Abstract
Dendritic cells (DCs) are a family of leukocytes that initiate T- and B-cell immunity against pathogens. Migration of antigen-loaded DCs from sites of infection into draining lymphoid tissues is fundamental to the priming of T-cell immune responses. In humans, the major peripheral blood DC (PBDC) types, CD1c+ DCs and interleukin 3 receptor–positive (IL-3R+) plasmacytoid DCs, are significantly expanded in vivo with the use of Flt3 ligand (FL). DC-like cells can also be generated from monocyte precursors (MoDCs). A detailed comparison of the functional potential of these types of DCs (in an autologous setting) has yet to be reported. Here, we compared the functional capacity of FL-expanded CD1c+ PBDCs with autologous MoDCs in response to 3 different classes of stimuli: (1) proinflammatory mediators, (2) soluble CD40 ligand trimer (CD40L), and (3) intact bacteria (Escherichia coli). Significant differences in functional capacities were found with respect to changes in phenotype, migratory capacity, cytokine secretion, and T-cell stimulation. MoDCs required specific stimuli for the expression of functions. They responded vigorously to CD40L or E coli, expressing cytokines known to regulate interferon-γ (IFN-γ) in T cells (IL-12p70, IL-18, and IL-23), but required prostaglandin E2 (PGE2) during stimulation to migrate to chemokines. In contrast, PBDCs matured in response to minimal stimulation, rapidly acquired migratory function in the absence of PGE2-containing stimuli, and were low cytokine producers. Interestingly, both types of DCs were equivalent with respect to stimulation of allogeneic T-cell proliferation and presentation of peptides to cytotoxic T lymphocyte (CTL) lines. These distinct differences are of particular importance when considering the choice of DC types for clinical applications.
Introduction
Dendritic cells (DCs) are rare bone marrow–derived cells, involved in antigen capture, processing, and presentation. DCs are uniquely able to prime a naive T-cell response.1,2 Because of their critical role in orchestrating the immune response, there is increasing interest in using DCs as cellular vaccine adjuvants in the immunotherapy of cancer.3,4 A variety of soluble factors and pathogen signals are known to activate DCs.1,2 Thus, the maturation state of vaccine-loaded DCs will probably be critical for their regulation of appropriate T-cell immune responses. Three main sources of DCs have been used in clinical trials: DCs derived from (1) CD34+ progenitor cells, (2) CD14+ monocytes, and (3) peripheral blood DC precursors. Generation of CD34+-derived DCs requires 10 to 28 days in in vitro culture,5,6 while DCs generated in vitro from CD14+ monocytes (MoDCs) require 5 to 7 days.7,8 Although their physiologic relevance in vivo remains unclear, MoDCs are the major DC type used in vaccine-based clinical studies.3,4 MoDCs have also been used to establish many of the biologic paradigms of DC function.1,2 An alternative, and perhaps more physiological, source of DCs in humans is provided by the immature DC populations found in peripheral blood.9,10 At least 2 peripheral blood DC (PBDC) populations, constituting fewer than 1% of total mononuclear cells, exist in human peripheral blood: CD1c+ PBDCs and interleukin 3 receptor–positive (IL-3R+) plasmacytoid DCs (PDCs).9-13 Several cytokines are known to expand the number of these PBDC types in vivo, including granulocyte colony-stimulating factor (G-CSF) and Flt-3 ligand (FL).9,14,15 FL expands both human CD1c+ PBDCs and IL-3R+ PDCs9,10,14-18 and has antitumor effects in animal models.19-21 It has been suggested that the CD1c+ PBDC subset in peripheral blood is related to the CD14-derived dermal DCs and to germinal center DCs.6,22,23 Both of these types of DCs appear to be of myeloid origin and can differentiate into Langerhans cells in the presence of transforming growth factor–β (TGF-β).12,24 However, little is currently known of the functional differences between the CD1c+ PBDC and MoDC types (eg, antigen uptake capacity, migration, cytokine secretion, and regulation of T-cell function).
Few direct comparisons of DC types have been reported. Comparisons of CD34+-derived DCs and MoDCs suggest that CD34+-derived DCs may be superior at activating low-frequency, peptide-specific cytotoxic T lymphocytes.25-28 Other studies have reported that IL-3R+ PDCs are functionally different from MoDCs.13,29-36 However, few of these studies have directly compared DC functions in an autologous setting; most have compared DC types among allogeneic donor sources. Thus, the degree to which donor variation contributes to the observed functional differences may be significant.
We performed a clinical trial that evaluated FL (to expand PBDC numbers) with or without peptide vaccination in patients with malignant melanoma (M.J. Shackleton et al, submitted manuscript, 2003). The present study describes the functional analysis of FL-expanded CD1c+ PBDCs isolated from these patients and compares them with autologous MoDCs. Furthermore, CD1c+ PBDCs and autologous MoDCs from healthy donors were also compared to exclude the possibility that functional differences among DC types from cancer patients were due to the cancer itself or that alterations in DC behavior were due to FL administration. We found major differences between the responses of MoDCs and CD1c+ PBDCs toward 3 different classes of physiologic stimuli with respect to migratory function, cytokine production, and regulation of T-cell function.
Materials and methods Media
DCs were cultured in RPMI 1640 (Trace Biosciences, Melbourne, Australia) supplemented with 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 60 mg/L penicillin G, 12.6 mg/L streptomycin, 2 mM L-glutamine, 1% nonessential amino acids, and 10% heat-inactivated fetal calf serum (FCS) (CSL, Melbourne, Australia) in a 5% CO2 incubator. Mixed leukocyte reactions (MLRs) were performed in Iscove modified Dulbecco medium (IMDM) (Gibco, Grand Island, NY) with 5% pooled normal human serum (gift from the Victorian Tissue Typing Service, Royal Melbourne Hospital, Melbourne, Australia) in a 10% CO2 incubator.
Monoclonal antibodies and cytokines
Flow cytometric analysis of DCs and T cells was performed with the following monoclonal antibodies: fluorescein isothiocyanate (FITC)– conjugated immunoglobulin G1 (IgG1) isotype control; phycoerythrin (PE)–conjugated IgG1 isotype control; anti-CD1a; anti-CD1c; anti-CD1d; anti-CD45RA; anti-CD80; anti-CD83; anti-CD86; anti-CD123 (IL-3Rα); anti–human leukocyte antigen DR (anti–HLA-DR); anti–macrophage mannose receptor (anti-MMR); anti-CXCR3; anti-CD3; anti-CD8; anti– interferon-γ (anti–IFN-γ) (all from BD Biosciences Pharmingen, San Diego, CA); anti–CC chemokine receptor 6 (anti-CCR6) (R&D Systems, Minneapolis, MN); and anti–blood dendritic cell antigen 2 (anti–BDCA-2) and anti–BDCA-3 (Miltenyi Biotech, Auburn, CA). The following recombinant human cytokines were added to DC cultures: tumor necrosis factor–α (TNF-α) (10 ng/mL); IL-4 (500 U/mL) (both Peprotech, Rocky Hill, NJ); granulocyte-macrophage CSF (GM-CSF) (40 ng/mL) (Schering-Plough, Sydney, Australia); and IFN-α2a (1000 IU/mL) (Roferon-A; Roche Products, Sydney, Australia). Prostaglandin E2 (PGE2) (1 μM final concentration) was purchased from Sigma Chemical (St Louis, MO). Soluble CD40L trimer (CD40L) (1 μg/mL final concentration) was a kind gift from Amgen (Seattle, WA).
Cell sources
CD1c+ PBDCs and monocytes were isolated (1) from peripheral blood mononuclear cells (PBMCs) of patients with stage II, III, or IV melanoma enrolled in a phase 1 clinical study (LUD-97-012) (M.J. Shackleton et al, submitted manuscript, 2003) receiving 14 consecutive days of FL (Amgen) (25 μg/kg/d) alone or in combination with peptide vaccines or (2) from buffy packs from healthy donors provided by the Australian Red Cross Blood Bank (Southbank, Melbourne, Australia). In the present study, the various types of DCs were examined from patients with minimal residual disease to exclude the possible issues of advanced cancer on decreasing the functional capacity of DCs via the release of immunosuppressive cytokines. Blood for monocyte isolation was taken prior to administration of FL, and on day 15 for CD1c+ PBDC isolation. The Protocol Review Committee of the Ludwig Institute for Cancer Research and the Human Research Ethics Committee of the Austin and Repatriation Medical Centre (Heidelberg, Victoria, Australia) approved the protocol, and informed consent was obtained from all patients.
CD14+ monocytes were affinity purified by means of the MACS CD14 isolation kit (Miltenyi Biotech) and cultured (7 days) in RPMI/10% FCS (5 × 105/mL) with GM-CSF (40 ng/mL) and IL-4 (500 U/mL) in 24-well plates to generate MoDCs (more than 95% of cultured cells). On day 7, all wells were pooled and readjusted to a DC concentration of 5 × 105/mL. Maturation-inducing factors were added on day 7, and cells and supernatants were harvested on day 8 or 9 for functional assessment. Cytokines and other stimuli in the present study (eg, TNF-α, IFN-α2a, CD40L, PGE2, and intact Escherichia coli) were titrated, and the concentrations used in the Figures represent those found to be optimal.
Enrichment of CD1c+ PBDCs from FL-treated patients and healthy volunteers
CD1c+ PBDCs were enriched from frozen PBMC samples obtained from the clinical trial (LUD-97-012) (M.J. Shackleton et al, submitted manuscript, 2003). After thawing, CD14+ monocytes, CD19+ B cells, and CD3+ T cells were depleted by means of immunomagnetic beads (MACS; Miltenyi Biotech) according to the manufacturer's instructions. This depletion procedure routinely yielded greater than 60% CD1c+CD14– HLA-DR+ PBDCs as assessed by fluorescence-activated cell sorter (FACS). The enriched PBDCs were then stained with anti-CD1-FITC (Biosource, Camarillo, CA), anti-CD123–PE (IL-3Rα), and anti–HLA-DR–allophycocyanin (APC) (both BD Biosciences Pharmingen) and sorted as a CD1c+CD123loHLA-DR+ population on a MoFlo cell sorter (94% to 98% purity) (Cytomation, Fort Collins, CO). Sorted CD1c+ PBDCs were then cultured in 24-well plates (5 × 105 per well) in RPMI/10% FCS for 2 days with various combinations of stimuli prior to assessment of function. In some experiments, CD1c+ PBDCs and autologous CD14+ monocytes were positively selected by means of magnetic bead isolation. PBMCs were sequentially treated with anti-CD14 beads (MACS; Miltenyi Biotech) and CD14+ monocytes (greater than 96% purity) cultured in GM-CSF and IL-4 for the generation of MoDCs. The residual PBMCs were then incubated with anti–BDCA-1 (anti-CD1c) beads (MACS; Miltenyi Biotech) and CD1c+ PBDCs were isolated (greater than 97% purity).
Cell migration assay
Assays were performed as previously described.37 Briefly, lower chambers of Transwell plates (8.0-μm pore size) (Costar, Corning, NY) were filled with 500 μL RPMI/10% FCS with or without chemokines: CCL21 (macrophage inflammatory protein 3–β [MIP-3β]) (300 ng/mL); CCL19 (6Ckine) (100 ng/mL); or CXCL12 (stromal cell–derived factor 1-alpha [SDF-1α; 30 ng/mL]) (all from Peprotech). DCs (1 to 2 × 104) were added in 50 μL RPMI/10% FCS into the upper chamber. After 2 hours, cells in the lower chambers were harvested, concentrated to 50 μL volumes in Eppendorf tubes, and counted microscopically with a hemocytometer. Each stimulation condition was performed in triplicate wells.
RNA isolation and cDNA synthesis
Total RNA was isolated from MoDCs and CD1c+ PBDCs by means of an RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. In brief, cells were lysed and homogenized in lysis buffer containing guanidine isothiocyanate and β-mercaptoethanol. Then, 70% ethanol was added to the samples, and the RNA immobilized on spin columns and eluted in RNase-free water. We used 0.16 μg total RNA to synthesize cDNA with 1 μg random hexamers (Promega, Madison, WI), 1 mM deoxynucleoside triphosphates (dNTPs) (Amersham Pharmacia Biotech, Piscataway, NJ), 2 U RNAse inhibitor (Promega), 5 mM MgCl2 (Applied Biosystems, Foster City, CA), 1 × polymerase chain reaction (PCR) buffer (Applied Biosystems), and 2 U Moloney murine leukemia virus (M-MLV) reverse transcriptase (Life Technologies, Rockville, MD) in a 20-μL reaction, for 60 minutes at 42°C. The enzyme was inactivated at 95°C for 5 minutes. One microliter of the resulting 20 μL cDNA was used for real-time PCR quantitation.
Quantitative real-time PCR
Predeveloped assay reagents (PDARs) for IL-12p35, IL-12p40, and IL-18 were obtained from Applied Biosystems and used in multiplex reactions with 18S rRNA PDAR (Applied Biosystems) for normalization. Primers and probe for IL-23p19 were designed with the use of Primer Express software, version 1.5a (Applied BioSystems). Gene expression levels were quantitated by means of ABI Prism 7700 Sequence Detection System (Applied Biosystems). PCR reactions were set up in 96-well plates (25 μL per reaction) according to the manufacturer's instructions and analyzed by means of the SDS program, version v1.7 (Applied BioSystems). Relative expression was calculated by the ΔCt method and is expressed relative to a calibrator, in this case the GM-CSF/IL-4 DC control as previously described.37
Cytokine ELISAs
Cytokine secretion by stimulated DCs or by allogeneic T cells was measured by cytokine enzyme-linked immunosorbent assays (ELISAs). Cytokine ELISA kits were purchased for IL-2, IL-5, IL-6, IFN-α, IL-10, IL-12p70 (Opteia; BD Biosciences Pharmingen), IL-18 (MBL, Nagoya, Japan), and PGE2 (BioScientific, Gymea, New South Wales, Australia). Capture and horseradish peroxidase (HRP)–conjugated detection antibodies for IFN-γ ELISAs were a kind gift from CSL. PGE2, IFN-α, IL-6, IL-10, IL-12p70, IL-18, and IFN-γ ELISAs were performed on supernatant (SN) from DC cultures, and IFN-γ, IL-2, IL-5, and IL-10 ELISAs were performed on SN from allo-MLRs according to the manufacturer's instructions with the use of Maxisorp plates (Nunc, Roskilde, Denmark). The HRP substrate was tetramethylbenzidine (TMB) peroxidase (KPL, Gaithersburg, MD); the color reaction was terminated by adding 100 μL ortho-phosphoric acid (1 M). Plates were read in a Thermomax microplate reader (BioMediq, Doncaster, Victoria, Australia).
T-cell purification and mixed leukocyte reaction
Allogeneic CD2+ T lymphocytes were obtained by rosetting PBMCs with aminoethylisothiouronium (AET)–treated sheep red blood cells. T cells were between 88% and 95% pure on the basis of CD3 staining. Varying numbers of DCs were cultured in round-bottomed 96-well plates in triplicate with 105 allogeneic PBMCs for 5 days in IMDM with 5% human serum. After 5 days, 200 μL supernatants were harvested, and fresh medium containing 1 μCi (0.037 MBq) [3H]thymidine (DuPont, Sydney, MA) per well was added for 8 hours. Cells were transferred onto a glass fiber filter (Wallac, Turku, Finland), and [3H]thymidine incorporation was measured by means of an NXT TopCount Betaplate scintillation counter (Packard, Meriden, CT). In separate experiments, the CD2+ T cells (1 × 107) were labeled with 5-(and 6) carboxyfluorescein diacetate succinimidyl ester (CFSE) (0.01 mM) in serum-free phosphate-buffered saline (PBS) in the dark (10 minutes at room temperature. T cells were then washed and cultured (3 × 105) with immature or mature MoDCs (1 × 104) in round-bottomed 96-well plates in triplicate for 5 days. On day 5, cultures were restimulated with freshly matured MoDCs in the presence of 10 μg/mL Brefeldin A at 37°C for 8 hours. Cells were harvested, pelleted, and stained with anti-CD8–APC and CD3–Cy-Chrome (BD Biosciences Pharmingen), washed again, and then fixed with 1% paraformaldehyde (ProSciTech, Thuringowan, Australia)/PBS before staining with FITC-conjugated anti–IFN-γ (BD Biosciences Pharmingen)/0.2% saponin/PBS at 4°C overnight. Cells were then analyzed by means of FACS.
DC-peptide presentation to a cytotoxic T-lymphocyte (CTL) line
First, 6 × 10–6 to 6 × 10–12 M HLA-A2–restricted peptides NY-ESO-1b (amino acids 157 through 165, sequence SLLMWITQC) (Biological Production Facility, Ludwig Institute for Cancer Research, Heidelberg, Australia) and Epstein-Barr virus (EBV) (BMLF1 sequence amino acids 280-288, GLCTLVAML, Austin Research Institute, Melbourne, Australia) were treated at room temperature for 1 hour with 500 μM Tris (tris(hydroxymethyl)aminomethane) (2-carboxyethyl)–phosphine hydrochloride (TCEP) (Pierce, Rockford, IL) in cystine-free Dulbecco modified Eagle medium (Cys-free DMEM) (Gibco) to reduce dimerized peptides to monomeric form. MoDCs or CD1c+ PBDCs or the transporter associated with antigen processing (TAP)–deficient T2 cells were resuspended in Cys-free DMEM, and equal volumes were added to the reduced peptide and pulsed at room temperature for 30 minutes. The DCs or T2 cells were then washed once and resuspended in RPMI/10% FCS and 10 μg/mL Brefeldin A at a cell concentration of 1 × 106/mL. Then, 100 μL peptide-pulsed DCs or T2 cells were incubated with 100 μL peptide-specific T cells (APC-effector ratio of 1:1) at 37°C for 4 hours in a 96-well U-bottom plate. Cells were pelleted, stained with anti-CD8 Cy-Chrome, washed, and then fixed with 1% paraformaldehyde (ProSciTech)/PBS before staining with FITC-conjugated anti–IFN-γ/0.2% saponin/PBS at 4°C overnight. Cells were then analyzed by means of FACS.
Results
Cell morphology and culture of MoDCs and CD1c+ PBDCs
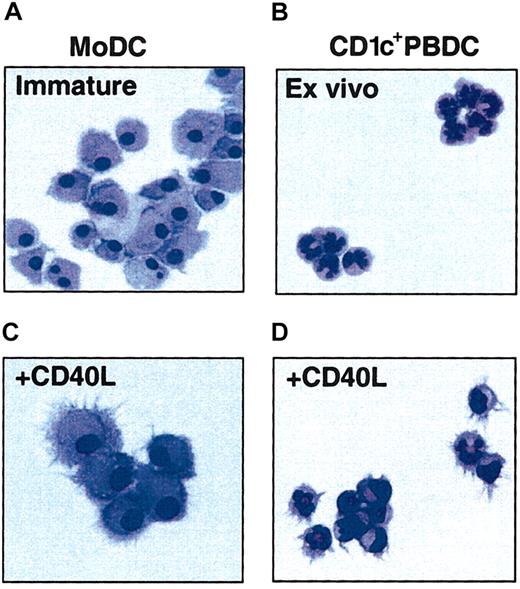
CD1c+ PBDCs were purified from the PBMCs of melanoma patients (with minimal residual disease) treated with FL by removal of lineage-positive cells by monoclonal antibody (mAb)–magnetic-activated cell sorting (MACS) bead depletion and cell sorting of lineage-negative cells on the basis of CD1b/c and HLA-DR expression to greater than 97% purity. CD1c+ PBDCs showed poor viability if cultured in medium alone, but viabililty was substantially improved when they were cultured with GM-CSF and IL-4. FL-expanded CD1c+ PBDCs were morphologically identical to their counterparts from untreated individuals with a typical multilobulated nuclear morphology (Figure 1A). To avoid issues relating to the myelopoietic effects of FL upon monocyte development in vivo,9 the present study generated autologous MoDCs from CD14+ monocytes isolated from blood samples taken prior to FL administration. Immature MoDCs (GM-CSF plus IL-4) were morphologically distinct from freshly isolated CD1c+ PBDCs, being larger with round or kidney-shaped nuclear morphology and more extensive cytoplasm (Figure 1B). Both FL-expanded CD1c+ PBDCs and MoDCs displayed morphologic features typical of mature DCs following stimulation with CD40L, including prominent dendritic processes (Figure 1C-D).
Morphology of immature and mature MoDCs and CD1c+ PBDCs. PBDCs were purified by negative depletion from the peripheral blood of patients treated with FL for 14 consecutive days and then FACS sorted to high purity (greater than 95%) on the basis of CD1c and HLA-DR expression. Autologous MoDCs were generated from blood taken prior to FL administration and cultured for 7 days prior to the parallel isolation of PBDCs. MoDCs were prepared by culturing purified CD14+ monocytes for 7 days in GM-CSF and IL-4. (A) Immature MoDCs. (B) Freshly sorted CD1c+ PBDCs. (C) MoDCs stimulated (second day) with CD40L. (D) CD1c+ PBDCs stimulated (second day) with CD40L. Figures are representative of more than 10 experiments. All photomicrographs are × 100 original magnification.
Morphology of immature and mature MoDCs and CD1c+ PBDCs. PBDCs were purified by negative depletion from the peripheral blood of patients treated with FL for 14 consecutive days and then FACS sorted to high purity (greater than 95%) on the basis of CD1c and HLA-DR expression. Autologous MoDCs were generated from blood taken prior to FL administration and cultured for 7 days prior to the parallel isolation of PBDCs. MoDCs were prepared by culturing purified CD14+ monocytes for 7 days in GM-CSF and IL-4. (A) Immature MoDCs. (B) Freshly sorted CD1c+ PBDCs. (C) MoDCs stimulated (second day) with CD40L. (D) CD1c+ PBDCs stimulated (second day) with CD40L. Figures are representative of more than 10 experiments. All photomicrographs are × 100 original magnification.
Phenotypic analysis and maturation of MoDCs and CD1c+ PBDCs
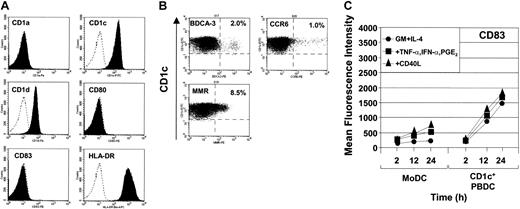
Freshly isolated CD1c+ PBDCs were phenotypically immature, expressing low levels of the maturation markers CD80, CD83 (Figure 2A), and CD86 (data not shown). Consistent with previous reports, CD1a was constitutively expressed on MoDCs but was not present on freshly isolated PBDCs (Figure 2A). In contrast, CD1c+ PBDCs, but not MoDCs, expressed CD1d. Both DC types expressed CD1c (Figure 2A) as well as CD1b, CD11c, CD13, CD33, and CD54 (data not shown), consistent with a putative myeloid origin. Whereas CD1c+ PBDCs spontaneously up-regulated the expression of CD80, CD83, and CD86 following overnight culture in medium containing GM-CSF and IL-4,38 MoDCs required maturation with specific combinations of stimuli.38 As previously reported, FL-expanded CD1c+ PBDCs showed heterogeneous expression of MMR.9 Additionally, discrete subpopulations within the CD1c+ PBDC gate were also detected on the basis of CCR6 and BDCA-3 expression (Figure 2B). The percentage expression for MMR, CCR6, and BDCA-3 suggests that multiple subpopulations are likely to exist. Interestingly, CD1c+ PBDCs up-regulated surface expression of CD83 (Figure 2C) and HLA-DR (data not shown) more rapidly than MoDCs, regardless of maturational stimulus. Furthermore, the mean fluorescence intensity of these markers was an order of magnitude greater for CD1c+ PBDCs compared with MoDCs (Figure 2C).
Surface marker expression of freshly isolated and matured CD1c+ PBDCs. (A) Surface antigen expression of maturation markers on CD1c+ PBDCs. Isotype control is marked by the broken-lined, unfilled histogram; surface staining of PBDCs is shown by filled histograms. (B) Surface expression of the chemokine receptor CCR6, BDCA-3, and MMR. Crosshairs reflect background settings based on the isotype-matched Ab controls. Data are representative of 3 separate experiments. (C) Surface expression of CD83 on CD1c+ PBDCs and MoDCs at 2, 12, and 18 hours following stimulation either with GM-CSF and IL-4 or with GM-CSF and IL-4 in combination with TNF-α, IFN-α, and PGE2 or with CD40L.
Surface marker expression of freshly isolated and matured CD1c+ PBDCs. (A) Surface antigen expression of maturation markers on CD1c+ PBDCs. Isotype control is marked by the broken-lined, unfilled histogram; surface staining of PBDCs is shown by filled histograms. (B) Surface expression of the chemokine receptor CCR6, BDCA-3, and MMR. Crosshairs reflect background settings based on the isotype-matched Ab controls. Data are representative of 3 separate experiments. (C) Surface expression of CD83 on CD1c+ PBDCs and MoDCs at 2, 12, and 18 hours following stimulation either with GM-CSF and IL-4 or with GM-CSF and IL-4 in combination with TNF-α, IFN-α, and PGE2 or with CD40L.
Induction of cytokine secretion by highly purified CD1c+ PBDCs and MoDCs
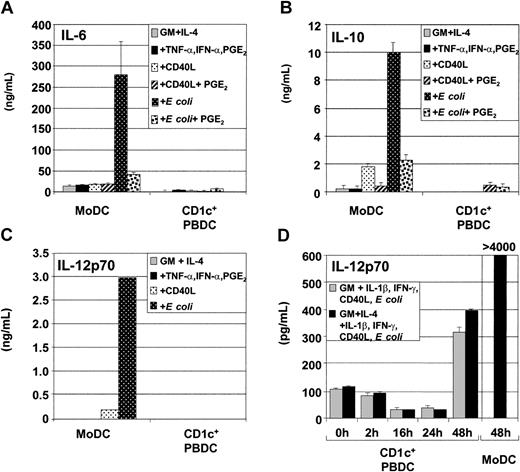
DCs produce several types of cytokines following stimulation with pathogen or CD40L, such as IL-6, IL-10, and IL-12p70. We compared cytokine secretion by CD1c+ PBDCs and MoDCs in response to different classes of physiologic stimuli. Figure 3A shows that MoDCs secreted considerably more IL-6 compared with CD1c+ PBDCs, particularly in response to E coli. Similarly, MoDCs secreted IL-10 in response to a range of stimuli, but produced the highest levels of IL-10 following stimulation with E coli (Figure 3B). As previously reported, the addition of PGE2 to CD40L or to E coli decreased the amount of IL-10 produced by MoDCs.37
Secretion of cytokines by immature and mature MoDCs and CD1c+ PBDCs. Immature MoDCs (GM-CSF plus IL-4) or freshly sorted CD1c+ PBDCs were stimulated for 2 days with TNF-α plus IFN-α plus PGE2; with CD40L in the presence or absence of PGE2; or with E coli in the presence or absence of PGE2. Cytokine ELISAs were performed on culture supernatant. Secretion by unstimulated or stimulated MoDCs or CD1c+ PBDCs is shown. (A) IL-6 secretion. (B) IL-10 secretion. (C) IL-12p70 secretion. Data represent the means ± standard errors of the means (SEMs) of triplicate cultures and are representative of 5 to 7 separate donors. (D) Kinetics of IL-12p70 production by CD1c+ PBDCs matured in vitro with GM-CSF or with GM-CSF plus IL-4 for the indicated times prior to stimulation (for 24 hours) with the combination of IL-1β, IFN-γ, CD40L, and intact E coli. MoDCs were stimulated for 48 hours with E coli. Data are representative of 4 separate experiments.
Secretion of cytokines by immature and mature MoDCs and CD1c+ PBDCs. Immature MoDCs (GM-CSF plus IL-4) or freshly sorted CD1c+ PBDCs were stimulated for 2 days with TNF-α plus IFN-α plus PGE2; with CD40L in the presence or absence of PGE2; or with E coli in the presence or absence of PGE2. Cytokine ELISAs were performed on culture supernatant. Secretion by unstimulated or stimulated MoDCs or CD1c+ PBDCs is shown. (A) IL-6 secretion. (B) IL-10 secretion. (C) IL-12p70 secretion. Data represent the means ± standard errors of the means (SEMs) of triplicate cultures and are representative of 5 to 7 separate donors. (D) Kinetics of IL-12p70 production by CD1c+ PBDCs matured in vitro with GM-CSF or with GM-CSF plus IL-4 for the indicated times prior to stimulation (for 24 hours) with the combination of IL-1β, IFN-γ, CD40L, and intact E coli. MoDCs were stimulated for 48 hours with E coli. Data are representative of 4 separate experiments.
IL-12p70 is critical for the induction of IFN-γ production by T cells. Bioactive IL-12p70 is composed of 2 subunits (IL-12p35 and IL-12p40). Another IL-12 family member, IL-23, has overlapping effects with IL-12p70 and is composed of IL-12p40 and the novel IL-23p19 subunit. We evaluated the expression of IL-12p70 and IL-23 by CD1c+ PBDCs and autologous MoDCs in order to evaluate the potential of these DC subpopulations to induce T-cell IFN-γ production. Figure 3C shows that MoDCs are potent producers of IL-12p70, especially following stimulation with E coli, whereas CD1c+ PBDCs are poor IL-12p70 producers, confirming previous results.37,38
Induction of cytokine secretion by CD1c+ PBDCs following initial in vitro culture prior to stimulation
Previous reports, as well as this study, indicate that freshly isolated CD1c+ PBDCs are relatively poor producers of cytokines following immediate stimulation.37,38 However, 2 studies have shown that CD1c+ PBDCs can produce IL-12p70 following in vitro stimulation. In both studies, PBDCs were initially cultured (thus matured) for at least 24 hours prior to stimulation with lipopolysaccharide (LPS) or CD40L.18,39 We, therefore, evaluated whether in vitro maturation of CD1c+ PBDCs enhanced their responsiveness to IL-12p70–inducing stimuli such as CD40L, intact E coli, or the combination of IL-1β, IFN-γ, CD40L, and E coli. Increased production of IL-12p70 was observed in response to the combination of GM-CSF, IL-1β, IFN-γ, CD40L, and E coli (Figure 3D), but not in response to GM-CSF plus CD40L or GM-CSF plus E coli (data not shown). Figure 3D also shows that CD1c+ PBDCs required prolonged in vitro culture (24 to 48 hours) prior to stimulation in order to produce increased levels of IL-12p70 (approximately 400 pg/mL). Shorter times of in vitro maturation (2 to 24 hours) prior to stimulation were not sufficient at enhancing IL-12p70–producing capacity. However, even under these optimized conditions, IL-12p70 production by CD1c+ PBDCs was consistently lower than that of MoDCs.
Figure 3D also demonstrates that the low production of cytokines by CD1c+ PBDCs shown in Figure 3A-C was not due to the attenuating effects of IL-4 since similar levels of IL-12p70 were produced regardless of whether IL-4 was present or absent from the stimulation cocktail.
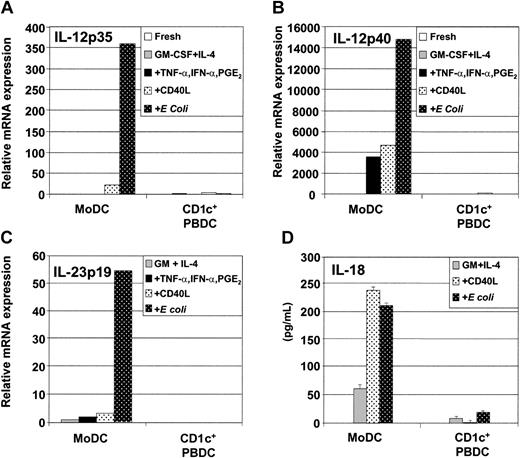
Using quantitative real-time PCR (qRT-PCR), we examined whether the low levels of IL-12p70 produced by CD1c+ PBDCs are due to low levels of IL-12p35 or IL-12p40 mRNA expression. Figure 4A-B demonstrates that for MoDCs, the levels of IL-12p35 mRNA expression correlated with IL-12p70 production by ELISA. Similarly, for CD1c+ PBDCs, we found that low IL-12p70 secretion correlated with low expression of IL-12p35 and IL-12p40 mRNA (Figure 4A-B). Finally, the novel IL-23p19 mRNA was neither constitutively expressed by freshly isolated CD1c+ PBDCs nor induced following stimulation. In contrast, immature MoDCs constitutively expressed IL-23p19 mRNA, which was further increased following stimulation with E coli (Figure 4C).
Secretion and mRNA expression of soluble factors by immature and mature MoDCs and CD1c+ PBDCs. Immature MoDCs (GM-CSF plus IL-4) or freshly sorted CD1c+ PBDCs were stimulated for 2 days with TNF-α plus IFN-α plus PGE2; with CD40L; or with intact E coli. Then, DCs were examined for mRNA expression by quantitative qRT-PCR as described in “Materials and methods.” (A) IL-12p35 expression. (B) IL-12p40 expression. (C) IL-23p19 expression. (D) Secretion of IL-18 by unstimulated or stimulated MoDCs or CD1c+ PBDCs. Data are representative of at least 3 separate experiments.
Secretion and mRNA expression of soluble factors by immature and mature MoDCs and CD1c+ PBDCs. Immature MoDCs (GM-CSF plus IL-4) or freshly sorted CD1c+ PBDCs were stimulated for 2 days with TNF-α plus IFN-α plus PGE2; with CD40L; or with intact E coli. Then, DCs were examined for mRNA expression by quantitative qRT-PCR as described in “Materials and methods.” (A) IL-12p35 expression. (B) IL-12p40 expression. (C) IL-23p19 expression. (D) Secretion of IL-18 by unstimulated or stimulated MoDCs or CD1c+ PBDCs. Data are representative of at least 3 separate experiments.
IL-18, like IL-12p70 and IL-23, can induce IFN-γ secretion by T cells. Production of IL-18 by CD1c+ PBDCs or MoDCs was investigated. Figure 4D shows that immature MoDCs (GM-CSF plus IL-4) constitutively produced low levels of bioactive IL-18 (approximately 50 pg/mL) and that secretion was increased upon stimulation with either CD40L or E coli (Figure 4D). In contrast, CD1c+ PBDCs were poor producers of bioactive IL-18 irrespective of the type of stimulus encountered.
Analysis of migratory capacity of MoDCs and CD1c+ PBDCs
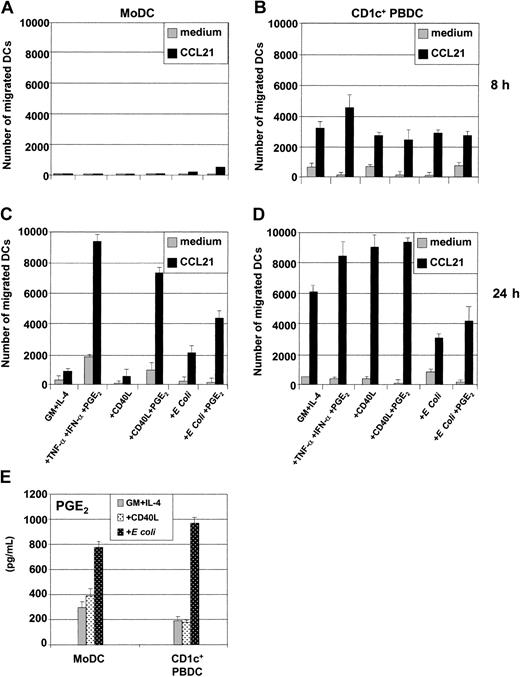
Migration of antigen-loaded DCs toward lymphoid organs is critical for the initiation of T-cell immunity and requires the expression of the chemokine receptor CCR7 to respond to the lymph node–directing chemokines CCL19 (MIP-3β or EBV ligand chemokine [ELC]) or CCL21 (6Ckine or secondary lymphoid tissue chemokine [SLC]). The migratory capacity of CD1c+ PBDCs and autologous MoDCs in response to CCL21 was assessed next. PGE2 is a critical regulator of migratory function in MoDCs.37,40 The addition of PGE2 reduced the ability of either CD40L or E coli to induce cytokine secretion in MoDCs (Figure 3A-C) while at the same time inducing MoDC migratory function (Figure 5C).37,40 In contrast, PGE2 was less critical for regulating these functions in CD1c+ PBDCs, which spontaneously migrated following maturation with all the classes of stimuli irrespective of the presence of PGE2 (Figure 5D).37,40 We next examined whether the kinetics with which CD1c+ PBDCs acquired migratory function paralleled that of MoDCs. As shown, CD1c+ PBDCs (Figure 5B,D) acquired migratory capacity in vitro more rapidly (8 hours) compared with autologous MoDCs (24 hours) (Figure 5A,C). The differing kinetics regarding acquisition of migratory function between FL-generated CD1c+ PBDCs and autologous MoDCs were also seen with DCs from healthy individuals (data not shown).
Migratory capacity of immature and mature MoDCs and CD1c+ PBDCs. Immature MoDCs (GM-CSF plus IL-4); freshly sorted CD1c+ PBDCs, or CD1c+ PBDCs were stimulated for 8 hours (A-B) or 24 hours (C-D) with TNF-α plus IFN-α plus PGE2, with CD40L in the presence or absence of PGE2, or with intact E coli in the presence or absence of PGE2. These were then loaded into the upper transwell chambers and examined for their capacity to migrate toward either medium alone or CCL21 present in the lower transwell chambers. The y-axis shows the number of DCs migrating through the transwell membrane (8 μm) after 2 hours. Data in panels A-D are representative of 4 separate experiments. (E) Secretion of PGE2 by unstimulated or stimulated MoDCs or CD1c+ PBDCs. Data are representative of 5 separate experiments.
Migratory capacity of immature and mature MoDCs and CD1c+ PBDCs. Immature MoDCs (GM-CSF plus IL-4); freshly sorted CD1c+ PBDCs, or CD1c+ PBDCs were stimulated for 8 hours (A-B) or 24 hours (C-D) with TNF-α plus IFN-α plus PGE2, with CD40L in the presence or absence of PGE2, or with intact E coli in the presence or absence of PGE2. These were then loaded into the upper transwell chambers and examined for their capacity to migrate toward either medium alone or CCL21 present in the lower transwell chambers. The y-axis shows the number of DCs migrating through the transwell membrane (8 μm) after 2 hours. Data in panels A-D are representative of 4 separate experiments. (E) Secretion of PGE2 by unstimulated or stimulated MoDCs or CD1c+ PBDCs. Data are representative of 5 separate experiments.
It has been proposed that MoDCs depend upon exogenous PGE2 as a consequence of IL-4's blocking endogenous PGE2 production by immature MoDCs.41 Alternatively, CD1c+ PBDCs, which are efficient migratory cells in the absence of PGE2-containing stimuli, may secrete higher levels of PGE2 in culture and thus not depend upon exogenous PGE2 to acquire migratory function. To address these possibilities, we examined the levels of PGE2 produced in culture SN by the 2 DC types. As shown in Figure 5E, MoDCs and CD1c+ PBDCs constitutively secreted comparable levels of PGE2 in vitro, and these levels were further increased following stimulation with E coli. Although not conclusive, these data argue that the differences in migratory capacity between MoDCs and CD1c+ PBDCs are not simply due to differences in the endogenous production of PGE2.
Comparison of T-cell stimulatory capacity of MoDCs and CD1c+ PBDCs
Mature DCs are the most efficient stimulators of naive T cells. We investigated the relative ability of differentially matured CD1c+ PBDCs or autologous MoDCs to stimulate the proliferation and cytokine secretion of alloreactive T cells in an MLR. CD1c+ PBDCs and autologous MoDCs were equally effective in stimulating allo–T-cell proliferation (Figure 6). However, MoDCs required prior activation with various physiologic stimuli to induce maximal T-cell proliferation. In this regard, immature MoDCs (GM-CSF plus IL-4) were, on a per cell basis, 10 to 100 times less efficient at inducing T-cell proliferation than mature MoDCs (Figure 6). In contrast, CD1c+ PBDCs induced T-cell proliferation equivalent to that seen with MoDCs irrespective of the stimulation conditions. This is consistent with the fact that CD1c+ PBDCs fully mature in culture without the need for further stimulation.
Induction of T-cell proliferation by immature and mature MoDCs and CD1c+ PBDCs. Immature MoDCs (GM-CSF plus IL-4) or freshly sorted CD1c+ PBDCs were stimulated for 2 days with the indicated stimuli, washed, and used as stimulators (1 × 104) of alloreactive T cells (1 × 105) in the MLR. On day 5 of the MLR, supernatants were harvested, and fresh medium containing 1 μCi (0.037 MBq) [3H]thymidine was added to each well for 8 hours. Proliferation of T cells stimulated with graded numbers of MoDCs (A) or CD1c+ PBDCs (B) is shown. Data represent the means ± SEMs of triplicate wells. This figure is representative of experiments from 5 separate donors.
Induction of T-cell proliferation by immature and mature MoDCs and CD1c+ PBDCs. Immature MoDCs (GM-CSF plus IL-4) or freshly sorted CD1c+ PBDCs were stimulated for 2 days with the indicated stimuli, washed, and used as stimulators (1 × 104) of alloreactive T cells (1 × 105) in the MLR. On day 5 of the MLR, supernatants were harvested, and fresh medium containing 1 μCi (0.037 MBq) [3H]thymidine was added to each well for 8 hours. Proliferation of T cells stimulated with graded numbers of MoDCs (A) or CD1c+ PBDCs (B) is shown. Data represent the means ± SEMs of triplicate wells. This figure is representative of experiments from 5 separate donors.
T-cell proliferation and cytokine secretion induced by MoDCs and/or CD1c+ PBDCs
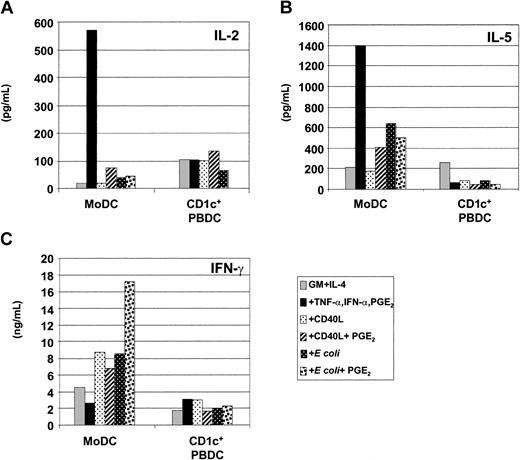
Next, we assessed DC-mediated cytokine secretion by alloreactive T cells in a separate series of experiments. Induction of IFN-γ by CD4+ T cells was assessed by intracellular cytokine secretion (ICS) with the use of immature or matured MoDCs. Here, T-cell proliferation could be examined in parallel by labeling CD3+ T cells with CFSE prior to coculture with DCs for 5 days. After 5-day stimulation, T-cell proliferation and IFN-γ secretion were assessed by FACS analysis by gating on CD3+CD8– T cells during analysis. Similar functional profiles were noted for CD8+ T cells (data not shown). The majority of CFSE-labeled T cells cultured in the absence of MoDCs died over the course of the culture. The few surviving T cells maintained much of their CFSE level, indicating that little T-cell division occurred in the absence of stimulation with APC (data not shown). As shown in Figure 7, immature MoDCs (GM-CSF plus IL-4) were poor stimulators of CD4+ T-cell division as well as IFN-γ secretion. MoDCs that secreted the highest levels of IL-12p70 (ie, those matured with CD40L or E coli) also induced the highest proportion of IFN-γ– secreting CD4+ T cells (12% and 20%, respectively). Furthermore, not all IFN-γ–producing CD4+ T cells had maximally divided, as could be seen from the proportion of T cells with low CFSE labeling. In contrast, migratory-type MoDCs (TNF-α plus IFN-α plus PGE2) induced few CD4+ T cells to secrete IFN-γ (1% to 2%), with the majority of these maximally dividing, as could be seen from reduction of CFSE labeling. Finally, although CD40L-plus-PGE2–matured MoDCs induced fewer IFN-γ–producing CD4+ T cells (1.5%), E coli-plus-PGE2–matured MoDCs remained potent inducers of T-cell IFN-γ (14.8%) as compared with MoDCs matured with E coli alone (20%) (Figure 7).
Induction of T-cell proliferation and IFN-γ secretion by immature and mature MoDCs. Immature MoDCs (GM-CSF plus IL-4) were stimulated for 2 days with the indicated stimuli and used as stimulators (3 × 104) of CFSE-labeled CD3+ alloreactive T cells (3 × 105) in the MLR. On day 5 of the MLR, T cells were restimulated with the identically conditioned MoDCs for 8 hours in the presence of Brefeldin A, and CD4+ T cells were assessed for intracellular IFN-γ secretion and proliferation by FACS analysis by gating on CD3+CD8– T cells. Percentages indicate the percent IFN-γ+CD4+ T cells within the gated region. Data are representative of experiments from 7 separate donors. Similar profiles were observed when CD3+CD8+ T cells were examined.
Induction of T-cell proliferation and IFN-γ secretion by immature and mature MoDCs. Immature MoDCs (GM-CSF plus IL-4) were stimulated for 2 days with the indicated stimuli and used as stimulators (3 × 104) of CFSE-labeled CD3+ alloreactive T cells (3 × 105) in the MLR. On day 5 of the MLR, T cells were restimulated with the identically conditioned MoDCs for 8 hours in the presence of Brefeldin A, and CD4+ T cells were assessed for intracellular IFN-γ secretion and proliferation by FACS analysis by gating on CD3+CD8– T cells. Percentages indicate the percent IFN-γ+CD4+ T cells within the gated region. Data are representative of experiments from 7 separate donors. Similar profiles were observed when CD3+CD8+ T cells were examined.
The ability of MoDCs and CD1c+ PBDCs to stimulate T-cell cytokine secretion was also assessed by measuring IL-2, IL-5, and IFN-γ in MLR culture SN by ELISA. MoDCs were more potent inducers of T-cell cytokines than autologous CD1c+ PBDCs, inducing T cells to secrete higher levels of IL-2, IL-5, and IFN-γ (Figure 8A-C). Once again, stimuli that induced maximal IL-12p70 and/or IFN-γ production by MoDCs (ie, CD40L or E coli) correlated with their capacity to induce the highest levels of IFN-γ by T cells (Figure 8C). Furthermore, MoDCs matured with TNF-α, IFN-α, and PGE2 induced higher levels of IL-2 and IL-5 production in alloreactive T cells (Figure 8A-B). Interestingly, MoDCs matured with E coli plus PGE2 expressed a mixed functional profile: that is, MoDCs with efficient migratory capacity (Figure 5A,C) and induction of high levels of IFN-γ by T cells (Figure 8C). Finally, despite negligible production of IL-12p70 or IFN-γ by CD1c+ PBDCs, these DCs did induce IL-2, IL-5, and IFN-γ by allogeneic T cells, albeit less efficiently than autologous MoDCs (Figure 8A-C).
Induction of T-cell cytokine secretion by immature and mature MoDCs and CD1c+ PBDCs. Immature MoDCs (GM-CSF plus IL-4) or freshly sorted CD1c+ PBDCs were stimulated for 2 days with the indicated stimuli, washed, and used as stimulators (1 × 104) of alloreactive T cells (1 × 105) in an MLR. On day 5 of the MLR, supernatants were harvested and T-cell cytokine secretion was measured by ELISA. (A) IL-2 production. (B) IL-5 production. (C) IFN-γ production. Data for panels A-C are representative of experiments from 5 separate donors.
Induction of T-cell cytokine secretion by immature and mature MoDCs and CD1c+ PBDCs. Immature MoDCs (GM-CSF plus IL-4) or freshly sorted CD1c+ PBDCs were stimulated for 2 days with the indicated stimuli, washed, and used as stimulators (1 × 104) of alloreactive T cells (1 × 105) in an MLR. On day 5 of the MLR, supernatants were harvested and T-cell cytokine secretion was measured by ELISA. (A) IL-2 production. (B) IL-5 production. (C) IFN-γ production. Data for panels A-C are representative of experiments from 5 separate donors.
Presentation of synthetic peptide to CTL lines by MoDCs and CD1c+ PBDCs
Finally, to assess antigen presentation to T cells, different populations of DCs were used in a peptide-antigen (peptide-Ag) presentation assay. In this assay, peptide-specific T cells were induced to produce IFN-γ following coculture with peptide-loaded DCs. The peptides tested were HLA-A2–restricted peptides derived from the tumor-associated antigen NY-ESO-1 (NY-ESO-1b157-165) and the viral antigen EBV BLMF-1 (BMLF-1280-288). Short-term CTL lines were generated (2% to 5% peptide specific, as assessed by peptide tetramer analysis) following culture of PBMCs for 7 to 10 days with the respective peptides and used as responders in the assays. As shown in Figure 9, both CD1c+ PBDCs and autologous MoDCs were equivalent, at the cell level, to TAP-deficient T2 cells at presenting NY-ESO-1b157-165 and EBV BMLF-1280-288 to peptide-specific CTL lines as assessed by intracellular IFN-γ staining. Importantly, both DC types could present peptides at between the 10–7 and 10–9 M range, indicating that both types of DCs were efficient at presenting limiting concentrations of peptide to CTL lines.
Peptide presentation and induction of T-cell IFN-γ secretion by immature and mature MoDCs and CD1c+ PBDCs. Immature MoDCs (GM-CSF plus IL-4) or freshly sorted CD1c+ PBDCs were stimulated for 18 hours with the indicated stimuli, washed, pulsed with the indicated peptides, and used as stimulators (1 × 104) of peptide-specific CTL lines as described in “Materials and methods.” T cells were assessed for intracellular IFN-γ secretion by flow cytometry. (A) NY-ESO-1b157-165 presentation to NY-ESO-1b–specific CTL line. (B) EBV BMLF-1280-288 presentation to EBV-specific CTL line. Data are presented as the percentage of all CD8+ T cells positive for intracellular IFN-γ staining. Data are representative of 3 separate donors.
Peptide presentation and induction of T-cell IFN-γ secretion by immature and mature MoDCs and CD1c+ PBDCs. Immature MoDCs (GM-CSF plus IL-4) or freshly sorted CD1c+ PBDCs were stimulated for 18 hours with the indicated stimuli, washed, pulsed with the indicated peptides, and used as stimulators (1 × 104) of peptide-specific CTL lines as described in “Materials and methods.” T cells were assessed for intracellular IFN-γ secretion by flow cytometry. (A) NY-ESO-1b157-165 presentation to NY-ESO-1b–specific CTL line. (B) EBV BMLF-1280-288 presentation to EBV-specific CTL line. Data are presented as the percentage of all CD8+ T cells positive for intracellular IFN-γ staining. Data are representative of 3 separate donors.
Discussion
The clinical application of DCs requires a detailed understanding of their functional potential and how best to manipulate this for optimal vaccine delivery and immune induction. Both PBDCs and MoDCs are currently being evaluated in anticancer immunotherapy trials.3,4,42 This study provides the first detailed, direct comparison of these 2 DC populations by comparing autologous DC types under identical conditions. Both FACS-sorted CD1c+ PBDCs and highly purified MoDCs were isolated from melanoma patients (with minimal residual disease) participating in a clinical trial evaluating FL as a vaccine adjuvant (M.J. Shackleton et al, submitted manuscript, 2003). Both DC types were cultured in the same media, containing GM-CSF and IL-4 for optimal viability.7,29 A variety of cancers have been shown to affect the generation of functionally mature MoDCs.43,44 Indeed, we found that MoDCs and PBDCs from patients with later stage, metastatic disease receiving FL expressed reduced functional capacities such as the ability to mature in response to in vitro stimulation and the ability to stimulate T cells. Furthermore, some of these patients expressed significant monocytosis following FL treatment as well as elevated serum levels of proinflammatory cytokines such as IL-6 (M.J. Shackleton et al, submitted manuscript, 2003). However, MoDCs and CD1c+ PBDCs used in the present studies were specifically derived from patients with minimal residual disease, and these DCs were found to be functionally similar to their counterparts from healthy individuals37,38,45 (also found in data not shown). Several important findings were made in the present study. First, CD1c+ PBDCs and autologous MoDCs are phenotypically and functionally distinct DCs, differing in their migratory ability and their capacity to secrete specific cytokines, including IL-6, IL-10, and IL-12p70. Second, the function of these 2 DC subtypes was regulated by different types of soluble mediators. Finally, although these 2 DC types were equivalent at presenting peptides and T-cell stimulation, they induced different levels of T-cell cytokines.
MoDCs and CD1c+ PBDCs are frequently considered to be similar cell populations.13,29-36 Although, the phenotypes of these 2 distinct DC subtypes are similar (eg, expression of CD4 and the myeloid markers CD11c, CD13, and CD33), there are several markers that distinguish them. For instance, MoDCs express CD1a but not CD1d, whereas CD1c+ PBDCs express CD1d but not CD1a. In addition, while the majority of MoDCs express the pattern recognition receptor MMR, only a subset (8% to 15%) of freshly isolated CD1c+ PBDCs expressed MMR. In this regard, CD1c+ PBDCs appear to be phenotypically heterogeneous, composed of distinct subsets expressing surface Ags not expressed on immature MoDCs (eg, CCR6 and/or BDCA-3). The percentage of CD1c+ PBDCs expressing MMR, CCR6, or BDCA-3 suggests that multiple subpopulations are likely to exist. It is unclear, however, whether these markers define distinct subsets or represent the same PBDC population at different stages of maturation. We and others have noted that freshly isolated FL-mobilized PBDCs are immature cells that mature rapidly (CD80+, CD83+, CD86+) following culture.10,16,38,46 The present study also indicates that this occurs with greater amplitude than for MoDCs. Although MoDCs and CD1c+ PBDCs express a similar repertoire of pathogen-recognition receptors (eg, MMR, DEC205 and Toll-like receptors),30,47,48 MoDCs produce higher levels of IL-1β, IL-6, IL-10, and IL-12p70 in response to pathogen signals.31,48
Several cytokines can induce IFN-γ in T cells, including IL-12p70, IL-23, and IL-18. Bioactive IL-12p70 is a heterodimeric cytokine composed of an inducible IL-12p35 subunit and a constitutively expressed IL-12p40 subunit. IL-12p40 can also homodimerize to form IL-12(p40)2, a putative antagonist of IL-12p70 function,49 or heterodimerize with a recently identified IL-23p19 subunit to form a novel cytokine, IL-23, which has overlapping biologic effects with IL-12p70.50 Furthermore, IL-18 has been shown to synergize with IL-12 and induce T-cell IFN-γ.51 Although others have reported that human DCs may express IL-18 mRNA,23,52 there are no reports of differential expression in MoDCs and CD1c+ PBDCs. We have shown that CD1c+ PBDCs are poor producers of IL-12p70, whereas MoDCs are prolific producers of this cytokine.37,38 The present study demonstrates that the differences in production of bioactive IL-12p70 by MoDCs and CD1c+ PBDCs are reflected at the gene level, with CD1c+ PBDCs expressing negligible IL-12p35 and p40 mRNA (even following stimulation) compared with MoDCs. Furthermore, in contrast to MoDCs, CD1c+ PBDCs did not express IL-23p19 mRNA (either constitutively or following stimulation), nor did they secrete bioactive IL-18 following stimulation. Interestingly, 2 reports indicate that CD1c+ PBDCs can produce high levels of IL-12p70.18,39 Both studies initially cultured the CD1c+ PBDCs in GM-CSF prior to stimulation. The present study confirms the need for maturation prior to stimulation but shows (1) that CD1c+ PBDCs require at least 24- to 48-hour maturation prior to stimulation and (2) that significant IL-12p70 is induced only by the combination of IL-1β, IFN-γ, CD40L, and E coli. However, even under these optimized conditions, CD1c+ PBDCs were still lower producers of IL-12p70 compared with MoDCs. It is unclear whether the prolonged in vitro culture enhanced sensitivity of PBDCs to inducers of IL-12p70 or induced their differentiation into MoDC-like cells. Omission of IL-4 from the CD1c+ PBDC culture conditions did not enhance stimuli-induced IL-12p70 secretion, suggesting that the potential attenuating effects of IL-4 are not the reason for the low cytokine-secreting capacity of PBDCs following stimulation. In any case, these data indicate that MoDCs have the potential to produce at least 3 cytokines known to induce IFN-γ in T cells (IL-12p70, IL-18, and IL-23), whereas CD1c+ PBDCs were poor producers of these cytokines. This probably reflects the reduced ability of CD1c+ PBDCs to induce T-cell cytokine production in vitro. Interestingly, Osada et al53 have reported IL-12–independent induction of T-cell IFN-γ by PBDCs, suggesting that PBDCs can produce as-yet-unidentified IFN-γ– inducing factors.
Although MoDCs and CD1c+ PBDCs express a similar repertoire of chemokine receptors,54-56 chemokine receptor expression by MoDCs is not predictive of their migratory function.37,40 Major differences in migratory capacity were observed in these 2 DC types. CD1c+ PBDCs migrated to chemokines shortly after culture (8 to 12 hours), requiring only minimal in vitro manipulation (eg, GM-CSF and IL-4), whereas MoDCs required prolonged culture (24 hours) with PGE2-containing stimuli. PGE2 appears to regulate MoDC migratory function via cyclic adenosine monophosphate (cAMP)/protein kinase A activation.37,38 Interestingly, migratory-type MoDCs (ie, matured with PGE2-containing stimuli) exhibit a functional profile similar to CD1c+ PBDCs (ie, migratory, low IL-12p70 production, and induction of IL-2 by T cells). PGE2 has also been shown to abrogate IL-12p70 secretion by MoDCs.57,58 Zelle-Rieser et al41 also implicate IL-4–mediated suppression of endogenous PGE2 production by MoDCs for the maturation-enhancing effects of exogenous PGE2. However, we found that PGE2 production by immature MoDCs (GM-CSF plus IL-4) was comparable to levels produced by CD1c+ PBDCs and that both DC populations increased PGE2 production following stimulation. Thus, differences in the endogenous levels of PGE2 in CD1c+ PBDCs and MoDCs cannot completely explain their different migratory capacity. An alternative is that CD1c+ PBDCs are the product of a stimulation history completely distinct from that of MoDCs in vivo. In particular, cAMP analogs, which can replace the ability of PGE2 to induce MoDC migration,37 are present in serum (eg, vasoactive intestinal peptide59 or sympathomimetics60 ). It is possible that freshly isolated CD1c+ PBDCs have already been exposed to cAMP-inducing serum factors in situ and are thus presensitized to migrate upon minimal in vitro stimulation. Work to address these specific questions is ongoing.
Analysis of the ability of each DC type to induce T-cell function revealed that MoDCs and CD1c+ PBDCs were equivalent at inducing alloreactive T-cell proliferation and were as efficient as TAP-deficient T2 cells at presenting peptides to CTL lines. As reported, however, MoDCs required prior maturation with specific stimuli to induce efficient T-cell stimulation,53,61 whereas CD1c+ PBDCs (which spontaneously mature in vitro without additional stimuli) efficiently induce T-cell stimulation. Major differences were also observed in the type and quantity of cytokines the DC populations induced T cells to secrete, paralleling differences in IL-12p70 production by the DC types. Stimuli that maximally induced IL-12p70 (eg, CD40L and E coli) preferentially skewed T cells toward the production of IFN-γ (Figures 7,8C). In contrast, PGE2-containing stimuli (eg, TNF-α plus IFN-α plus PGE2, or CD40L plus PGE2) induced lower levels of IFN-γ and increased IL-2 and IL-5 production by T cells. The one exception was the combination of E coli plus PGE2. This combination resulted in MoDCs' expressing a mixed functional profile: that is, migratory-type MoDCs (Figure 5A,C) that also secreted high levels of IFN-γ (Figure 3A) and induced high levels of IFN-γ by T cells (Figures 7,8C). This suggests that migratory function and cytokine secretion can be coexpressed by MoDCs in the context of pathogen signals and that this class of stimulus can override some of the attenuating affects of PGE2 upon MoDC cytokine secretion. Interestingly, E coli–derived LPS was, in our hands, a suboptimal stimulus for inducing these functional changes in MoDCs, compared with the intact E coli pathogen (data not shown). This probably reflects the more complex array of pattern-recognition receptors that would recognize intact E coli (eg, Toll-like receptors 2, 4, and 6 and C-type lectins) as compared with LPS, which would be recognized through the Toll-like receptor 4 (TLR-4)/CD14 complex (reviewed by Medzhitov62 ).
Our clinical trial found that FL expanded the number of immature CD1c+ and IL-3R+ PBDCs in patients with melanoma (M.J. Shackleton et al, submitted manuscript, 2003) However, discernible immune responses were infrequent and clinical responses rare. Similarly, a recent study suggested that vaccines using FL as an adjuvant did not enhance T-cell proliferative responses but did increase the precursor frequency of IFN-γ– secreting HER-2/neu–specific T cells.46 In contrast, Fong et al10 demonstrated immunological and clinical responses using FL-mobilized PBDCs pulsed with a carcinoembryonic antigen (CEA)– derived peptide ex vivo. The ex vivo enrichment and culture step was noted to induce PBDC maturation, again highlighting the importance of the maturational state of DCs to the T-cell immune outcome. A recent review emphasizes that immature DCs may induce T-cell tolerance or anergy.63 If this is true for immature PBDCs, then the work of Fong et al and the results presented here suggest that ex vivo maturation of FL-generated PBDCs may enhance their immune potency and minimize their potential to dampen immune response induction. The present study provides crucial information to optimally manipulate PBDCs in vitro to produce cells with defined functional characteristics. Given that not all DC types or all stages of their maturation will be appropriate for the initiation of immune responses, it is critical that the most appropriate type or stage of DC is matched with the clinical aim.
Prepublished online as Blood First Edition Paper, May 8, 2003; DOI 10.1182/blood-2002-12-3854.
Supported by the Sylvia and Charles Viertel Foundation, a program grant from the Australian National Health and Medical Research Council (NH&MRC), and the Ludwig Institute for Cancer Research. M.J. was supported by the Stewardson Family Trust; M. Schnurr was supported by a grant of the Dr Mildred Scheel Stiftung. I.D.D. was supported in part by an Australian NH&MRC Career Development Award; T.L. was supported by a fellowship from The Cancer Council Victoria, Australia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 6. Induction of T-cell proliferation by immature and mature MoDCs and CD1c+ PBDCs. Immature MoDCs (GM-CSF plus IL-4) or freshly sorted CD1c+ PBDCs were stimulated for 2 days with the indicated stimuli, washed, and used as stimulators (1 × 104) of alloreactive T cells (1 × 105) in the MLR. On day 5 of the MLR, supernatants were harvested, and fresh medium containing 1 μCi (0.037 MBq) [3H]thymidine was added to each well for 8 hours. Proliferation of T cells stimulated with graded numbers of MoDCs (A) or CD1c+ PBDCs (B) is shown. Data represent the means ± SEMs of triplicate wells. This figure is representative of experiments from 5 separate donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/5/10.1182_blood-2002-12-3854/6/m_h81734846006.jpeg?Expires=1769129464&Signature=P7wzbmgdYlPQjF80jGUIYFYJ--ol3SdgMJJ83q9RZ7-wtzgXOaZ9soJ6gkwZ4-dLUN7lhcS6WyPPARkRhZeAcSE6l5wiVr26HySV-qppRLFyvOBVetmFoFCx0qmeiypmdJgfbXQQVq8XKKCTo4kkeKcjuEp47YCKcqk4T-kDhYaBQ-MH5BV5EbqMi5tOLuzVTln7igLmecwDnjoCLtD93EqOReiQqsvRnOkz1IvBuBMkIglOjNuefHOIlk2H3cHkDdgGAJRH9FSVc5NHiJOZUw~Fl92F2pkbBIShQQQVqaqPNHxV8~DXWLGvFfkUbfov2SSF1n4XleCZ8hXz~mSZrQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal