Abstract
Mantle cell lymphoma (MCL) is rarely cured with standard-dose chemotherapy. From January 1997 to February 2000, 28 previously untreated advanced-stage MCL patients younger than 61 years of age were treated at 9 Italian hematologic departments with 3 cycles of standard-dose debulking chemotherapy followed by a high-dose rituximab-supplemented sequence (R-HDS) including intravenous administration of high-dose cyclophosphamide, high-dose cytarabine, high-dose melphalan, and high-dose mitoxantrone plus melphalan. Study end points included toxicity, clinical and molecular response rates, long-term event-free survival (EFS), and overall survival (OS) rates, as well as the ability to harvest tumor-free peripheral blood stem cells. Optimal amounts of polymerase chain reaction–negative (PCR-negative) CD34+ cells were collected from all 20 informative patients. One patient died of toxicity. All 27 patients assessable for response achieved a complete response (CR), of which 24 remain in continuous complete remission (CCR) after a median follow-up of 35 months. Three patients had transient evidence of PCR-detectable disease in the bone marrow. The OS and EFS rates at 54 months were 89% and 79%, respectively. These results compare with the 42% OS rate and the 18% EFS rate observed in 35 age-matched historic controls treated with standard-dose chemotherapy at the participating centers. The use of rituximab in combination with high-dose chemotherapy represents a very effective in vivo purging method. The R-HDS regimen can be safely applied in a multicenter hematology setting and leads to long-term EFS and OS in the majority of patients with an otherwise incurable disease.
Introduction
Mantle cell lymphoma (MCL) is a CD5+, CD20+ malignancy, characterized by a t(11:14) translocation causing overexpression of cyclin D1 and cell-cycle dysregulation.1,2 It is now recognized by the Revised European-American Classification of Lymphoid Neoplasms (REAL) and the World Health Organization (WHO) classification systems for non-Hodgkin lymphomas (NHLs)3,4 as a distinct clinical entity, which comprises around 5% of all cases of NHLs. Patients with MCL typically present with disseminated disease that frequently includes bone marrow (BM), peripheral blood (PB), and splenic involvement.5 Complete response (CR) to standard chemotherapy regimens is poor (in the order of 50% or less)6,7 with progression typically occurring within just one year after diagnosis and an overall survival (OS) of only 3 years.6,7
Patients with MCL are clearly in need of an alternative treatment to conventional chemotherapy, and therefore high-dose chemotherapy with autologous hematopoietic stem cell transplantation has been considered for these patients. While some pilot studies have given encouraging results,8-10 long-term follow-up has generally revealed a poor clinical outcome, with no evidence for long-term remission.11 On the assumption that reinfusion of lymphoma-contaminated stem cell harvests might contribute to relapse, attempts have been made to improve remission duration by purging the stem cell graft of tumor cells both in vitro12,13 or in vivo.14 However, immunologic purging failed to eradicate polymerase chain reaction (PCR)–detectable disease in the vast majority of patients, with little evidence of improvement over patients reinfused with nonpurged cells.12-14 In conclusion, available evidence supports the need for more effective purging methods as well as for better induction therapy to enhance the complete response rate before autografting tumor-free hematopoietic stem cells.
In a previous study from our group, 6 consecutive patients with MCL at diagnosis have been treated with multiple cycles of high-dose chemotherapy given in combination with the monoclonal antibody rituximab.15 All 6 patients achieved a CR (including a PCR-negative BM status) and yielded PCR-negative PB stem cell harvests, thus providing the proof of principle that in vivo purging is feasible and that the chemo-immunotherapy strategy adopted is effective against this highly resistant lymphoma type. This initial series has been subsequently implemented with 2 more patients from our unit and 20 additional patients from 8 other Italian centers treated with the same protocol. In the present multicenter study we report results after 54 months follow-up of a retrospective analysis of these 28 patients, who underwent high-dose chemo-immunotherapy and in vivo–purged PB stem cell autografting for advancedstage MCL at diagnosis. In contrast with historic controls, a significant number of patients remain disease free and in molecular remission after completing the program. This suggests that highdose chemo-immunotherapy may contribute to improved outcome in selected (ie, younger than 61 years and clinically fit) patients with mantle cell lymphoma.
Patients and methods
Selection of patients
Between January 1997 and February 2000, 28 patients with MCL at diagnosis treated at 9 Italian hematologic departments were included in this high-dose sequential chemotherapy study. Study protocol had been approved by the institutional ethics committee of each participating center. Eligibility criteria included written informed consent, patient age of 60 years or younger, absence of severe organ dysfunction not due to tumor, and no previous viral infections (hepatitis B or C, or human immunodeficiency virus). All pathologic materials were reviewed by a single pathologist at the Milan Cancer Institute using a combination of morphologic, immunophenotypic (CD20+, CD5+, CD23-), cytogenetic [t(11;14)(q13;q32)], and molecular (bcl-1 rearrangement) criteria. Diffuse histology, nodular histology, and blastic variant were present in 75%, 7%, and 18% of the cases, respectively.
For controls, we reviewed the medical records of all adult patients with MCL who were seen between May 1985 and December 1999 at the same 9 centers participating in the present study. Patients were eligible for inclusion in the control group if they had a pathologic confirmed diagnosis of MCL, were aged 60 years or younger, and had completed a full course (at least 6 cycles) of CHOP (cyclophosphamide + vincristine + prednisone) or CHOP-like chemotherapy. Two patients, who had received high-dose myeloablative chemotherapy as second-line chemotherapy, were excluded. A total of 35 adult patients met the latter criteria and served as historic controls. Diffuse histology was present in 86% of the cases and blastic variant in 14%.
Treatment schedule
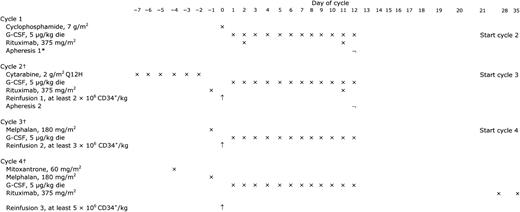
The treatment program, including its schema, has been described in detail previously.15 As outlined in Figure 1, after an initial standard-dose phase consisting of 2 to 3 cycles of either doxorubicinor cisplatin-containing chemotherapy, all patients were assigned to receive a rituximabsupplemented 4-step high-dose sequence (R-HDS) including the following: intravenous administration of high-dose cyclophosphamide (7 g/m2), high-dose cytarabine (2 g/m2 every 12 hours for 6 consecutive days), high-dose melphalan (180 mg/m2), and high-dose mitoxantrone plus melphalan (60 mg/m2 and 180 mg/m2, respectively). Rituximab was infused intravenously for a total of 6 doses, as previously indicated (twice after cyclophosphamide, twice after cytarabine, and twice after the final myeloablative step).15 Because of medical decision or patient refusal, yet in the absence of compelling medical contraindications, 4 patients did not complete the tandem myeloablative transplantation program. Among the latter, 3 patients (nos. 11, 13, and 15) received 1 cycle of mitoxantrone plus melphalan, and 1 patient (no. 20) received 1 cycle of melphalan only.
HDS treatment schema. G-CSF indicates granulocyte colony-stimulating factor; ×, administration; ¬, apheresis; ↑, reinfusion. *If quantitatively adequate (ie, ≥10 × 106/kg) and PCR-negative, the post–cyclophosphamide leukapheresed cells (apheresis 1) were used for all 3 subsequent reinfusions. Alternatively, 2 × 106/kg of them were reinfused after cytarabine, while the postcytarabine harvested cells (apheresis 2) were used for reinfusions 2 and 3, respectively. †Cycles 2, 3, and 4 were started as soon as both hematologic and nonhematologic toxicities from prior the cycle had disappeared (usually on day 21 after cycle 1 and 2, and on day 28 after cycle 3).
HDS treatment schema. G-CSF indicates granulocyte colony-stimulating factor; ×, administration; ¬, apheresis; ↑, reinfusion. *If quantitatively adequate (ie, ≥10 × 106/kg) and PCR-negative, the post–cyclophosphamide leukapheresed cells (apheresis 1) were used for all 3 subsequent reinfusions. Alternatively, 2 × 106/kg of them were reinfused after cytarabine, while the postcytarabine harvested cells (apheresis 2) were used for reinfusions 2 and 3, respectively. †Cycles 2, 3, and 4 were started as soon as both hematologic and nonhematologic toxicities from prior the cycle had disappeared (usually on day 21 after cycle 1 and 2, and on day 28 after cycle 3).
Leukaphereses were performed during growth-factor–expanded mobilization of progenitor cells occurring after administration of both high-dose cyclophosphamide (median 1 procedure, range 1-3), and high-dose cytarabine (median 1, range 1-3). On the first recovery day after chemotherapy when the CD34+ cell count reached at least 0.02 × 109/L, the patients received 1 dose of rituximab; 24 hours later, they underwent one or more once-daily leukaphereses until the target number of CD34+ cells was collected. Mononuclear cells were harvested with use of a novel automated leukapheresis system (Auto PBSC Spectra; COBE, Lakewood, CO), as previously described.16
To hasten hematopoietic recovery and to reduce hematologic toxicity, each patient received 3 progenitor/stem cell reinfusions, both after the severely myelotoxic, yet nonmyeloablative course of high-dose cytarabine (reinfusion 1, Figure 1), and after the 2 subsequent myeloablative courses of melphalan and mitoxantrone plus melphalan (reinfusion 2 and 3, respectively). The minimum target dose of CD34+ cells per kg of body weight to be reinfused after each of the 3 autografts were 2 × 106, 3 × 106, and 5 × 106, respectively. The timing and number of CD34+ cell collections, as well as the choice of the different products to be reinfused, were guided by real-time assessment of circulating progenitor counts17 and by results of overnight PCR analysis. Briefly, if the first harvest during the postcyclophosphamide recovery phase was both quantitatively adequate (ie, yielded ≥ 10 × 106 CD34+ cells/kg) and PCR-negative, the harvested cells were used for all 3 subsequent reinfusions, and the additional leukaphereses performed after cytarabine administration were kept as backup. If the CD34+ cells harvested after cyclophosphamide were either less than 10 × 106/kg, PCR-positive, or PCR unknown (probe unavailable), the postcyclophosphamide harvested cells were kept as backup, and the postcytarabine leukapheresed cells were used for reinfusion 2 and 3, irrespective of PCR-status (negative, positive, or unknown). Thus, the goal was to reinfuse, whenever possible, optimal amounts of PCR-negative cells harvested after cyclophosphamide treatment.
The supportive care given during the entire course of R-HDS therapy (including administration of hematopoietic growth factors) was described in detail previously,15 with additional measures prompted by the common occurrence of herpes simplex virus and/or cytomegalovirus (CMV) reactivation. Briefly, starting after the high-dose cyclophosphamide treatment, all patients received acyclovir or a derivative as prophylaxis and were monitored weekly for the presence of CMV pp65–positive white blood cells for a period of 3 months after the final autograft. Patients becoming positive for CMV pp65 antigen received a course of gancyclovir, with or without foscarnet, as treatment. Patients with hypogammaglobulinemia received intravenous gammaglobulins.
Patient evaluation and response criteria
Before treatment, all patients were evaluated by physical examination, blood chemistry profile, blood cell counts, chest x-ray, total body computed tomographic scan, BM aspirate and biopsy, and immunophenotypic analysis of BM and PB cells. When a molecular probe was available, molecular analysis of minimal residual disease (MRD) in BM and PB cells was performed. Additional examinations, such as gallium scan, positron emission tomography, nuclear magnetic resonance, or bipedal lymphography were performed as needed to determine the extent of the disease. Whenever possible, the patients were re-evaluated before the start of each of the 4 high-dose chemotherapy cycles and one month after completion of the final high-dose chemotherapy treatment. Follow-up restaging was scheduled every 6 months after transplantation or as clinically indicated for the first 2 years and yearly thereafter.
CR and partial response (PR) were defined according to the recommendations of the International Workshop.18 Molecular complete response was defined as the absence of neoplastic cells within PB and/or BM mononuclear cells as demonstrated by seminested PCR assay to identify bcl-1/IgH translocations or clonal IgH rearrangements, respectively. Toxicity was evaluated according to the WHO criteria.
Molecular monitoring of MRD
Molecular monitoring of MRD was accomplished by assessing DNA samples from PB, BM, and leukapheresis products with use of either seminested PCR amplification of the bcl-1/IgH translocation or seminested amplification of clonal rearrangement of IgH genes, essentially as described by Corradini et al14 and Andersen et al.12 The limit of detection of MRD was reproducible at the level of 105 for both rearrangements. The progenitor cells to be autografted were processed, cryopreserved, thawed, and reinfused as described previously.19
Statistical analysis
OS was measured from diagnosis until death. Event was defined as progression, relapse of disease, death in remission, or toxic death. Event-free survival (EFS) was calculated from the day of starting chemotherapy to date of event, or to the date when the patient was last known to be alive and disease-free. OS and EFS curves were calculated according to the Kaplan and Meier method.20 The log-rank test was used to compare event-free and overall survival duration between patient groups. To account for the longer follow-up of the control patients, and to prevent later events in the control group to negatively influence curve comparisons, all control patients were censored at 54 months. Thus, the 2 additional relapses and the 8 additional deaths that did occur in the control group at later time points were not counted in the event-free and survival analysis, respectively.
Results
Patient characteristics
The characteristics of the 28 R-HDS patients and the 35 controls, are listed in Table 1. The presence of bcl-1/IgH or clonal IgH rearrangement was detected in 11 and 9, respectively, of the 28 patients, while no molecular probe was available for the remaining 8 patients (29%) and for all the controls. As expected for a disease that occurs predominantly in males,21 the female sex was underrepresented in both arms. While the age of the study population was younger (median, 48.5 years) than the typical mantle-cell patient population (median slightly above 60 years), it was superimposable to the age of the historic control group (median, 48.8 years). The majority of patients had stage IV disease by virtue of morphologic BM involvement, with other extranodal sites in approximately half of the patients. The most common extranodal site of disease was the gastrointestinal tract. The presence of molecular and/or immunophenotypic markers for cyclin D1 deregulation was documented in 89% of the R-HDS patients and in 68% of the controls. Molecular lymphoma involvement in both BM and PB was detected by PCR in all 20 R-HDS patients for whom a molecular probe was available. According to the International Prognostic Index (IPI),22 36% of patients had 1 risk factor (none had zero factors), 50% had 2 or 3 factors, and 14% had 4 or 5 factors. The corresponding figures in the control group were 17%, 77%, and 6%, respectively. Thus, even if stratification by IPI is of limited value in MCL, 64% of the R-HDS patients and 77% of the controls had an unfavorable presentation, with 2 or more risk factors present.23 None of the differences in the characteristics of the patients was statistically significant (Fisher exact test).
Patient characteristics
Parameter . | Patients n (%) . | Controls n (%) . |
|---|---|---|
| Total no. | 28 | 35 |
| Sex | ||
| Male | 24 (86) | 26 (74) |
| Female | 4 (14) | 9 (26) |
| Age, y, median | 48.5 | 48.8 |
| Range | 23-65 | 28-60 |
| Molecular rearrangement | ||
| bcl1 | 11 | ND |
| IgH | 9 | ND |
| Probe not available | 8 | 35 |
| Cyclin D1 expression | ||
| Positive | 18 | 19 |
| Negative | 3 | 9 |
| Not done | 7 | 7 |
| Cyclin D1 and bcl1 status | ||
| One or both markers available | 26 | 28 |
| Cyclin D1 and/or bcl1 positive | 23 (89) | 19 (68) |
| CD20+/CD5+/CD23- immunophenotype | 28 (100) | 35 (100) |
| Ann Arbor Stage III-IV | 28 (100) | 35 (100) |
| B symptoms | 11 (39) | 14 (40) |
| Mass larger than 10 cm | 8 (29) | 7 (20) |
| LDH, abnormal | 10 (36) | 9 (26) |
| IPI | ||
| 0-1 | 10 (36) | 6 (17) |
| 2-3 | 14 (50) | 27 (77) |
| 4-5 | 4 (14) | 2 (6) |
| Sites of involvement | ||
| No. of nodal sites, median, inclusive of spleen | 5.5 | 4.4 |
| Extranodal sites, exclusive of BM | 13 (46) | 17 (48) |
| BM involvement, histology | 26 (93) | 22 (62) |
| BM involvement, PCR | 20 (100*) | ND |
| Lymphoma cells in PB, immunophenotype | 16 (57) | ND |
| Lymphoma cells in PB, PCR | 20 (100*) | ND |
Parameter . | Patients n (%) . | Controls n (%) . |
|---|---|---|
| Total no. | 28 | 35 |
| Sex | ||
| Male | 24 (86) | 26 (74) |
| Female | 4 (14) | 9 (26) |
| Age, y, median | 48.5 | 48.8 |
| Range | 23-65 | 28-60 |
| Molecular rearrangement | ||
| bcl1 | 11 | ND |
| IgH | 9 | ND |
| Probe not available | 8 | 35 |
| Cyclin D1 expression | ||
| Positive | 18 | 19 |
| Negative | 3 | 9 |
| Not done | 7 | 7 |
| Cyclin D1 and bcl1 status | ||
| One or both markers available | 26 | 28 |
| Cyclin D1 and/or bcl1 positive | 23 (89) | 19 (68) |
| CD20+/CD5+/CD23- immunophenotype | 28 (100) | 35 (100) |
| Ann Arbor Stage III-IV | 28 (100) | 35 (100) |
| B symptoms | 11 (39) | 14 (40) |
| Mass larger than 10 cm | 8 (29) | 7 (20) |
| LDH, abnormal | 10 (36) | 9 (26) |
| IPI | ||
| 0-1 | 10 (36) | 6 (17) |
| 2-3 | 14 (50) | 27 (77) |
| 4-5 | 4 (14) | 2 (6) |
| Sites of involvement | ||
| No. of nodal sites, median, inclusive of spleen | 5.5 | 4.4 |
| Extranodal sites, exclusive of BM | 13 (46) | 17 (48) |
| BM involvement, histology | 26 (93) | 22 (62) |
| BM involvement, PCR | 20 (100*) | ND |
| Lymphoma cells in PB, immunophenotype | 16 (57) | ND |
| Lymphoma cells in PB, PCR | 20 (100*) | ND |
ND indicates not done; and LDH, lactate dehydrogenase
Twenty of 20 informative patients
CD34+ cell harvesting and PCR assessment of leukapheresis products
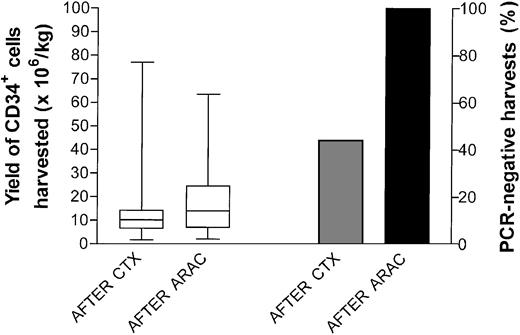
Figure 2 shows the yield (left panel) and the PCR analysis (right panel) of the CD34+ cells harvested after cyclophosphamide and cytarabine, respectively. Quantitatively, no significant differences were observed in the total number of CD34+ cells harvested from the 28 patients after the 2 treatments, thus confirming the surprising lack of impairment that a prior and close cyclophosphamide infusion had on postcytarabine mobilization.15,24 A molecular assessment on leukapheresed cells was carried out in 17 patients following cyclophosphamide and in 19 patients following cytarabine. Following cyclophosphamide, 4 patients with clonal IgH rearrangement and 3 with bcl-1/IgH rearrangement yielded PCR-negative harvests for an overall 42% of negative cases. Conversely, all 19 postcytarabine leukaphereses analyzed (100%) were lymphoma-free as documented by IgH (9 cases) or bcl-1/IgH rearrangement analysis (10 cases). As already pointed out, a reproducible sensitivity level of 10-5 for both probes was observed.
CD34+ cell harvesting and PCR assessment of leukapheresis products. Yield (left y-axis) and PCR analysis (right y-axis) of the CD34+ cells harvested after cyclophosphamide (CTX) and cytarabine (ARAC). Each patient underwent the minimum number of procedures sufficient to complete the therapeutic program. Boxes extend from the 25th to the 75th percentile, with the horizontal line indicating the median. Whiskers extend from the largest to smallest values.
CD34+ cell harvesting and PCR assessment of leukapheresis products. Yield (left y-axis) and PCR analysis (right y-axis) of the CD34+ cells harvested after cyclophosphamide (CTX) and cytarabine (ARAC). Each patient underwent the minimum number of procedures sufficient to complete the therapeutic program. Boxes extend from the 25th to the 75th percentile, with the horizontal line indicating the median. Whiskers extend from the largest to smallest values.
Detection of MRD in bone marrow samples
Figure 3 shows the results of PCR analysis for the 20 patients in whom a probe for IgH (9 patients) or a bcl-1/IgH rearrangement (11 patients) was available for evaluation. The analysis was carried out on BM samples obtained at diagnosis, after standard-dose chemotherapy, after cyclophosphamide, after cytarabine, after the second autografting (day 0), and at the indicated follow-up times. Following the final autografting, all but 1 patient (no. 18, •) achieved a molecular remission. At a median observation time for molecular analysis of 21 months (range, 4 to 47 months), all 20 of the informative patients were in clinical and molecular remission. A transient PCR positivity was documented in 3 patients (nos. 3, 5, and 18).
Detection of MRD in bone marrow samples. Results of PCR analysis for the 20 patients in whom a probe for IgH (9 patients) or a bcl-1/IgH rearrangement (11 patients) was available. The analysis was carried out on bone marrow samples obtained at diagnosis, after standard-dose chemotherapy, after cyclophosphamide, after cytarabine, after the second autografting (day 0), and at the indicated follow-up times. • indicates PCR-positive; ○, PCR-negative. UPN indicates unique patient number.
Detection of MRD in bone marrow samples. Results of PCR analysis for the 20 patients in whom a probe for IgH (9 patients) or a bcl-1/IgH rearrangement (11 patients) was available. The analysis was carried out on bone marrow samples obtained at diagnosis, after standard-dose chemotherapy, after cyclophosphamide, after cytarabine, after the second autografting (day 0), and at the indicated follow-up times. • indicates PCR-positive; ○, PCR-negative. UPN indicates unique patient number.
Toxicity
The high response rate seen with R-HDS was not accompanied by unacceptable toxicity. As expected with high-dose chemotherapy, all patients had grade 4 hematologic adverse events, but, in general, the safety profile of this regimen indicates that R-HDS is a feasible treatment for younger patients with MCL. One toxic death was seen, but this was a cardiac event that occurred in a patient with prior history of tachyarrhythmias and may not have been related to study treatment. Reported adverse events of grade 3 or higher are shown in Table 2. Of note, 6 patients (21%), experienced reactivation of CMV. This otherwise rare event in the autologous stem cell transplantation setting occurred within the interval between cytarabine infusion and 3 months after the final autograft. In all instances CMV reactivation promptly responded to anti-CMV therapy (gancyclovir with or without foscarnet). No cases of secondary myelodysplasia or acute myelogenous leukemia have been reported to date.
Main toxicities by course
. | Cyclophosphamide . | Cytarabine . | Melphalan . | Mitoxantrone and melphalan . | Overall . |
|---|---|---|---|---|---|
| Grade 5 | |||||
| Cardiac | — | — | — | 4 | 4 |
| Grade 4 | |||||
| Hematologic | 100 | 100 | 100 | 100 | 100 |
| Mucositis | — | — | 4 | 8 | 11 |
| Grade 3 | |||||
| FUO/doc infection | 11 | 14 | 21 | 21 | 64 |
| Hepatic | — | 4 | — | — | — |
| Neurologic | — | 8 | — | — | — |
| CMV reactivation | — | — | 7 | 17 | 21 |
. | Cyclophosphamide . | Cytarabine . | Melphalan . | Mitoxantrone and melphalan . | Overall . |
|---|---|---|---|---|---|
| Grade 5 | |||||
| Cardiac | — | — | — | 4 | 4 |
| Grade 4 | |||||
| Hematologic | 100 | 100 | 100 | 100 | 100 |
| Mucositis | — | — | 4 | 8 | 11 |
| Grade 3 | |||||
| FUO/doc infection | 11 | 14 | 21 | 21 | 64 |
| Hepatic | — | 4 | — | — | — |
| Neurologic | — | 8 | — | — | — |
| CMV reactivation | — | — | 7 | 17 | 21 |
— indicates not applicable; FUO, fever of unknown origin; and doc, documented infection
Response and survival
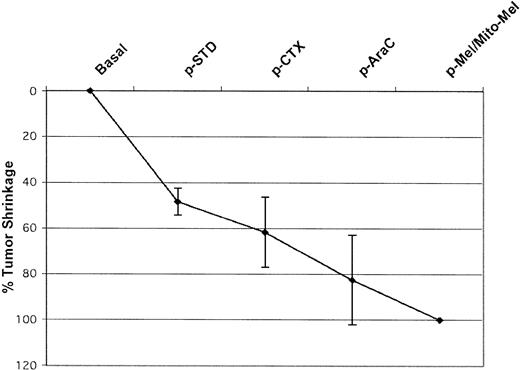
After the final transplantation, all 27 study patients evaluable for tumor response were in clinical CR (100% CR rate). The one remaining patient died of toxicity without evidence of disease (NED) on day +15 after transplantation (ie, too early for response evaluation). For 12 patients it was possible to accurately measure tumor shrinkage and to assess response at various time points during the treatment, thus helping define the contribution of the various steps to the final overall outcome. As shown in Figure 4, all phases were followed by a measurable reduction in tumor size. Despite a dramatic initial tumor shrinkage (half of the patients experienced a 50% tumor reduction following standard-dose induction therapy), a NED status was achieved late in the course of the treatment schedule. In fact, the number of patients who become NED was none after standard-dose chemotherapy, none after high-dose cyclophosphamide, 6 (50%) after high-dose cytarabine, and 6 after the final autografting. Of the 35 patients in the historic control group treated with CHOP or CHOP-like chemotherapy, only 13 (37%) achieved a CR and 11 a PR, for an overall response rate (RR) of 69%. As shown in Table 1, in 57% of the patients lymphoma cells were detected in the PB by immunophenotypic analysis, with absolute counts of CD20+, CD5+, CD23- cells ranging from 10/μL to as high as 41 000/μL. Of note, during the hematologic recovery following the standard-dose induction chemotherapy cycles, these patients showed a marked increase in the number of circulating lymphoma cells at a time when solid tumor masses were shrinking. This chemotherapy-elicited leukemization, already reported by Jacquy et al,25 was no longer evident after subsequent high-dose chemotherapy cycles.
Tumor size reduction after each phase of R-HDS program. Patients were re-evaluated before the start of each of the 4 high-dose chemotherapy cycles. All phases were followed by a measurable tumor size reduction with CR in 50% of patients after high-dose cytarabine (HD-Ara-C) and 50% after the final myeloablative phase(s). p-STD indicates after standard dose chemotherapy; p-CTX, after high-dose cyclophosphamide; p-AraC, after high-dose cytarabine; and p-Mel/Mito-Mel, after high-dose melphalan and after high-dose mitoxantrone/melphalan.
Tumor size reduction after each phase of R-HDS program. Patients were re-evaluated before the start of each of the 4 high-dose chemotherapy cycles. All phases were followed by a measurable tumor size reduction with CR in 50% of patients after high-dose cytarabine (HD-Ara-C) and 50% after the final myeloablative phase(s). p-STD indicates after standard dose chemotherapy; p-CTX, after high-dose cyclophosphamide; p-AraC, after high-dose cytarabine; and p-Mel/Mito-Mel, after high-dose melphalan and after high-dose mitoxantrone/melphalan.
Of the 28 study patients, there was 1 acute in-hospital treatmentrelated death from cardiac arrhythmia and 3 relapses at 9, 13, and 31 months. Of the 3 patients who relapsed, 2 relapsed in sites of prior disease and 1 in the central nervous system only. Two of the relapsed patients have died because of disease progression 14 and 3 months following relapse. Twenty-four patients remain in continuous complete remission (CCR) with a median follow-up for the entire study of 35 months (range, 21 to 54 months). The Kaplan-Meier estimate of the percentage of patients alive or event-free at 54 months was 89% (95% confidence intervals [CI], 78% to 100%), and 79% (95% CI, 58% to 100%), respectively (Figure 5). Of the 35 historic control patients, 11 never obtained a CR and progressed shortly after completion of CHOP or CHOP-like chemotherapy, 8 relapsed, and 16 remain in CCR with a median follow-up of 51 months (range, 16 to 54 months). For these patients the 54-month OS rate was 42% (95% CI, 24% to 59%), and the 54-month EFS was 18% (95% CI, 5% to 31%), respectively (Figure 5). When the results of the 28 study-group patients were compared with the 35 historic controls, there was a significant advantage for the high-dose–treated patients in terms of both EFS (P < .0001) and OS (P = .027). Since patients without molecular, cytogenetic, or immunohistochemical evidence of cyclin D1 deregulation might have a better prognosis, a subset analysis was carried out limited to patients (23 in the R-HDS group and 19 in the control group) with cyclin D1 overexpression and/or bcl1 rearrangement. The results were hardly distinguishable from the results in the whole group, with a 54-month EFS rate of 87% versus 26% (P = .034), and an OS rate of 87% versus 18% (P < .0001) in favor of the R-HDS arm.
Overall survival and event-free survival of patients treated with R-HDS versus conventional chemotherapy. The Kaplan-Meier estimate of the percentage of patients alive or event-free at 54 months was 89% (95% confidence intervals [CI], 78% to 100%) and 79% (95% CI, 58% to 100%). For the 35 historic control patients, 54-month OS rate was 42% (95% CI, 24% to 59%) and the 54-month EFS was 18% (95% CI, 5% to 31%). There was a significant advantage for the R-HDS–treated patients in terms of both EFS (P < .0001) and OS (P = .027).
Overall survival and event-free survival of patients treated with R-HDS versus conventional chemotherapy. The Kaplan-Meier estimate of the percentage of patients alive or event-free at 54 months was 89% (95% confidence intervals [CI], 78% to 100%) and 79% (95% CI, 58% to 100%). For the 35 historic control patients, 54-month OS rate was 42% (95% CI, 24% to 59%) and the 54-month EFS was 18% (95% CI, 5% to 31%). There was a significant advantage for the R-HDS–treated patients in terms of both EFS (P < .0001) and OS (P = .027).
Discussion
Because of its invariably poor outcome, MCL has become a preferred target for new treatment approaches. These include the use of new drugs, aggressive leukemia-like regimens,10 and high-dose myeloablative chemotherapy or chemo-radioimmunotherapy26 followed by either autologous or allogeneic stem cell transplantation, as reviewed by Sweetenham.27 The main goals of our study were as follows: (1) to confirm the original observation15 that the combination of highdose chemotherapy and rituximab is an efficient in vivo purging strategy in MCL; and (2) to assess in a multicenter setting the feasibility, toxicity, and long-term efficacy of a high-dose sequential treatment that included a tandem transplantation, as well as the use of rituximab (R-HDS), as induction treatment in a larger series of consecutive patients with newly diagnosed, advanced-stage MCL.
Eradication of PCR-detectable disease in stem cell products is very difficult to obtain in MCL. The recent finding that rituximab and combination chemotherapy28 transiently cleared PB and the BM of detectable tumor cells in 36% of 25 informative patients has been obtained from the analysis of small samples of BM and/or PB and might not predict the ability to obtain negative harvests. In fact, it has been shown that the number of progenitor cells harvested is directly proportional to lymphoma contamination of mobilized stem cells.29 In the few published instances in which the molecular analysis was performed on preparative harvests, both ex vivo12 and in vivo,14 the purging methods used have generally failed; a 12% success rate has been reported for both. In contrast, the results in Figure 2 show that all 20 informative patients treated with R-HDS yielded very large amounts of CD34+ cells, devoid of mantle cell contamination. Of note, this very high eradication rate was obtained in patients starting with an overt leukemic PB pattern in 57% of the cases and showing (as observed previously by Jacquy et al25 ), a massive lymphoma-cell mobilization following the initial 3 courses of conventional chemotherapy. These results confirm and extend our original observation15 that the combination of high-dose chemotherapy and rituximab represents a very effective in vivo purging method in this otherwise hard to purge disease. The role of a successful purging in autologous stem cell transplantation has yet to be established. However, the observation that eradication of PCR-detectable lymphoma cells from BM harvests was associated with a markedly reduced relapse rate,30 makes the harvest of uncontaminated products a worthwhile and appealing objective. This is particularly relevant if purging can be achieved with a simple and highly reproducible in vivo method like the one described here.
One patient with a prior history of cardiac arrhythmia died suddenly on day 14 after mitoxantrone and melphalan, following an otherwise uneventful recovery. In all remaining patients, acute toxicity attributable to the treatment was limited and consisted mainly of severe but short-term pancytopenia after each high-dose course (Table 1). The only acute toxicity that could not be anticipated from our previous experience with similar HDS regimens, was CMV reactivation, which occurred in 21% of the patients here and very infrequently in the past. This suggests a higher degree of immunosuppression of the present intensified schedule, in which cytarabine, rituximab, and a tandem transplant were added. Early diagnosis through pp65-positive white blood cell monitoring and timely treatment with antiviral drugs prevented the appearance of signs and symptoms of overt CMV infection in all patients. After a median follow-up of 35 months, no secondary malignancies or myelodysplasias have been observed in this previously untreated study population. The manageable toxicity of the program, at least in this relatively young patient population, was best attested by its applicability in this multicenter setting. In fact, 24 of the 28 study patients could complete the program as scheduled, while 4 patients received only 1 of the 2 programmed myeloablative courses because of patient refusal or clinical decision.
Of the 27 patients eligible for response assessment, a 100% CR rate was observed and was durable in 24 patients with a projected EFS rate at 4 to 5 years of 79%. In addition, we observed molecular remission in the BM of all 20 patients for which a molecular probe was available. Seventeen of these 20 patients have remained in molecular remission throughout the period of molecular follow-up, while 3 have shown a transient PCR-positivity (Figure 3).
An accurate appraisal of the clinical efficacy of this approach is difficult in the absence of a randomized control group. Moreover, as for other similar intensive regimens, it cannot be safely applied to elderly patients (older than 60 years of age or so) who are not candidates for this approach. However, the present results are very different from the results invariably obtained after standard-dose anthracycline-based chemotherapy, with a CR rate less than 50%,6 and a median survival of only 3 years.31 More recent studies using rituximab in combination with CHOP chemotherapy failed to achieve an improved CR rate or to prolong the time to progression.28 This finding, at variance with aggressive B-cell lymphoma in the elderly,32 indicates that the addition of rituximab to CHOP is unable to significantly improve the clinical outcome of this disease. In the present study we have compared the results of R-HDS with a historic control population consisting of 35 adult patients younger than 61 years of age who had received a full course of anthracyclinebased chemotherapy at the same centers participating in the present study. Allowing for the limitations of studies using historic controls, the R-HDS regimen was associated with higher CR (100% vs 37%), EFS (79% vs 18%), and OS rates (89% vs 42%). These results did not change when only patients with cyclin D1 expressions and/or bcl1 rearrangement were compared. Our figures also compare favorably with the results from studies that used high-dose chemotherapy as first-line treatment in MCL. In general, the best results were reported for studies on limited numbers of patients and short follow-up8,33 or on patients selected for having achieved a CR following standard-dose induction chemotherapy.34 Most series analogous to ours in terms of long-term follow-up duration (median over 2 years) and without patient selection according to initial complete response have shown progression-free survival rates ranging from 40% to 55% with overall survival rates of 50% to 88%.8,11,35,36 Our results are very similar to those reported by Khouri et al10 for 25 chemotherapy-naive patients who received an aggressive multiagent acute lymphoblastic leukemia–like induction therapy (Hyper-CVAD [hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone] and high-dose methotrexate/cytarabine) followed by stem cell transplantation. After a median follow-up of 25 months, the 3-year EFS rate of the latter study was 72% with a 3-year OS rate of 92%. More recently the same group reported encouraging results on 36 previously untreated MCL patients younger than 66 years of age, treated with rituximab-supplemented Hyper-CVAD, and without stem cell transplantation if the patients had achieved a CR with 6 courses of the treatment. The CR rate was 92% and, after a median follow-up of 14 months, the 2-year EFS was 80%, and the 2-year OS rate 87%.37 If confirmed after a longer follow-up, these results could suggest that, when supplemented with rituximab, an intensive leukemia-like treatment also might be curative in MCL. However, its applicability, efficacy, and toxicity compared with stem cell–supported approaches would require ad hoc prospective studies. Today, the reinfusion of hematopoietic progenitors is simply a convenient and effective type of supportive therapy increasingly being used also after nonmyeloablative treatments (like high-dose cytarabine, as used here). The absence of autografting should not represent per se a critical issue in decision making.
In conclusion, our regimen achieved complete and durable responses in a very high proportion of patients, thus confirming in a relatively large population of patients that rituximab-supplemented high-dose chemotherapy has a role in the initial management of MCL in a relatively young patient population (up to 60 years of age). We are presently implementing a phase 2 multicenter trial to prospectively substantiate these findings, while the precise role of this high-dose approach awaits direct comparison with novel, competing strategies.26,37
Prepublished online as Blood First Edition Paper, March 27, 2003; DOI 10.1182/blood-2002-08-2476.
Supported in part by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC) and Ministero della Sanità, Italy, ICS 030.1/RF 96.278. A.M.G. is the Chair of Medical Oncology, University of Milan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank staff at the various Istituto Nazionale Tumori Units for expert patient care; Marco Milanesi and Paolo Longoni for technical assistance; Dr Attilio Gabbas for referring patients with MCL; Dr Alessandra Cuttica; and Dr Enrica Gamba from Roche Spa, Milan, Italy.


![Figure 5. Overall survival and event-free survival of patients treated with R-HDS versus conventional chemotherapy. The Kaplan-Meier estimate of the percentage of patients alive or event-free at 54 months was 89% (95% confidence intervals [CI], 78% to 100%) and 79% (95% CI, 58% to 100%). For the 35 historic control patients, 54-month OS rate was 42% (95% CI, 24% to 59%) and the 54-month EFS was 18% (95% CI, 5% to 31%). There was a significant advantage for the R-HDS–treated patients in terms of both EFS (P < .0001) and OS (P = .027).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/2/10.1182_blood-2002-08-2476/6/m_h81434656005.jpeg?Expires=1769148784&Signature=ZC7hSwotwt-sGQ2HaknUpr2MQSaNYs5Tk3hKIcQb79mbrm7xWSho3LG2ZaFAyE1dW6Ku8PBn385AquyV2wDntNHZneEOi75XECu-kpAgGtwtLc6HwH-UuyAIumVp3ebbQJK9PN3FsMiq83PcgQExyIcycgrSZnwU~3YNCRKfjh1TILxXzv4pyOuG8KfVxAyP7KBETjDnbGMMfn4o~i88Ukrhm8dijHUbLlxGhp7v7feeBJhvtKSYkqxAGxoUcolHb-y43m5swDsMXnBaPf4ENej6xRJpCHwnfKz7P8hol1napWTFJbhl1XQ9rBgFxLbTrOPBg8orBBf5hEsdZIxzKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal