Abstract
It has been established that amelioration of murine immune thrombocytopenia purpura (ITP) by IVIg is dependent on the inhibitory receptor FcγRIIB. Co-cross-linking of the FcγRIIB with the B-cell receptor complex or with FcϵRI in mast cells results in cell inhibition, which is mediated by recruitment of the inositol phosphatase SHIP1 to the cytoplasmic tail of the FcγR. The FcγRIIB can also associate with protein tyrosine phosphatase SHP-1 as a potential secondary target of the receptor. Alternatively, homoaggregation of FcγRIIB can induce a proapoptotic state in B cells that is dependent on the presence of Bruton tyrosine kinase (Btk), a kinase also expressed in monocytes. We sought to determine if these signaling pathways may direct IVIg-mediated FcγRIIB-dependent regulation of in vivo monocyte function in a murine model of ITP in which IVIg functions in an FcγRIIB-dependent manner. We demonstrate that mice deficient in SHIP1, SHP-1, and Btk respond to the ameliorating effects of IVIg with the same kinetics as control mice. We conclude that IVIgmediated inhibitory pathways operating via monocyte FcγRIIB may involve a transmembrane signaling pathway different from that of B cells.
Introduction
The inhibitory low-affinity receptor for immunoglobulin G (IgG), FcγRIIB, is widely expressed by hematopoietic cells such as B cells, mast cells, and monocytes. Two main FcγRIIB-mediated inhibitory signaling pathways in B cells have been identified.
The first pathway is initiated by co-cross-linking of the FcγRIIB with the B-cell receptor complex, which leads to a dominant-negative signal, resulting in cell inactivation. This event delivers a signal through a motif in the cytoplasmic tail of FcγRIIB, the immunoreceptor tyrosine-based inhibitory motif (ITIM; for a review, see Ravetch and Lanier1 ). SHIP1 binds directly, via its SH2 domain, to the phosphorylated receptor ITIM of FcγRIIB. This same inhibitory pathway also blocks antigen-induced mast cell degranulation by recruitment of SHIP1 to the ITIM via co-crosslinking of FcγRIIB with the activating high-affinity IgE receptor, FcϵRI.2-4 Evidence also indicates that ITIM-dependent FcγRIIB-mediated immune suppression could involve the tyrosine phosphatase SHP-1.5-7
The second major inhibitory pathway mediated by FcγRIIB in B cells is ITIM independent and involves immune complex-mediated homoaggregation of FcγRIIB.1,8 This event leads to delivery of a proapoptotic signal to the B cell. It has been shown that signaling via FcγRIIB homoaggregation is dependent on the presence of Bruton tyrosine kinase (Btk), a B-cell kinase also expressed in monocytes.9
We10 and others11,12 have shown that intravenous immunoglobulin (IVIg) can successfully ameliorate thrombocytopenia in a murine model of immune thrombocytopenia purpura (ITP), a disease state that is mediated by monocyte phagocytosis of opsonized platelets.13,14 Samuelsson et al11 recently demonstrated that the acute activity of IVIg in preventing murine ITP requires the expression of FcγRIIB, and we have shown that the activity of IVIg in treating murine ITP is not dependent on the presence of B or T cells, nor anti-idiotype reactivity of IVIg.10 To determine if the FcγRIIB-dependent activity of IVIg uses the ITIM-dependent inhibitory pathway (SHIP1/SHP-1) versus the homoaggregation (Btk)-dependent signaling pathway, we used mice genetically deficient for these key signaling molecules. We found that FcγRIIB knockout (KO) mice rendered thrombocytopenic were refractory to IVIg treatment. Mice lacking FcγRIIB-dependent signaling mediators including SHIP1, SHP-1, and Btk, however, all responded to IVIg treatment, indicating that either the known FcγRIIB signaling pathways are not used by IVIg for its effect or that monocytes use another inhibitory pathway via FcγRIIB.
Study design
Mice
Wild-type, heterozygous, and homozygous SHIP1 KO mice were as previously described15 and bred at the British Columbia Cancer Agency (Vancouver, BC, Canada). SHP-1-deficient motheaten (me/me) mice and control littermates were bred at the Mt Sinai Hospital Samuel Lunenfeld Research Institute (Toronto, ON, Canada). Btk KO mice, FcγRIIB KO mice, and C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME).
Induction and reversal of ITP
Thrombocytopenia was induced by intraperitoneal injection of 2 μg rat antimouse integrin αIIb antibody (PharMingen, Mississauga, ON, Canada) as previously described.10 The following day, mice were injected with 2 g/kg human serum albumin (HSA) control protein, followed by 2 g/kg IVIg (Bayer, Elkhart, IN) on day 2. Platelet counts were assessed as previously described.10
Results and discussion
It is well accepted that inhibition of cell function through the inhibitory receptor FcγRIIB requires recruitment of the inositol phosphatase SHIP1 to the receptor ITIM in both B cells1,3,16-19 and mast cells.2-4 The expression of FcγRIIB on monocytes can lead to the down-regulation of phagocytosis,20 although the pathway by which FcγRIIB down-modulates monocyte function remains unclear. To better understand this inhibitory event in vivo, we examined IVIg-mediated amelioration of ITP, which uses FcγRIIB to alleviate thrombocytopenia.11 ITP was the first autoimmune disease successfully treated with IVIg, and IVIg is currently used to treat many autoimmune diseases.21-23 We have shown that IVIg can successfully ameliorate a murine model of antibody-induced thrombocytopenia with similar doses and kinetics as in humans with ITP.10 We investigated the signaling mediators used by IVIg through analyzing IVIg treatment of ITP in mice genetically deficient for primary signaling molecules juxtaposed to FcγRIIB.
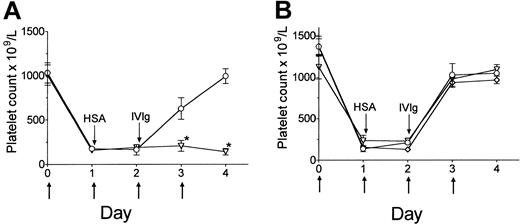
Mice injected with antiplatelet antibody became thrombocytopenic by day 1 after injection (Figure 1A). Treatment of mice with 2 g/kg HSA (control protein) did not affect ITP. However, treatment of mice with 2 g/kg IVIg successfully reversed the disease in wild-type mice (Figure 1A). In separate experiments, thrombocytopenic wild-type mice treated with HSA alone for the duration of the experiment displayed no increase in platelet count (data not shown). In contrast to wild-type mice, IVIg had no effect in FcγRIIB KO mice (Figure 1A); platelet counts in these mice remained low throughout the course of the experiment. These findings confirm that in our model system, the activity of IVIg in treating ITP is completely dependent on FcγRIIB expression. In addition, the complete lack of any elevation in platelet counts in FcγRIIB KO mice following IVIg treatment (Figure 1A, day 2 versus days 3 and 4) suggests that “competitive” reticuloendothelial system (RES) blockade per se does not significantly contribute to IVIg-mediated amelioration of thrombocytopenia.
IVIg requires expression of FcγRIIB but not SHIP1 to ameliorate murine ITP. (A) FcγRIIB KO (▿) or control wild-type (○) mice (C57BL/6) were injected with 2 μg anti-integrin αIIb antibody on days 0, 1, 2, and 3. The arrow (↑) denotes injection of anti-integrin αIIb antibody. All mice received an injection of 2 g/kg HSA control protein on day 1, followed by 2 g/kg IVIg on day 2 (↓, denotes injection of HSA or IVIg). Mice were bled daily for platelet enumeration. (B) SHIP1 heterozygous KO (⋄) and homozygous KO (▿) mice and littermate control (○) mice were treated as described in panel A. Data are expressed as mean ± SEM; n = 10 mice/data point. *P < .001 versus control.
IVIg requires expression of FcγRIIB but not SHIP1 to ameliorate murine ITP. (A) FcγRIIB KO (▿) or control wild-type (○) mice (C57BL/6) were injected with 2 μg anti-integrin αIIb antibody on days 0, 1, 2, and 3. The arrow (↑) denotes injection of anti-integrin αIIb antibody. All mice received an injection of 2 g/kg HSA control protein on day 1, followed by 2 g/kg IVIg on day 2 (↓, denotes injection of HSA or IVIg). Mice were bled daily for platelet enumeration. (B) SHIP1 heterozygous KO (⋄) and homozygous KO (▿) mice and littermate control (○) mice were treated as described in panel A. Data are expressed as mean ± SEM; n = 10 mice/data point. *P < .001 versus control.
The inositol phosphatase SHIP1 preferentially binds the FcγRIIB ITIM and it has been established that SHIP1 is the primary negative signaling pathway used by FcγRIIB in B cells1,3,16-18 and mast cells.2-4 SHIP1 can negatively regulate FcγR-mediated phagocytosis24 and overexpression of SHIP1 in murine macrophages can inhibit phagocytosis.25 Thus, we surmised that SHIP1 expression might play a key role in monocyte FcγRIIB-dependent inhibition by IVIg and hypothesized that SHIP1 KO mice would be unresponsive to IVIg treatment. We found, however, that IVIg was able to exert its effects in both homozygous and heterozygous SHIP1 KO mice to the same extent as littermate controls (Figure 1B). Thus the FcγRIIB-dependent activity of IVIg in immune thrombocytopenia is not dependent on the expression or activity of SHIP1.
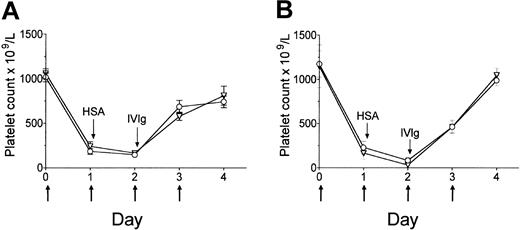
The tyrosine phosphatase SHP-1 can dephosphorylate multiple immunoreceptor-regulated substrates, leading to cell inactivation.5,6 SHP-1 has been shown to bind FcγRIIB under extreme conditions such as receptor superclustering6 or hyperphosphorylation.7 We examined whether this enzyme is a possible mediator in the pathway by which IVIg exerts its protective effect. Administration of IVIg to thrombocytopenic SHP-1-deficient mice resulted in an increase in platelet counts, indistinguishable from that of control littermates (Figure 2A). This suggests that like SHIP1, SHP-1 is not required for the FcγRIIB-mediated activity of IVIg. The FcγRIIB ITIM contains 2 distinct, albeit overlapping, binding sites for SHIPs and SHPs17 ; thus there exists the possibility that a redundancy may exist in the SHIP/SHP families. However, evidence suggests that FcγRIIB ITIM binds to SHIP proteins, but not SHP-1 or SHP-2 in vivo.26
Mice deficient for SHP-1 or Btk respond to IVIg treatment of ITP. Mice were treated as described in Figure 1A. (A) Littermate control (○), SHP-1-deficient (me/me; ▿) mice; n = 6 except for day 4, where n = 3 for SHP-1-deficient mice. (B) Btk KO (▿) and control wild-type (○) mice (C57BL/6) were treated as in Figure 1A; n = 10 mice/data point. Data are expressed as mean ± SEM.
Mice deficient for SHP-1 or Btk respond to IVIg treatment of ITP. Mice were treated as described in Figure 1A. (A) Littermate control (○), SHP-1-deficient (me/me; ▿) mice; n = 6 except for day 4, where n = 3 for SHP-1-deficient mice. (B) Btk KO (▿) and control wild-type (○) mice (C57BL/6) were treated as in Figure 1A; n = 10 mice/data point. Data are expressed as mean ± SEM.
Because these major known ITIM-dependent signaling intermediates did not appear to be required for activity of IVIg, we questioned whether IVIg therapy resulted in ITIM-independent FcγRIIB-mediated inhibition. This inhibitory pathway focuses on delivery of a proapoptotic signal generated through FcγRIIB homoaggregation27 and is dependent on the presence and expression of the Tec family kinase Btk,8 a B-cell kinase also expressed in monocytes.9 IVIg has been shown to induce apoptosis in multiple cell types, including monocytes.28 IVIg ameliorated thrombocytopenia in both Btk KO mice and control mice (Figure 2B), indicating that the activity of IVIg is not dependent on a Btk-dependent proapoptotic event.
We demonstrate that FcγRIIB-dependent IVIg activity is not reliant on the established signaling pathways downstream of FcγRIIB. Mice lacking the normal FcγRIIB downstream signaling mediators, SHIP1, SHP-1, or Btk, all responded to IVIg treatment of ITP as successfully as control mice. This suggests that inhibitory signaling through FcγRIIB on monocytes may use a different pathway than B cells. Indeed, a recent report has demonstrated that SHIP-dependent inhibition of monocyte function can occur independently of FcγRIIB, a mechanism distinct from that used by B-cell expressed FcγRIIB.24 It may be that inhibition of monocyte function by IVIg occurs via an FcγRIIB signaling pathway unique to monocytes.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2003-01-0023.
Supported by The Bayer-Canadian Blood Services-Héma Québec Partnership Fund and the National Cancer Institute of Canada with funds from the Terry Fox Foundation (R.K.H.). C.D.H. was a recipient of a BC Health Research Foundation Joint Scholarship and holds a Canadian Institutes of Health Research (CIHR) New Investigator Scholarship. K.A.S. is a CIHR Senior Scientist.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Alison F Starkey, Mr Hoang Le-Tien, Mr Davor Brinc, and Dr Vinayakumar Siragam for assistance and helpful discussion; Dr John W Semple for critical review of the manuscript; and Ms Carolyn Bateman of the British Columbia Cancer Agency Joint Animal Facility, Ms Michele Deverill of the Samuel Lunenfeld Research Institute, and the St Michael's Hospital Research Vivarium staff.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal