 67 | M | 12 | 11 | 18 | 18 | ||
| 18 | 54 | M | 7 | 8 | 98 | 96 | ||
| 19 | 61 | M | 61 | 13 | 94 | 98 | ||
| 20 | 65 | F | 53 | 15 | 75 | 59 | ||
| 21 | 77 | M | 40 | 14 | 86 | 196 | ||
| 22 | 78 | F | 1 | 4 | 8 | 10 | ||
| 23 | 77 | F | 39 | 41 | 41 | 92 | ||
| 24 | 67 | F | 6 | 7 | 73 | 41 | ||
| 25 | 73 | M | 7 | 7 | 16 | 16 | ||
| 26 | 65 | F | 1 | 10 | 6 | 10 | ||
| 27 | 62 | M | 37 | 17 | 76 | 75 | ||
| 28 | 62 | F | 1 | 7 | 8 | 10 | ||
| 29 | 63 | M | 75 | 28 | 76 | 45 | ||
| 30 | 61 | M | 18 | 21 | 32 | 29 | ||
| 31 | 72 | F | 75 | 35 | 88 | 67 | ||
| 32 | 67 | F | 76 | 15 | 77 | 88 | ||
| 33 | 58 | M | 66 | 26 | 93 | 80 | ||
. | . | . | Basal CD38 . | . | CD38/IL-2 . | . | ||
|---|---|---|---|---|---|---|---|---|
| Patient no. . | Age, y . | Sex . | % . | MFI . | % . | MFI . | ||
| 1 | 65 | M | 71 | 13 | ND | ND | ||
| 2 | 63 | F | 8 | 6 | ND | ND | ||
| 3 | 52 | F | 89 | 51 | 98 | 155 | ||
| 4 | 55 | M | 55 | 24 | 93 | 123 | ||
| 5 | 68 | M | 58 | 36 | 70 | 254 | ||
| 6 | 63 | M | 4 | 12 | 33 | 48 | ||
| 7 | 69 | M | 1 | 13 | ND | ND | ||
| 8 | 76 | M | 62 | 36 | 88 | 260 | ||
| 9 | 66 | F | 7 | 16 | 77 | 209 | ||
| 10 | 49 | M | 14 | 14 | 6 | 7 | ||
| 11 | 74 | M | 76 | 35 | 94 | 73 | ||
| 12 | 64 | M | 82 | 40 | 98 | 128 | ||
| 13 | 81 | F | 1 | 12 | 13 | 60 | ||
| 14 | 74 | F | 70 | 33 | 88 | 261 | ||
| 15 | 54 | F | 34 | 10 | 59 | 27 | ||
| 16 | 61 | M | 2 | 4 | 8 | 11 | ||
| 17 | 67 | M | 12 | 11 | 18 | 18 | ||
| 18 | 54 | M | 7 | 8 | 98 | 96 | ||
| 19 | 61 | M | 61 | 13 | 94 | 98 | ||
| 20 | 65 | F | 53 | 15 | 75 | 59 | ||
| 21 | 77 | M | 40 | 14 | 86 | 196 | ||
| 22 | 78 | F | 1 | 4 | 8 | 10 | ||
| 23 | 77 | F | 39 | 41 | 41 | 92 | ||
| 24 | 67 | F | 6 | 7 | 73 | 41 | ||
| 25 | 73 | M | 7 | 7 | 16 | 16 | ||
| 26 | 65 | F | 1 | 10 | 6 | 10 | ||
| 27 | 62 | M | 37 | 17 | 76 | 75 | ||
| 28 | 62 | F | 1 | 7 | 8 | 10 | ||
| 29 | 63 | M | 75 | 28 | 76 | 45 | ||
| 30 | 61 | M | 18 | 21 | 32 | 29 | ||
| 31 | 72 | F | 75 | 35 | 88 | 67 | ||
| 32 | 67 | F | 76 | 15 | 77 | 88 | ||
| 33 | 58 | M | 66 | 26 | 93 | 80 | ||
ND indicates not determined.
CD38 signaling in B-CLL patients
The first step was to verify whether ligation of CD38 in B-CLL cells was followed by biologic events. To this aim, purified B-CLL cells were incubated with an agonistic anti-CD38 mAb followed by use of an RαMIg as a cross-linker, scoring short-term (Ca2+ fluxes), medium-term (apoptosis), and long-term (proliferation and differentiation) events.
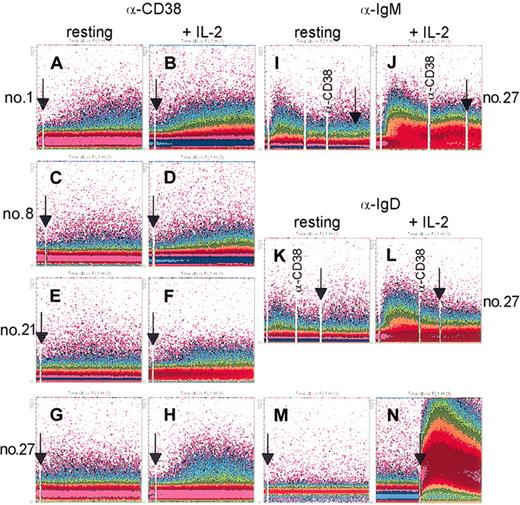
Results indicate that CD38 ligation induced minor Ca2+ responses in 2 of 8 patients examined (nos. 1 and 8). The response was low, apparent only in a minority of cells, and depended on the addition of a cross-linker (Figure 1A,C). Although minimal, this effect was specific because a nonagonistic anti-CD38 mAb was unable to mobilize Ca2+ (Figure 1M), as was an irrelevant mAb. Moreover, CD19 and CD5 expressions were constantly more than 95%, ruling out the possibility of T or NK responses. Finally, the limited amplitude of the signal could not be attributed to technical pitfalls; the cells were efficiently labeled in every experiment, as witnessed by the maximal Ca2+ mobilization following the addition of A23187 (Figure 1N).
mAb ligation of CD38 induces Ca2+ waves in purified B-CLL cells. B-CLL cells purified from patients 1, 8, 21, and 27 were loaded with the fluorescent indicator Fluo 3-am and preincubated (10 minutes at room temperature) with an agonistic anti-CD38 mAb (A,C,E,G). The cells were then washed and analyzed continuously at 37°C using a FACSort, while a RαMIg was added as cross-linker (arrows) 10 seconds after starting the analysis. IgM and IgD ligation using specific polyclonal antibodies induced Ca2+ responses, as expected, whereas no synergy was observed when adding the anti-CD38 mAb (I,K). The same cells were cultured for 3 days in the presence of 100 IU/mL IL-2 and re-evaluated for Ca2+ mobilization on CD38 cross-linking (B,D,F,H), as well as for IgM (J) and IgD (L). A nonagonistic anti-CD38 mAb was unable to mobilize Ca2+ ions (M). Appropriate dye loading by the cells was checked by the addition of the ionophore A23187 (N, arrow). Data are presented as density plot of the shift in the Fluo 3-am fluorescence (y-axis) over a 512-second period (x-axis).
mAb ligation of CD38 induces Ca2+ waves in purified B-CLL cells. B-CLL cells purified from patients 1, 8, 21, and 27 were loaded with the fluorescent indicator Fluo 3-am and preincubated (10 minutes at room temperature) with an agonistic anti-CD38 mAb (A,C,E,G). The cells were then washed and analyzed continuously at 37°C using a FACSort, while a RαMIg was added as cross-linker (arrows) 10 seconds after starting the analysis. IgM and IgD ligation using specific polyclonal antibodies induced Ca2+ responses, as expected, whereas no synergy was observed when adding the anti-CD38 mAb (I,K). The same cells were cultured for 3 days in the presence of 100 IU/mL IL-2 and re-evaluated for Ca2+ mobilization on CD38 cross-linking (B,D,F,H), as well as for IgM (J) and IgD (L). A nonagonistic anti-CD38 mAb was unable to mobilize Ca2+ ions (M). Appropriate dye loading by the cells was checked by the addition of the ionophore A23187 (N, arrow). Data are presented as density plot of the shift in the Fluo 3-am fluorescence (y-axis) over a 512-second period (x-axis).
CD38 signaling failed to induce detectable effects on apoptosis and did not influence proliferation. Indeed, the effects were negligible and were not statistically significant (see relevant histogram bars in Figures 6 and 7A).
CD38 ligation in the presence of IL-2 triggers B-CLL proliferation. B-CLL cells purified from 12 different CD38+ patients were cultured for 3 days in the presence of the indicated agents. The simultaneous presence of an agonistic anti-CD38 mAb and of IL-2 increases 3H-thy incorporation. Analysis of purified B cells from 3 CD38– patients (nos. 13, 16, and 28) indicates that CD38 is unable to deliver significant growth signals either alone or in association with IL-2. Error bars indicate SD.
CD38 ligation in the presence of IL-2 triggers B-CLL proliferation. B-CLL cells purified from 12 different CD38+ patients were cultured for 3 days in the presence of the indicated agents. The simultaneous presence of an agonistic anti-CD38 mAb and of IL-2 increases 3H-thy incorporation. Analysis of purified B cells from 3 CD38– patients (nos. 13, 16, and 28) indicates that CD38 is unable to deliver significant growth signals either alone or in association with IL-2. Error bars indicate SD.
CD38 ligation in the presence of IL-2 prolongs B-CLL survival but does not alter the apoptosis rate. (A) PI staining of B-CLL cells purified from 8 different patients (nos. 3, 8, 12, 14, 19, 20, 27, and 33) and cultured for 48 hours in the presence of the indicated treatment. IL-2 is the only agent able to confer mild protection from apoptosis, whereas the anti-CD38 mAb is ineffective. IgM was included as control and strongly promoted apoptosis. (B) Purified B-CLL cells from 8 patients (nos. 3, 8, 12, 14, 19, 20, 27, and 33) were cultured in presence of IL-2, the agonistic anti-CD38 mAb, or a combination of the two and evaluated weekly to monitor cell survival by the trypan blue exclusion test. Exposure to anti-CD38 mAb and IL-2 resulted in a substantial improvement in cell viability, already visible after 1 week and lasting for over 4 weeks.
CD38 ligation in the presence of IL-2 prolongs B-CLL survival but does not alter the apoptosis rate. (A) PI staining of B-CLL cells purified from 8 different patients (nos. 3, 8, 12, 14, 19, 20, 27, and 33) and cultured for 48 hours in the presence of the indicated treatment. IL-2 is the only agent able to confer mild protection from apoptosis, whereas the anti-CD38 mAb is ineffective. IgM was included as control and strongly promoted apoptosis. (B) Purified B-CLL cells from 8 patients (nos. 3, 8, 12, 14, 19, 20, 27, and 33) were cultured in presence of IL-2, the agonistic anti-CD38 mAb, or a combination of the two and evaluated weekly to monitor cell survival by the trypan blue exclusion test. Exposure to anti-CD38 mAb and IL-2 resulted in a substantial improvement in cell viability, already visible after 1 week and lasting for over 4 weeks.
Cytokine modulation of CD38 expression in the B-CLL study group
The refractoriness of B-CLL cells to CD38-mediated signaling could reflect a generalized anergic condition, likely attributable to impairment of the main signaling pathways or of the BCR complex. Alternatively, the low epitope density of CD38 may result in dilution of the molecule on the surface, consequently preventing its localization in specific membrane patches and/or dimeric or oligomeric organization. This working hypothesis was tested by assessing whether the expression of CD38 could be increased on incubation with different agents and by determining its organization on the cell surface. Purified B-CLL cells were cultured in the presence of IL-2, IL-4, IL-6, IL-21, and GM-CSF and the surface expression of CD38 was evaluated after specific intervals of time. The results represented in Figure 2 indicate that IL-2 constantly up-modulated CD38 in B-CLL cells purified from the CD38+ subgroup of patients. The effect could be highlighted in every CD38+ patient and was predominant in the CD38+ fraction in the patients in whom a CD38+ and a CD38– subpopulation of neoplastic cells could be identified (patient nos. 5 and 32 in Figure 2). IL-2 was generally unable to up-modulate CD38 in the CD38– cells, with the exceptions of patients 9, 18, and 24. The effects were time and dose dependent, with the increase in surface expression becoming detectable 30 hours after the addition of IL-2 and peaking at 72 hours. The optimal IL-2 concentration was 100 IU/mL, although a marked up-regulation started to become evident with 10 IU/mL IL-2 (not shown). No modification in terms of CD38 surface density occurred when culturing the cells with complete medium for 3 days (not shown). The same experimental conditions failed to induce modifications of the surface expression of CD5, CD20, IgM, IgD, and CD31. An increase in surface expression of CD19 was recorded in most patients, as well as of HLA class I and class II (not shown). No detectable effects on CD38 expression were observed using IL-4, IL-6, IL-21, or GM-CSF. Table 1 shows the increase in percentage and MFI on IL-2 treatment of purified B-CLL cells.
IL-2 up-regulates CD38 expression in purified B-CLL cells. Cytofluorographic analysis of CD38 expression (gray histograms) in purified B cells from 24 CLL patients. B cells were purified from peripheral blood mononuclear cells by negative selection and stained with an anti-CD38 mAb followed by an FITC-conjugated GαMIg. The same cells were cultured for 3 days in the presence of 100 IU/mL IL-2 and re-evaluated for CD38 expression (open histograms). Analysis was carried out using a FACSort and scoring at least 5000 events/sample. The no. label refers to Table 1.
IL-2 up-regulates CD38 expression in purified B-CLL cells. Cytofluorographic analysis of CD38 expression (gray histograms) in purified B cells from 24 CLL patients. B cells were purified from peripheral blood mononuclear cells by negative selection and stained with an anti-CD38 mAb followed by an FITC-conjugated GαMIg. The same cells were cultured for 3 days in the presence of 100 IU/mL IL-2 and re-evaluated for CD38 expression (open histograms). Analysis was carried out using a FACSort and scoring at least 5000 events/sample. The no. label refers to Table 1.
CD38 signaling in IL-2–treated CD38+ B-CLL cells
Purified B-CLL cells cultured for 3 days in RPMI 1640 medium plus 5% FCS in the presence of 100 IU/mL IL-2 showed an increase in CD38 expression (Figure 2) and acquired the ability to transduce Ca2+-modulating signals on ligation. Panels B and D in Figure 1 show a net increase in cytoplasmic Ca2+ levels in the B-CLL cells purified from the 2 patients who showed minimal responses in resting conditions (see “CD38 signaling in B-CLL patients”). Further, IL-2 effects on CD38-mediated Ca2+ fluxes were also apparent in the cells purified from patients completely unresponsive in resting conditions (Figure 1F,H). The Ca2+ response on CD38 ligation: (1) started approximately 50 seconds after the addition of an RαMIg, (2) rose slowly, while (3) elevated Ca2+ levels were maintained until the end of recording. The Ca2+ wave was invariably limited in amplitude and lower than the one obtained on IgM triggering in resting conditions (Figure 1I). Similarly to what has been described for CD38, IL-2 also significantly increased IgM- and IgD-mediated Ca2+ fluxes (Figure 1J,L). Addition of anti-CD38 mAb followed by a cross-linker after IgM and IgD ligation did not significantly modify the Ca2+ profiles, suggesting that CD38 controls an independent signaling pathway.
CD38 is laterally associated with the BCR complex and with CD19 in B-CLL cells
The working hypothesis was that CD38 signaling is dependent on both the number of surface molecules and the membrane organization, with signaling likely depending on a physical association with the BCR complex. The strategy devised to detect the lateral association was to induce CD38 expression by exposing B-CLL cells to 100 IU/mL IL-2 for 3 days and then to perform cocapping experiments. Antibody-mediated capping is an energy-dependent redistribution of cell surface molecules to a single pole of the cell. In general, only molecules bound by the antibody will redistribute to the area of the cap, unless they have a particular association with other structures, in turn induced to cocap to the same area. The results indicate that CD79α and CD79β are constantly present in the CD38 caps, implying that CD38 lies close to the main signaling apparatus of the B-cell membrane (Figure 3). Moreover, a direct association between CD38 and surface IgM and, although less marked, between CD38 and IgD could be highlighted using the same technique (Figure 3). No cocapping was observed with HLA class I, used as an isotype-matched control (not shown). A summary of data acquired from a large number of cells purified from 8 patients is presented in Table 2.
CD38 is laterally associated with the BCR complex. B cells purified from 8 different CD38+ CLL patients were cultured for 3 days in the presence of 100 IU/mL IL-2 to increase CD38 expression. CD38 capping was induced on ligation with the IB4 mAb followed by binding with a TRITC-conjugated GαMIg for 30 minutes at 37°C (red). After blocking the experiment with cold PBS plus 0.1% NaN3, directly FITC-labeled mAbs to CD79α, CD79β, IgM, and IgD were added for 20 minutes at 4°C (green). The cells were then fixed and analyzed with an Olympus 1 × 70 microscope using the ANALYSIS software. The panels on the right show the merge of the 2 images. Original magnification × 60.
CD38 is laterally associated with the BCR complex. B cells purified from 8 different CD38+ CLL patients were cultured for 3 days in the presence of 100 IU/mL IL-2 to increase CD38 expression. CD38 capping was induced on ligation with the IB4 mAb followed by binding with a TRITC-conjugated GαMIg for 30 minutes at 37°C (red). After blocking the experiment with cold PBS plus 0.1% NaN3, directly FITC-labeled mAbs to CD79α, CD79β, IgM, and IgD were added for 20 minutes at 4°C (green). The cells were then fixed and analyzed with an Olympus 1 × 70 microscope using the ANALYSIS software. The panels on the right show the merge of the 2 images. Original magnification × 60.
Cocapping of CD79α, CD79β, IgM, and IgD with CD38
Cap/cocap . | Total cells, no. . | Caps, % . | Cocaps, % . |
|---|---|---|---|
| CD38/CD79α | 103 | 88 | 80 |
| CD38/CD79β | 97 | 81 | 78 |
| CD38/IgM | 106 | 92 | 65 |
| CD38/IgD | 98 | 79 | 44 |
Cap/cocap . | Total cells, no. . | Caps, % . | Cocaps, % . |
|---|---|---|---|
| CD38/CD79α | 103 | 88 | 80 |
| CD38/CD79β | 97 | 81 | 78 |
| CD38/IgM | 106 | 92 | 65 |
| CD38/IgD | 98 | 79 | 44 |
Table shows cumulative data from the different experiments described in Figure 3. The number of caps and cocaps are presented as a percentage of the cells analyzed. Cells exhibiting partial redistribution of the surface molecule detected by the primary capping antibody were excluded from the analysis.
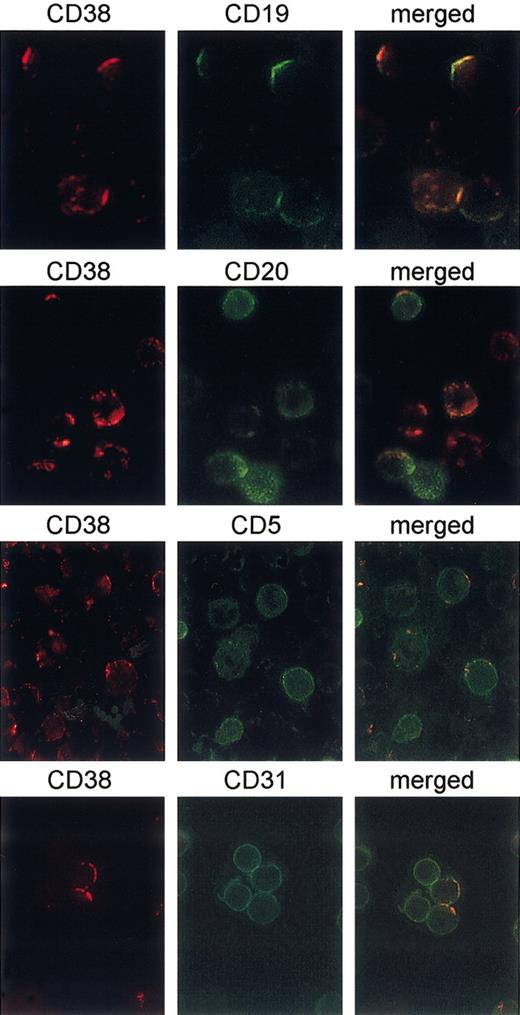
Further, CD38 proved to be laterally associated with CD19, as highlighted by using the same techniques (Figure 4). No association could be observed between CD38 and CD20, CD38 and CD5, and CD38 and CD31 (Figure 4). Table 3 shows cumulative data from 6 different experiments.
CD38 is laterally associated with CD19, but not with CD5, CD20, and CD31. B cells purified from 6 different CD38+ CLL patients were cultured for 3 days in the presence of 100 IU/mL IL-2 to increase CD38 expression. CD38 capping was induced on ligation with the IB4 mAb followed by binding with a TRITC-conjugated GαMIg for 30 minutes at 37°C (red). After blocking the experiment with the addition of cold PBS plus 0.1% NaN3, directly FITC-conjugated mAbs to CD19, CD5, CD20, and CD31 were added for 20 minutes at 4°C (green). The cells were then fixed and analyzed with an Olympus 1 × 70 microscope using the ANALYSIS software. The panels on the right show the merge of the 2 images. Original magnification × 60.
CD38 is laterally associated with CD19, but not with CD5, CD20, and CD31. B cells purified from 6 different CD38+ CLL patients were cultured for 3 days in the presence of 100 IU/mL IL-2 to increase CD38 expression. CD38 capping was induced on ligation with the IB4 mAb followed by binding with a TRITC-conjugated GαMIg for 30 minutes at 37°C (red). After blocking the experiment with the addition of cold PBS plus 0.1% NaN3, directly FITC-conjugated mAbs to CD19, CD5, CD20, and CD31 were added for 20 minutes at 4°C (green). The cells were then fixed and analyzed with an Olympus 1 × 70 microscope using the ANALYSIS software. The panels on the right show the merge of the 2 images. Original magnification × 60.
Cocapping of CD19, CD20, CD5, and CD31 with CD38
Cap/cocap . | Total cells, no. . | Caps, % . | Cocaps, % . |
|---|---|---|---|
| CD38/CD19 | 112 | 92 | 56 |
| CD38/CD20 | 97 | 81 | 38 |
| CD38/CD5 | 121 | 94 | 34 |
| CD38/CD31 | 106 | 83 | 22 |
Cap/cocap . | Total cells, no. . | Caps, % . | Cocaps, % . |
|---|---|---|---|
| CD38/CD19 | 112 | 92 | 56 |
| CD38/CD20 | 97 | 81 | 38 |
| CD38/CD5 | 121 | 94 | 34 |
| CD38/CD31 | 106 | 83 | 22 |
Table shows cumulative data from 6 different experiments described in Figure 4. The number of caps and cocaps are presented as a percentage of the cells analyzed. Cells exhibiting partial redistribution of the surface molecule detected by the primary capping antibody were excluded from the analysis.
CD38 and the BCR complex are colocalized within the rafts in B-CLL cells
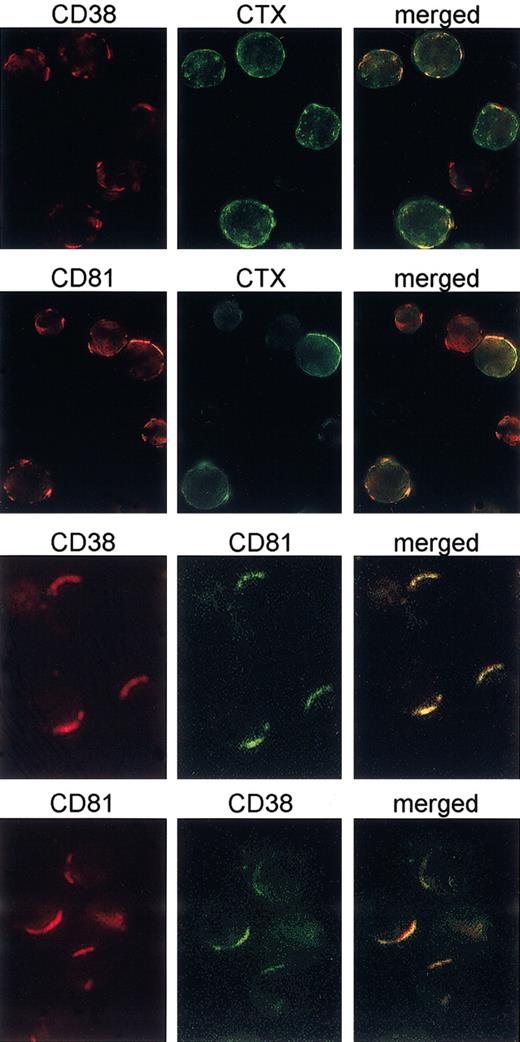
The signaling potential of CD38 has recently been shown to depend on its localization in membrane microdomains,24 where the main signaling receptors of T and B lymphocytes are recruited on activation. The results shown in Figure 5 indicate that mAb ligation drives CD38 to the membrane microdomains, as witnessed by the presence of GM1 ganglioside in the CD38 patches stained by CTX-FITC. This association is statistically significant. Raft localization of CD38 on mAb binding was also confirmed by the finding of a strong lateral association with CD81, a tetraspanin family member, reported as constitutively localizing within the membrane microdomains.25,26 Results indicate that CD81 may be found in the CD38 caps and vice versa, confirming that the association is bidirectional (Figure 5). Moreover, the association is specific, with no cocapping observed with HLA class I using CD38 or CD81 (not shown). Lateral associations between CD79α and CD81, CD79β and CD81, IgM and CD81, and IgD and CD81 were also observed, offering indirect evidence that the microdomains simultaneously harbor CD38 and the BCR complex (Table 4). This is also in line with the reported finding of a microdomain compartmentalization of the BCR on ligand binding and activation.27,28
CD38 is recruited in the membrane rafts on mAb binding. B cells purified from 8 different CD38+ CLL patients were cultured for 3 days in the presence of 100 IU/mL IL-2 to increase CD38 expression. The experiments were performed as described in Figures 3 and 4. The mAb ligation drives CD38 to the membrane microdomains, as witnessed by the presence of GM1 ganglioside (stained by CTX-FITC) in the CD38 patches. Further, capping of the CD38 molecules induces cocapping of CD81 (a microdomain resident molecule), but not of HLA class I. Original magnification × 60.
CD38 is recruited in the membrane rafts on mAb binding. B cells purified from 8 different CD38+ CLL patients were cultured for 3 days in the presence of 100 IU/mL IL-2 to increase CD38 expression. The experiments were performed as described in Figures 3 and 4. The mAb ligation drives CD38 to the membrane microdomains, as witnessed by the presence of GM1 ganglioside (stained by CTX-FITC) in the CD38 patches. Further, capping of the CD38 molecules induces cocapping of CD81 (a microdomain resident molecule), but not of HLA class I. Original magnification × 60.
Lateral association between CD38, CD81, CD79α, CD79β, IgM, IgD, CTX, and HLA class I
Cap/cocap . | Total cells, no. . | Caps, % . | Cocaps, % . |
|---|---|---|---|
| CD38/CTX | 98 | 73 | 54 |
| CD38/CD81 | 96 | 90 | 76 |
| CD81/CTX | 98 | 82 | 59 |
| CD81/CD38 | 94 | 87 | 78 |
| CD81/CD79α | 96 | 79 | 65 |
| CD81/CD79β | 100 | 91 | 86 |
| CD81/IgM | 104 | 89 | 67 |
| CD81/IgD | 97 | 75 | 61 |
| CD81/HLA class I | 98 | 81 | 5 |
Cap/cocap . | Total cells, no. . | Caps, % . | Cocaps, % . |
|---|---|---|---|
| CD38/CTX | 98 | 73 | 54 |
| CD38/CD81 | 96 | 90 | 76 |
| CD81/CTX | 98 | 82 | 59 |
| CD81/CD38 | 94 | 87 | 78 |
| CD81/CD79α | 96 | 79 | 65 |
| CD81/CD79β | 100 | 91 | 86 |
| CD81/IgM | 104 | 89 | 67 |
| CD81/IgD | 97 | 75 | 61 |
| CD81/HLA class I | 98 | 81 | 5 |
The results of the lateral association experiments between CD79α and CD81, CD79β and CD81, IgM and CD81, and IgD and CD81, are summarized. Cumulative data from the different experiments are shown. The number of caps and cocaps are presented as a percentage of the cells analyzed. Cells exhibiting partial redistribution of the surface molecule detected by the primary capping antibody were excluded from the analysis.
The appearance of Ca2+ fluxes and the occurrence of raft localization on CD38 mAb ligation in IL-2–treated B-CLL cells is highly reminiscent of the conditions observed in T cells,24 where CD38 behaves as an active signaling receptor.
Effects of CD38 signaling on proliferation in B-CLL cells treated with IL-2
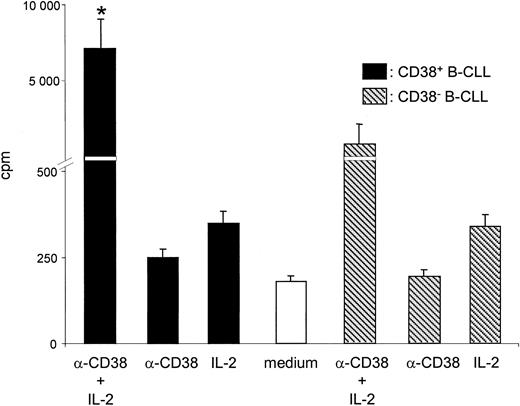
The lateral association experiments suggest that the signaling potential of CD38 becomes functional only on raft localization and likely in virtue of its proximity with the BCR. This finding prompted the next step in our investigation, which was analysis of the biologic effects elicited on IL-2 exposure and CD38 ligation. Purified CD38+ B-CLL cells from 8 patients (nos. 3, 8, 12, 14, 19, 20, 27, and 33) were cultured in the presence of an agonistic anti-CD38 mAb, IL-2, or a combination of the two, with an irrelevant isotype-matched mAb used as control. Triggering with either anti-CD38 alone or IL-2 alone induced a small but constant increase in 3H-thy incorporation, which, however, did not reach statistical significance (Figure 6). The simultaneous presence of the 2 agents induced a prominent proliferative response in all the CD38+ patients tested. These effects were reproducible using an F(ab′)2 preparation of the IB4 mAb, witnessing the specificity of the signal (not shown). No synergy was observed when using an irrelevant isotype-matched control (not shown). The results from the analysis of purified CD38– B-CLL cells from 3 patients (nos. 13, 16, and 28) indicate that CD38 is unable to deliver growth signals either alone or in association with IL-2 (Figure 6). A significant 3H-thy incorporation (mean cpm, 3450) was observed in IL-2–treated B-CLL cells from patient 9, and is likely attributable to the strong induction of CD38 expression on IL-2 exposure (Figure 2).
Effects of CD38 signaling on apoptosis and cell survival in IL-2–treated B-CLL cells
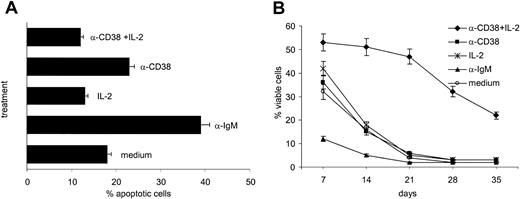
Ligation of CD38 in B-CLL cells purified from 8 different patients (nos. 3, 8, 12, 14, 19, 20, 27, and 33) does not offer protection from apoptosis, as indicated by PI staining after 24, 48, and 72 hours of treatment. Similarly, no significant cooperation between CD38 and IL-2 could be detected, whereas IL-2 alone conferred only mild protection from apoptosis. Panel A in Figure 7 shows the percentage of dead cells after 48 hours, selected as the most representative time point. IgM was included as control and strongly promoted apoptosis. Simultaneous staining with annexin V–FITC confirmed these results (not shown).
Evaluation of long-term survival provided a contrasting picture, with CD38 and IL-2 working together to sustain cell viability for a mean period of over 4 weeks (Figure 7B). Purified B-CLL cells from 8 patients (nos. 3, 8, 12, 14, 19, 20, 27, and 33) were cultured in the presence of IL-2, the agonistic anti-CD38 mAb, or a combination of the two and evaluated every 3 days to monitor cell survival. Results indicate that after a mean of 14 days, about 90% of untreated cells were dead, as were the samples treated with the anti-CD38 mAb or IL-2 alone. IgM cross-linking diminished viability with about 10% of residual live cells after 1 week. Exposure to anti-CD38 mAb and IL-2 resulted in a substantial improvement in cell viability already visible after 1 week and lasting for over 4 weeks (Figure 7B).
These apparently contradictory results may be explained by hypothesizing that the combined effects of CD38- and IL-2–mediated signaling involve only a subset of cells, whereas the remaining population is unaffected. Moreover, such effects are likely to become detectable beyond the time intervals where apoptosis was evaluated, thus explaining the absence of results in terms of short-term cell death.
Whither the CD38-triggered B-CLL cells?
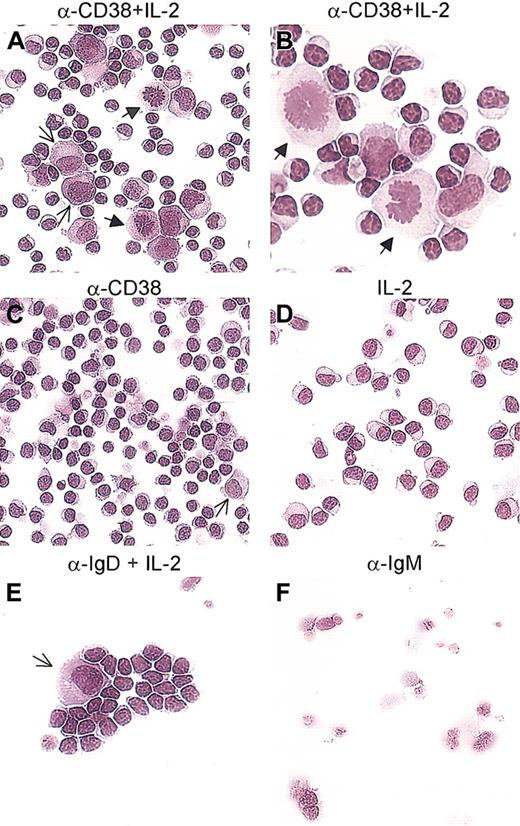
Purified B-CLL cells, cultured for 5 days in the conditions described, were evaluated for number and morphology. The first observation is that CD38 ligation alone induces 2% to 5% of the whole population to increase in size and acquire a blastlike morphology (Figure 8C, arrow). The remaining population of B-CLL cells does not display detectable morphologic differences as compared with the untreated samples and is composed of a discrete population of apoptotic cells and a minor fraction with the morphology of resting lymphocytes (Figure 8C). The presence of IL-2 in the medium prevents the formation of apoptotic figures and the general situation is that of mildly activated lymphocytes with an expanded cytosol (Figure 8D).
CD38 ligation in the presence of IL-2 induces differentiation of B-CLL cells. B-CLL cells obtained from 10 different patients were exposed to the anti-CD38 mAb (C), IL-2 (D), or a combination of the two (A-B). A population of cells with an enlarged cytosol and a light perinuclear zone, with the characteristics of plasmablasts, was observed after culture with anti-CD38+ IL-2 (A, thin arrows). Several blasts were present in every sample tested (A-B, arrows). Ligation of IgD with a polyclonal antibody in the presence of IL-2 was included as control and induced the appearance of plasmablasts (E, arrow). Samples treated with an anti-IgM polyclonal antibody were primarily apoptotic or necrotic (F). Panels A, C, D-F: original magnification × 20. Panel B: original magnification × 40.
CD38 ligation in the presence of IL-2 induces differentiation of B-CLL cells. B-CLL cells obtained from 10 different patients were exposed to the anti-CD38 mAb (C), IL-2 (D), or a combination of the two (A-B). A population of cells with an enlarged cytosol and a light perinuclear zone, with the characteristics of plasmablasts, was observed after culture with anti-CD38+ IL-2 (A, thin arrows). Several blasts were present in every sample tested (A-B, arrows). Ligation of IgD with a polyclonal antibody in the presence of IL-2 was included as control and induced the appearance of plasmablasts (E, arrow). Samples treated with an anti-IgM polyclonal antibody were primarily apoptotic or necrotic (F). Panels A, C, D-F: original magnification × 20. Panel B: original magnification × 40.
Giemsa staining of B-CLL cells cultured for 5 days with CD38 and IL-2 revealed increased cell size, as measured using the ANALYSIS software (Table 5). This result owes to the appearance of a population of cells characterized by an enlarged cytosol and a perinuclear light zone (Figure 8A, thin arrows). These features are shared by plasmablasts. Moreover, several mitoses per high power field were scored in all patients analyzed (Figure 8A-B, block arrows). Ligation of IgD with a polyclonal antibody in the presence of IL-2 was included as the control and shows, as expected,29 a few plasmablast-like cells (Figure 8E, arrow) together with resting lymphocytes. Finally, IgM cross-linking was a potent apoptotic trigger (Figure 8F). Table 5 shows the mean arbitrary area values obtained by scoring the 5 largest cells per high-power field (× 40).
Morphologic analysis of B-CLL cells obtained from 10 different patients
. | No. 3 . | No. 5 . | No. 8 . | No. 12 . | No. 15 . | No. 19 . | No. 33 . | No. 27 . | No. 14 . | No. 16 . |
|---|---|---|---|---|---|---|---|---|---|---|
| α-CD38 + IL-2 | 218 | 309 | 289 | 328 | 313 | 298 | 324 | 265 | 176 | 148 |
| α-CD38 | 111 | 137 | 121 | 177 | 133 | 109 | 188 | 137 | 114 | 122 |
| IL-2 | 133 | 112 | 147 | 184 | 123 | 119 | 146 | 146 | 184 | 144 |
| Untreated | 99 | 107 | 112 | 132 | 103 | 98 | 134 | 126 | 122 | 114 |
. | No. 3 . | No. 5 . | No. 8 . | No. 12 . | No. 15 . | No. 19 . | No. 33 . | No. 27 . | No. 14 . | No. 16 . |
|---|---|---|---|---|---|---|---|---|---|---|
| α-CD38 + IL-2 | 218 | 309 | 289 | 328 | 313 | 298 | 324 | 265 | 176 | 148 |
| α-CD38 | 111 | 137 | 121 | 177 | 133 | 109 | 188 | 137 | 114 | 122 |
| IL-2 | 133 | 112 | 147 | 184 | 123 | 119 | 146 | 146 | 184 | 144 |
| Untreated | 99 | 107 | 112 | 132 | 103 | 98 | 134 | 126 | 122 | 114 |
The results of the morphologic analyses are supported by the numerical representation of the mean arbitrary area values obtained by scoring the 5 largest cells at a × 40 magnification, after analyzing at least 3 different fields (ANALYSIS software).
Discussion
Human CD38 is a multifunctional protein that belongs to the growing number of molecules independently performing as ectoenzymes and as receptors. Although its enzymatic activities and signaling pathway are understood in some detail, the true biologic function exerted in vivo by CD38 remains elusive. Interest in the molecule was refueled by independent reports of its involvement in a heterogeneous number of human diseases, which include HIV infection,30 diabetes, prostatic carcinoma, acute promyelocytic leukemia,12 and, most recently, B-CLL.
CD38 expression splits the B-CLL patient population into 2 groups and represents a dependable negative prognostic marker. Clues about the function of the molecule in these cells were gathered from comparative evaluation of the gene expression profiles of the 2 populations by microarray analyses.31,32 Initially disappointing, the results showed that CLL is characterized by a common gene expression “signature,” which does not reflect Ig mutational status or CD38 expression. This observation suggests that the cell of origin or the mechanisms of transformation (or both) may be common to all CLL patients. More recent analyses led to the identification of a set of genes differentiating CD38+ and CD38– B-CLL33 and IgVH unmutated and mutated.34 The majority of these genes are involved in signaling and regulation of cell survival.
The present work suggests that the acquisition by CD38 of signaling capacities in B-CLL cells may help explain the worse prognosis of CD38+ B-CLL patients. We relied on a conventional approach to establish receptor properties and exposed CD38+ B-CLL cells to mAb-mediated signaling. The results were initially unexpected because only in a minority of cells was CD38 ligation followed by the elicitation of detectable signals, at least under the experimental conditions adopted. This can be accounted for, however, if the signaling feature of CD38 depends on its reaching a specific threshold of molecules/cell and a specific membrane organization, as recent independent evidence from studies on monocytes35 implies. We therefore hypothesized that the dulled responses of the CD38+ B-CLL cells to anti-CD38–mediated signaling were due to their low levels of CD38 expression in terms of surface density and/or of molecular dimerization or polymerization. When IL-2 was added to the purified B-CLL cells, CD38 expression increased in time- and dose-dependent ways. The effect was mostly evident in CD38+ B-CLL cases; further, B-CLL patients with discrete CD38+ and CD38– populations featured a response predominantly within the CD38+ population. The appearance of Ca2+ fluxes following mAb ligation indicates that CD38 had become a signaling molecule in IL-2–exposed B-CLL cells. Based on previous experience with CD38, it seems reasonable to postulate that the enabling effects of IL-2 on CD38 signaling may involve the rearrangement of the surface of B-CLL cells, with modification of the expression and recruitment of selected molecules involved in signal transduction. The finding of increased Ca2+ waves mediated by IgM and IgD cross-linking after IL-2 treatment provides indirect evidence for this hypothesis.
The low epitope density of CD38 in resting B-CLL cells makes it difficult to evaluate membrane localization with any accuracy; however, IL-2 exposure and mAb treatment at 37°C induced CD38 to clearly localize in the same patches as the GM1 ganglioside. Indirect evidence in favor of raft localization of CD38 is derived from the lateral associations displayed with CD81, a tetraspanin family member constitutively harbored in membrane microdomains. These data are in line with previous results obtained in the T-cell lineage.24,36 Moreover, IL-2–treated B-CLL cells featured strong and highly reproducible lateral associations of CD38 with CD79α and CD79β, respectively. A direct colocalization with IgM and IgD confirmed the close proximity between CD38 and the BCR complex. Moreover, CD38 was found to be laterally associated with CD19, suggesting that the molecule could form part of the so-called positive receptor complex on the B-cell membrane.28 This situation is reminiscent of what is described for CD38 in T cells19 and in NK cells,20 where the membrane contiguity with CD3 and CD16, respectively, proved to be a prerequisite for CD38 signaling. One point that merits further investigation is the relationship of CD38 with an alternative form of CD79β, recently reported to diminish the apoptosis signals.37
After establishing the membrane organization of the molecule, attention was turned to understanding the nature of CD38 signaling in B-CLL cells. Results indicate that CD38, along with IL-2, induces a strong proliferative response, likely shifting the balance from lack of apoptosis to active proliferation. A correlation between CD38 expression and the proliferative potential of B cells was recently made by Tangye and colleagues while investigating the mechanisms underlying memory B-cell proliferation and differentiation into immunoglobulin-secreting cells.38 The CD38+ population was highly proliferative, likely representing the immediate precursor of long-lived plasma cells, and displayed the ability to proliferate without CD40L engagement.39 In contrast with these observations, CD38 expression seems to correlate with the presence of immunoglobulin unmutated genes and thus with an immature, pregerminal center differentiation stage of the cells. However, this point is still open to debate.40
B-CLL cells cultured with IL-2 and ligated with CD38 remain vital in culture for long periods of time (mean survival of > 4 weeks). During this period, the cells acquire morphologic features reminiscent of plasmablasts, as witnessed by (1) an increased size, (2) the presence of a large cytoplasm with a light perinuclear zone, and (3) the mitotic figures.
The conclusions of this work are that (1) CD38 is a receptor in B-CLL cells and (2) that it transduces activation/differentiation signals. It is true that the current approach is limited in its reliance on IL-2 to induce CD38 signaling; this, however, does not stand in the way of our findings for examination of circulating B-CLL cells, only takes into consideration a fraction of the neoplastic cells, which is not necessarily identical to the ones in the BM and in the peripheral lymphoid organs. Therefore, it is reasonable to surmise that B-CLL cells present in the BM or in the lymph nodes are exposed to IL-2 generated by a T-cell reaction and, as a consequence, up-regulate CD38.41 This would imply the existence of a defect in the generation of a T-cell response in this subset of patients and may explain the finding of a similarity between Ig-mutated and Ig-unmutated B-CLL cells. In other words, the difference in clinical course and prognosis might have to take into account cells other than the neoplastic clone. In line with this hypothesis, IL-2 is reported as able to modulate the response to purine- and chlorambucil-based therapeutic regimens.42 Alternatively, it may simply indicate that B-CLL cells modulate surface expression according to transit or localization in organs or districts (blood, BM, lymph nodes, among others). These cells reach the CD38 signaling threshold, which implements the signaling pathway leading to proliferation and differentiation, a mechanism already demonstrated for other proteins.43 This reservoir of B-CLL cells may add to the tumor burden and may be responsible for the clinical course.
In our view, the most promising finding of the present work pertains to the appearance of a distinct population of plasmablasts. This implies that synergistic signaling through a surface receptor such as CD38 and a soluble factor such as IL-2 may induce proliferation and differentiation of B-CLL cells. Further, one could hypothesize that by acting with its counterreceptor, CD38 may be responsible for the acquisition of growth properties accounting for poor prognosis. The only counterreceptor thus far described for CD38 is CD31, which is highly expressed by subsets of stromal cells and by the B cells themselves.17
Results by Morabito et al44 indicate that more than 50% of all CLL cases express CD31, as is the case for what has been described in normal and pathologic plasma cells.45 In this study, CD31 was shown to be unaffected by treatment with IL-2, suggesting that only the stromal cells expressing CD31 play a role in the cross-talk between the 2 molecules. Not less important is the notion that both CD3846 and CD3147 are polymorphic in the white population. CD38 signaling might be hampered by the presence of CD31 on the same B-CLL cell. Interactions between CD38 and CD31 simultaneously present on the tumor cells could lead to modulation or steric modifications of both molecules, consequently making them unavailable to other ligands and therefore unresponsive to signaling.
Taking into account all the present evidence, one could devise a model in which the neoplastic transformation hits a B cell before or after the germinal center. When this occurs prior to Ig rearrangement, the cell still expresses CD38, whose signaling properties may be counteracted by the expression by the same cell of CD31, a potent inhibitor of apoptosis.48 As a result of the interactions between the B cells and the environment (represented by stromal cells and by soluble factors) CD38 could be up-regulated, reach its signaling threshold, and start delivering proliferation/differentiation signals. In this respect, the events recorded in vitro may represent a magnification of in vivo events, which happen only in a minority of B-CLL patients undergoing the transformation known as Richter syndrome. And this is what we are currently testing.
Prepublished online as Blood First Edition Paper, May 22, 2003; DOI 10.1182/blood-2003-03-0989.
Supported by grants from Associazione Italiana Ricerca Cancro; “Fondo Incentivazione Ricerca Base,” Ministero Instruzione, Università e Ricerca; “ex-40%,” Ministero Università Ricerca Scientifica Technologica, and from the 2002 Health Strategic Research Project (Ministero della Salute). The Fondazione Internazionale Ricerche Medicina Sperimentale (FIRMS) and Compagnia di SanPaolo provided valuable financial contributions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
S.D. is a student in the PhD Program in Human Genetics, University of Torino Medical School, Torino, Italy. Thanks are given to Dr R. Villela (Barcelona, Spain) and to Dr T. Musso (Torino, Italy) for providing reagents and to Dr M. Ferrarini (Genova, Italy) for helpful discussion. The help of Drs M. Coscia and M. Foglietta (Torino, Italy) is gratefully acknowledged. We are indebted to Dr M. Capris (Olympus, Italy) for assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal