Abstract
We have shown previously that primary dendritic cells and monocytes express equal levels of CD14 but are distinguishable by the presence of CD2 on dendritic cells. CD2 is known to mediate the activation of T and natural killer (NK) cells through its interaction with CD58. CD2 epitopes recognized by anti-T111, -T112, and -T113 monoclonal antibodies (mAbs) are present on dendritic cells. Here we show that CD2 engagement significantly increases class II, costimulatory (CD40, CD80, CD86), adhesion (CD54, CD58), and CCR7 molecule expression on primary dendritic cells. Conversely, minimal or no change in the expression of the above antigens occurs on monocyte-derived dendritic cells, because these molecules are already maximally expressed. However, both kinds of dendritic cells release interleukin-1β (IL-1β) and IL-12 after CD2 engagement. Lastly, interference with dendritic cell CD2–T-cell CD58 engagement decreases naive CD4+CD45RA+ T-cell proliferation. Collectively, our results suggest another role of the CD2-CD58 pathway that allows nonimmune and immune cells to interact directly with dendritic cells and initiate innate and adaptive immune responses.
Introduction
Dendritic cells (DCs) are unique antigen-presenting cells (APCs) of bone marrow origin1 characterized by their dendritic morphology and high expression of major histocompatibility complex (MHC) class II molecules.2,3 DCs are efficient inducers of T-cell activation,4 originally distinguished from monocytes (Mo's) by their potent allogeneic mixed lymphocyte reaction (allo-MLR) capacity3 and later discovered to be responsible for initiation of primary immune responses in vivo.4 DCs are found in most solid tissues of the body, comprising less than 1% of the total cell content,5 while blood primary CD2+ DCs (pDCs) may represent up to 3% to 4% of the circulating peripheral blood mononuclear cells (PBMCs).6 In addition to their potent T-cell stimulatory capacity, DCs survey many tissues for evidence of damage or signs of infection, but the mechanisms by which this occurs are unknown.4 Because of difficulties in enriching human DCs, monocyte-derived dendritic cells (MDDCs) now serve as the primary source of DCs for most studies. MDDCs are generated from CD14+ myeloid cells (Mx's) cultured for 7 days in the presence of granulocytemacrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) cytokines.7 Phenotypically, MDDCs express high levels of class II, costimulatory, and adhesion molecules and are also more efficient at T-cell activation than macrophages.
We previously described CD2 on the surface of DCs and used this antigen to distinguish and sort pDCs from pMo's from the blood.6 CD2 is a 50 to 55 kDa glycoprotein that is expressed on T and natural killer (NK) cells and distinguishes them from other leukocytes.8,9 CD2, a member of the immunoglobulin (Ig) superfamily, is structurally similar to its principal ligand, lymphocyte function-associated antigen-3 (LFA-3) (CD58)10 ; the 2 molecules interact through similar amino terminal ligand-binding regions.11 CD58, a 55 to 70 kDa glycoprotein, is expressed on both hematopoietic and nonhematopoietic cells.10 The interaction between CD2 on T cells and CD58 on APCs is intimately involved in T cell–specific antigen recognition.10,12 Importantly, CD2 engagement with a pair of anti-CD2 monoclonal antibodies (mAbs)13 or by CD58 in conjunction with an anti-CD2 mAb14 provides an activation signal. Signaling through CD2 induces cytoskeletal reorganization,15 lowers the threshold for T-cell activation through the T-cell receptor (TCR)/CD3 complex,16 stimulates NK cell cytotoxicity,17 and enhances IL-12 responsiveness of activated T cells.18 These findings led us to postulate that the engagement of CD2 on DCs could affect expression of cell surface molecules and/or the production of inflammatory cytokines.
Molecular and functional studies of human CD2 identified 3 distinct epitopes on T and NK cells that are detected by the mAbs anti-T111, anti-T112, and anti-T113.13 Anti-T111 mAb binds to the NH2-terminal domain of CD2 and blocks adhesion to CD58.13 T112 binds to a region of CD2 that is not involved in CD58 binding but induces the reorientation of the D1 region in relation to D2 of the CD2 extracellular segment and the exposure of a cryptic epitope recognized by the T113 mAb.13 Pairs of these CD2 mAbs induce T-cell proliferation and transmembrane Ca2+ flux13 and, in the case of NK cells, killing of target cells.19-22 Similarly, CD58 binding, in conjunction with certain CD2-specific mAbs, induces T-cell proliferation,10,23 but efficient signal transduction through CD2 requires either the binding of the TCR to MHC class I or II molecules or the binding of antibody to FcγRIII (CD16) on T and NK cells, respectively.17,22 Although the role of CD2 on T and NK cells is well studied, the function of this molecule on DCs is not well characterized. Therefore, the objectives of the present study were to characterize CD2 epitopes on DCs and to investigate whether CD2 is capable of mediating DC activation.
Materials and methods
Generation of PBMC subsets
Approval for this study was granted by The Center for Blood Research Institutional Review Board, and informed consent was provided according to the Declaration of Helsinki. PBMCs were isolated from buffy coats from healthy volunteers (Division of Transfusion Therapy, Children's Hospital, Boston, MA) as previously described.6 The PBMCs were layered over a 14.5% wt/vol discontinuous Metrizamide gradient (Sigma Chemical, St Louis, MO) and centrifuged (Sorvall RT 6000; DuPont, Wilmington, DE) at 650g for 10 minutes to separate the PBMCs into low-(pDC and pMo) and high-(T, B, and NK cells) density fractions. The low-density population was depleted of contaminating leukocytes with anti-CD3, -CD19, and -CD56 immunomagnetic beads (Miltenyi Biotec, Auburn, CA). This negatively selected population was more than 98% CD14+ and negative for CD3+ T cells and CD56+ NK cells as determined by fluorescence-activated cell sorter (FACS) analysis. These CD14+ Mx's served as our source of pDCs, pMo's, and MDDCs. The pDCs were CD2-selected DCs (about 90% pure), and the pMo's were obtained from the CD2-depleted eluate. CD3+ T cells were also negatively selected by depleting B cells, Mx's, and NK cells from the high-density population with anti-CD14, -CD19, -CD56, and –HLA-DR immunomagnetic beads (Miltenyi Biotec), whereas CD4+CD45RA+ T-cell enrichment required the addition of anti-CD8 and -CD45R0 immunomagnetic beads (Miltenyi Biotec).
Culture media
To produce mature pDCs and pMo's, CD14+ Mx's were plated at a density of 2 × 106 to 5 × 106 cells per well in 6-well plates and maintained in RPMI 1640 medium (Cellgro; Fisher Scientific, Pittsburgh, PA, and Gibco, Gaithersburg, MD) supplemented with 10% heat-inactivated pooled human serum (PHS) (Nabi, Boca Raton, FL), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco) and cultured for 5 days. To generate immature MDDCs, CD14+ Mx's were cultured for 7 days at a similar concentration in the presence of 1000 units of GM-CSF and of IL-4 (R&D Systems, Minneapolis, MN), while mature MDDCs were generated by adding tumor necrosis factor-α (TNF-α) (10 ng/mL) during the last 2 days of GM-CSF/IL-4 culture.
Measurement of calcium mobilization
To evaluate the triggering of Ca2+ flux ([Ca2+]i) by CD2 cross-linking, we adapted a previously described method.24 In brief, 5 × 106 CD14+ Mx's or CD3+ T cells were incubated with Indo-1 (10 μM) (Molecular Probes, Junction City, OR) in 1 mL of 1% PHS culture medium for 45 minutes at 37°C. Following incubation, samples were washed twice in warm RPMI, resuspended in 1% PHS, and incubated for an additional 30 minutes at 37°C. Changes in [Ca2+]i in Indo-1–loaded samples were assessed with an EPICS V flow cytometer (Coulter, Hialeah, FL). The baseline levels were determined by measuring unstimulated Indo-1–loaded samples for 1 minute, followed by sequential addition of anti-T112 (1Old-4C1 [dilution 1:100]) and anti-T113 (1 mono2A6 [dilution 1:100]) in 1% PHS. Goat antimouse IgG mAb (dilution 1:100) and the Ca2+ ionophore A23187 (5 μM) (Molecular Probes) were added to augment the response and serve as a positive control, respectively.
Activation of DCs with anti-CD2 mAb
After 36 to 120 hours of culture for CD14+ Mx's and 7 days of culture for MDDCs, cells were harvested and washed 3 times to remove dead cell debris and residual cytokines. After washing, MDDCs and enriched pDCs and pMo's were incubated at a concentration of 5 × 105 cells per milliliter in 10% PHS with mouse IgG2a, the irrelevant antibody control (IR), and anti-T112, anti-T111 (SFCI3Pt2H9), and/or anti-T113. To detect cell surface class II (HLA-DR and -DQ [Beckman Coulter, Fullerton, CA]), costimulatory (CD40, CD80, and CD86 [BD PharMingen, San Diego, CA]), adhesion (CD50, CD54, and CD58 [Beckman Coulter]), DC-specific (CD1a and CD83 [Beckman Coulter]), and CCR7 (R&D Systems) antigens, corresponding fluorescein- or phycoerythrin-conjugated mAbs were used. Supernatants were collected and stored frozen at –20°C until tested.
RT-PCR analysis of class II gene expression
To assess class II (HLA-DP and -DM) gene expression in DCs, mRNA was extracted using Dynabead Oligo (dT)25 (Dynal, Great Neck, NY) from day 5–cultured pDCs (105) and pMo's (105) treated with or without anti-T111 or anti-T113 mAbs for an additional 24 hours. The purified mRNA was incubated with Moloney murine leukemia virus (MMLV) reverse transcriptase (RT) (Promega, Madison, WI) and random hexanucleotide primers for 1 hour at 42°C. The resulting cDNA was amplified using AmpliTaq Gold (Applied Biosystems/Roche, Branchburg, NJ). Each 50 μL polymerase chain reaction (PCR) contained 1 × PCR Gold Buffer, 25 mM MgCl2, 0.25 units of AmpliTaq Gold, 200 μM deoxyribonucleoside triphosphate (dNTP) (Promega), and 500 ng cDNA in sterile water. Three PCR reactions were set up for each cDNA, and 20 pmol of primer pairs corresponding to HLA-DP (forward: 5′-ACGGCGTTACTGATGGTGCTGCTC-3′; reverse: 5′-TGAATGCTGCCT-GGGTAGAAATCC-3′), HLA-DM (forward: 5′-AGATGACCTGCAAAACCACAC-3′; reverse: 5′-GGCCCAAATCCTTCCACAG-3′), and β-actin (forward: 5′-CGTGGACATC-CGTAAAGACC-3′; reverse: 5′-ACATTCTGCTGGAAGGTGGAC-3′) were used. Thermocycling conditions were denaturing for 1 minute at 94°C, annealing for 30 seconds at 60°C, and extension for 1 minute at 72°C for 30 cycles. After completion, an additional extension for 10 minutes at 72°C was performed. The PCR products were separated by electrophoresis on 2% agarose gels (FMC BioProducts, Rockland, ME).
ELISA
Supernatants from culture samples were thawed and assayed for IL-1β and IL-12 cytokines by enzyme-linked immunosorbent assay (ELISA). Ultra-Sensitive kits were used according to the manufacturer's instructions (Biosource International, Camarillo, CA). The lower limits of detection for the IL-12 and IL-1β kits were 0.8 pg/mL and 0.12 pg/mL, respectively.
CHO transfectant assays
Untransfected Chinese hamster ovary (CHO-NEO) or CHO cells transfected with and expressing CD58 (CHO-58) (Dana-Farber Cancer Institute, Boston, MA) were constructed as described previously.10,25 Untransfected and transfected CHO cells were cultured in Dulbecco modified Eagle medium (DMEM) (Gibco) supplemented with 10% fetal calf serum (FCS) (Cellgro), F12 nutrient mixture (Gibco), 1% HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 1% penicillin-streptomycin, 1% glutamine, and 50 mg/mL G418 (Gibco). The pDCs and pMo's (2 × 106) were spread over the CHO monolayer and cultured for 24 hours in 6-well tissue culture plates.
Mixed lymphocyte reaction
The pDCs and pMo's were cultured and isolated as previously described. Enriched populations were cultured in the presence of blocking buffer, which prevents nonspecific binding of mAbs, for 30 minutes at 4°C and pulsed with either anti-T111 or -T112 mAbs for an additional 30 minutes at 4°C. Following this incubation, samples were washed to remove residual mAbs and resuspended in 10% PHS culture medium. Each preparation (104) of pDCs and pMo's was cocultured with alloreactive CD4+CD45RA+ naive T cells (105) in a 96-well round bottom plate for 120 hours. Sixteen hours prior to the completion of the assay, [3H]thymidine was added to the cultures to assess the level of naive T-cell proliferation. The percent proliferation values were calculated by using the following formula: ([pulsed experimental value – T-cell background value]/[unpulsed experimental value – T-cell background value] × 100).
Endocytosis
Cytokine-differentiated immature and mature DCs were harvested after 7 days of culture, resuspended in 10% PHS medium, and incubated an additional 24 hours at 37°C in the presence of IR, lipopolysaccharide (LPS) (1 μg/mL; Sigma), CD40L (10 ng/mL), or anti-T111. Prior to terminating the assay, 1 mg/mL fluorescein isothiocyanate (FITC)–T70–dextran (Sigma) was added to each culture and the cells were incubated an additional 10 minutes before cold 1% PHS–phosphate buffered saline (PBS) was added. Samples were washed 3 times in termination buffer and immediately analyzed on a FACSCalibur (BD Biosciences) flow cytometer. Data are presented as expression index: (% positive population × mean fluorescence intensity [MFI]). Results are representative of 3 independent experiments.
Internal cytokine assay
In brief, CD4+CD45RA+ T cells (104/mL) and autologous pDCs (104/mL) were cultured in the absence or presence of tetanus toxoid (TT; 10 μg/mL; Connaught Laboratories, Willowdale, ON) or gag protein (10 μg/mL; NIH AIDS Research and Reference Reagent Program, Rockville, MD) for 5 days. T cells were restimulated with phorbol myristate acetate (PMA) (5 ng/mL; Sigma) and ionomycin (500 ng/mL; Sigma) for 6 hours, and monensin (1.3 μM) was added 4 hours before the reaction was terminated. Samples were subsequently collected, washed, fixed with perm/fix solution (BD PharmMingen), and internally stained with anti–IL-2 (BD PharmMingen) and anti–interferon-γ (anti–IFN-γ) (BD PharmMingen) mAbs. Samples were washed 3 times in rinse buffer and immediately analyzed on a FACSCalibur (BD Biosciences) flow cytometer. Results are presented as expression index and are similar to findings in 3 independent studies.
Statistical analyses
Data are presented as mean value ± SEM. Unpaired Student t test was used for analysis of statistical significance of data retrieved from within experiments unless otherwise stated. P values less than .05 were considered statistically significant.
Results
Phenotypic and functional properties of CD2 on pDCs
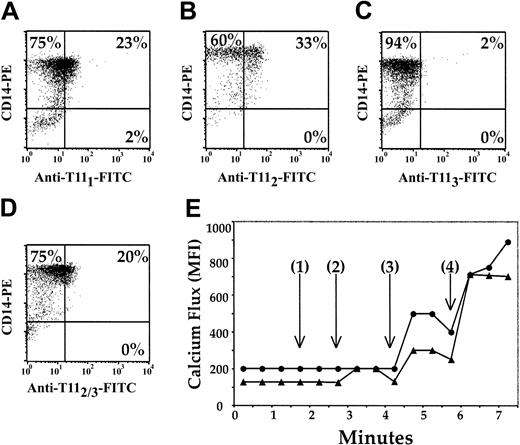
We previously reported the universal presence of CD2 on circulating pDCs. Here, we tested for the presence of 3 distinct CD2 epitopes, T111, T112, and T113, on mature CD14+ Mx's with anti-T111 (Figure 1A), anti-T112 (Figure 1B), and anti-T113 (Figure 1C-D) mAbs. Anti-T111 and anti-T112 identified CD2 on 23% and 33% of the CD14+ Mx's (Figure 1A-B), respectively, while none of the CD14+ Mx's stained positive for CD2 with anti-T113 (Figure 1C). However, when CD14+ Mx's were incubated for 30 minutes with anti-T112 and subsequently stained with anti-T113 (Figure 1D), 20% of the CD14+ Mx's were CD2+. Because these studies were performed at 4°C and in medium that lacked Mg2+ and Ca2+, the conformational change in CD2 that exposes the T113 epitope was not temperature-, Ca2+-, or Mg2+-dependent.
Phenotypic and functional mapping of the CD2 antigen on pDCs. CD14+ Mx's were enriched from PBMCs by negative selection and were negative for CD3, CD19, and CD56. The remaining population was more than 93% positive for CD14 (A-D). The Mx cells were further stained with anti-T111 (A), anti-T112 (B), or anti-T113 (C-D) mAbs. Mx's (▴) and CD3+ T cells (•) were incubated sequentially with anti-T112, anti-T113, and goat antimouse mAbs (E), and changes in intracellular Ca2+ were measured. Time represents (1) the addition of T112, (2) the addition of anti-T113, (3) the addition of goat antimouse IgG, and (4) the addition of A23187 Ca2+ ionophore. The results are representative of 3 experiments.
Phenotypic and functional mapping of the CD2 antigen on pDCs. CD14+ Mx's were enriched from PBMCs by negative selection and were negative for CD3, CD19, and CD56. The remaining population was more than 93% positive for CD14 (A-D). The Mx cells were further stained with anti-T111 (A), anti-T112 (B), or anti-T113 (C-D) mAbs. Mx's (▴) and CD3+ T cells (•) were incubated sequentially with anti-T112, anti-T113, and goat antimouse mAbs (E), and changes in intracellular Ca2+ were measured. Time represents (1) the addition of T112, (2) the addition of anti-T113, (3) the addition of goat antimouse IgG, and (4) the addition of A23187 Ca2+ ionophore. The results are representative of 3 experiments.
Because previous reports have demonstrated that CD2 ligation induces activation of T and NK cells,13 we measured [Ca2+]i mobilization, an early intracellular activation event, in CD14+ Mx's and CD3+ T cells after CD2 ligation with anti-T112 and anti-T113 mAb pairs (Figure 1E). Samples were pulsed with anti-T112 (Figure 1E [1]), and there was virtually no increase in [Ca2+]i. The subsequent addition of T113 (Figure 1E [2]) induced a 69% increase in [Ca2+]i in CD14+ Mx's. Further addition of goat antimouse IgG (Figure 1E [3]), which cross-linked the anti-T112 and anti-T113 mAbs previously bound to CD2, increased [Ca2+]i in CD14+ Mx's by 95% above the baseline. Similar increases in [Ca2+]i were also demonstrated with enriched CD2-sorted pDCs (data not shown).
These results were compared with CD3+ T cells from the same donor (Figure 1E). No increase in [Ca2+]i was seen upon addition of anti-T112 or anti-T113 mAbs (Figure 1E [1] and [2]) to T cells. However, when goat antimouse IgG was added (Figure 1E [3]), the level of [Ca2+]i increased to 180% above baseline in the T-cell sample. Furthermore, similar findings were demonstrated when Jurkat cells were assessed (data not shown). Collectively, these results strongly suggest that CD2 is a functional molecule on pDCs.
Ligation of CD2 on pDCs induces changes in cell surface antigen expression
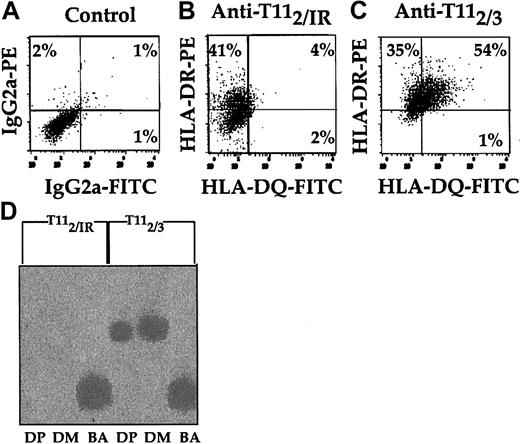
Because CD2 ligation induced increased [Ca2+]i, we investigated the effect of CD2 ligation on pDC cell surface antigen expression. Immunofluorescence analysis of HLA-DR and HLA-DQ expression revealed that both molecules were up-regulated with anti-CD2 mAb pairs (Figure 2A-C). HLA-DR levels increased 2-fold, while HLA-DQ levels increased 9-fold. This up-regulation of class II molecules on pDCs was dependent on the simultaneous binding of CD2 by anti-T112 and either anti-T113 (Figure 2C) or anti-T111 mAbs (data not shown) and did not occur with anti-T112 alone (Figure 2B) or on Mo's (data not shown). To determine whether transcription of HLA-DP and -DM genes was altered by CD2 ligation, equal numbers of pDCs were incubated with either anti-T112 and IR or anti-T112 and anti-T113 (Figure 2D). Following 24 hours of incubation, cDNA was generated and PCR was performed on equal amounts of cDNA for HLA-DM, HLA-DP, and β-actin. The pDCs cultured in the presence of anti-T112 and anti-T113 mAbs had higher levels of HLA-DM and HLA-DP mRNA than samples cultured in the presence of IR and anti-T112 mAbs. Thus, as seen with HLA-DR and -DQ surface expression, anti-T113 also induced HLA-DP and -DM gene activation in pDCs (Figure 2D). These results provide further evidence that engagement of CD2 on pDCs increased class II molecule expression.
Induction of class II antigens of pDCs detected by immunofluorescence and molecular analysis after CD2 ligation. CD14+ Mx's were precultured for 36 hours in 10% PHS, followed by anti-T112 enrichment of pDCs. The enriched pDCs were resuspended in fresh 10% PHS at a final concentration of 106 cells per milliliter for 12 additional hours in the presence of an IR control (B) or anti-T113 (C). Afterward, 2-color analysis of each DC sample was performed with anti–HLA-DR and -DQ mAbs. The quadrant boundaries were set using isotype controls (A). RT-PCR (D) was used to assess HLA-DP, HLA-DM, and β-actin gene expression of pDCs that were cultured in the presence of either anti-T112 and IR control or anti-T112 and anti-T113 mAbs. The results are representative of 3 or more studies. Percentages in panels A-C indicate the percentage of cells expressing antigen in that quadrant.
Induction of class II antigens of pDCs detected by immunofluorescence and molecular analysis after CD2 ligation. CD14+ Mx's were precultured for 36 hours in 10% PHS, followed by anti-T112 enrichment of pDCs. The enriched pDCs were resuspended in fresh 10% PHS at a final concentration of 106 cells per milliliter for 12 additional hours in the presence of an IR control (B) or anti-T113 (C). Afterward, 2-color analysis of each DC sample was performed with anti–HLA-DR and -DQ mAbs. The quadrant boundaries were set using isotype controls (A). RT-PCR (D) was used to assess HLA-DP, HLA-DM, and β-actin gene expression of pDCs that were cultured in the presence of either anti-T112 and IR control or anti-T112 and anti-T113 mAbs. The results are representative of 3 or more studies. Percentages in panels A-C indicate the percentage of cells expressing antigen in that quadrant.
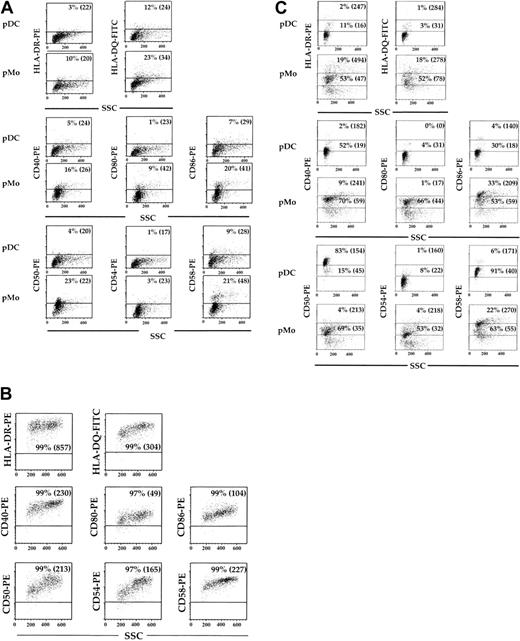
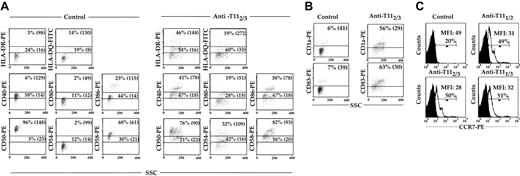
The pDCs, pMo's, and MDDCs were assessed for their expression of class II antigens (HLA-DR and HLA-DQ), costimulatory antigens (CD40, CD80, and CD86), and adhesion molecules (CD50, CD54, and CD58) after 5 days of culture in 10% PHS. These cell surface antigens were expressed on no more than 12% (P < .02) and 23% (P < .04) of pMo's (Figure 3A), while they were found on more than 96% (P < .02) of the MDDCs (Figure 3B). When pDCs, pMo's, and MDDCs were recultured in fresh medium for 24 hours, the expression of all the above-mentioned cell surface antigens on pDCs and pMo's discordantly increased (Figure 3C), while no significant change was noted in MDDCs (data not shown). More than 90% (P < .03) of the pDCs expressed CD50 and CD58 adhesion molecules, while minimal increases of other activation antigens and a decrease in cellular side scatter (a reflection of cellular activity) were noted (Figure 3C). In contrast, a much greater percentage of the above-mentioned activation antigens was expressed by pMo's, and no significant change in cellular side scatter was noted (Figure 3C). Because the levels of some activation antigens on pDCs did not increase significantly after reculturing, all of the analyzed cell surface antigens on pDCs were reexamined after culture in the presence of IR or the anti-T112/3 mAb pair. The levels of HLA-DR and HLA-DQ expressed by pDCs cultured with the anti-T112/3 pair increased 2- to 3-fold compared with control cultures (Figure 4A). The levels of costimulatory antigens CD40 and CD80 increased 2- to 3-fold respectively, while only the high-expressing CD86 population increased by 65% (P < .04). More than 96% of the control samples expressed high levels of CD50, but upon CD2 engagement the percentage of CD50hi pDCs decreased from 96% to 76% (P < .04) and their MFI decreased from 148 to 90 (P < .04) (Figure 4A). In contrast, the baseline levels of CD54 were low (14%) but increased almost 9-fold after culture with anti-CD2 pairs (Figure 4A). Even though not as dramatic as the increase in CD54 expression, the percentage of CD58hi pDCs increased by 21% (P < .03) with an accompanying 53% (P < .04) increase in MFI. In addition, DC-specific antigens, CD83 and CD1a (Figure 4B), and CCR7 (Figure 4C) increased after CD2 engagement 9- and 2.5-fold, respectively. Collectively, these findings also correlated with the increase in cellular side scatter and suggest that engagement of pDCs by CD2 not only up-regulates class II antigens but also induces additional modifications in pDC phenotypic properties associated with cellular activation. In contrast to pDCs, MDDCs demonstrated no appreciable change in cell surface marker expression (data not shown).
Phenotypic characteristics of mature pDCs, pMo's, and MDDCs. CD14+ Mx's were cultured in 10% PHS for 5 days, separated into mature pDCs and pMo's, and analyzed by flow cytometry for MHC class II, costimulatory, and adhesion molecules (A). Analysis of the same surface antigens on GM-CSF/IL-4—generated MDDCs was also performed (B). The pDCs and pMo's were recultured for 24 hours in fresh media, and surface expression of the above molecules was reassessed (C). Dot plots were divided into upper and lower regions, which represent high and low MFI populations. Quadrant boundaries were set using isotype control. Percentages indicate the percentage of cells within the gated region. Values within parentheses represent the MFI of the high or low populations. Results are representative of data from more than 3 experiments.
Phenotypic characteristics of mature pDCs, pMo's, and MDDCs. CD14+ Mx's were cultured in 10% PHS for 5 days, separated into mature pDCs and pMo's, and analyzed by flow cytometry for MHC class II, costimulatory, and adhesion molecules (A). Analysis of the same surface antigens on GM-CSF/IL-4—generated MDDCs was also performed (B). The pDCs and pMo's were recultured for 24 hours in fresh media, and surface expression of the above molecules was reassessed (C). Dot plots were divided into upper and lower regions, which represent high and low MFI populations. Quadrant boundaries were set using isotype control. Percentages indicate the percentage of cells within the gated region. Values within parentheses represent the MFI of the high or low populations. Results are representative of data from more than 3 experiments.
Induction of cell surface antigen expression after DC CD2 engagement. The pDCs were isolated and cultured for 24 hours in the presence of either IR control or the anti-T112/3 mAb pair (A-B), or else anti-T111/2, -T112/3, or -T111/3 mAb pairs (C). Quadrant boundaries were set using isotype control. Percentages indicate the percentage of cells within the gated region. Cultures were harvested, stained, and analyzed by flow cytometry; the data shown are similar to those of 3 other experiments. Values in parentheses indicate the MFI of the high and low populations. Closed histograms indicate CCR7 expression on pDCs cultured with IR, and open histograms indicate CCR7 expression on pDCs cultured with mAb pairs.
Induction of cell surface antigen expression after DC CD2 engagement. The pDCs were isolated and cultured for 24 hours in the presence of either IR control or the anti-T112/3 mAb pair (A-B), or else anti-T111/2, -T112/3, or -T111/3 mAb pairs (C). Quadrant boundaries were set using isotype control. Percentages indicate the percentage of cells within the gated region. Cultures were harvested, stained, and analyzed by flow cytometry; the data shown are similar to those of 3 other experiments. Values in parentheses indicate the MFI of the high and low populations. Closed histograms indicate CCR7 expression on pDCs cultured with IR, and open histograms indicate CCR7 expression on pDCs cultured with mAb pairs.
Effects of CD2 engagement on DC cytokine production and endocytosis
Our preliminary studies demonstrated that activation of pDCs by CD2 ligation influenced the expression of key cell surface markers that are intimately involved in T-cell activation. This prompted us to investigate the effects of CD2 ligation on the regulation of key innate and adaptive immune cytokines, IL-1β and IL-12. The pDCs (Figure 5A) and MDDCs (Figure 5B) were cultured in the presence or absence of anti-T111 mAb for 6 hours, 12 hours, 24 hours, 48 hours, and 72 hours. Control samples produced no appreciable levels of IL-1β or IL-12, whereas anti-T111 induced high levels of release of both cytokines by pDCs and MDDCs. Furthermore, anti-T111 had a more pronounced effect on MDDCs than on pDCs, inducing the release of 75- and more than 100-fold higher levels of IL-1β and IL-12, respectively.
Effect of CD2 engagement on DC cytokine production and endocytic activity. (A-B) Freshly isolated pDCs (A) and GM-CSF– and IL-4–differentiated MDDCs (B) were cultured in the presence (▴ [IL-12] and ▪ [IL-1β]) or absence (♦ [IL-12] and * [IL-1β]) of anti-CD2 (T-111 and T-112) mAbs for 24 hours. The supernatants were assayed for IL-1β and IL-12 by ELISA, and results are typical of 3 similar experiments. (C) Endocytic activity of immature (▪) and mature (□) MDDCs was assessed. MDDCs, previously cultured for 24 hours in the absence or presence of either LPS or anti-CD2, were cultured with FITC-dextran for 30 minutes, and the amount of probe internalized was measured by FACS analysis. Expression indices for samples were determined, and data are presented as mean ± SEM. Findings were representative of 3 similar experiments.
Effect of CD2 engagement on DC cytokine production and endocytic activity. (A-B) Freshly isolated pDCs (A) and GM-CSF– and IL-4–differentiated MDDCs (B) were cultured in the presence (▴ [IL-12] and ▪ [IL-1β]) or absence (♦ [IL-12] and * [IL-1β]) of anti-CD2 (T-111 and T-112) mAbs for 24 hours. The supernatants were assayed for IL-1β and IL-12 by ELISA, and results are typical of 3 similar experiments. (C) Endocytic activity of immature (▪) and mature (□) MDDCs was assessed. MDDCs, previously cultured for 24 hours in the absence or presence of either LPS or anti-CD2, were cultured with FITC-dextran for 30 minutes, and the amount of probe internalized was measured by FACS analysis. Expression indices for samples were determined, and data are presented as mean ± SEM. Findings were representative of 3 similar experiments.
Collectively, the previous findings suggested that CD2 engagement of DCs induced cell surface antigen and cytokine expression profiles suggestive of maturation. To further verify this point, we investigated whether CD2 engagement reduced the ability of DCs to capture exogenous antigens. FITC-dextran was used to assess mannose receptor–mediated endocytosis, which was analyzed by flow cytometry. As shown in Figure 5C, endocytic activity of untreated mature DCs was more than 50% lower (P < .05) than untreated immature DCs. In contrast, anti-CD2–treated mature DCs retained their endocytic ability with levels equal to the untreated-immature sample, while the LPS-treated sample was comparable to untreated-mature controls.
CHO cell transfectants expressing CD58 can activate pDCs
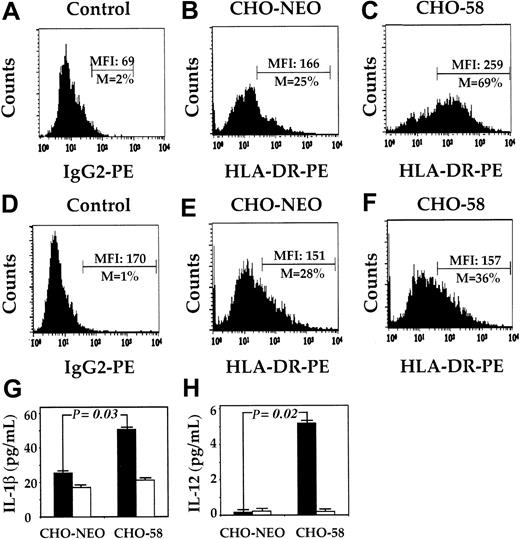
To investigate directly whether pDCs could be activated by CD2-CD58 interaction, we cultured pDCs in the presence of CHO-NEO or CHO-58 cells (Figure 6). When pDCs were cocultured with CHO-NEO (nontransfected) cells, the level of expression of HLA-DR did not increase over that with pDCs cultured alone (Figure 3C). In contrast, when pDCs were cultured in the presence of CHO-58 cells, the level of HLA-DR increased more than 2.5-fold. As expected, no significant change in the expression of HLA-DR was noted on pMo's cocultured with either CHO cell population compared with pMo's cultured alone (Figure 3C).
CD58-transfected CHO cells induced pDC-specific increases in class II expression and IL-1β and IL-12 production. The pDCs (A-C) and pMo's (D-F) were cultured overnight in the presence of CHO-NEO (B,E) or CHO-58 (C,F). These samples were assessed by immunofluorescence analysis for the expression of HLA-DR, and the results were compared with isotype controls (A,D). The supernatants from pDCs (▪) and pMo's (□) cocultured in either CHO-NEO or CHO-58 were harvested, and IL-1β (G) and IL-12 (H) levels were measured; data are presented as mean ± SEM. The results are representative of at least 3 experiments.
CD58-transfected CHO cells induced pDC-specific increases in class II expression and IL-1β and IL-12 production. The pDCs (A-C) and pMo's (D-F) were cultured overnight in the presence of CHO-NEO (B,E) or CHO-58 (C,F). These samples were assessed by immunofluorescence analysis for the expression of HLA-DR, and the results were compared with isotype controls (A,D). The supernatants from pDCs (▪) and pMo's (□) cocultured in either CHO-NEO or CHO-58 were harvested, and IL-1β (G) and IL-12 (H) levels were measured; data are presented as mean ± SEM. The results are representative of at least 3 experiments.
We also investigated CD58-mediated induction of cytokine production. Analysis of supernatants from pDCs and pMo's cocultured with CHO-NEO and CHO-58 revealed a 2-fold increase in IL-1β (Figure 6G) and a 26-fold increase in IL-12 (Figure 6H) in the DC/CHO-58 cocultures compared with DC/CHO-NEO. In contrast to DC/CHO-58 cocultures, there was no significant increase in IL-1β or IL-12 levels in pMo/CHO-58 cocultures. No increase in IL-1β or IL-12 was observed when DCs were cocultured with CHO58/K34A (data not shown), which possesses a single amino acid substitution in D1 of CD58 that prevents its binding to CD2. These findings are consistent with our T11 mAb pair results, and they further suggest that CD2 is a functional molecule on DCs.
CD2-CD58 engagement is required for efficient CD4+CD45RA+ T-cell activation
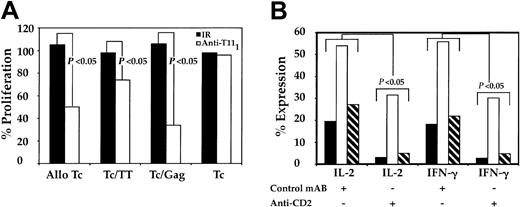
The role of CD2-CD58 engagement in pDC-induced activation of T cells was evaluated. Because immature and mature MDDCs lack the ability to activate autologous CD4+CD45RA+ (data not shown), pDCs served as the primary DCs for this portion of the study. The pDCs were initially pulsed with either anti-T111 mAb, which is known to block CD2-CD58 interaction, or an IR, which is known not to interfere with CD2-CD58 engagement. Both samples were washed to remove residual mAb, cocultured with autologous or allogeneic CD4+CD45RA+ T cells for 5 days, and assessed for T-cell proliferation. IR-pulsed pDCs induced T-cell proliferation similar to that of unpulsed pDCs, while the anti-T111–pulsed pDCs were less efficient. T-cell proliferation dropped by 50% in alloreactive cocultures, while in autologous cocultures T-cell proliferation decreased between 20% and 68% (P < .04) and was dependent on the type of soluble antigen used (Figure 7A). We further evaluated the requirement of CD2-CD58 interaction on antigen-independent and -dependent T-cell cytokine production (Figure 7B). The pDCs, treated as noted above, were cultured with autologous T cells in the absence or presence of either HIV-1 gag or TT antigens. Cocultures containing anti-T111–blocked pDCs had lower intracellular levels of both IL-2 and IFN-γ cytokines compared with unblocked control pDCs. Interference of pDC CD2-CD58 engagement decreased T-cell intracellular cytokine levels by 42% to 86% (P < .03). These results support our previous findings and further indicate that CD2 on pDCs is important for pDC-mediated T-cell activation.
Effect of CD2 blockade on DC-induced CD4+CD45RA+ T-cell activation and cytokine production. The pDCs were cultured at 4°C in the presence of anti-T111 or an IR mAb. After 30 minutes, preparations were washed 3 times and resuspended in culture medium. These pulsed and unpulsed pDCs (104) were separately cocultured with CD4+CD45RA+ T cells (105), and their ability to induce allogeneic and autologous T-cell (Tc) proliferation was measured (A). Results are expressed as the percent proliferation and are typical of data obtained from 3 similar experiments. Parallel samples were cultured, as stated in “Materials and methods,” harvested, and stained with anti–IL-2 and anti–IFN-γ (B). In panel B, ▪ indicates control; □, Gag; and ▧, TT. P values were calculated from intracellular cytokine data from 3 independent experiments.
Effect of CD2 blockade on DC-induced CD4+CD45RA+ T-cell activation and cytokine production. The pDCs were cultured at 4°C in the presence of anti-T111 or an IR mAb. After 30 minutes, preparations were washed 3 times and resuspended in culture medium. These pulsed and unpulsed pDCs (104) were separately cocultured with CD4+CD45RA+ T cells (105), and their ability to induce allogeneic and autologous T-cell (Tc) proliferation was measured (A). Results are expressed as the percent proliferation and are typical of data obtained from 3 similar experiments. Parallel samples were cultured, as stated in “Materials and methods,” harvested, and stained with anti–IL-2 and anti–IFN-γ (B). In panel B, ▪ indicates control; □, Gag; and ▧, TT. P values were calculated from intracellular cytokine data from 3 independent experiments.
Discussion
In this study, we investigated the phenotypic and functional characteristics of the CD2 antigen and changes induced by its engagement on pDCs and MDDCs. Our results show that the CD2 antigen expressed on pDCs and MDDCs is structurally similar to that expressed on T and NK cells, as determined by the binding characteristics of anti-T111, anti-T112, and anti-T113 mAbs, which recognize 3 distinct CD2 regions.13 CD2 engagement by anti-CD2 mAbs increased synthesis and expression of MHC class II (HLA-DR, -DQ, -DP, and -DM), costimulatory (CD40, CD80, and CD86), and adhesion (CD54, CD58) molecules. DC-specific markers, CD1a and CD83, which are associated with maturation, were simultaneously up-regulated, which is in contrast to reports of CD83 up-regulation and CD1a down-regulation upon maturation induced by TNF-α.26 Another important cell surface antigen, CCR7, which is a receptor needed for efficient migration of immune cells to regional lymph tissue, was also up-regulated. In addition to changes in phenotypic markers, activation of DCs via CD2 induced secretion of key immune response cytokines, IL-1β and IL-12, and increased endocytic activity of mature DCs. However, interference of CD2-CD58 engagement prevented efficient DC activation of naive T cells. These findings suggest that CD2 is a functional antigen on DCs, not merely a developmental remnant, which allows immunologic crosstalk among a variety of different cell types. Furthermore, this CD2-CD58 pathway is needed for efficient naive T-cell activation and is possibly the means by which nonimmune tissue transmits the danger signal to immune tissue.
CD58 is present on all hematopoietic cells as well as on human vascular endothelium, vascular smooth muscle, and fibroblasts.27,28 Little is known about the role of CD58 on nonlymphoid tissues, because most studies investigating the CD2-CD58 relationship have focused on the interaction of CD2 on T cells with CD58 on APCs.11,13,29-31 Our current finding that CD58-transfected CHO cells activate pDCs in the absence of any other human cell surface antigen suggests that nonlymphoid as well as lymphoid tissues could influence pDC function. Interestingly, tumors with high levels of CD58 expression are not as malignant as those tumors that express low levels of CD58,32 raising the possibility that DCs recognize aberrant cell growth33 through this CD2-CD58 pathway. Furthermore, increased levels of CD58 are found in some patients with autoimmune disease.21 In these patients, the onset of autoimmunity may be associated with CD58 up-regulation and ligation of CD2 on DCs and the subsequent autocrine release of IL-1β that increases the release of IL-12, which, in turn, activates T cells.
We have demonstrated a novel effect following interaction of anti-CD2 mAbs or CD58 with DCs. To explain this novel effect, our model proposes a principal functional link between CD2 on DCs and cells capable of expressing CD58. Gallucci and Matzinger34 suggest the existence of a danger signal triggered by damaged tissue and identified by DCs. Viral and bacterial infections are known to increase expression of CD58.34 This might represent the signal required to trigger the early events of innate and adaptive immunity. Furthermore, our findings generate a new focus on the functional significance of CD2, uncovering a third immune cell CD2-CD58 interaction pathway capable of influencing not only the presence of receptors but also optimizing the immune response. Because our model is focused on CD2-induced IL-12/1L-1β cytokine production, class II and costimulatory/adhesion molecule up-regulation, and naive T-cell activation, it may yield valuable information regarding the signaling pathways and how they differ from CD2 signaling in T and NK cells.
In view of the fact that our method of DC enrichment involves positive selection, 2 considerations were of potential concern. It is possible that engagement of CD2 with an mAb during selection could induce DC activation or inhibit the induction of T-cell proliferation. The latter consideration is important, because positive selection of pDCs or pMo's with anti–HLA-DR or anti-CD14 mAbs inhibits the ability of these APCs to activate T cells.6 Our data support our hypothesis that the method by which DCs are enriched can influence their functional properties. In our case, blocking CD2-CD58 interaction decreased cytokine production and subsequent naive CD4 T-cell activation. However, enrichment with nonblocking mAbs did not activate DCs or interfere with their ability to activate autologous or alloreactive T cells.
It has been proposed that efficient activation of T cells requires the presence of mature CD83+ DCs. To investigate DC-induced T-cell proliferation, many groups have used the forced differentiation of Mo's into MDDCs by prolonged culturing with GM-CSF and IL-4 cytokines.7,26 This method generates adequate quantities of immature DCs (CD83–/+) requiring additional culturing with CD40L, cytokines (IL-1β, IL-15, TNF-α), prostaglandins, or LPS to induce the mature CD83+ phenotype. DCs with this phenotype express maximal levels of class II antigens, costimulatory molecules (CD40, CD80, CD86), and intercellular adhesion molecule-1 (ICAM-1), which facilitate physical contact with T cells via corresponding ligands. This direct cell-cell contact is responsible for the production of various inflammatory cytokines (IL-6, IL-10, IL-12) involved in T-cell responsiveness and the skewing of T cells toward Th1 or Th2 profiles. It is known35 that CD40-CD40L engagement between DCs and T cells is key to activating the signaling pathway for adaptive immunity. The engagement of CD40 on MDDCs increases the expression of ICAM-1 and costimulatory molecules (CD80, CD86).35 In the present studies, CD2 engagement of immature and mature MDDCs did not alter the surface expression of such molecules (data not shown). In contrast, we observed significant increases in the surface expression of class II and costimulatory molecules on pDCs; however, a discordant effect was shown for adhesion molecule expression. The cell surface density of both CD54 and CD58, as measured by MFI, increased upon CD2 engagement, while that of CD50 decreased. However, the percentages of pDCs expressing CD50 and CD58 were similar, while the percentage of CD54+ cells increased significantly upon CD2 engagement. An effect unique to activation of pDCs by CD2 engagement was the up-regulation of class II gene transcription and translation not previously demonstrable for MDDCs activated by CD40 ligation.
The aforementioned molecules are of critical importance in inducing T-cell activation, increasing IL-12 responsiveness, and developing Th1 profiles.18,30,36 Two adhesion pathways, CD2-CD58 and ICAM-1–LFA-1,37 enable efficient activation of memory T cells by DCs, Mo's, and B cells and bias memory T cells toward the Th1 phenotype.38 In contrast, only the ICAM-1–LFA-1 pathway efficiently activates naive T cells and biases this lymphocyte subset toward the Th1 profile.37 Our data suggest that the CD2-CD58 pathway is involved in naive T-cell activation; however, the cellular locations of these molecules are reversed (CD2 on DCs and CD58 on T cells). Interference with this pathway decreased production of IL-2 and IFN-γ cytokines and suppressed alloreactive and autologous T-cell proliferation.
Several groups have reported the need for IL-1β, IL-4, or IFN-γ to optimize the release of IL-12 from MDDCs.39,40 IL-1β has more recently41,42 been demonstrated as the potent inducer of IL-12 by MDDCs. When CD2 on pDCs or MDDCs was engaged, optimal levels of IL-12 were generated without exogenous IL-4 or IL-1β cytokines. Whether the optimization of IL-12 release was directly related to CD2 ligation or indirectly related to IL-1β release is presently under investigation.
From a clinical standpoint, the effectiveness of injected DC-based vaccines is dependent on the expression of key surface antigens such as MHC class II, costimulatory (CD40, CD80, CD86), and adhesion (CD54, CD58) molecules and the release of IL-1β and IL-12 cytokines, which are known to provide primary, secondary, and tertiary signals to the T cells. The cell signal generated by CD40 engagement is crucial for the release of a variety of inflammatory cytokines. However, the method of DC preparation dictates the types of cytokines released after engagement. For example, CD40 ligation of MDDCs induces IL-6, IL-10, and IL-12 release, while in CD1b/c+ DCs only the secretion of IL-12 increases but not that of IL-6 or IL-10.35 This difference in cytokine expression profile suggests that MDDCs are different from CD1b/c+ DCs and more similar to CD2+ pDCs. Even though the level of IL-12 secreted by CD2-engaged pDCs is much lower than that of CD2-engaged MDDCs, results support the possibility that efficient IL-12 release requires the presence of IL-1β in the microenvironment of the DCs.
There are important clinical implications of the current findings for DC-based AIDS and tumor vaccines. For example, such vaccines could be phenotypically and functionally modified by CD2 engagement prior to vaccination of patients. Because CD2 engagement increases the release of IL-12 without the need for additional cytokines, this may improve the ability of DC-based vaccines to promote antigen-specific T-cell responsiveness and lessen the side effects associated with systemic use of cytokines to help boost immunity. Because DC tumor vaccines hold considerable promise for the treatment of oncologic disorders, these cellular interactions need to be further understood.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2002-07-2206.
Supported by the National Heart, Lung, and Blood Institute; National Institute of Allergy and Infectious Diseases; and National Cancer Institute (HL 29583, AI 35630, and CA 09141) of the National Institutes of Health (NIH). Supported in part by the Center for AIDS Research at Harvard Medical School (AIDS Training Grant AI 07382), The Robert Wood Johnson Foundation, US Department of Defense grant DAMD17, and Center for AIDS Research grant P30 AI 28691 (K.C.). D.G. is an Elizabeth Glaser Scientist supported by the Pediatric AIDS Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs D. Dubey, Z. Husain, and J. Lieberman for discussion and critical review of the manuscript. We also thank Louise Viehmann, Lori Siniski, and Shawn Turnquist for excellent assistance with manuscript preparation.

![Figure 5. Effect of CD2 engagement on DC cytokine production and endocytic activity. (A-B) Freshly isolated pDCs (A) and GM-CSF– and IL-4–differentiated MDDCs (B) were cultured in the presence (▴ [IL-12] and ▪ [IL-1β]) or absence (♦ [IL-12] and * [IL-1β]) of anti-CD2 (T-111 and T-112) mAbs for 24 hours. The supernatants were assayed for IL-1β and IL-12 by ELISA, and results are typical of 3 similar experiments. (C) Endocytic activity of immature (▪) and mature (□) MDDCs was assessed. MDDCs, previously cultured for 24 hours in the absence or presence of either LPS or anti-CD2, were cultured with FITC-dextran for 30 minutes, and the amount of probe internalized was measured by FACS analysis. Expression indices for samples were determined, and data are presented as mean ± SEM. Findings were representative of 3 similar experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/5/10.1182_blood-2002-07-2206/6/m_h81734838005.jpeg?Expires=1769099197&Signature=MVQNCMjb3ULKlty5FzFhsrxLkbaTHrOMDf5cc1ojMaPY7zxzhT0riJe--EfqxerFRpuCsimiZF4f9GwIQmj~tajSQPpmWRI5I~kkfz~TgpaG6gGZpYTQq7hSMSZy-xu8niTdTZgZDnxectlRSOvoJPCQODfZ5Kai3FcwwlZIWYRLLvBivXIKQf-M~4pP3JY5ayclBRTpu0SfZdH87s14aX91Ktoq4fAa2yyJIcjb~S0e7k2~vBXvtmEvTXhQPaL19IzyefENONAVeLF8b3v4opihIt5EAWhZFvlv3WCbsU1LM~8fmnh0Zl8xRRhCSGd72lb2XXQy-B9J45Fxv7mOdQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal