Abstract
Successful gene therapy of β-thalassemia will require replacement of the abnormal erythroid compartment with erythropoiesis derived from genetically corrected, autologous hematopoietic stem cells (HSCs). However, currently attainable gene transfer efficiencies into human HSCs are unlikely to yield sufficient numbers of corrected cells for a clinical benefit. Here, using a murine model of β-thalassemia, we demonstrate for the first time that selective enrichment in vivo of transplanted, drug-resistant HSCs can be used therapeutically and may therefore be a useful approach to overcome limiting gene transfer. We used an oncoretroviral vector to transfer a methylguanine methyltransferase (MGMT) drug-resistance gene into normal bone marrow cells. These cells were transplanted into β-thalassemic mice given nonmyeloablative pretransplantation conditioning with temozolomide (TMZ) and O6-benzylguanine (BG). A majority of mice receiving 2 additional courses of TMZ/BG demonstrated in vivo selection of the drug-resistant cells and amelioration of anemia, compared with untreated control animals. These results were extended using a novel γ-globin/MGMT dual gene lentiviral vector. Following drug treatment, normal mice that received transduced cells had an average 67-fold increase in γ-globin expressing red cells. These studies demonstrate that MGMT-based in vivo selection may be useful to increase genetically corrected cells to therapeutic levels in patients with β-thalassemia.
Introduction
Gene therapy is an attractive approach for a number of human lympho-hematopoietic disorders caused by single gene defects.1 In the last few years, several murine models of hematopoietic disorders, including β-thalassemia and sickle cell disease, have been successfully corrected using oncoretroviral or lentiviral vectors to target hematopoietic stem cells (HSCs).2-6 The success of these preclinical studies has relied on highly efficient gene transfer into murine HSCs and myeloablative conditioning of transplant recipients to insure high-level engraftment of transduced cells. However, current gene transfer efficiencies into human HSCs will likely be inadequate to achieve successful gene therapy for the hemoglobin disorders, particularly since there is only a modest selective advantage for corrected cells.7,8 In contrast, recently reported human trials for the severe combined immunodeficiency disorders have demonstrated that efficient stem cell gene transfer and myeloablation is not required when there is a powerful selective advantage to corrected cells.9,10
Despite recent improvements, levels of HSC-targeted retroviral gene transfer in nonhuman primate models and in human clinical marking protocols are significantly lower than those obtained in mice.11-14 Levels of genetically marked blood cells up to 5% to 10% have been obtained in only a small number of human patients, and long-term consistent marking at these levels has not yet been reported. Although lentiviral vectors appear to be a promising new approach for improving human stem cell gene transfer efficiency, it is unknown whether they will prove superior to oncoretroviral vectors in the clinical context.15-17 Additionally, the desire to avoid myeloablative conditioning in the setting of a gene therapy trial for patients with nonmalignant hematopoietic disorders would further reduce the proportion of engrafting genetically corrected cells due to dilution with residual endogenous HSCs. Therefore, an effective strategy to selectively enrich genetically corrected autologous HSCs to therapeutic levels in vivo is highly desirable. One approach that has been proposed is to incorporate a drug-resistance gene into the therapeutic vector.18 Following transplantation, treatment with a stem cell toxic drug could then be used to enrich the drug-resistant, genetically modified cells, while simultaneously eradicating the endogenous, abnormal stem cell population.
Toward this goal, we have evaluated an in vivo selection system based on the methylguanine methyltransferase (MGMT) drug-resistance gene for the treatment of β-thalassemia, a prevalent, inherited red blood cell disorder characterized by severe anemia due to deficiency of β-globin expression. MGMT is an alkyltransferase that functions to repair cellular DNA damage at the O6 position of guanine.19 The cytotoxicity of methylating agents such as temozolomide (TMZ) can be averted by MGMT-mediated removal of the drug-induced O6 adduct. Variant MGMT proteins with specific amino acid changes from the wild-type protein, such as Pro140Lys and Gly156Ala, retain significant activity and possess the useful property of resistance to inactivation by O6-benzylguanine (BG).20,21 BG can be used to inactivate endogenous MGMT and further enhance alkylator-mediated cell death. Due to their low-level expression of endogenous MGMT, HSCs may be particularly sensitive to the cytotoxic effect of O6 adduct formation induced by TMZ and other alkylating agents.22 Consistent with this is the recent demonstration by several groups that retroviral-mediated transfer and expression of variant MGMT genes can be used for selection of genetically modified HSCs in normal mice.23-25
Here we report the first successful use of MGMT-based in vivo selection of drug-resistant hematopoietic cells to ameliorate an animal model of a human genetic disorder. Following nonmyeloablative conditioning of β-thalassemic mice, in vivo selection of a small population of transplanted normal cells expressing the MGMT vector resulted in significant and sustained hematologic improvement. In additional experiments, incorporation of an MGMT expression cassette into a γ-globin lentiviral vector allowed an average 67-fold enrichment of γ-globin–expressing red blood cells (RBCs), further demonstrating the utility of this system in the context of a therapeutic vector.
Materials and methods
Mice
Twelve- to 20-week-old male and female β-thalassemic mice26 backcrossed onto the C57BL/6J and B6.C-TyrcH1b Hbbd/By (referred to as HW80) backgrounds7 were used as bone marrow (BM) donors and recipients as indicated. Female C57BL/6J, HW80, and C57BL/6J Ly5.1 (B6.SJL-Ptrpca Pep3b/BoyJ) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and used between 8 and 14 weeks of age.
MGMT expression vectors, gene transfer, and transplantation procedures
The MSCV-P140K-IR-GFP (murine stem cell virus–Pro140Lys–internal ribosome entry site–green fluorescent protein) (encoding human MGMT with a proline to lysine amino acid change at position 140) and MFG-ΔMGMT (encoding human MGMT with a glycine to alanine amino acid change at position 156) vectors and vector producer cells have been described previously.25,27 The MFG-ΔMGMT producer cells were a gift from Dr Stanton L. Gerson (Case Western Reserve University, Cleveland, OH). We have previously described methods for oncoretroviral gene transfer into murine BM cells.28 The d432β-γ MSCV Pro140Lys MGMT–posttranscriptional regulatory element (PRE) lentiviral vector was generated by inserting an MSCV Pro140Lys MGMT-PRE cassette (Figure 5A) into the previously described d432β-γ lentiviral vector5 (details available upon request). Lentiviral vector particles were generated and BM cells from 5-fluorouracil–treated mice were transduced as previously described.5 For the generation of mice having predominantly β-thalassemic hematopoiesis but a low level of chimerism with MGMT-transduced normal cells, HW80 mice were irradiated with 11 Gy and injected with BM cell mixtures of 1.0 × 105 to 4 × 105 MGMT/GFP-transduced, normal C57BL/6J Ly 5.1 BM cells and 1.6 × 106 to 1.9 × 106 β-thalassemic C57BL/6J BM cells. The competitive repopulation assay used C57BL/6J Ly 5.1 and HW80 (Ly 5.2) normal mice and was performed by mixing pooled BM volume equivalents from 3 TMZ/BG-treated mice (Ly 5.1) with pooled BM from 3 untreated animals (Ly 5.2) in a 1:1 ratio and then transplanting this mixture into irradiated (11 Gy) recipients. Thirteen weeks after transplantation, donor contribution to lymphocytes and granulocytes was determined by fluorescence-activated cell sorting (FACS) analysis (FACSCalibur, Becton Dickinson Immunocytochemistry Systems, San Jose, CA) for the Ly 5.1 and 5.2 markers. The observed donor contribution or hematopoietic activity of BM from treated animals was normalized to that of untreated Ly 5.1 BM competed with the same competitor Ly 5.2 cells. Secondary transplantation experiments were performed by transplanting the BM cells flushed from the hind limbs of a primary animal into 3 secondary animals. Secondary spleen colony-forming cell (CFU-S) assays were performed by transplanting 5 × 104 to 1 × 105 BM cells from a primary recipient into C57BL/6J mice that had received 9.5 Gy. Discrete, well-separated splenic colonies were dissected and analyzed 13 days after transplantation.
In vivo selection of γ-globin–expressing RBCs. (A) Diagram of the self-inactivating γ-globin/MGMT lentiviral vector. The γ-globin expression cassette is in reverse transcriptional orientation relative to the provirus and is driven by a 130-bp β-globin promoter in conjunction with DNase I hypersensitive site (HS) fragments HS4 (445 bp), HS3 (898 bp), and HS2 (374 bp) from the β-globin locus control region. The MSCV LTR-driven Pro140Lys MGMT also contains the PRE from the woodchuck (WC) hepatitis virus. cPPT indicates central polypurine tract/DNA flap; RRE, Rev-responsive element; and Ex, exon. Arrows delineate the transcriptional orientation of the expression cassettes. (B) FACS analysis for human γ-globin expression (HbF-PE fluorescence) in the RBCs of mice that received transplants of γ-globin/MGMT–transduced BM cells. Analysis of a representative control, untreated animal is shown in the top left (baseline) and right (at the end of the observation period) panels. Shown in the panels below are analyses of drug-treated animals at baseline (left column) and after completion of 3 courses of TMZ/BG (right column). The percentage of γ-globin–expressing cells is shown for each histogram. Solid lines indicate the γ-globin staining profile for each experimental animal, whereas dotted lines indicate the staining profile of a negative control mouse.
In vivo selection of γ-globin–expressing RBCs. (A) Diagram of the self-inactivating γ-globin/MGMT lentiviral vector. The γ-globin expression cassette is in reverse transcriptional orientation relative to the provirus and is driven by a 130-bp β-globin promoter in conjunction with DNase I hypersensitive site (HS) fragments HS4 (445 bp), HS3 (898 bp), and HS2 (374 bp) from the β-globin locus control region. The MSCV LTR-driven Pro140Lys MGMT also contains the PRE from the woodchuck (WC) hepatitis virus. cPPT indicates central polypurine tract/DNA flap; RRE, Rev-responsive element; and Ex, exon. Arrows delineate the transcriptional orientation of the expression cassettes. (B) FACS analysis for human γ-globin expression (HbF-PE fluorescence) in the RBCs of mice that received transplants of γ-globin/MGMT–transduced BM cells. Analysis of a representative control, untreated animal is shown in the top left (baseline) and right (at the end of the observation period) panels. Shown in the panels below are analyses of drug-treated animals at baseline (left column) and after completion of 3 courses of TMZ/BG (right column). The percentage of γ-globin–expressing cells is shown for each histogram. Solid lines indicate the γ-globin staining profile for each experimental animal, whereas dotted lines indicate the staining profile of a negative control mouse.
Drug treatment with TMZ and BG
Pre- and posttransplantation drug treatment of mice with TMZ (Schering-Plough, Madison, New Jersey) at 66 mg/kg by gastric lavage and BG at 15 mg/kg intraperitoneally (except for the first treatment course in the initial chimera study where BG was given at 30 mg/kg) was performed as previously described.25 BG was provided as a gift from Dr R. C. Moschel (National Cancer Institute, Frederick, MD).
Hematologic analysis
Blood samples were obtained from anesthetized mice by retro-orbital puncture and analyzed using an automated blood cell analyzer as described.7 Methods for preparing peripheral blood (PB) smears, hemoglobin (Hb) cellulose acetate gel electrophoresis, and flow cytometric analysis of GFP-expressing blood cells have all been described elsewhere.7 Reticulocyte counts were estimated as described by using FACS analysis to determine the proportion of red cells staining with the RNA binding dye thiazole orange (Aldrich, Milwaukee, WI).29 The data were collected using a FACSCalibur and analyzed using the Cell Quest System (Becton Dickinson Immunocytochemistry Systems).
DNA analysis
Southern blot analysis of BM, spleen, and spleen colony tissue was carried out using 10 to 15 μg DNA cut with the indicated restriction enzyme. A full-length Pro140Lys MGMT cDNA fragment was used as the probe. Average MGMT vector copy number in BM and spleen was estimated by comparison to the hybridization signal of NIH 3T3 cells transduced with the MFG-ΔMGMT vector (NIH 3T3 Gly156Ala) which have an estimated average copy number of 6.
FACS analysis of RBCs for expression of human γ-globin
Five to 10 μL blood was used to fix, permeabilize, and stain RBCs with a biotinylated monoclonal antibody against the human γ-globin chain (Perkin-Elmer Wallac, Norton, OH) as previously described.7 In brief, 5 μL monoclonal antibody was incubated for 30 minutes on ice with permeabilized cells. After washing, cells were then incubated in a 1:200 streptavidin-phycoerythrin (PE) secondary reagent (Southern Biotechnology, Birmingham, AL) in order to identify cells stained with the primary antibody. RBCs were gated on by light-scatter characteristics and analyzed for PE fluorescence using a FACSCalibur.
Statistical analysis
The probability of a statistically significant difference between the mean values of 2 data sets was determined by a 2-tailed Student t test using InStat 2.03 software from Apple (Cupertino, CA).
Results
Therapeutic in vivo selection in β-thalassemic/normal BM chimeras
To test whether TMZ/BG drug treatment could enrich genetically normal, drug-resistant cells to therapeutic levels in the setting of β-thalassemia, we initially generated murine BM chimeras having the majority of their hematopoiesis derived from β-thalassemic stem cells but with low levels of normal, MGMT-transduced cells. Normal BM cells transduced with an MSCV retroviral vector encoding both the Pro140Lys MGMT variant and the GFP25 were mixed in small proportions with mock-transduced β-thalassemic BM cells (see “Materials and methods”) and then transplanted into lethally irradiated normal mice. Following hematopoietic reconstitution, PB from these animals was analyzed for both standard hematologic parameters and the proportion of MGMT/GFP+ RBCs. At 6 weeks after transplantation, the animals were anemic (Figure 1A) and PB smears showed the presence of characteristic β-thalassemic red blood cell abnormalities.
Amelioration of anemia in mice with β-thalassemic hematopoiesis after in vivo selection of Pro140Lys MGMT/GFP-transduced hematopoietic cells. (A) PB Hb concentrations (g/dL) are shown for the control, untreated mice (▪) and the group of mice treated with TMZ/BG (▴). Mice not surviving the treatment are indicated (⋄). Values at 6 weeks (baseline), 18 weeks (6 weeks after the final drug treatment), and 30 weeks (12 weeks following the final drug treatment) after transplantation are shown. The mean baseline values of Hb concentration for the entire group of treated mice and the 5 mice that responded to drug treatment did not statistically differ from those of the untreated group of animals (P = .55 and P = .52, respectively). (B) Percentage of GFP+ RBCs at the indicated times after transplantation for the control, untreated mice (▪) and the mice treated with TMZ/BG (▴). Mice not surviving the treatment are indicated (⋄). The mean baseline values of GFP+ RBCs for the entire group of treated mice and the 5 mice that responded to drug treatment did not statistically differ from those of the untreated group of animals (P = .61 and P = .99, respectively). (C) In vivo selection of Pro140Lys MGMT/GFP+ RBCs in TMZ/BG-treated mice. FACS histograms for GFP expression in RBCs are shown for the indicated drug-treated mice. The percentage of GFP+ cells is indicated in the upper right corner of each histogram. The left panels show pretreatment baseline analyses and the right panels show posttreatment analyses at 18 weeks after transplantation. The solid lines indicate the profiles for the experimental mice, whereas the dotted lines indicate the profile for a normal, negative control mouse.
Amelioration of anemia in mice with β-thalassemic hematopoiesis after in vivo selection of Pro140Lys MGMT/GFP-transduced hematopoietic cells. (A) PB Hb concentrations (g/dL) are shown for the control, untreated mice (▪) and the group of mice treated with TMZ/BG (▴). Mice not surviving the treatment are indicated (⋄). Values at 6 weeks (baseline), 18 weeks (6 weeks after the final drug treatment), and 30 weeks (12 weeks following the final drug treatment) after transplantation are shown. The mean baseline values of Hb concentration for the entire group of treated mice and the 5 mice that responded to drug treatment did not statistically differ from those of the untreated group of animals (P = .55 and P = .52, respectively). (B) Percentage of GFP+ RBCs at the indicated times after transplantation for the control, untreated mice (▪) and the mice treated with TMZ/BG (▴). Mice not surviving the treatment are indicated (⋄). The mean baseline values of GFP+ RBCs for the entire group of treated mice and the 5 mice that responded to drug treatment did not statistically differ from those of the untreated group of animals (P = .61 and P = .99, respectively). (C) In vivo selection of Pro140Lys MGMT/GFP+ RBCs in TMZ/BG-treated mice. FACS histograms for GFP expression in RBCs are shown for the indicated drug-treated mice. The percentage of GFP+ cells is indicated in the upper right corner of each histogram. The left panels show pretreatment baseline analyses and the right panels show posttreatment analyses at 18 weeks after transplantation. The solid lines indicate the profiles for the experimental mice, whereas the dotted lines indicate the profile for a normal, negative control mouse.
Mice that received transplants were randomly assigned to receive either drug treatment with TMZ and BG or no treatment. Baseline Hb concentration (treated group, 101 ± 2 g/L [10.1 ± 0.2 g/dL] versus control group, 102 ± 1 g/L [10.2 ± 0.1 g/dL], P = .55; Figure 1A) and the percentage of MGMT/GFP+ RBCs (treated group, 7.5% ± 1.6% versus control group, 8.3% ± 1.5%, P = .99; Figure 1B) did not significantly differ between the 2 groups. The treated group received two 5-day courses of TMZ and BG 5 weeks apart beginning 7 weeks after transplantation. Eight of 12 drug-treated animals responded with increases in MGMT/GFP+ RBCs after the first treatment (mean of 7.5% ± 1.6% increasing to 56% ± 6%). In contrast, MGMT/GFP+ RBCs decreased in the control mice (mean of 8.3% ± 1.5% decreasing to 3.1% ± 0.9%, n = 8). Six weeks following the second treatment with TMZ/BG (18 weeks after transplantation), the mean number of MGMT/GFP+ RBCs increased further to 69% ± 7.7% in 5 responding animals that survived (Figure 1B-C). Increases in MGMT/GFP-transduced PB myeloid (baseline 4.1% ± 2.8% increasing to 45% ± 15%) and lymphoid cells (baseline of 2.2% ± 0.8% increasing to 38% ± 9%) were also observed in these mice following drug treatment. In contrast, MGMT/GFP+ RBCs remained unchanged in the untreated control group (3.1% ± 1.0%; with significant difference from responding animals, P < .0001) over the same time interval (Figure 1B). Similarly, PB MGMT/GFP+ myeloid and lymphoid cells remained low in the untreated animals (3.1% ± 0.4% and 2.3% ± 0.5%, respectively).
Concomitant with in vivo selection of the MGMT/GFP+ cells, there was a significant increase in the PB Hb concentration (Figure 1A). The responding animals in the treated group had a mean Hb concentration of 113 ± 4 g/L (11.3 ± 0.4 g/dL), whereas animals in the untreated group had a mean Hb concentration of 95 ± 2 g/L (9.5 ± 0.2 g/dL) (significant difference with P < .002) at 18 weeks after transplantation. PB smears from the treated animals showed replacement of β-thalassemic RBCs with morphologically normal RBCs. In contrast, the RBCs of animals in the untreated control group showed the characteristic abnormalities of β-thalassemia, including poorly hemoglobinized cells of widely varying sizes and shapes.
In this experiment, 2 nonresponding and 1 responding animal in the treatment group died due to hematopoietic toxicity. Two other responding animals died acutely during the 5-day period of drug administration due to complications of the gastric lavage procedure in one instance and from neurotoxicity attributable to the polyethylene glycol used as a vehicle for BG in the other instance. Acute deaths were not observed in the subsequent experiments described below due to improved lavage technique and reduction in the polyethylene glycol concentration.
Sustained hematologic improvement in animals that received primary and secondary transplants
Thirty weeks after transplantation, the responding, drug-treated animals showed persistent hematologic improvement and high levels of transduced RBCs (Figure 1A-B). These animals had a mean Hb level of 109 ± 2 g/L (10.9 ± 0.2 g/dL), suggesting that in vivo selection of primitive cells with long-term hematopoietic activity had occurred (Figure 1A). In contrast, the mean Hb level in the control group decreased further to 88 ± 2 g/L (8.8 ± 0.2 g/dL) (significant difference from above group with P < .002), reflecting progressively ineffective β-thalassemic erythropoiesis in these animals. To further evaluate whether selection occurred at the level of the repopulating HSCs, 2 representative animals from both the drug-treated and untreated control groups were killed and secondary BM transplantations were performed. Twelve weeks after transplantation, the levels of MGMT/GFP+ RBCs averaged 60% and 99% in secondary recipients of BM from drug-treated donors (Figure 2A). High levels of PB MGMT/GFP+ myeloid and lymphoid cells (> 80%) were also observed in both sets of secondary animals that received BM transplants from the drug-treated donors. These mice displayed a mean Hb concentration of about 120 g/L (12 g/dL) (Figure 2B) and their RBCs had normal morphology. In contrast, the proportion of MGMT/GFP+ RBCs and leukocytes in animals derived from untreated, control animals was approximately 2% or less (Figure 2A). These animals displayed severe anemia (Figure 2B) and the characteristic blood smear findings of β-thalassemia. These data confirmed that the phenotypic improvement in drug-treated animals resulted from selection of HSCs transduced with the MGMT vector.
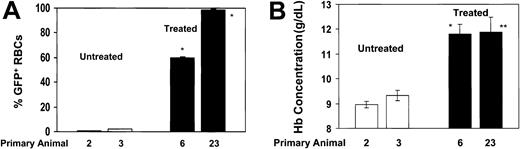
Secondary animals that received BM-cell transplants from responding, primary drug-treated animals demonstrate persistence of high levels of Pro140Lys MGMT/GFP+ RBCs and a corrected hematologic phenotype. Secondary transplantation experiments were performed by transplanting the BM cells flushed from the hind limbs of a primary animal into 3 secondary animals. (A) The percentages of Pro140Lys MGMT/GFP+ RBCs are shown in animals that received secondary transplants derived from control untreated animals (□; animals 2 and 3) and secondary animals derived from drug-treated animals that demonstrated in vivo selection (▪; animals 6 and 23). * indicates P < .0001 versus both control, untreated data sets. (B) PB Hb concentration in the same secondary recipients as shown in (A). * indicates P < .0084 versus both control, untreated data sets; **P = .038 versus untreated control 2 and P = .055 versus untreated control 3. Analysis was performed at 12 weeks after transplantation and all values represent the mean ± SEM.
Secondary animals that received BM-cell transplants from responding, primary drug-treated animals demonstrate persistence of high levels of Pro140Lys MGMT/GFP+ RBCs and a corrected hematologic phenotype. Secondary transplantation experiments were performed by transplanting the BM cells flushed from the hind limbs of a primary animal into 3 secondary animals. (A) The percentages of Pro140Lys MGMT/GFP+ RBCs are shown in animals that received secondary transplants derived from control untreated animals (□; animals 2 and 3) and secondary animals derived from drug-treated animals that demonstrated in vivo selection (▪; animals 6 and 23). * indicates P < .0001 versus both control, untreated data sets. (B) PB Hb concentration in the same secondary recipients as shown in (A). * indicates P < .0084 versus both control, untreated data sets; **P = .038 versus untreated control 2 and P = .055 versus untreated control 3. Analysis was performed at 12 weeks after transplantation and all values represent the mean ± SEM.
Therapeutic in vivo selection in β-thalassemic recipient mice following nonmyeloablative conditioning
In order to more closely approximate a clinical trial in which nonmyeloablated patients receiving transduced cells might undergo subsequent in vivo selection, we next tested whether MGMT-based selection could be therapeutically beneficial in the context of transplantation of drug-resistant cells into nonmyeloablated β-thalassemic animals. To first evaluate the toxicity of the drug regimen in β-thalassemic mice that have pre-existing anemia, these animals along with control C57BL/6 normal mice (n = 3 in each group) were treated with the 5-day course of TMZ (66 mg/kg/d) and BG (15 mg/kg/d). Both groups of mice demonstrated transient decreases in their baseline hematocrits. No animals died and hematocrits recovered to baseline values in all cases. In order to establish the degree of reduction in HSC mass achieved with a conditioning course of TMZ and BG, a competitive repopulation BM transplantation assay was used. Normal C57BL/6 Ly5.1 mice treated with TMZ/BG showed an approximate 80% decrease in BM stem cell activity when competed against untreated HW80 Ly 5.2 normal BM (see “Materials and methods”; data not shown). TMZ and BG treatment therefore provides a relatively nontoxic method to reduce the numbers of endogenous HSCs without full ablation of hematopoiesis.
Based on these results, β-thalassemic mice received a 5-day pretransplantation conditioning course of TMZ and BG followed on day 6 by transplantation with normal C57BL/6 BM cells transduced with a retroviral vector encoding only the Gly156Ala MGMT variant.27 Since the C57BL/6 donor and the β-thalassemic (THAL) recipient on the HW80 background differ at the H1b minor histocompatibility locus, this design also modeled a possible treatment approach in which normal allogeneic HSCs are transduced and subsequently selected in nonablated patients. Three weeks after receiving transduced cells, mice were randomly assigned to either undergo treatment with 2 additional TMZ/BG courses 5 weeks apart (n = 10) or to receive no further treatment (n = 5). Six weeks after completion of the treatment phase (14 weeks after transplantation), PB from both the treated and untreated control groups was analyzed for the presence and level of donor-derived red cells and hematologic parameters. An estimate of the proportion of RBCs derived from the transduced donor graft (“single” Hb type) and that of the host β-thalassemic RBCs (“diffuse” Hb type) was made using cellulose acetate gel electrophoresis (Figure 3A). Six of 10 animals in the treated group showed nearly complete conversion to the hemoglobin type of the MGMT-transduced, C57BL/6 (B6) donor graft. Although the 4 nonresponding animals survived the drug treatment, these animals presumably failed to engraft with MGMT-expressing cells. In contrast to the significant frequency and levels of engrafted donor cells in the drug-treated animals, only 1 of 5 animals that received transduced cells without additional TMZ/BG treatment courses showed any detectable donor red cell contribution (Figure 3A). Twenty-one weeks after transplantation, sustained in vivo selection of the donor graft was observed in 4 surviving, responding animals. However, little or no donor hemoglobin was observed in the 3 surviving, untreated control animals. Although treated animal 235 demonstrated robust in vivo selection of donor cells, death later occurred due to BM failure. This was likely due to the absence of vector-expressing cells with long-term repopulating activity.
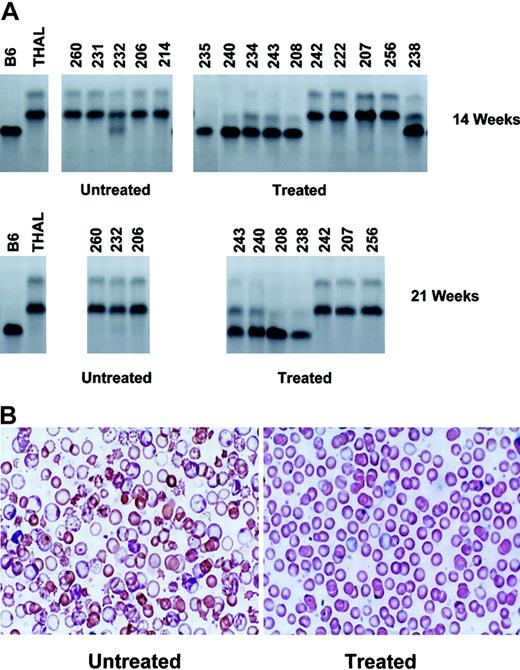
In vivo selection of transplanted MGMT-transduced normal BM cells results in sustained hematologic improvement in nonmyeloablated β-thalassemic mice. (A) Cellulose acetate gel electrophoresis of red cell lysates from donor C57BL/6 (B6), recipient β-thalassemic (THAL), and untreated control and drug-treated β-thalassemic recipient animals 14 weeks (top row) and 21 weeks (bottom row) after transplantation with 7 ×106 MGMT-transduced, nucleated BM cells. The MGMT-transduced, donor B6 graft has the “single” hemoglobin pattern, while the recipient THAL has the “diffuse” pattern. Mouse identification numbers are shown at the top of each lane. (B) Wright-Giemsa–stained blood smears are shown for a representative control, untreated animal (left) and for a drug-treated animal that demonstrated in vivo selection of transduced cells (right). Original magnification, × 250.
In vivo selection of transplanted MGMT-transduced normal BM cells results in sustained hematologic improvement in nonmyeloablated β-thalassemic mice. (A) Cellulose acetate gel electrophoresis of red cell lysates from donor C57BL/6 (B6), recipient β-thalassemic (THAL), and untreated control and drug-treated β-thalassemic recipient animals 14 weeks (top row) and 21 weeks (bottom row) after transplantation with 7 ×106 MGMT-transduced, nucleated BM cells. The MGMT-transduced, donor B6 graft has the “single” hemoglobin pattern, while the recipient THAL has the “diffuse” pattern. Mouse identification numbers are shown at the top of each lane. (B) Wright-Giemsa–stained blood smears are shown for a representative control, untreated animal (left) and for a drug-treated animal that demonstrated in vivo selection of transduced cells (right). Original magnification, × 250.
Concomitant with in vivo selection of donor cells, the mean PB Hb concentration of the responding, drug-treated animals 14 weeks after transplantation was increased compared with that of the untreated control group (114 ± 3 g/L versus 98 ± 4 g/L [11.4 ± 0.3 g/dL versus 9.8 ± 0.4 g/dL]; P = .0097). Furthermore, blood smears from the treated, responding animals demonstrated replacement of thalassemic red cells with those derived from the normal donor graft (Figure 3B). In contrast, mice that received no treatment displayed the characteristic blood smear findings for β-thalassemia. Persistent hematologic correction at 21 weeks after transplantation was observed in the responding, treated animals compared with the untreated control group (mean Hb level of 117 ± 3 g/L versus 92 ± 5 g/L [11.7 ± 0.3 g/dL versus 9.2 ± 0.5 g/dL]; P = .004). Also indicative of disease amelioration, the responding, treated animals displayed a significant reduction at that time in the PB reticulocyte count (4.2% ± 0.6%) relative to that of the untreated control group (28.2% ± 3.8%; significant difference with P = .0007).
If in vivo selection in these animals occurred at the level of a pluripotent repopulating cell, both BM myeloid cells and lymphoid cells from the spleen should be significantly enriched in cells containing the MGMT provirus. In order to test this prediction, Southern blot analysis of BM and spleen DNA from animals from the drug-treated and control groups was performed to determine the presence of the MGMT vector genome. The BM DNA from responding, drug-treated animals (208, 243) showed a strongly hybridizing band of the expected size of the unrearranged provirus (Figure 4A). We estimated the average vector copy number per BM cell from animals 208 and 243 to be 1.4 and 1.8, respectively. Two treated, nonresponding animals (207 and 256) as well as a control nontreated animal (232) did not show a significant BM DNA signal. Similar results were obtained when spleen DNA of these animals was analyzed, although the estimated average vector copy numbers (0.54 and 0.5, respectively) were somewhat lower (Figure 3A).
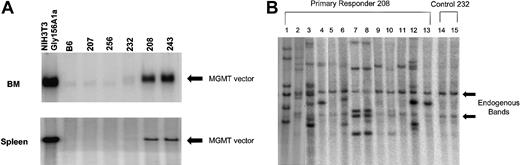
High levels of proviral DNA in the BM and spleen of primary, drug-treated, animals and in clonal spleen colonies of mice that received secondary transplants. (A) Southern blot analysis using an MGMT probe to detect the Gly156Ala MGMT vector in BM and spleen DNAs digested with EcoRV, which liberates a near unit length proviral fragment. Analysis of DNA samples from NIH 3T3-Gly156Ala–transduced cells, control B6, treated nonresponders (207, 256) untreated control (232), and treated responders (208, 243) are shown. The black arrow indicates the near unit length provirus of correct molecular size. (B) Southern blot analysis of spleen colony DNAs digested with BamHI, which cuts once within the proviral DNA and liberates a proviral-genomic DNA junctional fragment for each proviral integration. Lanes 1 through 13 represent distinct DNAs from secondary spleen colonies derived from animals transplanted with BM cells of animal 208. Lanes 14 and 15 represent DNA from spleen colonies harvested from animals that received BM-cell transplants of control animal 232. The arrows indicate the endogenous MGMT hybridizing sequences, which were similarly observed in normal mouse spleen DNA (data not shown). Unique patterns are observed for lanes 1, 2, 3, 4, 6, 12, and 13, whereas similar patterns are observed for lanes 7, 8, 10, and also for lanes 9 and 11. Some patterns may reflect additional integration events into daughter cells derived from a transduced parental cell (eg, 4 and 13).
High levels of proviral DNA in the BM and spleen of primary, drug-treated, animals and in clonal spleen colonies of mice that received secondary transplants. (A) Southern blot analysis using an MGMT probe to detect the Gly156Ala MGMT vector in BM and spleen DNAs digested with EcoRV, which liberates a near unit length proviral fragment. Analysis of DNA samples from NIH 3T3-Gly156Ala–transduced cells, control B6, treated nonresponders (207, 256) untreated control (232), and treated responders (208, 243) are shown. The black arrow indicates the near unit length provirus of correct molecular size. (B) Southern blot analysis of spleen colony DNAs digested with BamHI, which cuts once within the proviral DNA and liberates a proviral-genomic DNA junctional fragment for each proviral integration. Lanes 1 through 13 represent distinct DNAs from secondary spleen colonies derived from animals transplanted with BM cells of animal 208. Lanes 14 and 15 represent DNA from spleen colonies harvested from animals that received BM-cell transplants of control animal 232. The arrows indicate the endogenous MGMT hybridizing sequences, which were similarly observed in normal mouse spleen DNA (data not shown). Unique patterns are observed for lanes 1, 2, 3, 4, 6, 12, and 13, whereas similar patterns are observed for lanes 7, 8, 10, and also for lanes 9 and 11. Some patterns may reflect additional integration events into daughter cells derived from a transduced parental cell (eg, 4 and 13).
To evaluate whether primitive hematopoietic cell clones were selected by the drug treatment, a secondary CFU-S assay was performed. Thirteen days after BM cells from primary treated and control mice were transplanted into irradiated mice, clonal splenic hematopoietic colonies were obtained. The DNA from 12 of 13 colonies derived from treated animal 208 contained the vector genome as judged by the presence of one or more vector-host genomic DNA junction fragments on Southern blot analysis (Figure 4B, lanes 1-13). These vector-host genomic DNA fragments reflect unique proviral integration sites. At least 6 different clones, as distinguished by different patterns of vector integration, gave rise to the colonies derived from the BM of the drug-treated animal (Figure 4B). In contrast, none of the colonies (23 examined) derived from the untreated control mice no. 232 (Figure 4B, lanes 14-15) and 206 contained vector DNA (data not shown). These data demonstrate that efficient selection of primitive BM cells containing the vector occurred and that multiple transduced clones can be selected by the drug treatment.
In vivo selection of cells transduced with a dual gene γ-globin/MGMT lentiviral vector
The above findings demonstrate proof that in vivo selection of genetically normal cells can significantly improve the β-thalassemic condition. We sought to extend these findings by testing a dual gene lentiviral vector encoding both the Pro140Lys MGMT drug resistance gene and a therapeutic human γ-globin expression cassette. An MSCV–long-terminal repeat (LTR)–driven Pro140Lys MGMT expression cassette was incorporated into a self-inactivating lentiviral vector containing a human γ-globin genomic cassette driven by the β-globin promoter and regulatory elements from the β-globin locus control region (Figure 5A). We have recently shown that this vector is capable of therapeutic expression in β-thalassemic mice.5 The dual gene vector genome was transmitted without rearrangement into NIH 3T3 cells and both genes were functional in murine erythroleukemia cells (data not shown).
To assess whether selection using this vector could be accomplished in vivo, normal C57BL/6 BM cells transduced with a low vector concentration (multiplicity of infection of ∼1) were transplanted into lethally irradiated HW80 mice. RBCs expressing human γ-globin were present at levels of 2% or less in all animals (n = 11) 8 weeks after transplantation (Figure 5B, left panels, and data not shown). Seven randomly chosen animals were treated with 3 cycles of TMZ/BG over a period of 4 months. Four untreated animals served as controls. Marked increases in γ-globin–expressing red cells were observed in 5 of 7 drug-treated animals (Figure 5B, right panels). The mean level of γ-globin–expressing red cells increased from 1.0% ± 0.2% to 67% ± 15% in these mice. One animal displayed a 2900-fold increase in γ-globin–expressing cells (0.03% increasing to 88%). One responding animal exhibited an increase in γ-globin–expressing red cells from 1.5% to 32% but died after the second drug treatment along with 2 other nonresponding animals. In contrast to the drug-treated animals, all 4 nontreated animals showed a decline in human γ-globin–positive cells (baseline mean of 1.0% ± 0.04% decreasing to 0.4% ± 0.2% 4 months later). Despite the significant levels of antibody staining for human γ-globin described above, appreciable accumulation of human γ2/murine α2 tetramers in the normal erythrocytes, as assessed by cellulose acetate gel electrophoresis, was not observed (data not shown). This finding is consistent with previous globin transgenic and gene transfer studies in mice indicating poor incorporation of human globin chains into chimeric hemoglobin tetramers in the absence of a pre-existing deficiency of endogenous mouse β-globin chains3-5,30,31 (D.A.P., unpublished observations, October 2001). Thus, definitive evidence of therapeutic levels of vector-encoded γ-globin will require testing in the β-thalassemic model.
Discussion
Effective gene therapy for β-thalassemia in humans will require sustained, high-level expression of a globin transgene in a substantial number of stem cell–derived, developing erythroid precursors. Recent studies have described successful treatment of mouse models of both β-thalassemia and sickle cell disease using lentiviral-mediated gene transfer of globin genes.3-6 However, disease correction was dependent on transduction of most or all HSCs in combination with myeloablative conditioning of transplant recipients, which allowed high-level engraftment with transduced cells. Obtaining similar results in humans is currently not possible considering the relative resistance of human stem cells to gene transfer and the possible use of nonmyeloablative conditioning for human patients. Together, these factors will likely lead to low-level engraftment of corrected cells.
Using a murine hematopoietic disease model, we provide here the first definitive evidence that in vivo selection of normal transplanted stem cells containing a retroviral-transferred MGMT drug resistance gene can ameliorate a disease phenotype. This was due to both the elimination of the diseased endogenous β-thalassemic HSCs and the selection of the transduced HSCs. Furthermore, using a γ-globin/MGMT dual gene lentiviral vector, we also present the first proof that the MGMT selection system can be used to obtain substantial in vivo enrichment of cells coexpressing a therapeutic gene. Both of these findings confirm the predictions, based on previous work,23-25 that this selection system has significant potential therapeutic application in increasing corrected cell chimerism to clinically relevant levels.
Overall, successful in vivo selection occurred in a majority of animals in this work (19 of 29; 66%). Following cytotoxic drug treatment, nontherapeutic, low levels of transplanted, normal cells were significantly enriched in β-thalassemic/normal HSC chimeras with resulting disease amelioration. Secondary transplantation studies confirmed that selection occurred at the level of the HSC. In other experiments, therapeutic in vivo selection was also obtained in the setting of nonmyeloablative conditioning of β-thalassemic recipients. Following infusion of transduced cells and subsequent drug treatment to enact in vivo selection, a majority of animals showed resolution of anemia with nearly complete, stable conversion of their red cell compartment to cells derived from the transduced, donor graft. In contrast, untreated control animals displayed no significant engraftment of donor cells and remained anemic. Secondary CFU-S experiments revealed efficient polyclonal selection of primitive cells. In addition to modeling therapeutic selection of genetically corrected cells, our results also provide evidence that posttransplantation selection of drug-resistant, minormismatched cells from an allogeneic donor may be a potential approach to obtain therapeutic levels of donor cell chimerism in the nonmyeloablative setting.
We have recently demonstrated the therapeutic capacity of a γ-globin lentiviral vector in the same model of murine β-thalassemia intermedia used in this work.5 In that study, hematologic correction was dependent on achieving high levels of γ-globin–expressing red cells (∼80%) and required transduction of most HSCs with 1 or more copies of the vector. Similarly, others have also found that hematologic correction of murine β-thalassemia using β-globin vectors required on average 80% to 100% expressing cells, with each transduced cell containing an average of 0.8 to 3 proviral copies.3,4,6 Using a novel γ-globin/MGMT lentiviral vector, we found that γ-globin–expressing cells can be increased from very low levels (∼1%) to levels in excess of 80% using MGMT-mediated in vivo selection. This suggests that this approach might alleviate the need for high-level gene transfer and myeloablative conditioning in order to achieve a clinical benefit for β-thalassemia. In addition, in vivo selection, through selection of clones with proviral integration sites favorable to globin expression, may diminish the variability of expression that has been observed for globin lentiviral vectors.4,5
An important consideration regarding the ultimate use of the MGMT selection system in humans is whether it can be used for stem cell selection with acceptable toxicity. In our mouse studies, despite no prophylactic antibiotic support, 18 (62%) of 29 treated animals survived the experimental manipulations overall, while 13 (68%) of 19 responding animals survived. Deaths occurred in some cases due to technical issues, while in other cases, deaths were due to BM failure, sometimes despite evidence of in vivo selection. In the latter instances, it is possible that the absence of engrafted, vector-expressing stem cells resulted in mortality despite in vivo selection of progenitors with limited expansion. Supportive of this idea was polymerase chain reaction (PCR) analyses of BM DNA from several nonresponding, nonmyeloablated animals, which showed the absence of MGMT-transduced cells (D.A.P., unpublished observations, August 2001). In the nonmyeloablative experiments in particular, initial engraftment may have been compromised by the minor histocompatibility mismatch between the donor and recipient. Overall, these results are consistent with those from one in vivo selection study in humans using the multidrug resistance–1 protein where the failure to achieve in vivo selection was also attributed to the lack of adequate engraftment of vector-expressing stem cells.32 Our data support the idea that a threshold level of engraftment of vector-expressing HSCs is required for successful in vivo selection. The development of strategies to increase initial engraftment levels will be important for achieving therapeutic results.
Although of concern, the toxicity observed in the mouse model is not necessarily reflective of that which will occur in humans. Both phase 1 and phase 2 studies have documented the safe administration of the alkylating agent carmustine and BG in cancer patients.19,33 The expected hematopoietic toxicity was observed but otherwise the regimen was well tolerated. Several human studies have also documented the safe use of orally administered TMZ34,35 and phase 1 studies evaluating TMZ in combination with BG are in progress. These data suggest that the short-term side effects of TMZ administration, in the setting of appropriate monitoring and supportive care, are well tolerated in humans. Despite the absence of data in humans regarding the long-term risks of treatment with TMZ, a potential concern is the associated risk of leukemia and myelodysplasia that has been observed with the use of other alkylating agents.36 However, we did not observe any cases of leukemia in our treated mice. In addition, others have shown that transgenic expression of MGMT is strongly protective of genotoxic damage in a murine model of lymphomagenesis.37 Nevertheless, the risk of using TMZ in patients with nonmalignant hematologic disorders will need to be weighed with both the natural history of the specific patient population under consideration for therapy, as well as the risk associated with other treatment options, including myeloablative transplantation, which has a known significant secondary malignancy rate.38
In conclusion, our studies demonstrate that the MGMT selection system can be used to increase a minor population of genetically normal or globin-expressing cells to therapeutically relevant levels for β-thalassemia. This approach holds promise to overcome therapeutically limiting human stem cell gene transfer efficiencies. In addition, modulation of allogeneic donor cell chimerism using MGMT-based in vivo selection in the minitransplantation setting could also be considered. In this regard, successful in vivo selection of MGMT-transduced hematopoietic cells has recently been reported in a dog model for allogeneic transplantation.39 Further work in mice and in large animal transplantation models will ultimately determine the feasibility of using the MGMT selection system in the context of therapeutic vectors for the hemoglobinopathies and other hematopoietic disorders.
Prepublished online as Blood First Edition Paper, March 27, 2003; DOI 10.1182/blood-2003-03-0677.
Supported in part by National Heart, Lung, and Blood Institute (NHLBI) grant KO8 HL04205 (D.A.P.), NHLBI Program Project PO1 HL53749 (B.P.S., A.W.N.), Cancer Center Support (CORE) grant CA-21765 (A.W.N.), and the American Lebanese Syrian Associated Charities.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ``advertisement'' in accordance with 18 U.S.C. section 1734.
We thank Dr Tom Hope (The Salk Institute, La Jolla, CA) for the gift of the WC hepatitis PRE DNA and Dr Stanton Gerson (Case Western Reserve University, Cleveland, OH) for the MFG-ΔMGMT retroviral vector producer cells. We thank the laboratory of Dr Richard Ashmun at St Jude for expert technical assistance in flow cytometry studies and Mike Straign and Joe Emmons of the St Jude Animal Resource Center for performing hematologic analyses.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal