Abstract
It is unknown whether the severity, timing, and quality of graft-versus-host disease (GVHD) may be different after nonmyeloablative as compared with myeloablative hematopoietic stem cell transplantation (HSCT). Therefore, GVHD incidence, morbidity of skin, liver, and gut, requirements for immunosuppressive therapy, and survival were retrospectively analyzed in 44 patients who underwent nonablative HSCT and 52 who underwent ablative HSCT (median ages, 56 and 54 years, respectively). The nonablative transplantation regimen consisted of low-dose total body irradiation (TBI), preceded in some patients by fludarabine administration and followed in all patients by immunosuppression with mycophenolate mofetil (MMF) and cyclosporine (CSP). Those who underwent myeloablative HSCT were prepared with different TBI- and non-TBI–containing regimens and received CSP plus methotrexate or MMF for GVHD prophylaxis. The cumulative incidence of grades II-IV acute GVHD was lower after nonablative transplantation (64% vs 85%; P = .001), but there were no differences in the cumulative incidence of chronic GVHD requiring treatment (73% vs 71%; P = .96). Nonablative transplantation was associated with the delayed initiation of steroid treatment for GVHD (0.95 months vs 3.0 months; P < .001) and with the use of fewer systemic immunosuppressants in the first 3 months after transplantation (P ≤ .04). This corresponded to more prevalent skin and more severe gut morbidity 6 to 12 months after nonablative transplantation. Our results show that nonablative HSCT is associated with a syndrome of acute GVHD occurring after day 100 in many patients. This “late-onset acute GVHD” should be taken into consideration in the design of prospective studies comparing GVHD resulting from the two types of transplantation procedures.
Introduction
In nonmyeloablative hematopoietic stem cell transplantation (HSCT), graft-versus-tumor effects have replaced high-dose cytotoxic therapy as the conceptual basis for treating underlying malignancies.1-8 Potent immunosuppression administered before and after transplantation has allowed a major reduction in pretransplantation cytotoxic therapy without compromising hematopoietic donor cell engraftment. This averts major regimen-related toxicities, making it possible to treat older and medically infirm patients who are at a high risk for complications after treatment with conventional transplantation regimens.1,9,10
Graft-versus-host disease (GVHD) has long been recognized as a serious and frequent complication of conventional ablative allogeneic HSCT. Although graft-versus-host effects have an important role in eradicating malignant cells, GVHD has remained a major determinant of posttransplantation morbidity, quality of life, and survival, especially when long-term immunosuppressive therapy is required to control this complication.11-13 Our clinical understanding of GVHD is largely based on experience gained during decades of ablative HSCT in which a syndrome of acute GVHD can be distinguished from chronic GVHD. Acute GVHD is characterized by inflammatory dermatitis, hepatitis, and enteritis and usually develops within the first 3 months of HSCT, whereas chronic GVHD is characterized by oral and ocular sicca and fibrotic complications affecting a wide spectrum of organs, usually with onset 3 months or more after transplantation.12,14 The incidence, severity, quality, and timing of GVHD after nonablative HSCT have not yet been systematically analyzed.
The immunobiology of nonablative HSCT differs from that of ablative HSCT in several important respects. First, nonablative conditioning appears to cause only limited tissue damage in the recipient, which may translate into less inflammatory cytokine release. A “cytokine storm,” which has been described after ablative conditioning therapy, has been proposed to provide a proinflammatory milieu for the development of GVHD.15-19 Second, studies with animals have shown that the development of transient mixed donor-host chimerism may facilitate the establishment of mutual tolerance, which, in turn, may down-regulate GVH activities.20,21 Third, the type and duration of immunosuppressive agents administered after nonablative conditioning differ from those used after ablative HSCT.1,2,4,6,10,22 Fourth, the number and function of recipient antigen-presenting cells may be higher after nonablative HSCT than after ablative HSCT. These cells may play a major role in the initiation of GVH responses early after HSCT.23,24 The net effects of these fundamental differences between nonablative and ablative transplantation regimens with respect to the clinical presentation of GVHD remain to be defined.
In the present study, we retrospectively analyzed data from 2 age-matched cohorts of patients who underwent nonablative or ablative HSCT from HLA-matched related and unrelated donors for the treatment of hematologic malignancies or renal cell cancer. We compared the incidence and severity of acute and chronic GVHD between the 2 transplantation groups based on conventional grading criteria. We then systematically compared morbidity involving the skin, liver, and gut as prototypic GVHD target organs and analyzed requirements for systemic immunosuppressive agents as treatment for GVHD during the first year after transplantation.
Patients, materials, and methods
Patients
Patients who underwent nonmyeloablative or myeloablative HSCT at the Fred Hutchinson Cancer Research Center between March 2000 and September 2001 were included in this study. Patients signed forms approved by the Institutional Review Board documenting informed consent to participate in the clinical trials. Because patients undergoing nonablative HSCT have typically been older than patients undergoing myeloablative HSCT, only those aged 50 to 65 years were included in this study. Two patients who underwent nonablative HSCT were excluded from analysis because of pre-existing liver cirrhosis, which would have confounded the analysis. All HSCTs were performed from related or unrelated donors who were serologically matched for HLA-A, -B, and -C and were allele-matched for HLA-DRB1 and -DQB1. Details regarding diagnoses, donor types, stem cell sources, preparative regimens, and immunosuppression after transplantation are listed in Table 1. Median patient ages were 56 years (range, 50-65 years) for the 46 patients in the nonablative group and 54 years (range, 50-64 years) for the 52 patients in the ablative group. Five patients in the nonablative group, who had undergone transplantation from unrelated donors, rejected their grafts. Morbidity and immunosuppressive data for these patients were censored at the time of rejection. All other patients had stable donor engraftment during follow-up. The median follow-up times among surviving patients were 13.9 months in the nonablative group and 11.1 months in the ablative group (P = .09; Wilcoxon rank sum test), respectively.
Characteristics of patients undergoing nonmyeloablative and myeloablative HSCT
Patient characteristics . | Nonablative transplantation; n = 44 . | Ablative transplantation; n = 52 . |
|---|---|---|
| Sex, female/male, % | 36/64 | 56/44 |
| Median age, y (range) | 56 (50-65) | 54 (50-64) |
| Disease, n (%) | ||
| CML | 8 (18) | 8 (15) |
| CLL | 5 (11) | 0 |
| MDS | 3 (7) | 23 (44) |
| AL in remission | 6 (14) | 7 (13) |
| AL in relapse | 4 (9) | 7 (13) |
| Lymphoma | 7 (16) | 1 (2) |
| Myeloma | 9 (20) | 0 |
| Myelofibrosis | 0 | 6 (12) |
| RCC | 2 (5) | 0 |
| Prior autologous transplantation (%) | 8 (16) | 0 |
| Donor,* n (%) | ||
| Related | 24 (55) | 30 (58) |
| Unrelated | 20 (45) | 22 (42) |
| Stem cell source, n (%) | ||
| PBSC | 38 (86) | 41 (79) |
| BM | 6 (14) | 10 (19) |
| Both | 0 | 1 (2) |
| Conditioning, n (%) | ||
| Flu + TBI, 2 Gy | 38 (86) | 0 |
| TBI, 2 Gy | 6 (14) | 0 |
| Bu + Cy | 0 | 32 (62) |
| Bu + Flu | 0 | 13 (25) |
| Cy + TBI, more than 10 Gy | 0 | 7 (13) |
| After transplantation immunosuppression, n (%) | ||
| MMF + CSP | 44 (100) | 7 (13) |
| MTX + CSP | 0 | 45 (87) |
| Donor lymphocyte infusions (%) | 1 (2) | 0 |
| Rejections, n (%) | 5 (11) | 0 |
Patient characteristics . | Nonablative transplantation; n = 44 . | Ablative transplantation; n = 52 . |
|---|---|---|
| Sex, female/male, % | 36/64 | 56/44 |
| Median age, y (range) | 56 (50-65) | 54 (50-64) |
| Disease, n (%) | ||
| CML | 8 (18) | 8 (15) |
| CLL | 5 (11) | 0 |
| MDS | 3 (7) | 23 (44) |
| AL in remission | 6 (14) | 7 (13) |
| AL in relapse | 4 (9) | 7 (13) |
| Lymphoma | 7 (16) | 1 (2) |
| Myeloma | 9 (20) | 0 |
| Myelofibrosis | 0 | 6 (12) |
| RCC | 2 (5) | 0 |
| Prior autologous transplantation (%) | 8 (16) | 0 |
| Donor,* n (%) | ||
| Related | 24 (55) | 30 (58) |
| Unrelated | 20 (45) | 22 (42) |
| Stem cell source, n (%) | ||
| PBSC | 38 (86) | 41 (79) |
| BM | 6 (14) | 10 (19) |
| Both | 0 | 1 (2) |
| Conditioning, n (%) | ||
| Flu + TBI, 2 Gy | 38 (86) | 0 |
| TBI, 2 Gy | 6 (14) | 0 |
| Bu + Cy | 0 | 32 (62) |
| Bu + Flu | 0 | 13 (25) |
| Cy + TBI, more than 10 Gy | 0 | 7 (13) |
| After transplantation immunosuppression, n (%) | ||
| MMF + CSP | 44 (100) | 7 (13) |
| MTX + CSP | 0 | 45 (87) |
| Donor lymphocyte infusions (%) | 1 (2) | 0 |
| Rejections, n (%) | 5 (11) | 0 |
CML indicates chronic myeloid leukemia; CLL, chronic lymphocytic leukemia; MDS, myelodysplastic syndrome; AL, acute leukemia; RCC, renal cell carcinoma; PBSC, peripheral blood stem cells; BM, bone marrow; Flu, fludarabine; Bu, busulfan; Cy, cyclophosphamide
HLA-matched
Preparative regimens
Patients in the nonmyeloablative group received low-dose total body irradiation (TBI; 2 Gy) alone (14%) or in combination with fludarabine (30 mg/m2 body surface area per day for 3 consecutive days) (86%). Those in the myeloablative group received busulfan (4 mg/kg per day for 4 consecutive days) and cyclophosphamide (60 mg/kg per day for 2 consecutive days) (62%), busulfan (4 mg/kg per day for 4 consecutive days), and fludarabine (30 mg/m2 body surface area per day for 4 consecutive days) (25%) or cyclophosphamide (60 mg/kg per day for 2 consecutive days) and fractionated TBI (12 Gy) (13%). Busulfan levels were targeted to 600 to 900 ng/mL in all except for one patient in whom levels were only tracked.
Prophylaxis against GVHD: nonmyeloablative group
Related donors. Mycophenolate mofetil (MMF) (15 mg/kg orally every 12 hours; days 0-27) and cyclosporine (CSP) (6.25 mg/kg orally every 12 hours; start day -3) were given to all patients after transplantation. Because of evolving treatment protocols, the duration of CSP given after transplantation was gradually extended. Sixteen (67%) patients received CSP until day 35, followed by a taper until day 56; 5 (21%) patients received CSP until day 56, followed by a taper until day 180; and 3 (12%) patients were given CSP until day 80, followed by a taper until day 180.
Unrelated donors. All patients received MMF (15 mg/kg orally every 12 hours) from day 0 through day 40, with subsequent taper to day 96. In addition, CSP (6.25 mg/kg orally every 12 hours) was given until day 100, followed by a taper until day 177. Tapering schedules of immunosuppressive agents were modified at the discretion of the attending physicians for the treatment of GVHD or persistent or recurrent malignancy.
Prophylaxis against GVHD: ablative group
In the ablative group, most patients were given CSP plus methotrexate (MTX), as described previously.25 Four patients with related donors and 3 patients with unrelated donors received CSP in combination with MMF. CSP was given at a dose of 1.5 mg/kg intravenously or 6.25 mg/kg orally every 12 hours, day -1 to day +60 (when given with MTX) or day +50 (when given with MMF); then it was tapered until day +180. MMF was given at 15 mg/kg intravenously every 8 hours from day 0 through day 27. Tapering schedules of immunosuppressive agents were modified at the discretion of the attending physicians for the reasons described.
GVHD grading and treatment
Diagnosis and clinical grading of acute and chronic GVHD were performed according to established criteria.12,14,26 Treatment decisions were based on the attending physician's assessment of the severity of acute GVHD, and initial treatment usually consisted of prednisolone (1-2 mg/kg per day; taper started after 14 days). In addition, the administration of CSP, MMF, or both was usually resumed at full doses. Different modalities, such as T-cell–directed monoclonal antibodies, were used for the treatment of steroid-refractory acute GVHD as part of clinical studies. Extensive chronic GVHD was usually treated with prednisolone with or without alternate-day CSP.27
Information regarding the administration of systemic immunosuppressive treatment for GVHD was collected retrospectively. The initiation and duration of corticosteroid therapy and the total number of systemic immunosuppressive agents used across time were recorded. Because of differences in the duration of MMF (27 or more days) and MTX (11 days) administration for GVHD prophylaxis in recipients in the nonablative and ablative groups, these agents were counted as “systemic immunosuppressants” only if they were given beyond the prescribed prophylaxis or if administration was resumed after the scheduled discontinuation of prophylaxis.
Donor lymphocyte infusion
Donor lymphocyte infusion (DLI) was a treatment option in the event of relapse or disease progression in patients without GVHD who had discontinued immunosuppressive treatment. No patient in the ablative group and only one patient in the nonablative group received DLI. The patient treated with DLI had progressive renal cell cancer and did not develop GVHD after DLI. This patient's skin, liver, and gut morbidity scores were zero at all time points over 12 months after transplantation.
Modulation of immunosuppression after relapse or progression of malignancy
If relapse or progression of malignancy occurred in patients who underwent nonablative or ablative transplantation and were still receiving immunosuppressive therapy but did not have GVHD, immunosuppressive therapy was rapidly tapered to induce graft-versus-tumor effects. Follow-up for patients in the ablative group was censored at the time of relapse, but no patient in this cohort acquired GVHD after relapse. Two of 44 patients in the nonablative group had GVHD after relapse. Because taper of immunosuppressive therapy was considered an integral part of nonmyeloablative transplantation if disease progression or relapse occurred, follow-up for patients was not censored at the time of disease progression or relapse.
Evaluation of morbidity involving the skin, liver, and gut
A 4-point scale was applied to evaluate morbidity involving the skin, liver, and gut after transplantation (Table 2). Morbidity grading was performed retrospectively by review of medical records. A peak morbidity score was determined for each organ system analyzed during each of the first 6 months after transplantation and at 3-month intervals between 7 to 9 and 10 to 12 months after transplantation. Data collection was discontinued in 5 patients in the nonablative group at the time of rejection. Skin morbidity was graded by determining the percentage of body surface area affected by rash. Liver morbidity was graded according to serum levels of total bilirubin and alkaline phosphatase, whichever had the higher score. Gastrointestinal morbidity was determined by the presence or absence of symptoms (anorexia, nausea, vomiting, or diarrhea) and the use of partial or complete parenteral nutrition. The overall morbidity grade was calculated by scoring the highest morbidity grade in any organ system. No attempt was made to record specific GVHD-related morbidity. Therefore, morbidity as determined by this approach included complications caused by regimen-related toxicity and infections.
Skin, liver, and gut morbidity scale
Score . | . | 0 . | 1 . | 2 . | 3 . |
|---|---|---|---|---|---|
| Skin | |||||
| BSA involved, * % | No changes | < 18 | 18-50 | > 50 | |
| Liver | |||||
| Total bilirubin, mg/mL | < 1.6 | 1.6-3.0 | 3.1-6.0 | > 6.0 | |
| Alkaline phosphatase, U/L | < 130 | 130-260 | 261-520 | > 520 | |
| Gut | |||||
| Symptoms† | Absent | Present | Present | Present | |
| Parenteral nutrition | Absent | Absent | Partial | Full |
Score . | . | 0 . | 1 . | 2 . | 3 . |
|---|---|---|---|---|---|
| Skin | |||||
| BSA involved, * % | No changes | < 18 | 18-50 | > 50 | |
| Liver | |||||
| Total bilirubin, mg/mL | < 1.6 | 1.6-3.0 | 3.1-6.0 | > 6.0 | |
| Alkaline phosphatase, U/L | < 130 | 130-260 | 261-520 | > 520 | |
| Gut | |||||
| Symptoms† | Absent | Present | Present | Present | |
| Parenteral nutrition | Absent | Absent | Partial | Full |
BSA indicates body surface area
Erythematous, lichenoid, sclerodermatous, or ichthyotic involvement
Anorexia, nausea, vomiting, or diarrhea
Infection prophylaxis
All patients received prophylactic antibiotics (ceftazidime or ciprofloxacin) when absolute neutrophil counts (ANCs) were less than 0.5 × 109/L. Prophylactic low-dose acyclovir was given for 1 year.28 Fluconazole (400 mg/d) was given to all patients from the start of conditioning to day 75 after transplantation.29 Prophylaxis against Pneumocystis carinii was administered with the use of trimethoprim-sulfamethoxazole as first-line treatment and dapsone as second-line treatment until day 180 after transplantation.30 Cytomegalovirus (CMV)–seronegative blood products were used for CMV-seronegative patients with CMV-seronegative donors. For all other patients, surveillance for CMV was performed with blood samples every 1 week to 2 weeks.28,31 In general, during the first 100 days after transplantation, all patients with CMV antigenemia at any level or with CMV viremia received ganciclovir induction therapy (5 mg/kg intravenously twice a day) for 7 to 14 days, followed by maintenance therapy until day 100. After day 100, pre-emptive therapy was recommended when the antigenemia test showed at least 5 positive cells per slide or when CMV viremia was detected by culture.
Statistical analysis
Demographic factors were summarized using percentages or median and range values. Continuously valued factors were compared between groups using Wilcoxan rank sum tests or Student t tests, whereas categorical factors were compared using χ2 analysis. Survival curves were evaluated using the method of Kaplan and Meier. Cumulative incidence curves were used for acute and chronic GVHD, initiation of prednisolone therapy, discontinuation of all immunosuppressive therapy, death or rejection during immunosuppressive therapy, and death with symptoms of GVHD.32 For all but the final 2 outcomes, death and rejection were considered competing risk events. For the final 2 outcomes, discontinuation of all immunosuppressive therapy and death without symptoms of GVHD constituted competing risk events, respectively. Time-to-event outcomes were compared using a log-rank test (to compare the hazards) or by comparing survival or incidence estimates at specific time points. Cumulative incidence of clinical extensive chronic GVHD was calculated only among the 40 and 43 patients who survived beyond day 70 in the nonmyeloablative and ablative groups, respectively. Median time to initiation of steroid treatment was calculated using the 50th percentile from the cumulative incidence curves.
Results
Conventional grading for acute and chronic GVHD
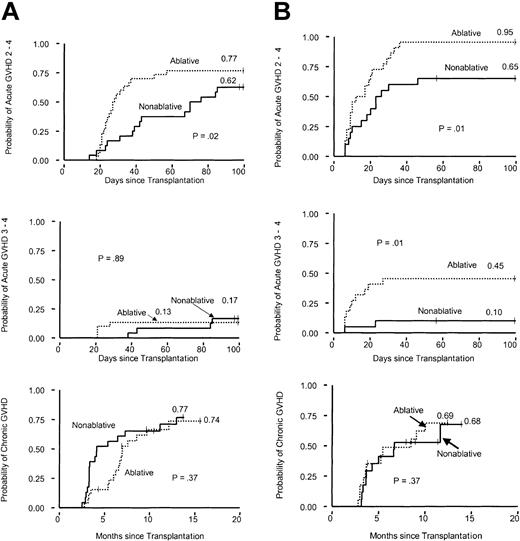
Related donors. The cumulative incidence of grades II-IV acute GVHD at day 100 was significantly lower among patients in the nonablative group than in the ablative group (P = .02) (Figure 1A). There was no difference in the cumulative incidences of grades III-IV acute GVHD between the 2 groups. There was also no significant difference between the 2 groups in the cumulative incidence of extensive chronic GVHD among patients who survived for at least 70 days.
Cumulative incidences of acute and extensive chronic GVHD after nonmyeloablative conditioning compared with myeloablative conditioning. (A) Related-donor transplantation. (B) Unrelated-donor transplantation.
Cumulative incidences of acute and extensive chronic GVHD after nonmyeloablative conditioning compared with myeloablative conditioning. (A) Related-donor transplantation. (B) Unrelated-donor transplantation.
Unrelated donors. The cumulative incidences of grades II-IV and grades III-IV acute GVHD to day 100 were lower among patients in the nonablative group than among those in the ablative group (P = .01; P = .01; log-rank test) (Figure 1B). The cumulative incidence of extensive chronic GVHD among patients who survived for at least 70 days was not significantly different between the 2 groups.
Morbidity involving the skin, liver, and gastrointestinal tract
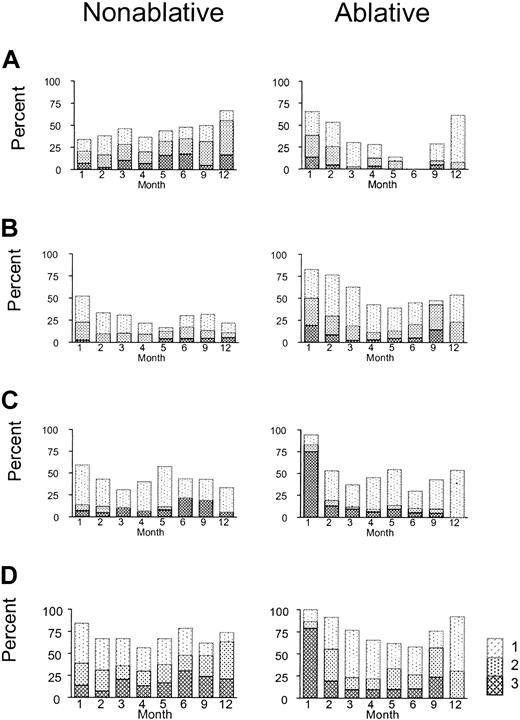
During the first month after transplantation, patients in the nonablative group appeared to experience less morbidity involving the skin, liver, and gastrointestinal tract than those in the ablative group, as expected (Figure 2). From 6 to 12 months after transplantation, however, skin morbidity appeared to be more prevalent and gastrointestinal morbidity more severe for patients in the nonablative group than in the ablative group (Figure 2A, C). Seven of 10 patients who underwent nonablative transplantation and who first began steroid treatment for rash after day 80 had acute inflammatory changes, such as maculopapular erythema. At all time points analyzed, liver morbidity appeared to be less severe in the nonablative group (Figure 2B). As shown in Figure 2D, overall morbidity gradually increased until 6 to 12 months after HSCT in the nonablative group, whereas a biphasic pattern was observed in the ablative group, with an early peak at 1 month and a second peak at 9 months. Because the shapes of the morbidity curves differed between groups, these data were summarized in a descriptive fashion rather than by a formal statistical comparison.
Morbidities involving the skin, liver, and gut after nonmyeloablative conditioning compared with myeloablative conditioning. Shown are proportions of patients who experienced no morbidity and morbidity grades 1, 2, and 3 at different time points during the 12 months after transplantation. (A) Skin; (B) liver; (C) gut; and (D) overall.
Morbidities involving the skin, liver, and gut after nonmyeloablative conditioning compared with myeloablative conditioning. Shown are proportions of patients who experienced no morbidity and morbidity grades 1, 2, and 3 at different time points during the 12 months after transplantation. (A) Skin; (B) liver; (C) gut; and (D) overall.
Treatment of GVHD
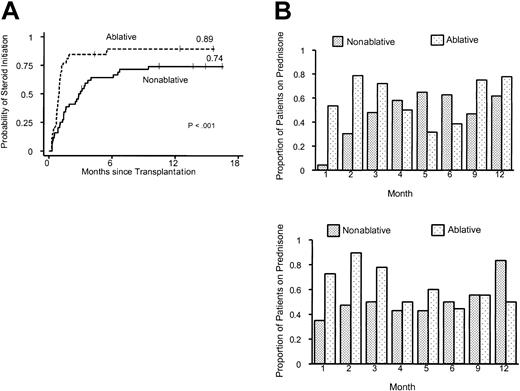
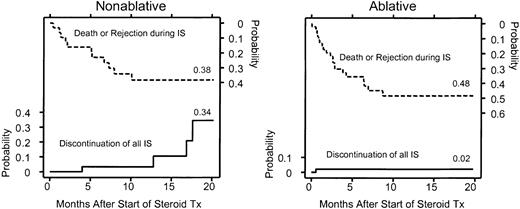
The median time to initiation of corticosteroids was 3.0 months in the nonablative group and 0.95 months in the ablative group (P < .001; log-rank test) (Figure 3A). Thus, the median time of onset of corticosteroid administration for treatment of GVHD was delayed by more than 2 months in the nonablative group. In addition, there was a strong suggestion that the proportion of patients who required steroids for treatment of GVHD was lower in the nonablative group (74% vs 89%; P = .06). Differences in GVHD prophylaxis between the nonablative group and the ablative group did not translate into differences in onset of GVHD. Comparison of the largest subgroups with uniform GVHD prophylaxis between nonablative (scheduled CSP taper days, 35-56; n = 16) and ablative (scheduled CSP taper days, 56-180; n = 26) HLA-matched sibling transplant recipients confirmed that the initiation of prednisolone treatment in the nonablative group was delayed by 2 months (median, 85.5 days [range, 16-287 days] vs 29.0 days [range, 21-167 days]).
Use of prednisolone for the treatment of GVHD after nonmyeloablative or myeloablative conditioning. (A) Time to initiation of prednisolone therapy for graft-versus-host disease. (B) Proportion of patients in the nonmyeloablative group (n = 44) and the myeloablative group (n = 52) continuing with prednisolone treatment for the first 12 months after transplantation. Upper panel, related donors; lower panel, unrelated donors.
Use of prednisolone for the treatment of GVHD after nonmyeloablative or myeloablative conditioning. (A) Time to initiation of prednisolone therapy for graft-versus-host disease. (B) Proportion of patients in the nonmyeloablative group (n = 44) and the myeloablative group (n = 52) continuing with prednisolone treatment for the first 12 months after transplantation. Upper panel, related donors; lower panel, unrelated donors.
Significantly smaller proportions of recipients in the nonablative group required steroids during each of the first 3 months after transplantation than in the ablative group (month 1, P < .001; month 2, P < .001; month 3, P = .02; χ2 tests) (Figure 3B). The pattern of lower steroid requirements in the nonablative group occurred in recipients of transplants from related and unrelated donors. The proportion of patients treated with steroids increased gradually during the first 5 months after nonablative HSCT from related donors and during the first 3 months after nonablative HSCT from unrelated donors. Differences in steroid requirements between the nonablative and ablative groups were not significant beyond the first 3 months after transplantation.
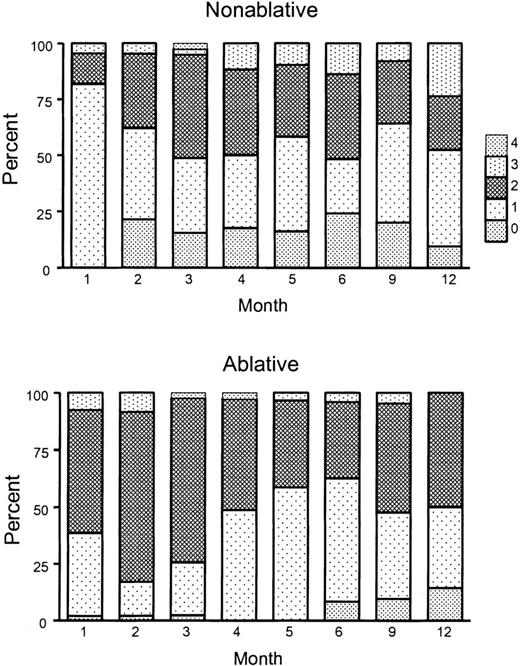
Distributions for the numbers of systemic immunosuppressive agents used for patients in the 2 transplantation groups across time are shown in Figure 4. The mean numbers of agents used per month increased from 1.23 to 1.47 between the first and the sixth months in the nonablative group, whereas the mean numbers decreased from 1.47 to 1.38 during the same period in the ablative group. Differences between groups were significant during each of the first 3 months after transplantation (month 1, P < .001; month 2, P < .001; month 3, P = .04; Wilcoxon rank sum test), but not after that time point.
Peak number of immunosuppressive agents used across time for the treatment of GVHD after nonmyeloablative or myeloablative conditioning. Shown are the proportions of patients treated with 0, 1, 2, 3, or 4 agents at different time points during 12 months after transplantation. Values at 1 to 6 months after transplantation represent peak numbers of immunosuppressive agents given during the respective month. Values at 9 and 12 months represent peak numbers of immunosuppressive agents given between 7 to 9 months and 10 to 12 months after transplantation. Upper panel, nonmyeloablative transplantation; lower panel, myeloablative transplantation.
Peak number of immunosuppressive agents used across time for the treatment of GVHD after nonmyeloablative or myeloablative conditioning. Shown are the proportions of patients treated with 0, 1, 2, 3, or 4 agents at different time points during 12 months after transplantation. Values at 1 to 6 months after transplantation represent peak numbers of immunosuppressive agents given during the respective month. Values at 9 and 12 months represent peak numbers of immunosuppressive agents given between 7 to 9 months and 10 to 12 months after transplantation. Upper panel, nonmyeloablative transplantation; lower panel, myeloablative transplantation.
To date, 4 of 32 patients who underwent nonablative HSCT and 1 of 46 patients who underwent ablative HSCT have discontinued all immunosuppression (Figure 5). These differences did not reach statistical significance. Also shown in Figure 5 are deaths and rejections during follow-up as competing risks for the discontinuation of immunosuppression. In one patient who underwent nonablative transplantation, immunosuppression was discontinued because of progressive renal cell carcinoma despite unresolved chronic skin GVHD. Two patients with multiple myeloma and one patient with AML, however, were in remission without evidence of GVHD when immunosuppressive treatment was discontinued. The patient who discontinued all systemic immunosuppressive therapy after ablative HSCT had relapsed AML without signs of GVHD.
Probability of discontinuation of all immunosuppressive agents after nonmyeloablative or myeloablative conditioning. Death and rejection during immunosuppressive therapy are shown as competing risks. The area between the curves represents patients continuing with immunosuppressive therapy for GVHD. Four patients who underwent nonmyeloablative HSCT and one who underwent myeloablative HSCT discontinued the use of all immunosuppressive agents during the follow-up period. The difference was not significant (P = .25; log-rank test). IS indicates immunosuppression.
Probability of discontinuation of all immunosuppressive agents after nonmyeloablative or myeloablative conditioning. Death and rejection during immunosuppressive therapy are shown as competing risks. The area between the curves represents patients continuing with immunosuppressive therapy for GVHD. Four patients who underwent nonmyeloablative HSCT and one who underwent myeloablative HSCT discontinued the use of all immunosuppressive agents during the follow-up period. The difference was not significant (P = .25; log-rank test). IS indicates immunosuppression.
Overall survival and cause of death related to GVHD
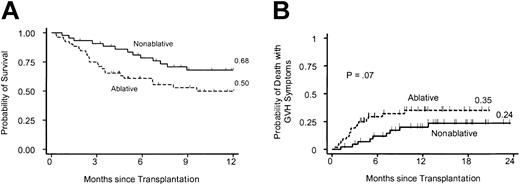
With median follow-up times of 13.9 months in the nonablative group and 11.1 months in the ablative group, there was a suggestion that overall survival at 1 year was superior in the nonablative group (68% vs 50%; P = .04) (Figure 6A). Cumulative incidence rates of death with manifestations of GVHD under treatment were 35% and 24% at 15 months for the ablative and nonablative group, respectively. Although the incidence was not significantly different (P = .27), there was a suggestion that deaths with persistent manifestations of GVHD occurred later in the nonablative group than in the ablative group (P = .07; log-rank test) (Figure 6B).
Survival after nonmyeloablative as compared with myeloablative conditioning. (A) Kaplan-Meier estimates of overall survival for cohorts of age-matched patients who underwent nonmyeloablative (n = 44) and myeloablative (n = 52) HSCT between March 2000 and September 2001 for hematologic malignancies and renal cell carcinoma. (B) Probabilities of death with manifestations of GVHD under treatment in the nonmyeloablative and myeloablative groups.
Survival after nonmyeloablative as compared with myeloablative conditioning. (A) Kaplan-Meier estimates of overall survival for cohorts of age-matched patients who underwent nonmyeloablative (n = 44) and myeloablative (n = 52) HSCT between March 2000 and September 2001 for hematologic malignancies and renal cell carcinoma. (B) Probabilities of death with manifestations of GVHD under treatment in the nonmyeloablative and myeloablative groups.
Discussion
Although regimen-related acute transplantation morbidity has decreased considerably with the introduction of nonablative preparative regimens for allografting, GVHD has remained a major cause of morbidity and mortality.1,2,4,6,22 Direct comparison of GVHD occurring after nonablative and ablative HSCT has been hampered mainly by 2 factors. First, patients undergoing nonablative HSCT are usually older than patients undergoing conventional HSCT, and increasing age has been associated with an increasing risk for GVHD after ablative HSCT.33,34 Second, the current GVHD grading system is based on decades of experience in the ablative setting, where a relatively clear temporal distinction between acute and chronic GVHD has historically recognized validity. This widely used grading system12,35 may not be the optimal tool for measuring GVHD after nonablative HSCT because clinical findings consistent with the syndrome of acute GVHD appear to occur well after day 100 in some recipients.
We used a scale that measured morbidity associated with acute and chronic GVHD in age-matched cohorts of patients at specified time points during the first year after transplantation. We found that peaks of skin and gastrointestinal morbidities occurred between 6 and 12 months after nonablative conditioning instead of during the first month after ablative conditioning. Morbidity grading reflected all causes, including regimen-related toxicity, infections, and GVHD, with regimen-related toxicity playing the predominant role during the first month after conditioning. Although an assessment of specific contributions from regimen-related toxicity, infections, and GVHD will undoubtedly be important in deciding how morbidity might be decreased in the future, we believe that a longitudinal, population-based, all-cause comparison provided an objective and informative platform for evaluating outcomes with the 2 types of transplantation.
Given the relatively nontoxic preparative regimen used, it is not surprising that morbidities of the skin, liver, and gastrointestinal tract were minimal during the first month after nonablative HSCT.1 Differences in early morbidity seemed to be most dramatic with regard to the gastrointestinal tract, where only 10% of patients in the nonablative group required full parenteral nutrition within the first month compared with 75% in the ablative group.
The strikingly delayed initiation of steroid treatment indicates a later onset of GVHD after nonmyeloablative conditioning. Considering the role that gastrointestinal damage appears to play in the initiation of experimental GVHD,15,16,18 it was also not surprising that decreased regimen-related toxicity translated into an overall decreased incidence of GVHD during the first 3 months in the nonablative group. However, the subsequent skin and gastrointestinal morbidity and the comparable use of immunosuppressive agents suggest that the development of GVHD was significantly delayed, but not prevented, in most patients in the nonablative group. This implies that the preserved integrity of an intestinal mucosal barrier, reduced inflammatory cytokine release, and transient mixed donor-host chimerism characteristic of nonablative conditioning did not translate into overall protection against GVHD.
The use of MMF instead of MTX as immunosuppressive therapy might have helped to delay the onset of GVHD after nonablative HSCT. However, preliminary results of a prospective trial in which MMF was given twice daily for 27 days in combination with CSP suggest that this combination is not superior to the standard regimen of MTX plus CSP for preventing GVHD after ablative HSCT from related and unrelated donors (R. Nash, unpublished data, August 2002). In the HLA-matched unrelated donor groups in our study, the cumulative incidence of severe (grades III-IV) acute GVHD reached 45% after ablative conditioning but only 10% after nonablative conditioning; the latter group received MMF for 40 days, followed by a taper until day 96. It is possible that the prolonged administration of MMF helped to delay the onset of GVHD after nonablative transplantation from HLA-matched unrelated donors.
A considerable proportion of patients in the nonablative group experienced clinical findings consistent with the syndrome of acute GVHD beyond 3 months from transplantation. In addition to inflammatory dermatitis, the clinical presentation in these patients often included nausea, vomiting, and diarrhea without detectable oral or ocular sicca or fibrotic manifestations usually considered pathognomonic for chronic GVHD after ablative HSCT. We therefore believe the term late-onset acute GVHD should be introduced into clinical practice. The evaluation of GVHD-associated morbidity in future studies of nonablative HSCT should be based on qualitative criteria, and the traditional day 100 cutoff for separation of acute from chronic GVHD should be abandoned. For this purpose, prospective data collection with qualitative descriptors of GVHD target-organ involvement will be required. This approach will also facilitate the evaluation of response after treatment.
Differences in survival and persistence of malignancy between the nonablative and ablative groups could have confounded the evaluation of morbidity between them. For example, the possible selection bias caused by a higher incidence of early transplantation-related mortality in the ablative group might have contributed to a lower prevalence of morbidity at later time points. Likewise, a higher incidence of persistent malignancy immediately after nonablative transplantation might have contributed to a higher prevalence of late GVHD-related morbidity caused by premature discontinuation of immunosuppression or less aggressive treatment of GVHD. In addition, the skewed disease distribution between the 2 groups and the absence of accounting for control of malignancy in our analysis preclude any definitive conclusions concerning the overall merits of one approach versus the other with respect to long-term disease-free survival.
The findings of this study cannot be extrapolated to other nonablative transplantation approaches under investigation.2,7,9,10,22 In addition to differences in hematopoietic and extrahematopoietic toxicities between different reduced-intensity regimens, the types and duration of immunosuppression after transplantation are not the same. Variations in regimen-related tissue damage and differences in immunosuppression after transplantation might translate into different patterns of GVHD. The unpredictable impact of reduced-intensity transplantation regimens on GVHD is illustrated by reports in which the frequencies of grades II-IV acute GVHD have ranged from 20% to 60% with HLA-matched related donors.1,2,6,9,10,22
In conclusion, nonablative HSCT was associated with a 2-month delay in onset of GVHD compared with conventional HSCT, which corresponded to right-shifted peaks of gastrointestinal and skin morbidities. The onset of erythema, nausea, vomiting, and diarrhea beyond 100 days after nonablative HSCT can best be described as late-onset acute GVHD, which underscores the importance of comprehensive long-term follow-up. Revision of current GVHD grading criteria emphasizing the quality of target-organ involvement and de-emphasizing temporal presentation will be needed to facilitate meaningful comparison of GVHD-associated morbidity after nonablative and ablative HSCT.
Prepublished online as Blood First Edition Paper, March 27, 2003; DOI 10.1182/blood-2002-08-2628.
Supported in part by National Institutes of Health grants CA78902, CA18221, CA18029, CA15704, HL36444, CA92058, and CA09515.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the research nurses Mary Hinds and Steve Minor, the trials coordinator Debbie Bassuk, and the staff at the Long-Term Follow-Up department of the Fred Hutchinson Cancer Research Center for their invaluable assistance with data collection, and we thank Helen Crawford and Bonnie Larson for typing the manuscript. We also thank the inpatient and outpatient nursing teams and support staff at the Fred Hutchinson Cancer Research Center and at the University of Washington Medical Center for the excellent care they provided to patients and families.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal