Abstract
A most distinctive feature of paroxysmal nocturnal hemoglobinuria (PNH) is that in each patient glycosylphosphatidylinositol-negative (GPI–) and GPI+ hematopoietic stem cells (HSCs) coexist, and both contribute to hematopoiesis. Telomere size correlates inversely with the cell division history of HSCs. In 10 patients with hemolytic PNH the telomeres in sorted GPI– granulocytes were shorter than in sorted GPI+ granulocytes in 4 cases, comparable in 2 cases, and longer in the remaining 4 cases. Furthermore, the telomeres of both GPI– and GPI+ hematopoietic cells were markedly shortened compared with age-matched controls. The short telomeres in the GPI– cells probably reflect the large number of cell divisions required for the progeny of a single cell to contribute a large proportion of hematopoiesis. The short telomeres of the GPI+ cells indicate that the residual hematopoiesis contributed by these cells is not normal. This epigenetic change is an additional feature shared by PNH and aplastic anemia.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired clonal disorder1 of the hematopoietic stem cell (HSC) characterized by intravascular hemolysis, venous thrombosis, and variable degrees of bone marrow failure.2,3 The exquisite susceptibility of PNH red cells to complement-dependent hemolysis is due to a somatic mutation of the X-linked PIG-A gene in HSCs,4,5 resulting in complete or partial deficiency of several glycosylphosphatidylinositol (GPI)–linked proteins (including CD59) from the surface of the blood cells that are the progeny of the mutated HSCs.6 Because the mutation is somatic, normal and mutant cells coexist in the blood of PNH patients.7,8 Very rare GPI– cells are present in healthy subjects,9 but only patients with PNH have a GPI– cell population so expanded that it contributes substantially to hematopoiesis. Therefore, in the pathogenesis of PNH this expansion is a necessary component. It has been surmised10-12 that this relative expansion results from negative selection against GPI+ (ie, normal) HSCs by an autoimmune process, akin to what is widely believed to be the pathogenetic basis for aplastic anemia (AA).13 One implication of this model is that the residual GPI+ cells in PNH are in fact not normal. Recently, Chen et al14 have reported their impaired growth and overexpression of Fas-receptor.
Telomeres of somatic cells become shorter with each cell division and therefore their size provides information on the mitotic history of cells.15,16 Accordingly, telomeres of hematopoietic cells become shorter with age,16-18 during in vitro cultures,19 and during the in vivo expansion that reconstitutes hematopoiesis after bone marrow transplantation.20 Ball et al21 have reported that total leukocyte counts from 68 AA patients (17 of whom had small PNH clones) and from 3 PNH patients had shortened telomeres. More recently Brümmendorf et al22 did not find any difference in telomere length between granulocytes from 6 PNH patients and those from healthy controls. However, neither of these studies has analyzed separately the GPI– and GPI+ blood cells that coexist in PNH patients.
Study design
Subjects
Blood samples from 10 patients with hemolytic PNH (median age 32 years; range 24-60 years) and from 45 healthy individuals (median age 34 years; range 16-73 years) were obtained under Memorial Sloan-Kettering Cancer Center institutional review board–approved protocols. We included only patients with primary classical PNH who had a large PNH population (GPI– granulocyte percentage greater than 30%), florid hemoglobinuria, and no severe cytopenias (Table 1).
Clinical and hematologic features of PNH patients
. | . | . | . | . | . | . | Size of PNH population, %* . | . | Absolute counts . | . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Sex/age . | Years from diagnosis . | Hb, g/L . | PMNs, × 109/L . | Plts, × 109/L . | Retics, % . | RBCs . | PMNs . | GPI+ PMNs, × 109/L . | GPI- PMNs, × 109/L . | Hemoglobinuria . | Thrombosis . | Treatment . | Transfusions . | ||
| A | F/24 | 6 | 101 | 2.3 | 130 | 4.5 | 39 | 80 | 0.46 | 1.84 | Yes | No | ATG | Occasional | ||
| B | F/27 | 7 | 108 | 4.2 | 115 | 3.9 | 30 | 90 | 0.42 | 3.78 | Yes | No | ATG | Occasional | ||
| C | M/40 | 2 | 118 | 3.2 | 137 | 3.4 | 13 | 32 | 2.18 | 1.02 | Yes | Cerebral | Supportive | Occasional | ||
| D | M/30 | 3 | 90 | 2.6 | 180 | 14.9 | 43 | 94 | 0.16 | 2.44 | Yes | Splenic | ATG | Occasional | ||
| E | F/37 | 7 | 82 | 1.9 | 115 | 8.2 | 12 | 59 | 0.78 | 1.12 | Yes | No | Supportive | Never | ||
| F | F/24 | 5 | 109 | 3.1 | 103 | 3.0 | 60 | 80 | 0.62 | 2.48 | Yes | Mesenteric | ATG | Occasional | ||
| G | F/34 | 11 | 124 | 2.3 | 74 | 2.1 | 23 | 40 | 1.38 | 0.92 | Yes | Budd-Chiari | Supportive | Never | ||
| H | F/45 | 7 | 97 | 1.3 | 160 | 5.2 | 18 | 77 | 0.30 | 1.00 | Yes | Pulmonar | Supportive | Occasional | ||
| I | F/28 | 10 | 106 | 8.4 | 62 | 5.4 | 72 | 96 | 0.34 | 8.06 | Yes | Budd-Chiari | Supportive | Never | ||
| J | F/60 | 9 | 70 | 3.0 | 229 | ND | 46 | 78 | 0.66 | 2.34 | Yes | No | Supportive | Occasional | ||
. | . | . | . | . | . | . | Size of PNH population, %* . | . | Absolute counts . | . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Sex/age . | Years from diagnosis . | Hb, g/L . | PMNs, × 109/L . | Plts, × 109/L . | Retics, % . | RBCs . | PMNs . | GPI+ PMNs, × 109/L . | GPI- PMNs, × 109/L . | Hemoglobinuria . | Thrombosis . | Treatment . | Transfusions . | ||
| A | F/24 | 6 | 101 | 2.3 | 130 | 4.5 | 39 | 80 | 0.46 | 1.84 | Yes | No | ATG | Occasional | ||
| B | F/27 | 7 | 108 | 4.2 | 115 | 3.9 | 30 | 90 | 0.42 | 3.78 | Yes | No | ATG | Occasional | ||
| C | M/40 | 2 | 118 | 3.2 | 137 | 3.4 | 13 | 32 | 2.18 | 1.02 | Yes | Cerebral | Supportive | Occasional | ||
| D | M/30 | 3 | 90 | 2.6 | 180 | 14.9 | 43 | 94 | 0.16 | 2.44 | Yes | Splenic | ATG | Occasional | ||
| E | F/37 | 7 | 82 | 1.9 | 115 | 8.2 | 12 | 59 | 0.78 | 1.12 | Yes | No | Supportive | Never | ||
| F | F/24 | 5 | 109 | 3.1 | 103 | 3.0 | 60 | 80 | 0.62 | 2.48 | Yes | Mesenteric | ATG | Occasional | ||
| G | F/34 | 11 | 124 | 2.3 | 74 | 2.1 | 23 | 40 | 1.38 | 0.92 | Yes | Budd-Chiari | Supportive | Never | ||
| H | F/45 | 7 | 97 | 1.3 | 160 | 5.2 | 18 | 77 | 0.30 | 1.00 | Yes | Pulmonar | Supportive | Occasional | ||
| I | F/28 | 10 | 106 | 8.4 | 62 | 5.4 | 72 | 96 | 0.34 | 8.06 | Yes | Budd-Chiari | Supportive | Never | ||
| J | F/60 | 9 | 70 | 3.0 | 229 | ND | 46 | 78 | 0.66 | 2.34 | Yes | No | Supportive | Occasional | ||
Hb indicates hemoglobin level; PMNs, granulocytes; Plts, platelets; Retics, reticulocytes; RBCs, red blood cells; Yes, recurrent episodes of macroscopic hemoglobinuria throughout several years of clinical history; F, female; M, male; ATG, antithymocyte globulin; and ND, not done.
The percentage of PNH PMNs reflects the relative size of the PNH cell population more accurately than the percentage of PNH RBCs because the latter will be grossly underestimated as a consequence of selective hemolysis; this underestimation may be further complicated by blood transfusion.
Immunomagnetic separation of GPI– from GPI+ granulocytes
Granulocytes, isolated as previously described,9,20 were incubated with 2 μg per 106 cells of the immunoglobulin M (IgM) monoclonal antibody (mAb) anti-CD16 (Leu-1b; Becton Dickinson, Heidelberg, Germany) and then with rat anti–mouse IgM microbeads according to manufacturer instructions (Miltenyi, Bergisch Gladbach, Germany). After 30 minutes on ice, GPI– (CD16–) cells were separated from GPI+ (CD16+) cells by a column in a strong magnetic field (MACS; Miltenyi). All separation steps were carried out strictly at 4°C.
Telomere length measurement
Statistical analysis
All data are expressed as mean ± SD. The expected-for-age telomere length (TRFE) has been estimated by linear regression of TRF against age of 45 healthy individuals: TRFE = 18 352 bp – 53 bp × age (years) (R2 = 0.12; P = .02). The 53-bp-per-year TRF loss is in agreement with previous reports.17,18,24 Wilcoxon rank sum test on paired samples, Kendall correlation, and Fisher exact test have been used when appropriate. Statistical significance was accepted for P < .05.
Results and discussion
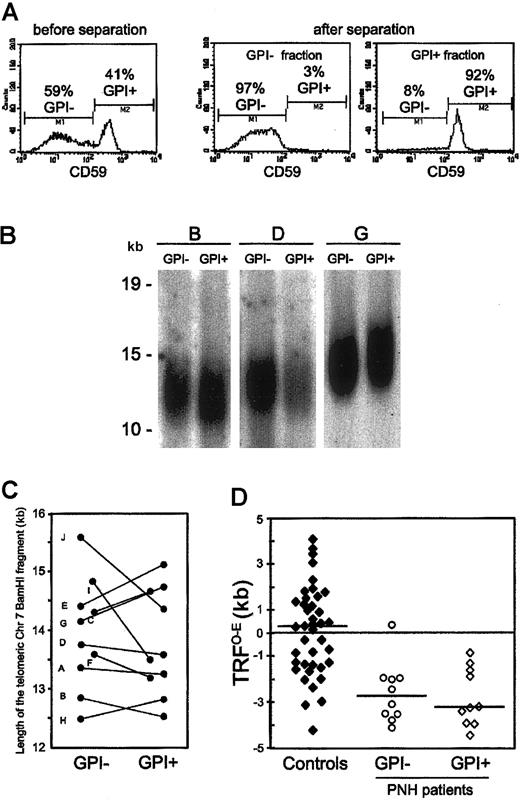
In order to study the dynamic relationship between the clonal GPI– hematopoiesis and the coexisting residual GPI+ hematopoiesis in each PNH patient, we compared the telomere length of both these populations from 10 patients with classical hemolytic PNH (Table 1). We measured side by side in each patient the telomere length of purified GPI– and GPI+ peripheral blood granulocytes (Figure 1A). Being terminally differentiated cells, these are likely to reflect the replicative history of HSCs. This internally controlled comparison showed that, overall, the average telomere length was similar in GPI– and GPI+ granulocytes: 13.9 ± 0.9 kilobase (kb) versus 13.8 ± 0.9 kb; P = .64 (Figure 1B-C). Nevertheless, we found different patterns in individual patients. The telomeres of GPI– granulocytes, compared with those of GPI+ granulocytes, were longer in 4 patients, similar in 2 patients, and shorter in the remaining 4 patients. We have investigated how these patterns relate to the patients' hematologic and biologic characteristics (Table 1). We have found that a difference in telomere length in favor of GPI– granulocytes correlates directly with the size of the PNH cell population (P = .032).
Telomere dynamics of GPI– and GPI+ granulocytes from patients with PNH. (A) Immunomagnetic separation of GPI– and GPI+ granulocytes from a patient with PNH (left panel). By staining with anti-CD59, one sees the efficient separation of GPI– (middle panel) and GPI+ (right panel) granulocytes. This technique enabled us to recover both the GPI– and the GPI+ granulocytes with a purity higher than 90%, as assessed by flow cytometry after staining with anti-CD59. (B) Southern blot analysis of the telomere length of GPI– and GPI+ granulocytes from 3 patients with PNH. Each patient is indicated by a capital letter (Table 1). Ten micrograms of high molecular weight genomic DNA, digested to completion with BamHI, was resolved on 1% agarose gel by field-inversion gel electrophoresis. After blotting, the filter was hybridized with TelBam8, a probe that is unique for the subtelomeric region of the long arm of chromosome 7.23 The length of the BamHI telomeric fragment was calculated from the densitometric profile. We have previously shown that by this method, in a side-by-side comparison, differences greater than 320 bp are significant.20 (C) Telomere length in GPI+ and in GPI– granulocytes from individual patients. The differences in telomere length between paired DNA samples of GPI– and GPI+ granulocytes from each PNH patient are shown by individual straight lines. Each patient is indicated by a capital letter (Table 1). (D) Scattergrams of the TRFO–E (difference between the observed telomere length, TRFO, and the expected-for-age telomere length, TRFE) in GPI– and GPI+ granulocytes from PNH patients, and in 40 age-matched healthy individuals (controls). The horizontal lines across each set of data points are the median values.
Telomere dynamics of GPI– and GPI+ granulocytes from patients with PNH. (A) Immunomagnetic separation of GPI– and GPI+ granulocytes from a patient with PNH (left panel). By staining with anti-CD59, one sees the efficient separation of GPI– (middle panel) and GPI+ (right panel) granulocytes. This technique enabled us to recover both the GPI– and the GPI+ granulocytes with a purity higher than 90%, as assessed by flow cytometry after staining with anti-CD59. (B) Southern blot analysis of the telomere length of GPI– and GPI+ granulocytes from 3 patients with PNH. Each patient is indicated by a capital letter (Table 1). Ten micrograms of high molecular weight genomic DNA, digested to completion with BamHI, was resolved on 1% agarose gel by field-inversion gel electrophoresis. After blotting, the filter was hybridized with TelBam8, a probe that is unique for the subtelomeric region of the long arm of chromosome 7.23 The length of the BamHI telomeric fragment was calculated from the densitometric profile. We have previously shown that by this method, in a side-by-side comparison, differences greater than 320 bp are significant.20 (C) Telomere length in GPI+ and in GPI– granulocytes from individual patients. The differences in telomere length between paired DNA samples of GPI– and GPI+ granulocytes from each PNH patient are shown by individual straight lines. Each patient is indicated by a capital letter (Table 1). (D) Scattergrams of the TRFO–E (difference between the observed telomere length, TRFO, and the expected-for-age telomere length, TRFE) in GPI– and GPI+ granulocytes from PNH patients, and in 40 age-matched healthy individuals (controls). The horizontal lines across each set of data points are the median values.
Next, we compared the telomere length of granulocytes from PNH patients with that from healthy individuals. As telomere length is extremely variable in the population,18,24 we resorted to calculating the difference between the telomere length observed (TRFO) in each individual and the expected-for-age telomere length (TRFE): TRFO–E (as defined by Ball et al21 ). In 40 age-matched healthy individuals (median age, 34 years; range, 21-63 years) the values of the TRFO–E were, as expected, quite variable (TRFO–E: 140 ± 1935 bp) and symmetrically distributed around the zero point (Figure 1D). By contrast, in both GPI– and GPI+ granulocytes from PNH patients, all but one TRFO–E values were below zero (TRFO–E: GPI–, –2563 ± 1306 bp; GPI+, –2730 ± 1256 bp; Figure 1D), and both TRFO–E distributions were significantly different from that in healthy individuals (healthy vs GPI–: P = .011; healthy vs GPI+: P = .001).
Thus, we have found in this study that both the GPI– and the GPI+ blood cells from patients with classical hemolytic PNH have shorter telomeres than blood cells from age-matched healthy individuals. Assuming that telomeres lose approximately 100 bp per cell division,17 the telomere shortening observed in PNH patients would be equivalent to approximately 25 extra cell divisions. The extreme telomere shortening in the GPI– blood cells is in keeping with the fact that in hemolytic PNH patients, just 1 clone, or a handful of clones supports a substantial proportion of hematopoiesis.25 It stands to reason that in order to achieve this task, which entails substantial expansion, these HSCs must perform a considerable number of extra mitotic divisions.
As for the shortening of telomeres found in the GPI+ blood cells, it is reminiscent of that reported in AA patients21,22 (it may be in fact even greater), indicating that in PNH patients the residual GPI+ hematopoiesis is not normal. A hypothetical explanation is that selective destruction of normal (GPI+) HSCs has left very few survivors. These must once again expand to support the residual GPI+ hematopoiesis in PNH patients. This hypothesis could also explain why different patterns are seen in different patients. This may depend on how far the PNH clone(s) has expanded at a particular point time, on whether damage to GPI+ cells is still on-going, etc. Indeed, telomeres being shorter in GPI+ than in GPI– granulocytes in patients with a larger PNH cell population may suggest that, because of an on-going damage, GPI+ HSCs are less able to contribute to hematopoiesis. Thus, telomere shortening could be regarded as an epigenetic marker of disease and one more feature that is shared by PNH and AA patients.
In summary, we have shown that severe telomere shortening affects GPI– and GPI+ hematopoiesis in patients with PNH roughly to the same extent. However, it is likely that these apparently similar epigenetic changes are caused by somewhat different mechanisms such as clonal expansion in the GPI– HSC and oligoclonal regeneration after selective destruction in the GPI+ HSC.
Prepublished online as Blood First Edition Paper, March 6, 2003; DOI 10.1182/blood-2003-01-0128.
Supported by grants from the National Institutes of Health (5RO1 HL56778-03), the Associazione Italiana per la Ricerca sul Cancro (AIRC), and the Ministero dell'Istruzione, dell'Università e della Ricerca.
Presented in part at the 41st annual meeting of the American Society of Hematology, New Orleans, LA. December 3-8, 1999.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ``advertisement'' in accordance with 18 U.S.C. section 1734.
We are very grateful to M. Bessler, M. De Angioletti, D. Tabarini, and K. Nafa for helpful suggestions, stimulating discussions, and support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal