Abstract
Resveratrol, an edible polyphenolic stilbene, has been reported to possess substantial antileukemic activities in different leukemia cell lines. We investigated whether resveratrol is active against fresh acute myeloid leukemia (AML) cells and its mechanism of action. Because interleukin 1β(IL-1β) plays a key role in proliferation of AML cells, we first tested the effect of resveratrol on the AML cell lines OCIM2 and OCI/AML3, both of which produce IL-1β and proliferate in response to it. Resveratrol inhibited proliferation of both cell lines in a dose-dependent fashion (5-75 μM) by arresting the cells at S phase, thus preventing their progression through the cell cycle; IL-1β partially reversed this inhibitory effect. Resveratrol significantly reduced production of IL-1β in OCIM2 cells. It also suppressed the IL-1β–induced activation of transcription factor nuclear factor κB (NF-κB), which modulates an array of signals controlling cellular survival, proliferation, and cytokine production. Indeed, incubation of OCIM2 cells with resveratrol resulted in apoptotic cell death. Because caspase inhibitors Ac-DEVD-CHO or z-DEVD-FMK partially reversed the antiproliferative effect of resveratrol, we tested its effect on the caspase pathway and found that resveratrol induced the activation of the cysteine protease caspase 3 and subsequent cleavage of the DNA repair enzyme poly (adenosine diphosphate [ADP]–ribose) polymerase. Finally, resveratrol suppressed colony-forming cell proliferation of fresh AML marrow cells from 5 patients with newly diagnosed AML in a dose-dependent fashion. Taken together, our data showing that resveratrol is an effective in vitro inhibitor of AML cells suggest that this compound may have a role in future therapies for AML.
Introduction
Acute myeloid leukemia (AML) is a hematologic malignancy characterized by abnormal proliferation of clonal myeloid cells. Despite extensive clinical research with numerous combinations of cytotoxic agents, the overall prognosis of patients with AML remains poor.1,2 Thus, the search for more effective agents continues.
Resveratrol (trans-3,4′-trihydroxystilbene),3 a polyphenolic phytoalexin found in the skin of red grapes, various other fruits, and root extracts of the weed Polygonum cuspidatum, has structural similarities to estradiol and diethylstilbestrol.4 In plants, resveratrol functions microbiologically as a phytoalexin that protects against fungal infections.3,5 Several studies within the last few years have shown that resveratrol induces the accumulation of p53 and p21 (WAF1/CIP1),6 inhibits ribonucleotide reductase7 and DNA polymerase,8 induces nitric oxide production, and suppresses cell growth by arresting cells at the S and G2 phases of the cell cycle.6 Resveratrol was found to inhibit growth and induce apoptosis in several human cancer cells,9 including mouse and human leukemia cell lines.10-14 Resveratrol was shown to induce apoptosis through both CD95-dependent6,15 and -independent16 mechanisms, by a mitochondrial permeability transition,17 and through induction of differentiation.14,18
The nuclear transcription factor NF-κB modulates the effects of various transcription factors responsible for proliferation of normal myeloid and leukemia cells,19 and its activation induces expression of various cytokines, including interleukin 1β (IL-1β).20 IL-1β itself plays a major role in stimulating proliferation of AML cells.21-24 We have recently found that IL-1β activates NF-κB in AML cells.25 Thus, this study focused on the effects of resveratrol on IL-1β–responsive AML cell lines and fresh bone marrow (BM) cells from 5 patients with newly diagnosed AML.
We found that resveratrol suppressed both the production of IL-1β and its effect on activation of NF-κB, inhibited AML cell proliferation, and activated caspase 3, thus inducing apoptotic cell death in AML cells.
Patients, materials, and methods
Cell lines
The AML cell lines OCI/AML326 and OCIM227 were kindly provided by M. D. Minden (Ontario Cancer Institute, Toronto, ON, Canada). OCI/AML3 was established from a patient with AML and OCIM2 from a patient with erythroleukemia. Both cell lines proliferate in the presence of culture medium and fetal calf serum (FCS) without exogenous growth factors. The cells were maintained in RPMI 1640 culture medium (Gibco, Grand Island, NY) supplemented with 10% FCS (Flow Laboratories, McLean, VA).
Patients
Bone marrow (BM) aspirates were obtained from 5 patients with newly diagnosed AML (Table 1). All studies were performed with the patients' informed consent and were approved by the Institutional Review Board.
Clinical data on AML patients
Patient no. . | Age, y. . | Sex . | Cytogenetic abnormality . | FAB category . | Hb level, g/dL . | WBC count, ×109/L . | Platelet count, ×109/L . | Blasts, % . | Blasts in BM, % . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | M | Dip,-Y | M5 | 7.3 | 234 | 43 | 61 | 71 |
| 2 | 54 | M | -5, -7 | M2 | 10.7 | 2.9 | 12 | 64 | 87 |
| 3 | 52 | F | Misc | M2 | 8.9 | 28.9 | 103 | 82 | 92 |
| 4 | 60 | F | -5, -7 | M1 | 8.3 | 5.9 | 27 | 93 | 97 |
| 5 | 75 | M | +8 | M5 | 7.2 | 200.5 | 74 | 63 | 86 |
Patient no. . | Age, y. . | Sex . | Cytogenetic abnormality . | FAB category . | Hb level, g/dL . | WBC count, ×109/L . | Platelet count, ×109/L . | Blasts, % . | Blasts in BM, % . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | M | Dip,-Y | M5 | 7.3 | 234 | 43 | 61 | 71 |
| 2 | 54 | M | -5, -7 | M2 | 10.7 | 2.9 | 12 | 64 | 87 |
| 3 | 52 | F | Misc | M2 | 8.9 | 28.9 | 103 | 82 | 92 |
| 4 | 60 | F | -5, -7 | M1 | 8.3 | 5.9 | 27 | 93 | 97 |
| 5 | 75 | M | +8 | M5 | 7.2 | 200.5 | 74 | 63 | 86 |
FAB indicates French-American-British; Hb, hemoglobin; WBC, white blood cells; BM, bone marrow; Misc, miscellaneous; Dip, diploid
Cell line clonogenic assay
The cell line clonogenic assay was performed as previously described.28 Briefly, OCI/AML3 and OCIM2 cells (2-4 × 104 cells/mL) were cultured in 0.8% methylcellulose (Fluka Chemical, Ronkonkoma, NY), 10% FCS, and RPMI 1640 medium in the presence of resveratrol (Sigma Chemical, St Louis, MO), which was dissolved in ethanol at a final concentration of less than 0.1%, with or without 100 ng/mL recombinant human IL-1β (molecular weight 17 500 Da; Boehringer Mannheim Biochemicals. Indianapolis, IN), and in the presence or absence of 50 μM of either the caspase inhibitor Ac-DEVD-CHO29 or the caspase inhibitor z-DEVD-FMK30 (Calbiochem, La Jolla, CA). The culture mixtures were placed in 35-mm Petri dishes (Nunc, Naperville, IL) in duplicate, triplicate, or quadruplicate and maintained at 37°C in humidified air with 5% CO2. Colonies were counted after 7 days by using an inverted microscope. A colony was defined as a cluster of more than 40 cells.
Cell cycle analysis
Cell cycle analysis was performed as previously described.31 In short, 5 × 106 OCIM2 cells were incubated with resveratrol and pelleted. The cell pellets were washed and resuspended in 2 mL 1% paraformaldehyde in phosphate-buffered saline (PBS). After incubation for 15 minutes at 4°C, the cells were washed twice in PBS, resuspended in 0.5 mL propidium iodide (PI) staining buffer (50 μg/mL PI, 10 μg/mL RNase in PBS), and then incubated for 1 hour at room temperature in the dark. Flow cytometry analysis was performed using FACSCaliber (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA). Data analysis was performed using CellQuest software (BDIS) and Modfit LT V2.0 (Verity Software House, Tosham, ME).
Western immunoblotting for detection of IL-1β
Cell lysates were assayed for protein concentrations with the BCA protein assay reagent kit (Pierce, Rockford, IL). Each set of paired samples was then adjusted to have the same protein concentration. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed by using a modification of the method of Laemmli.32 In brief, electrophoresis was conducted at a constant wattage (10 W) in running buffer cooled to 4°C. Stacking gels contained 4% (wt/vol) acrylamide, and separating gels contained 12% (wt/vol) acrylamide. Approximately 200 μL sample protein was loaded into each of the appropriate lanes. Proteins separated by SDS-PAGE were transferred to nitrocellulose membranes overnight at 30 V in a cooled (4°C) reservoir containing transfer buffer (25 mM Tris [tris(hydroxymethyl)aminomethane], 192 mM glycine, and 20% methanol, pH 8.3).33 The nitrocellulose membranes were then removed from the blot apparatus and placed in Ponceau S staining solution (0.5% Ponceau S and 1% glacial acetic acid in H2O) for 5 minutes to verify the equal loading of protein in control and treated samples.34
After verification of equal protein loading, the membranes were rinsed and immunoscreened. In brief, the membranes were blocked in Blotto (5% dried milk dissolved in 50 mM PBS) for at least 1 hour at room temperature and then washed 3 times in PBS plus 0.5% Tween 20. Next, the membranes were incubated for 1 hour with polyclonal rabbit anti–IL-1β antibodies (Endogen, Boston, MA) or with normal rabbit immunoglobulin G (IgG, used as a control) diluted 1:200 in PBS containing 0.5% Tween 20. After incubation, the membranes were subjected to three 15-minute rinses in PBS containing 0.5% Tween 20. Bound antibody was detected by using the enhanced chemiluminescence (ECL) Western blotting detection system (Amersham, Arlington Heights, IL). The membranes were incubated with antirabbit horseradish peroxidase-labeled antibody at a concentration of 1:2000 in PBS plus 0.5% Tween 20 at room temperature for 1 hour. After this incubation, the membranes were washed in PBS containing 0.5% Tween 20, and bound antibody was detected according to the ECL protocol. The chemiluminescence of the membranes was detected by exposure to X-OMAT AR5 x-ray film (Kodak, Rochester, NY) in stainless steel cassettes (Sigma Chemical).
Detection of membrane-bound IL-1β
To detect membrane-bound IL-1β on OCIM2 cells we used a flow cytometric assay as previously described.24 Briefly, OCIM2 cells were incubated with or without resveratrol for 2 hours, washed, and thereafter treated overnight with mouse antihuman IL-1β antibodies (Sigma Chemical) at 4°C. Then the cells were washed in PBS and treated with biotinylated antimouse antibodies (Vector Laboratories, Burlingame CA). The signal was amplified using fluorescein isothiocyanate (FITC)–labeled avidin and biotinylated antiavidin antibodies (Vector Laboratories), and detected by a second application of FITC-avidin. After staining, the cells were evaluated using the FACSCaliber flow cytometer (Becton Dickinson) as described (see “Cell cycle analysis”).
Electrophoretic mobility shift assay of NF-κB activation
To determine NF-κB activation, we carried out the electrophoretic mobility shift assay (EMSA) as previously described.35,36 Briefly, nuclear extracts prepared from OCIM2 cells (2 × 106/mL) treated with IL-1β were incubated with 32P-end-labeled 45-mer double-stranded NF-κB oligonucleotide (4 μg protein with 16 fmol DNA) from the human immunodeficiency virus long terminal repeat, 5′-TTGTTACAAGGGACTTTC CGCTGGGGACTTTC CAGGGA GGCGT GG-3′ (boldface indicates NF-κB binding sites), 15 minutes at 37°C, and the DNA-protein complex formed was separated from free oligonucleotide on 6.6% native polyacrylamide gels. A double-stranded mutated oligonucleotide, 5′-TTGTTACAACTCACTTTC CGCTGCTCACTTTCCAGGGAGG CGTGG-3′, was used to examine the specificity of binding of NF-κB to the DNA. The specificity of binding was also examined by competition with the unlabeled oligonucleotide. For super-shift assays, nuclear extracts prepared from IL-1–treated cells were incubated with antibodies against either p50 or p65 of NF-κB for 30 minutes at room temperature before the complex was analyzed by EMSA. Antibodies against cyclin D1 and preimmune serum were included as negative controls. The dried gels were visualized, radioactive bands were quantitated by using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA), and the data were analyzed by Imagequant software.36,37
Apoptosis assays
To quantify the percentage of cells undergoing apoptosis, we used annexin V–FITC (PharMingen, San Diego, CA) as previously described.38 Briefly, OCIM2 cells were incubated for 12 hours with or without 10 to 100 μM resveratrol. Then the cells were washed twice with cold PBS and resuspended in binding buffer (10 nM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 140 nM NaCl, 5 nM CaCl2, pH 7.4) at a concentration of 1 × 106 cells/mL. After incubation, 100 μL of the solution was transferred to a 5-mL culture tube, and 5 μL annexin V–FITC and 10 μL PI were added. The tube was gently vortexed and incubated for 15 minutes at room temperature in the dark. At the end of incubation, 400 μL binding buffer was added, and the cells were analyzed immediately by flow cytometry. Flow cytometric analysis was performed with a FACSCaliber using the CellQuest software (BDIS). Data were analyzed by CellQuest and Modfit LT V2.0 software (Verity Software House).
To further confirm that resveratrol induces apoptosis in AML cells we used the TdT-mediated deoxyuridine triphosphate (dUTP) nick-end labeling (TUNEL) apoptosis detection system (Promega, Madison, WI) as previously described.25 Briefly, 4% formaldehyde-treated cytospin cells were made permeable with 0.2% Triton-100 in PBS. After washing, slides were treated with equilibration buffer (supplied with the kit) and then incubated with TdT buffer (prepared according to the manufacturer's instructions) for 60 minutes. The staining reaction was terminated by treating the slides with 2 × standard sodium citrate (SSC) for 15 minutes. After washing, the slides were treated with antifade solution and then mounted on slides with glass coverslips and rubber cement. The slides were analyzed using a fluorescence microscope.
Western immunoblotting for detection of caspase 3, PARP, Bcl-2, bcl-XL, and XIAP
Cell lysates (from 5 × 105 cells) were used as described. The following antibodies were used to detect the relevant proteins: monoclonal mouse antihuman CPP32 (Transduction Laboratories, Lexington, KY) to detect uncleaved caspase 3, polyclonal rabbit antihuman CPP32 (PharMingen) to detect cleaved caspase 3, mouse antihuman poly (ADP ribose) polymerase (PARP; Upstate Biotechnology, Lake Placid, NY) to detect PARP, mouse antihuman Bcl-2 (Transcription Laboratories) to detect Bcl-2, rabbit antihuman Bcl-XL (Transcription Laboratories) to detect Bcl-XL, and mouse antihuman x-inhibitor of apoptosis protein (XIAP; Transcription Laboratories) to detect XIAP. Normal mouse IgG and normal rabbit serum cells were used as controls. To confirm detection of uncleaved caspase 3 and Bcl-2, Jurkat cells (American Type Culture Collection [ATCC], Rockville, MD) were used; to confirm detection of cleaved caspase and either PARP or XIAP, 3T3 cells (ATCC) and HeLa cell (ATCC) nuclear extracts, respectively, were used. To confirm detection of Bcl-XL, lysates of human endothelial cells were used. Bound antibodies were detected according to the ECL protocol (Amersham Life Sciences) as described.
Adherent cell fractionation
Low-density BM mononuclear cells were obtained by fractionation with Ficoll-Hypaque (Pharmacia, Piscataway, NJ). The cells were then incubated in plastic tissue culture dishes or flasks (Falcon Plastics, Becton Dickinson, Oxnard, CA) with 10% FCS in α medium (Gibco). The fractionation procedure was repeated until no cells adhered to tissue culture dishes. Nonadherent cells harvested in this way contained less than 3% monocytes as confirmed by the following techniques: (1) microscopic differential counting of at least 100 cells prepared with Wright stain and (2) nonspecific (α-naphthyl butyrate) esterase staining and immunocytochemical analysis with CD14 monoclonal antibodies (Becton Dickinson, San Jose, CA) to identify monocyte-promonocyte cells, as previously described.39,40
T-cell depletion
T cells were depleted from the nonadherent fraction by negative immunomagnetic selection.41 In a modification of this technique, nonadherent BM cells were incubated with CD3 monoclonal antibodies (Becton Dickinson) at a concentration of 1 μg/106 cells in PBS with 0.25% FCS for 30 minutes at 4°C. The labeled cells were washed 3 times and then incubated with goat antimouse IgG-conjugated immunomagnetic beads (Advanced Magnetics, Cambridge, MA) at 4°C for 60 minutes in an end-over-end rotation at a 20:1 bead-cell ratio. Immunomagnetic bead-rosetted cells were removed with a magnetic particle concentrator (Advanced Magnetics), and unrosetted cells remaining in suspension were harvested by a Pasteur pipette. In some experiments, this procedure was repeated twice. The T lymphocyte–depleted population contained less than 3% CD3 cells as assessed by an immunocytochemical technique performed on cytospun cells.39,40
AML blast colony assay
A previously described method was used to assay AML blast colony formation.42,43 Briefly, 1 × 105 nonadherent T cell–depleted BM cells were plated in 0.8% methylcellulose in α-medium supplemented with 10% FCS and 50 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF; Immunex, Seattle, WA). Resveratrol was dissolved in ethanol and added at the initiation of the cultures at concentrations ranging from 5 to 50 μM in the absence or presence of 100 ng/mL of IL-1β. The cultures were incubated in 35-mm Petri dishes in duplicate or triplicate for 7 days at 37°C in a humidified atmosphere of 5% CO2 in air. AML blast colonies were microscopically evaluated on day 7 of culture. A blast colony was defined as a cluster 20 or more cells. Individual colonies were plucked, smeared on glass slides, and stained to confirm their leukemic cell composition. (That the AML blast colony assay identifies blasts rather than normal progenitors had been demonstrated previously by cytogenetic analysis of colonies obtained by using this assay.44 )
Results
Resveratrol inhibits leukemia cell line colony proliferation
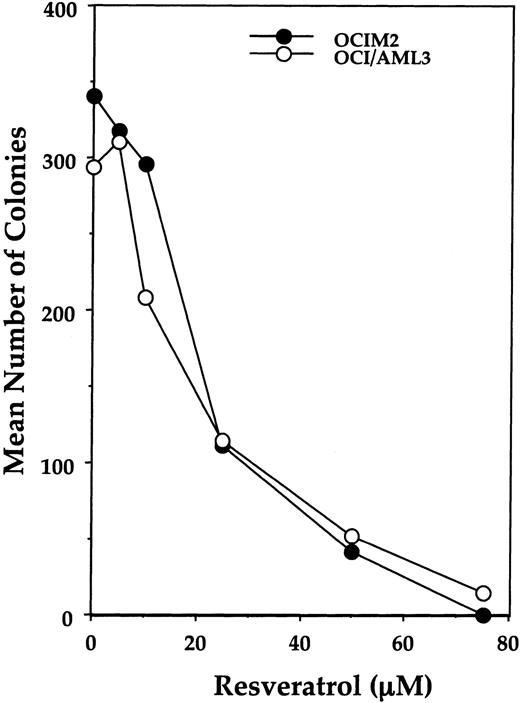
Because IL-1β plays a major role in stimulating AML cell proliferation,21-24 we began studying the effects of resveratrol on the IL-1–responsive cell lines OCI/AML3 and OCIM2. We found that resveratrol suppressed the colony-forming growth of both cell lines in a dose-dependent fashion at concentrations ranging from 10 to 75 μM (Figure 1). A concentration of 20 μM resveratrol reduced cell growth by more than 60%; at 75 μM, OCIM2 and OCI/AML3 colony proliferation was completely abolished.
Effect of resveratrol on OCIM2 and OCI/AML3 colony-forming cell proliferation. Each data point represents the mean colony number in duplicate cultures. Representative data from 1 of 3 identical experiments are depicted.
Effect of resveratrol on OCIM2 and OCI/AML3 colony-forming cell proliferation. Each data point represents the mean colony number in duplicate cultures. Representative data from 1 of 3 identical experiments are depicted.
Resveratrol causes accumulation of AML cells in S phase
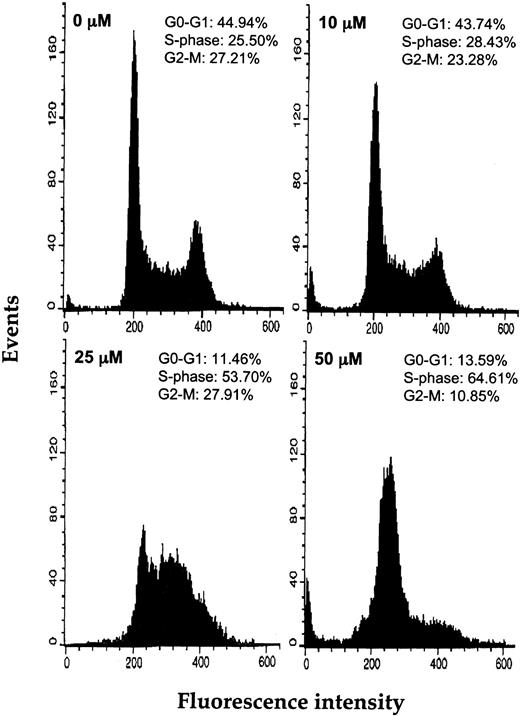
Because we recently found that resveratrol induces the accumulation of breast cancer cells in the S phase of the cell cycle,45 we analyzed the impact of resveratrol on the cell cycle progression of AML cells. Cell cycle analysis performed after 6-hour incubations of OCIM2 cells in increasing concentrations of resveratrol showed that this agent arrested the cells in the S phase of the cell cycle in a dose-dependent manner (Figure 2). After exposure to 50 μM resveratrol, 64.6% of the cells were arrested at the S phase, whereas only 10.9% were at the G2/M phase and 13.6% at the G0/G1 phase of the cell cycle.
Effect of resveratrol on the cell cycle status of OCIM2 cells. Depicted are the percentages of cells in the G0/G1, S, and G2/M phases of the cell cycle after 6 hours of incubation with increasing concentrations of resveratrol.
Effect of resveratrol on the cell cycle status of OCIM2 cells. Depicted are the percentages of cells in the G0/G1, S, and G2/M phases of the cell cycle after 6 hours of incubation with increasing concentrations of resveratrol.
IL-1β partially reverses the inhibitory effect of resveratrol
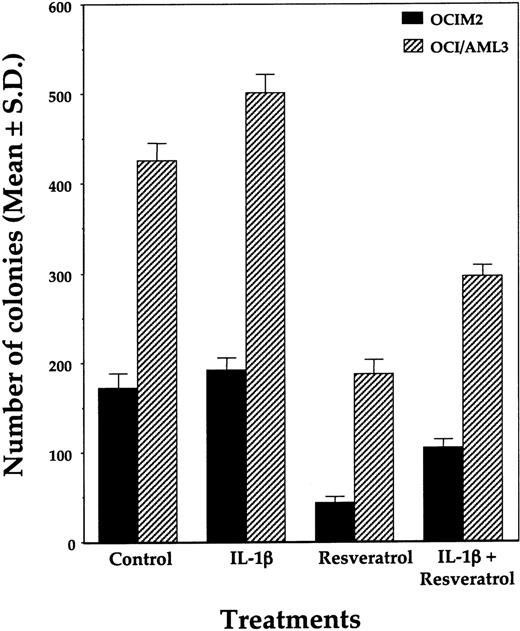
Because we previously found that OCIM2 and OCI/AML3 cells proliferate in response to IL-1β,24,46 we also tested whether IL-1β could modulate the inhibitory effect of resveratrol. We found that 100 ng/mL IL-1β added at the initiation of culture partially reversed the suppressive effect of resveratrol (Figure 3). This is in keeping with previous studies in which we found that (1) OCI/AML3 and OCIM2 cells produce large quantities of IL-1β, which maximally stimulates their proliferation in an autocrine fashion; (2) the addition of exogenous IL-1β could not significantly stimulate their further growth; and (3) IL-1β antibodies could suppress their growth.24,46 Our current results therefore suggested that resveratrol might suppress IL-1β production by AML cells.
Effects of IL-1β on resveratrol-induced inhibition of OCIM2 and OCI/AML3 colony-forming cell growth. Resveratrol (25 μM) and IL-1β (100 ng/mL) were added at initiation of the cultures. The means ± SDs of colony numbers from quadruplicate cultures are depicted.
Effects of IL-1β on resveratrol-induced inhibition of OCIM2 and OCI/AML3 colony-forming cell growth. Resveratrol (25 μM) and IL-1β (100 ng/mL) were added at initiation of the cultures. The means ± SDs of colony numbers from quadruplicate cultures are depicted.
Resveratrol inhibits IL-1β protein production
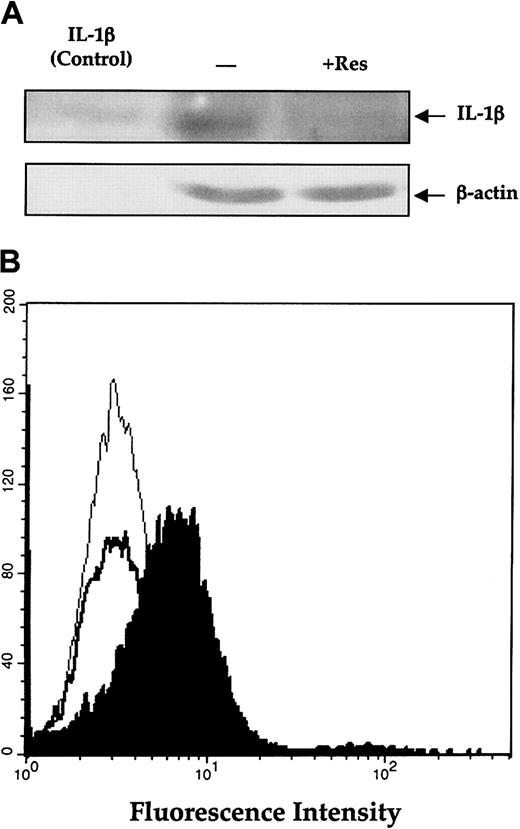
In light of these results and published data indicating that resveratrol inhibits NF-κB,3,47 the binding site known to be present in the IL-1β promoter,48,49 we hypothesized that resveratrol inhibits IL-1β. To test this idea, we incubated the cells with resveratrol and measured IL-1β protein levels in lysates of OCIM2 cells. Using Western immunoblotting to measure active (cleaved) IL-1β in OCIM2 cells, we found that resveratrol significantly suppressed production of mature IL-1β (Figure 4A).
Effect of resveratrol on IL-1β (A) Effect of resveratrol on the production of IL-1β by OCIM2 cells. Cells were incubated in RPMI 1640 supplemented with 10% FCS in the presence or absence of 25 μM resveratrol (Res). The amount of mature IL-1β protein produced by these cells was then assessed by Western immunoblotting. The arrows point to the 17.5-kDa mature IL-1β protein and β actin. The first lane (control) shows commercially available, pure, mature IL-1β protein, the second lane shows protein from cells incubated in tissue culture medium, and the third lane shows protein from cells incubated with resveratrol. (B) Effect of resveratrol on membrane-bound IL-1β. OCIM2 cells were incubated in the presence and absence of resveratrol as described. The level of membrane-bound IL-1β was measured by flow cytometry; thin line denotes isotypic control; filled histogram, untreated cells; and thick line, cells treated with resveratrol.
Effect of resveratrol on IL-1β (A) Effect of resveratrol on the production of IL-1β by OCIM2 cells. Cells were incubated in RPMI 1640 supplemented with 10% FCS in the presence or absence of 25 μM resveratrol (Res). The amount of mature IL-1β protein produced by these cells was then assessed by Western immunoblotting. The arrows point to the 17.5-kDa mature IL-1β protein and β actin. The first lane (control) shows commercially available, pure, mature IL-1β protein, the second lane shows protein from cells incubated in tissue culture medium, and the third lane shows protein from cells incubated with resveratrol. (B) Effect of resveratrol on membrane-bound IL-1β. OCIM2 cells were incubated in the presence and absence of resveratrol as described. The level of membrane-bound IL-1β was measured by flow cytometry; thin line denotes isotypic control; filled histogram, untreated cells; and thick line, cells treated with resveratrol.
Mature IL-1β is generated via cleavage of the 31-kDa inactive cytoplasmatic interleukin precursor by IL-1β–converting enzyme after association with the plasma membrane during secretion.50 Because OCIM2 cells produce IL-1β and proliferate in response to this cytokine, we decided to test the effect of resveratrol on membrane-bound IL-1. By doing so we measured both the secreted mature IL-1β and the previously secreted IL-1β that reattached to the cell. As shown in Figure 4B, resveratrol significantly reduced the level of membrane-bound IL-1β in OCIM2 cells, further confirming that resveratrol inhibits production of IL-1β.
Resveratrol inhibits IL-1β–induced NF-κB activation
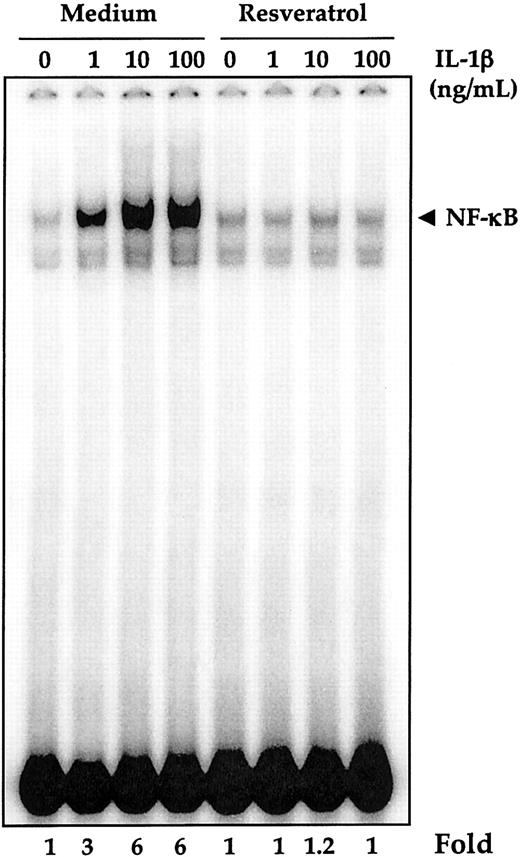
Because IL-1β production is regulated by NF-κB and the proliferation of AML cells is mediated through induction of NF-κB,25 we asked whether resveratrol also suppresses NF-κB activation. To determine this, OCIM2 cells (2 × 106 cells/mL) were preincubated with 50 μM resveratrol for 4 hours at 37°C and then treated with increasing concentrations of IL-1β. Nuclear extracts were prepared and tested for NF-κB activation by EMSA. As shown in Figure 5, IL-1β activated NF-κB in a dose-dependent manner (6-fold at 10 ng/mL), and exposure of OCIM2 cells to 50 μM resveratrol for 4 hours completely inhibited the IL-1–induced NF-κB activation. Resveratrol by itself, however, did not activate NF-κB. Because NF-κB is a family of proteins, various combinations of Rel/NF-κB protein can constitute an active NF-κB heterodimer that binds to a specific sequence in DNA.19,35 To show that the retarded band visualized by electrophoretic mobility shift assay (EMSA) in the IL-1β–treated cells was indeed the p50 and p65 subunits of NF-κB, we incubated nuclear extracts from IL-1–activated cells with antibodies to the p50 (NF-κB1) and the p65 (RelA) subunits of NF-κB. Both antibodies shifted the band to a higher molecular mass (data not shown), suggesting that the IL-1–activated complex consisted of p50 and p65 subunits. Neither preimmune serum nor anticyclin D1 had any effect. Addition of excess (100-fold) unlabeled NF-κB (cold oligo) caused complete disappearance of the band.
Inhibition of IL-1β–induced NF-κB activation by resveratrol. OCIM2 cells (× 106/mL) were preincubated with 50 μM resveratrol for 4 hours at 37°C and then treated with various concentrations of IL-1β for 1 hour. Nuclear extracts were prepared and tested for NF-κB activation, as described in “Patients, materials, and methods.”
Inhibition of IL-1β–induced NF-κB activation by resveratrol. OCIM2 cells (× 106/mL) were preincubated with 50 μM resveratrol for 4 hours at 37°C and then treated with various concentrations of IL-1β for 1 hour. Nuclear extracts were prepared and tested for NF-κB activation, as described in “Patients, materials, and methods.”
Resveratrol induces apoptosis in AML cells
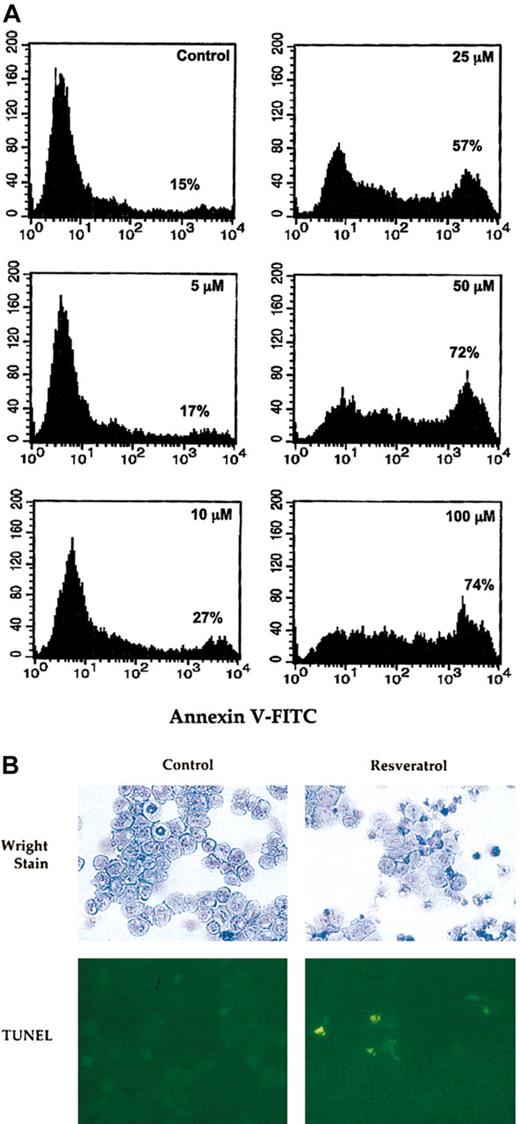
Because lack of NF-κB activation may abolish cellular proliferation and lead to apoptotic cell death,51,52 and because resveratrol was shown to induce apoptosis in HL60 cells,4 we hypothesized that resveratrol might have a similar effect on OCIM2 cells. To test this idea, OCIM2 cells at the peak of their growth were washed and then incubated in the presence or absence of 5, 10, 25, 50, or 100 μM resveratrol. Using annexin V–FITC, we demonstrated that resveratrol induced apoptosis in these AML cells in a dose-dependent fashion. The percentage of cells undergoing apoptotic cell death increased from 15% in the control culture to 74% after exposure to 100 μM resveratrol (Figure 6A). To validate these findings, we incubated AML blasts with 10 to 100 μM resveratrol. We stained cytospun cells with Wright stain to study morphologic changes and used the TUNEL assay to detect apoptotic cells. As shown in Figure 6B, 25 μM resveratrol induced morphologic changes consistent with AML cell differentiation and apoptotic cell death. A dose-response increase in apoptotic cells was detected on exposure of AML cells to higher concentrations (50-100 μM) resveratrol (data not shown).
Resveratrol induces apoptosis in AML cells. (A) OCIM2 cells were incubated in the absence (control) or presence of 5, 10, 25, 50, or 100 μM resveratrol. The fraction of cells undergoing apoptotic cell death was detected by annexin V–FITC. The percentages presented in each frame depict the dose-dependent increase in the apoptotic cell fraction. (B) AML cells were incubated with or without 25 μM resveratrol. Cytospun cells were stained with Wright stain and TUNEL. After exposure to resveratrol (Wright stain in right upper corner) several cytospun cells show larger cytoplasm and other cells show pyknotic nuclei. Dividing cells were not found (as shown in the control culture in left upper corner) and numerous yellow-appearing apoptotic cells were detected (right lower corner). Original magnification, × 10.
Resveratrol induces apoptosis in AML cells. (A) OCIM2 cells were incubated in the absence (control) or presence of 5, 10, 25, 50, or 100 μM resveratrol. The fraction of cells undergoing apoptotic cell death was detected by annexin V–FITC. The percentages presented in each frame depict the dose-dependent increase in the apoptotic cell fraction. (B) AML cells were incubated with or without 25 μM resveratrol. Cytospun cells were stained with Wright stain and TUNEL. After exposure to resveratrol (Wright stain in right upper corner) several cytospun cells show larger cytoplasm and other cells show pyknotic nuclei. Dividing cells were not found (as shown in the control culture in left upper corner) and numerous yellow-appearing apoptotic cells were detected (right lower corner). Original magnification, × 10.
Resveratrol induces apoptosis by activating the caspase pathway
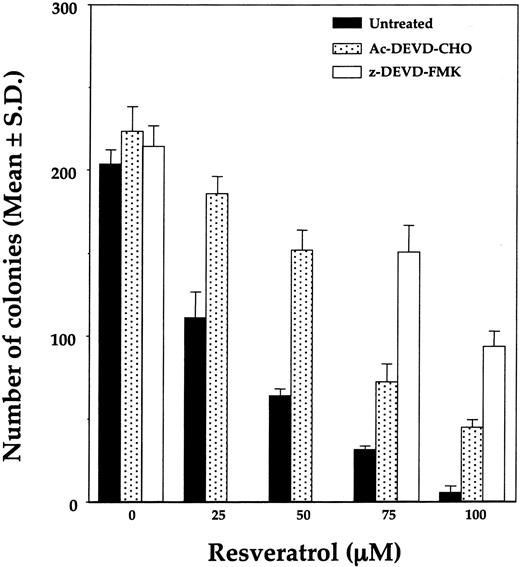
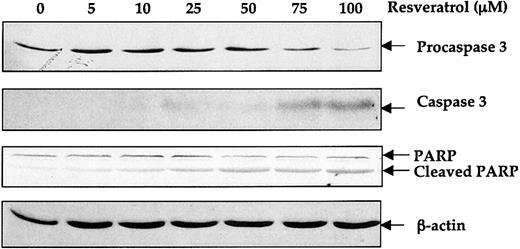
To determine the mechanism by which resveratrol induces apoptosis, we grew OCIM2 cells in a clonogenic assay with increasing concentrations of resveratrol in the absence or presence of caspase inhibitors Ac-DEVD-CHO or z-DEVD-FMK. As shown in Figure 7, both caspase inhibitors partially reversed the antiproliferative effect of resveratrol, with z-DEVD-FMK being more effective. To further determine whether resveratrol activates the caspase pathway, we incubated OCIM2 cells in the absence or presence of 5, 10, 25, 50, 75, or 100 μM resveratrol and then harvested the cells for Western immunoblot analysis. Because caspase 3 appears to be involved in the induction of apoptosis in leukemia cells,53-55 we measured the levels of procaspase 3 and cleaved caspase 3 in OCIM2 cells. As shown in Figure 8, incubation of OCIM2 cells with resveratrol reduced the levels of procaspase 3, and up-regulated the levels of the biologically active caspase and the inactivated (cleaved) form of the DNA-repair enzyme PARP,56-58 thereby activating the apoptotic cascade.
Effect of Ac-DEVD-CHO and z-DEVD-FMK on resveratrol-induced inhibition of OCIM2 colony growth. OCIM2 cells were incubated in the absence or presence of increasing concentrations of resveratrol, with or without one of the caspase inhibitors.
Effect of Ac-DEVD-CHO and z-DEVD-FMK on resveratrol-induced inhibition of OCIM2 colony growth. OCIM2 cells were incubated in the absence or presence of increasing concentrations of resveratrol, with or without one of the caspase inhibitors.
Effect of resveratrol on procaspase 3 and PARP cleavage. OCIM2 cells were incubated with increasing concentrations of resveratrol for 18 hours. Levels of procaspase 3, caspase 3, and uncleaved and cleaved PARP proteins were detected by Western immunoblotting. The results shown here demonstrate dose-dependent decreases in levels of procaspase 3 and PARP and increases in cleaved caspase 3 and cleaved PARP.
Effect of resveratrol on procaspase 3 and PARP cleavage. OCIM2 cells were incubated with increasing concentrations of resveratrol for 18 hours. Levels of procaspase 3, caspase 3, and uncleaved and cleaved PARP proteins were detected by Western immunoblotting. The results shown here demonstrate dose-dependent decreases in levels of procaspase 3 and PARP and increases in cleaved caspase 3 and cleaved PARP.
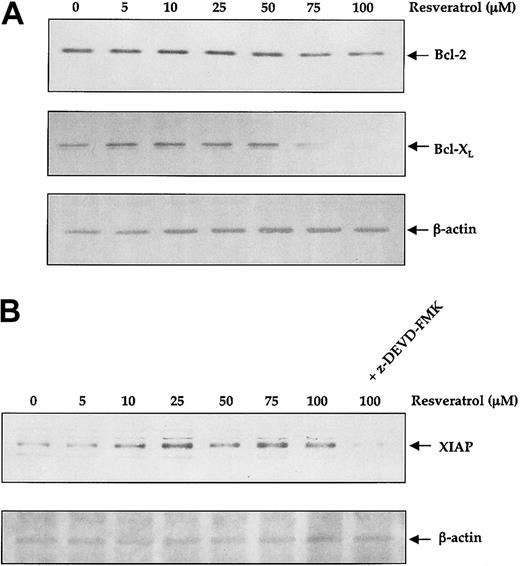
Resveratrol down-regulates Bcl-2 and Bcl-XL protein levels
Activation of NF-κB was found to induce the expression of Bcl-2 and Bcl-XL.59-62 Because we found that resveratrol inhibited IL-1β production and IL-1–induced activation of NF-κB, we asked whether resveratrol would affect Bcl-2 and Bcl-XL protein levels in the IL-1β–producing OCIM2 cells. Using Western immunoblot analysis, we found that resveratrol reduced the level Bcl-XL and, to a lesser extent, that of Bcl-2 in a dose-dependent manner (Figure 9A). Then we analyzed the effect of resveratrol on the apoptosis inhibitor XIAP. In contrast to its effect on Bcl-2 and Bcl-XL, resveratrol up-regulated XIAP protein levels. Because XIAP is induced by cellular stress,63 we asked whether caspase inhibition would abolish this effect. As shown in Figure 9B, z-DEVD-FMK completely reversed the resveratrol-induced up-regulation of XIAP, suggesting that expression of XIAP in OCIM2 cells is not mediated through NF-κB activation but rather through NF-κB–independent mechanisms.
Effect of resveratrol on Bcl-2, Bcl-X, and XIAP protein levels. OCIM2 cells were incubated with increasing concentrations of resveratrol for 18 hours. The levels of Bcl-2, Bcl-X, and XIAP were detected by Western immunoblotting. The results shown here demonstrate a dose-dependent decrease in the levels of Bcl-2 and Bcl-X (A), and a dose-dependent increase in the level of XIAP (B) that was completely reversed when the cells were incubated with both 100 μM resveratrol and 50 μM of the caspase inhibitor z-DEVD-FMK.
Effect of resveratrol on Bcl-2, Bcl-X, and XIAP protein levels. OCIM2 cells were incubated with increasing concentrations of resveratrol for 18 hours. The levels of Bcl-2, Bcl-X, and XIAP were detected by Western immunoblotting. The results shown here demonstrate a dose-dependent decrease in the levels of Bcl-2 and Bcl-X (A), and a dose-dependent increase in the level of XIAP (B) that was completely reversed when the cells were incubated with both 100 μM resveratrol and 50 μM of the caspase inhibitor z-DEVD-FMK.
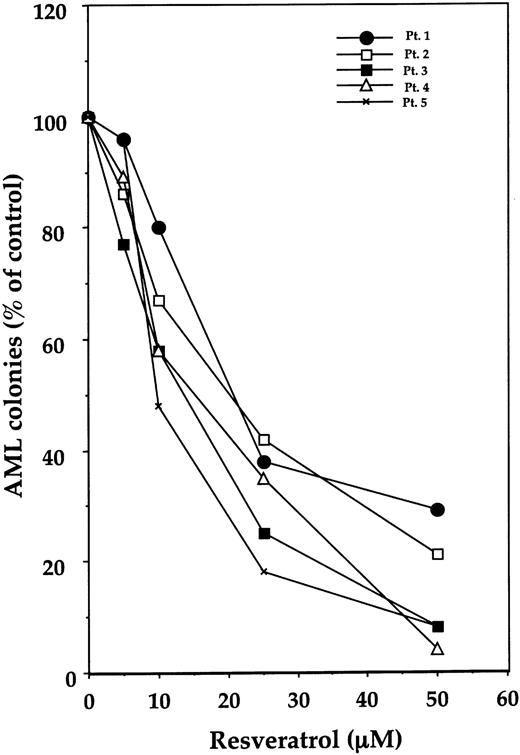
Resveratrol inhibits leukemia colony-forming cell proliferation of fresh AML cells
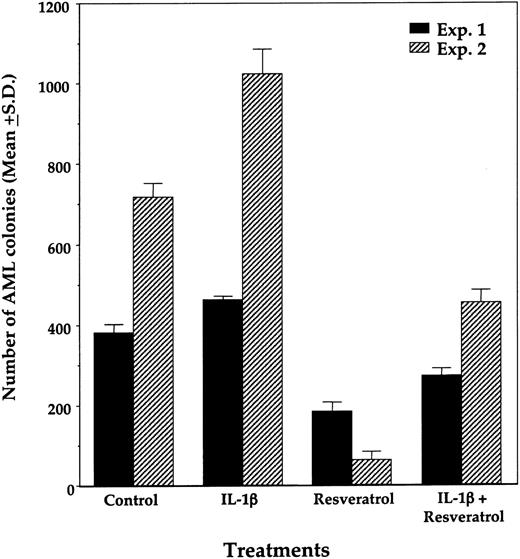
We then studied the effect of resveratrol on the proliferation of fresh AML colony-forming cells. For this, we used diagnostic BM cells from 5 patients with AML whose clinical characteristics are depicted in Table 1. As shown in Figure 10, resveratrol inhibited the growth of AML blast colony-forming cells in a dose-dependent manner in the samples studied. As it did in AML cell lines, IL-1β, when added at the initiation of culture, partially reversed the inhibitory effect of resveratrol (Figure 11).
Effect of resveratrol on leukemia colony-forming cell proliferation of fresh AML cells from 5 patients. After fractionation of adherent cells and depletion of T lymphocytes, remaining cells were cultured in a clonogenic assay with resveratrol at concentrations ranging from 5.0 to 50 μM. AML colonies are presented as percentages of control. (The mean numbers of colonies obtained from patients 1 through 5 in the absence of resveratrol were 65, 196, 153, 256, and 549, consecutively.)
Effect of resveratrol on leukemia colony-forming cell proliferation of fresh AML cells from 5 patients. After fractionation of adherent cells and depletion of T lymphocytes, remaining cells were cultured in a clonogenic assay with resveratrol at concentrations ranging from 5.0 to 50 μM. AML colonies are presented as percentages of control. (The mean numbers of colonies obtained from patients 1 through 5 in the absence of resveratrol were 65, 196, 153, 256, and 549, consecutively.)
Effect of resveratrol and IL-1β on proliferation of AML colony-forming cells. Data from triplicate cultures of BM samples from patient 1 (Exp 1) and patient 5 (Exp 2) are depicted. Resveratrol (50 μM) and IL-1β (100 ng/mL) were added at the initiation of culture. The means ± SDs of AML colony numbers from triplicate cultures are depicted.
Effect of resveratrol and IL-1β on proliferation of AML colony-forming cells. Data from triplicate cultures of BM samples from patient 1 (Exp 1) and patient 5 (Exp 2) are depicted. Resveratrol (50 μM) and IL-1β (100 ng/mL) were added at the initiation of culture. The means ± SDs of AML colony numbers from triplicate cultures are depicted.
Discussion
Resveratrol and flavonoids evolve from a common synthetic pathway in plants. First, hydroxy-cinnamoic acid is formed. Then, chalcone synthase uses 3 cinnamoil radicals to produces flavonoids, and pinosylvin synthase (stilbene synthase) uses 2 cinnamoil radicals to make trans-3,4′-trihydroxystilbene, known as resveratrol. Resveratrol is a phytoalexin used by plants to defend themselves from fungi. It is found in various fruits and vegetables and is abundant in grapes and red wine. The root extract, the weed Polygonum cuspidatum, has been used for centuries in Asian medicine as an anti-inflammatory drug.3-5 Within the last few years, resveratrol was found to induce antiproliferative and proapoptotic effects on neoplastic cells, including mouse and human leukemia cell lines.10-14 In most studies, including our own, resveratrol was used at concentrations ranging from 5 to 100 μM. Our recent data show that resveratrol is effective at similar concentrations in an animal model (B.B.A., unpublished data, October 2002). Recently, Goldberg and coworkers tested the absorption of resveratrol in healthy human subjects and, under their experimental conditions, achieved nanomolar serum concentrations.64
Several growth factors regulate hematopoietic cell survival by interfering with apoptotic signals.65-67 One of these is the cytokine IL-1β, a proinflammatory protein that has been implicated in early events in hematopoiesis. It induces production of various cytokines and synergizes with several growth factors in stimulating hematopoietic progenitor multiplication.48,68 In addition, as we and others have found, IL-1β plays an important role in AML cell proliferation (for a review, see Estrov et al68 ). Suppression of IL-1 production or inhibition of its interaction with the corresponding cellular receptors significantly inhibits AML progenitor cell growth.21-24,46,69 Furthermore, activation of NF-κB appears to be an important step in the molecular events leading to IL-1β production20,48,49 and, as a result, also appears to stimulate leukemia cell proliferation.
NF-κB is a ubiquitous transcription factor and a major regulator of the immune system by inducing expression of various inflammatory cytokines, including IL-1β.48,49 NF-κB exists in the cytoplasm as a heterotrimeric complex with the inhibitor IβBα (for a review, see Siebenlist et al20 ). Within minutes of activation by inflammatory agents such as IL-1β, IκBα undergoes phosphorylation, ubiquitination, and proteolytic degradation, thus releasing the NF-κB p50-p65 complex for translocation from the cytoplasm to the nucleus. Although activation of NF-κB induces cellular proliferation20 and protects cells from apoptosis,52,70 its inhibition enhances spontaneous apoptosis51 or apoptosis induced by various stimuli such as irradiation or cytotoxic drugs.52
In this light, we assumed that an effective NF-κB inhibitor such as resveratrol3,47 might suppress production of IL-1β, inhibit direct NF-κB–mediated leukemia cell proliferation,8 or both. Resveratrol has been previously shown to inhibit the growth of several leukemia cell lines, many of which are not IL-1 dependent.10-14 Because of the central role IL-1β plays in the proliferation of the cells of patients with AML, we chose to study the effects of resveratrol on IL-1–responsive AML cell lines and found that resveratrol suppressed the growth of the IL-1–responsive OCIM2 and OCI/AML3 cells in a dose-dependent manner and that IL-1β partially reversed this inhibitory effect. This growth inhibition was mediated through blocking progression of the leukemia cells through the S phase of the cell cycle, as previously described.9 Together, these results suggest that at least part of the resveratrol-induced suppression observed in the present study was mediated through inhibition of IL-1β production by resveratrol. Indeed, incubation of OCIM2 cells in the presence of resveratrol significantly reduced production of IL-1β protein. In addition, resveratrol significantly inhibited the IL-1β–induced NF-κB activity. Although IL-1β activated NF-κB in these cells, as it did in our previous study,25 resveratrol suppressed it in a dose-dependent fashion. Thus, resveratrol inhibited both IL-1β production and the IL-1β–mediated activation of NF-κB, resulting in additional reduction in production of IL-1β.
Because NF-κB activation suppresses the signals for cell death, and inhibition of NF-κB may result in apoptotic cell death,51,52 we tested the effect of resveratrol on induction of apoptosis. We found that treatment of OCIM2 leukemia cells with resveratrol induced apoptotic cell death. Our results agree with those of Jimi et al,71 who found that inhibition of NF-κB by oligodeoxynucleotides to p65 and p50 abolished the IL-1–induced survival of osteoclasts.
Because most cell types require activation of the caspase pathway if apoptosis is to occur, we also wondered whether resveratrol might activate that pathway in AML cells. We incubated OCIM2 cells with the caspase inhibitors Ac-DEVD-CHO and z-DEVDFMK and found that both inhibitors partially reversed the antiproliferative effect of resveratrol, suggesting that resveratrol activates the caspase pathway. We then studied the effect of resveratrol on caspase 3. Caspase 3 is a key executioner of apoptosis41-43 whose activation downstream in the apoptotic cascade is essential for leukemia cell apoptosis.53,54 Moreover, activation of caspase 3 results in cleavage of cellular substrates critical for cell survival, such as PARP.53,54 We used an approach similar to that in a previous study in which we tested the effect of resveratrol on tumor necrosis factor-induced cytotoxicity in U-937 cells.3 As we hoped, we found that resveratrol activated caspase 3 and consequently cleaved PARP. Interestingly, Barkett et al reported that caspase 3 cleaves human IκBα in vitro at a conserved Asp-Ser sequence, thus creating dominant inhibitor that prevents activation of NF-κB and thereby adding another death signal.72 Because expression of antiapoptotic molecules such as Bcl-2 and Bcl-XL was found to be induced by NF-κB,59-62 we asked whether resveratrol, which inhibits IL-1β–induced NF-κB activation, would reduce Bcl-2 and Bcl-XL levels in OCIM2 cells. We found that resveratrol down-regulated Bcl-XL and, to a lesser extent, Bcl-2 protein levels. However, resveratrol up-regulated XIAP levels, and this effect was completely reversed by z-DEVD-FMK, suggesting that in OCIM2 cells XIAP expression is likely mediated through an NF-κB–independent, stress-induced63 mechanism.
Similar to its effect on AML cell lines and comparable to the effect of other IL-1 inhibitors,11,22-24,46 resveratrol suppressed AML progenitor cell proliferation and had inhibitory effect partially reversed by IL-1β. These results indicate that inhibition of IL-1β production is part of the inhibitory mechanism of resveratrol in AML cells.
Taken together, our data suggest that resveratrol, through inhibition of NF-κB, suppression of IL-1β production, or both, may eliminate leukemia cells and become a potential agent in the treatment of AML.
Prepublished online as Blood First Edition Paper, April 10, 2003; DOI 10.1182/blood-2002-11-3550.
Supported in part by National Cancer Institute Grant No. PO1CA 55164 and by the Clayton Foundation for Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Kathryn Hale for editing the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal