Abstract
Intravenous administration of recombinant human factor IX (rhFIX) acutely corrects the coagulopathy in hemophilia B dogs. To date, 20 of 20 dogs developed inhibitory antibodies to the xenoprotein, making it impossible to determine if new human FIX products, formulations, or methods of chronic administration can reduce bleeding frequency. Our goal was to determine whether hemophilia B dogs rendered tolerant to rhFIX would have reduced bleeding episodes while on sustained prophylactic rhFIX administered subcutaneously. Reproducible methods were developed for inducing tolerance to rhFIX in this strain of hemophilia B dogs, resulting in a significant reduction in the development of inhibitors relative to historical controls (5 of 12 versus 20 or 20, P < .001). The 7 of 12 tolerized hemophilia B dogs exhibited shortened whole blood clotting times (WBCTs), sustained detectable FIX antigen, undetectable Bethesda inhibitors, transient or no detectable antihuman FIX antibody titers by enzyme-linked immunosorbent assay (ELISA), and normal clearance of infused rhFIX. Tolerized hemophilia B dogs had 69% reduction in bleeding frequency in year 1 compared with nontolerized hemophilia B dogs (P = .0007). If proven safe in human clinical trials, subcutaneous rhFIX may provide an alternate approach to prophylactic therapy in selected patients with hemophilia B. (Blood. 2003;102:4393-4398)
Introduction
Hemophilia B is an X-linked bleeding disorder caused by a quantitative or qualitative deficiency of the factor IX (FIX) protein. The incidence of hemophilia B is approximately 3 in 100 000 male births.1 Bleeding episodes are usually treated on demand with intravenous administration of recombinant human factor IX (rhFIX) or highly purified plasma-derived FIX concentrates. Strong supportive evidence indicates that prophylactic treatment at a dose of 25 to 40 IU/kg intravenously twice weekly in humans with hemophilia B decreases the frequency of bleeding episodes and the associated complications.2-6 It is likely that preventing bleeds with prophylactic administration is a more physiologic approach to the treatment of hemophilia than is treating bleeding once it has occurred. The clinical benefit of prophylactic treatment with FIX has not been established in an animal model of hemophilia B.
Currently, administration of FIX either as an on-demand or prophylactic basis requires venipuncture. The venipuncture procedure can be painful, difficult, and time consuming, resulting in delayed treatment and considerable stress for the patient. Both intratracheal7 and subcutaneous administration8,9 are being tested as alternative routes of administration to circumvent the requirement for venipuncture. Although both routes have been shown to provide some measure of correction of the hemophilic coagulopathy, it is unknown if either would reduce the frequency of bleeding over long periods of time when given prophylactically.
The Chapel Hill strain of hemophilia B dogs has been used in short-term preclinical studies to determine the safety and efficacy of rhFIX administered intravenously.10 These hemophilic dogs have no detectable FIX activity or antigen in plasma11 and exhibit a severe bleeding phenotype that closely mirrors the severe form of the human disease.12 The molecular defect in this strain of hemophilia B dogs is a missense point mutation in the catalytic domain of the FIX molecule that changes Gly379 to a Glu residue.13 Bleeding episodes are effectively managed by immediate and aggressive treatment by using normal canine plasma prepared from normal donor dogs. One unique feature of this colony is that dogs treated with normal canine plasma rarely develop inhibitory antibodies to infused canine factor IX. In contrast, all of the dogs that received human FIX developed inhibitory antihuman FIX antibodies within 14 days of exposure.10 If the immune response of these hemophilic dogs to rhFIX could be avoided, then the safety and efficacy of long-term administration of subcutaneous recombinant human FIX could be studied in this clinically relevant animal model.
Our goal was to determine whether hemophilia B dogs rendered tolerant to rhFIX would enjoy a reduction in the frequency of bleeding episodes while on sustained prophylactic rhFIX administered subcutaneously. Reproducible methods were developed for inducing tolerance to rhFIX in this strain of hemophilia B dogs. For this experiment, tolerance is defined as the absence of a detectable inhibitory antibody to rhFIX despite prolonged and repeated exposure to the xenoprotein. The frequency of bleeding events per year was then determined in the tolerized hemophilia B dogs on chronic prophylactic subcutaneous rhFIX maintained for 3.5 years and compared with nontolerized hemophilia B dogs that were treated on demand.
Materials and methods
Hemophilia B dogs
Hemophilia B dogs from the closed colony at the Francis Owen Blood Research Laboratory at the University of North Carolina in Chapel Hill were used in this study. This strain of hemophilia B dogs has been maintained continuously since 1966. All animals were treated according to standards in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication No. 85-23). The Institutional Animal Care and Use Committee of the University of North Carolina, Chapel Hill, approved all experiments.
Recombinant human FIX (rhFIX)
rhFIX (BeneFix) was prepared by Genetics Institute, Andover, MA, as previously described.14,15 Preparations of frozen liquid and lyophilized rhFIX ranged in activity from 690 IU/mL to 14 500 IU/mL. The specific activity of these preparations ranged from 240 to 290 IU/mg protein. The rhFIX was stored and reconstituted according to the manufacturer's specifications.16
Tolerance protocol: pilot studies, induction, interruptions in therapy, intravenous challenges, and chronic maintenance
An empiric 7-step protocol for inducing tolerance was devised that consisted of the following regimen shown on a timeline in Table 1: (1) obtaining baseline pretreatment samples; (2) administering rhFIX subcutaneously daily, days 1 to 60; (3) allowing first washout by administering no FIX from days 61 to 84; (4) administering a single dose of intravenous rhFIX at 50 IU/kg on day 85; (5) allowing second washout by administering no FIX from days 86 to 94; (6) administering a daily dose of intravenous rhFIX at 50 IU/kg for 14 days from days 96 to 109; (7) starting maintenance from day 112 forward (dose of rhFIX indicated in Table 1).
Antihuman factor IX antibody titers and Bethesda inhibitors for each hemophilia B dog receiving recombinant human FIX
| . | Study 1 . | . | . | . | . | . | . | . | Study 2 . | . | . | . | Study 3 . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Neonatal puppies (48 to 72 hours old) . | . | . | . | . | . | . | . | 7-week old puppies . | . | . | . | Neonatal puppies (48 to 72 hours old) . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | ||||||||||||||||||||||||||||
. | B02 . | . | B04 . | . | B03 . | . | B05 . | . | D41 . | . | D42 . | . | C19 . | . | C24 . | . | C27 . | . | C20 . | . | C22 . | . | C23* . | . | C25 . | . | C26* . | . | ||||||||||||||||||||||||||||
| Sex | M | F | M | F | F | F | M | F | F | M | M | F | F | F | ||||||||||||||||||||||||||||||||||||||||||
| Treatment and study day | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||
| Subcutaneous FIX, days 1-60 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 82.6 IU/kg/d | 1650 IU/kg/d | 82.6 IU/kg/d | 82.6 IU/kg/d | 82.6 IU/kg/3 × week | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 | — | — | — | — | 1.9 | 2 | 2.4 | 6 | 4 | 4 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||
| 29 | 1.8 | — | 1.8 | — | 3.8 | 3.7 | 5 | 3 | 3.2 | 3.5 | 4.3 | — | — | 2.5 | — | 2.5 | — | 1.5 | — | — | — | |||||||||||||||||||||||||||||||||||
| 43 | — | — | — | — | 4.3 | 4.8 | 4 | 10 | 3.7 | 3.7 | 4.2 | — | — | 2.2 | — | 2.1 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||
| 57 | — | — | — | — | 4.1 | 93 | 4.4 | 123 | 14 | 20 | 3.6 | 7 | 3.7 | 7 | 3.7 | 3 | — | — | 1.8 | — | 1.8 | — | — | — | — | — | ||||||||||||||||||||||||||||||
| 1st washout, days 61-84 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No FIX | No FIX | No FIX | No FIX | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| 67 | — | — | — | — | 3.8 | 4.4 | 3.2 | 5 | 3.8 | 18 | 3.2 | 11 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||
| 74 | — | — | — | — | 3.4 | 4.9 | 3.1 | 3.7 | 18 | 3.7 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||
| 80 | — | — | — | — | 3.2 | 4.8 | 2.4 | 3.7 | 2.9 | — | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||
| Intravenous FIX × one, day 85 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 50 IU/kg | 50 IU/kg | 50 IU/kg | 50 IU/kg | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| 85 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd washout, days 86-94 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No FIX | No FIX | No FIX | No FIX | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| 86 | — | — | — | — | 3.1 | 4.3 | 1.9 | 2.9 | 2.5 | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| 88 | — | — | — | — | 3.1 | 4.3 | 1.9 | 3 | 2.6 | 6 | 3.1 | 0.9 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||
| 91 | — | — | — | — | 3.1 | 3.9 | 1.9 | 3.0 | 3.2 | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Intravenous FIX × 14 days, days 96-109 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 50 IU/kg/d | 50 IU/kg/d | 50 IU/kg/d | 50 IU/kg/d | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| 95 | — | — | — | — | 3.2 | 3.7 | 2.3 | 10 | 1.9 | 3.2 | 3 | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||
| 101 | — | — | — | — | 3.0 | 3.0 | 2.5 | 3.2 | 1.9 | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||
| 108 | — | — | — | — | 2.9 | 3.2 | 3.3 | 25 | 1.9 | 0.6 | 1.9 | 9 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||
| Maintenance day 112 forward | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 82.6 IU/kg/d | 1650 IU/kg/d | 82.6 IU/k twice weekly | 82.6 IU/kg 3 times weekly† | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| 115 | — | — | — | — | 2.9 | 3.4 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
| 122 | — | — | — | — | 2.7 | 10 | 3.2 | 32 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||
| 129 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||||
| 149 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||||
| 178 | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||||
| . | Study 1 . | . | . | . | . | . | . | . | Study 2 . | . | . | . | Study 3 . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Neonatal puppies (48 to 72 hours old) . | . | . | . | . | . | . | . | 7-week old puppies . | . | . | . | Neonatal puppies (48 to 72 hours old) . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | ||||||||||||||||||||||||||||
. | B02 . | . | B04 . | . | B03 . | . | B05 . | . | D41 . | . | D42 . | . | C19 . | . | C24 . | . | C27 . | . | C20 . | . | C22 . | . | C23* . | . | C25 . | . | C26* . | . | ||||||||||||||||||||||||||||
| Sex | M | F | M | F | F | F | M | F | F | M | M | F | F | F | ||||||||||||||||||||||||||||||||||||||||||
| Treatment and study day | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Baseline | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||
| Subcutaneous FIX, days 1-60 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 82.6 IU/kg/d | 1650 IU/kg/d | 82.6 IU/kg/d | 82.6 IU/kg/d | 82.6 IU/kg/3 × week | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 | — | — | — | — | 1.9 | 2 | 2.4 | 6 | 4 | 4 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||
| 29 | 1.8 | — | 1.8 | — | 3.8 | 3.7 | 5 | 3 | 3.2 | 3.5 | 4.3 | — | — | 2.5 | — | 2.5 | — | 1.5 | — | — | — | |||||||||||||||||||||||||||||||||||
| 43 | — | — | — | — | 4.3 | 4.8 | 4 | 10 | 3.7 | 3.7 | 4.2 | — | — | 2.2 | — | 2.1 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||
| 57 | — | — | — | — | 4.1 | 93 | 4.4 | 123 | 14 | 20 | 3.6 | 7 | 3.7 | 7 | 3.7 | 3 | — | — | 1.8 | — | 1.8 | — | — | — | — | — | ||||||||||||||||||||||||||||||
| 1st washout, days 61-84 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No FIX | No FIX | No FIX | No FIX | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| 67 | — | — | — | — | 3.8 | 4.4 | 3.2 | 5 | 3.8 | 18 | 3.2 | 11 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||
| 74 | — | — | — | — | 3.4 | 4.9 | 3.1 | 3.7 | 18 | 3.7 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||
| 80 | — | — | — | — | 3.2 | 4.8 | 2.4 | 3.7 | 2.9 | — | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||
| Intravenous FIX × one, day 85 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 50 IU/kg | 50 IU/kg | 50 IU/kg | 50 IU/kg | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| 85 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2nd washout, days 86-94 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| No FIX | No FIX | No FIX | No FIX | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| 86 | — | — | — | — | 3.1 | 4.3 | 1.9 | 2.9 | 2.5 | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| 88 | — | — | — | — | 3.1 | 4.3 | 1.9 | 3 | 2.6 | 6 | 3.1 | 0.9 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||
| 91 | — | — | — | — | 3.1 | 3.9 | 1.9 | 3.0 | 3.2 | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||
| Intravenous FIX × 14 days, days 96-109 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 50 IU/kg/d | 50 IU/kg/d | 50 IU/kg/d | 50 IU/kg/d | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| 95 | — | — | — | — | 3.2 | 3.7 | 2.3 | 10 | 1.9 | 3.2 | 3 | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||
| 101 | — | — | — | — | 3.0 | 3.0 | 2.5 | 3.2 | 1.9 | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||
| 108 | — | — | — | — | 2.9 | 3.2 | 3.3 | 25 | 1.9 | 0.6 | 1.9 | 9 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||
| Maintenance day 112 forward | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 82.6 IU/kg/d | 1650 IU/kg/d | 82.6 IU/k twice weekly | 82.6 IU/kg 3 times weekly† | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| 115 | — | — | — | — | 2.9 | 3.4 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
| 122 | — | — | — | — | 2.7 | 10 | 3.2 | 32 | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||
| 129 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||||
| 149 | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||||
| 178 | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||||
Antihuman FIX antibody titers detected by ELISA are listed in the first column and the Bethesda inhibitor levels are listed in the second column for each dog. —, not detected. Gaps in data indicate either that it was not available, or that a given animal was no longer in the study.
C23 had one litter at 1.4 years into the study, and C26 had litters at 1.3 and 2 years into the study. The pregnancies were uncomplicated, and both dogs received only rhFIX to prevent bleeding.
Dogs C20, C22, C23, C25, and C26 were switched to 82.6 IU/kg twice weekly from day 178 through the remainder of the 3.5 years.
Assessment of correction of hemophilic coagulopathy and phenotype
Blood and plasma samples drawn while the dogs were receiving rhFIX were obtained just before administration of rhFIX. Blood samples during interruptions in therapy were obtained at scheduled intervals indicated in Table 1.
Whole blood clotting time (WBCT). The WBCT was performed by a 2-tube procedure at 28°C. Whole blood (1 mL) collected with a 1-mL syringe was distributed equally between 2 siliconized tubes (Vacutainer, no. 6431; Becton Dickinson, Rutherford, NJ). The first tube was tilted every 30 seconds. After a clot forms, the second tube is tilted and observed every 30 seconds. The end point is the clotting time of the second tube. The range of values for 6 nontolerized hemophilia B dogs and 6 healthy dogs was determined for reference.
Activated partial thromboplastin time (APTT). The APTT assays were determined in the ST4 coagulation instrument (Diagnostica Stago, Asnieres, France). Mixtures consisted of equal portions of partial thromboplastin (APTT reagent; bioMérieux, Durham, NC), 0.025 M CaCl2, and citrated test plasma. The range of values for 6 nontolerized hemophilia B dogs and 6 healthy dogs was determined for reference.
Human-specific FIX antigen ELISA. The concentration of rhFIX antigen was determined by a double monoclonal sandwich enzyme-linked immunosorbent assay (ELISA) that is specific for human FIX and does not cross-react with canine FIX.10 Standard curves were prepared by adding known amounts of rhFIX into canine hemophilia B plasma. The lower limit of detection of the assay is 25 ng/mL.
Antihuman FIX antibodies by ELISA and Bethesda inhibitor assay. Antihuman FIX antibody assays were performed by using 2 procedures: a direct binding ELISA7,16 with immobilized recombinant human FIX and enzyme-coupled anti-immunoglobulin reagents, and the Bethesda inhibitor assay. The ELISA titer of a given sample represents an arbitrary unit defined as the reciprocal dilution for that sample that would generate an optical density (OD) value equal to the cutpoint OD of the assay.7,16 The cutpoint OD is defined as twice the mean OD of the negative control wells. Titer values were then calculated by linear interpolation from values above and below the cutpoint OD in the dilution series using the following equation: Titer = DilnOD1 - ([(OD1 - ODcp)/(OD1 - OD2)] × [DilnOD1 - DilnOD2]) where ODcp = cutpoint OD, OD1 = sample OD value above the cutpoint OD in the dilution series, OD2 = sample OD value below the cutpoint OD in the dilution series, DilnOD1 = sample reciprocal dilution at OD1, and DilnOD2 = sample reciprocal dilution at OD2. These anti-FIX antibody data were then reported as the log of the interpolated value.
The Bethesda inhibitor assay was performed with an activated thromboplastin reagent and hemophilia B substrate at 37°C.7,10,17,18 One Bethesda unit (BU) of inhibitor per milliliter is defined as the residual FIX activity of 50% of the healthy control. Positive controls were obtained from hemophilia B dogs with known antihuman FIX antibodies confirmed in a second laboratory. Negative controls were obtained from nontolerized hemophilia B dogs in the colony repeatedly shown to be without inhibitors.
Frequency of bleeding events in tolerized and nontolerized hemophilia B dogs. The frequency of spontaneous bleeds and excess bleeding from minor trauma requiring replacement FIX treatment in the 5 tolerized and in the 11 nontolerized hemophilia B dogs was determined from the treatment records. Comparisons were made for the first year of life for both groups. The frequency between the end of year 1 and 3.5 years was also measured for the available dogs. Detection of spontaneous bleeding in hemophilia B dogs is done by clinical observation and/or monitoring dogs for a decrease in hematocrit. Clinical manifestations are variable and include swelling in joints or soft tissues, lameness, weakness consistent with bleeding in the central nervous system or entrapment of a peripheral nerve, and the development of a spontaneous hematoma. In addition, a change in behavior (eg, lethargy or poor appetite) may be the only manifestation of an occult bleed in the retroperitoneum, mediastinum, peritoneal cavity, or other similar location. In the case of suspected bleeding, the dog is monitored with frequent measurements of hematocrit. All bleeding events, clinical observations, and treatments are recorded daily in computer-based clinical records. In addition, 2 of the tolerized hemophilia B females were bred during the 3.5-year treatment period. Control of hemostasis was maintained by intravenous administration of rhFIX during surgical procedures, peripartum, and postpartum.
Clearance of rhFIX in tolerized hemophilia B dogs. After 3.5 years of receiving subcutaneous rhFIX, the half-life of an intravenous bolus of rhFIX (50 IU/kg) was determined in 2 situations. The first situation was while the tolerized dogs were on subcutaneous prophylaxis (ie, an intravenous dose of rhFIX was substituted for the next subcutaneous dose) and the second was after a 2-month washout during which no rhFIX was given. Plasma samples were drawn at baseline, 5 minutes, 30 minutes, 1, 4, 8, 24, 48, and 72 hours. FIX activity was determined as described.7,10
Results
Induction of tolerance in neonatal hemophilia B dogs and incidence of anti-FIX antibodies by ELISA and Bethesda inhibitor assay
Two pilot studies were performed to test the efficacy of an empiric method for inducing tolerance to rhFIX in the hemophilia B dogs and to identify a target dose for a larger study. In study 1, hemophilia B puppies were treated with subcutaneous rhFIX at a dose of 82.6 IU/kg (n = 2) or 1650 IU/kg (n = 2) daily, starting during the first 48 to 72 hours following birth through step 2 of the induction protocol (Table 1). These 4 puppies then had their dose of rhFIX adjusted as described to complete all 7 steps of the tolerance induction protocol. On day 29 in study 1, both neonatal dogs that received 82.6 IU/kg per day subcutaneously had an anti-hFIX antibody detectable by ELISA (Table 1, dogs B02 and B04). This anti-hFIX antibody on day 29 had no inhibitory activity detectable by Bethesda assay. Neither of these dogs had an anti-FIX antibody or detectable Bethesda inhibitor for the duration of the protocol (149 days). These 2 dogs were defined as tolerized. The 2 neonatal dogs in study 1 that received 1650 IU/kg per day subcutaneously developed anti-hFIX antibodies by ELISA by day 15 that were inhibitory by Bethesda assay (Table 1, dogs B03 and B05). These inhibitory anti-FIX antibodies persisted in these dogs for the duration of the protocol (122 days).
In study 2, 7-week-old hemophilia B dogs were started on rhFIX at 82.6 IU/kg (n = 2) daily (Table 1). These puppies completed step 2 of the protocol. Both 7-week-old dogs developed detectable Bethesda inhibitors by day 15 and were not analyzed beyond step 2 of the treatment protocol (60 days). The results of these 2 studies suggested that tolerance to rhFIX could be successfully induced in hemophilia B puppies by initiating therapy at 82.6 IU/kg per day subcutaneously in the neonatal period.
On the basis of the results from studies 1 and 2, study 3 was then initiated with 48- to 72-hour-old puppies from a single litter produced by a hemophilia B sire and dam (Table 1).19 These neonatal hemophilia B dogs received 82.6 IU/kg subcutaneously daily (n = 4) or 3 times a week (n = 4) for 60 days. These 8 puppies then had their dose of rhFIX adjusted as described to complete all 7 steps of the tolerance induction protocol. Five of 8 hemophilia B neonatal puppies completed the 7-step protocol and exhibited no neutralizing antihuman FIX antibodies and were defined as tolerized to rhFIX (Table 1). Three of these 5 tolerized dogs had detectable but low titer, self-limited antihuman FIX antibodies by ELISA between days 29 and 51 of the study (Table 1, dogs C22, C23, and C25). However, Bethesda assays performed on these study days found no detectable inhibitory activity for these antibodies. The 2 other tolerized dogs had no detectable anti-FIX antibodies by ELISA and also had no detectable Bethesda inhibitors (Table 1, dogs C20 and C26). All 5 tolerized hemophilia B dogs continued to exhibit immune tolerance through interruptions and intravenous challenge regimens (Table 1). The dose of rhFIX was adjusted to 82.6 IU/kg twice a week in all 5 tolerized dogs on day 178 through the rest of the 3.5 years.
The remaining 3 hemophilia B puppies in study 3 developed inhibitory anti-FIX antibodies during the first 60 days of exposure (Table 1, C19, C24, and C27). These inhibitor puppies also had positive ELISA results by day 29 and developed values that were up to 2 logs higher than detected in the tolerized dogs. Anti-hFIX antibodies were still detectable by both assays at the end of step 6 (day 108) when these 3 inhibitor dogs were excluded from further study. Overall, a significant reduction in inhibitors to rhFIX was achieved in the hemophilia B dogs receiving subcutaneous rhFIX relative to the historical controls that received intravenous rhFIX (5 of 12 versus 20 of 20, P < .001).10
Correction of hemophilic coagulopathy and phenotype
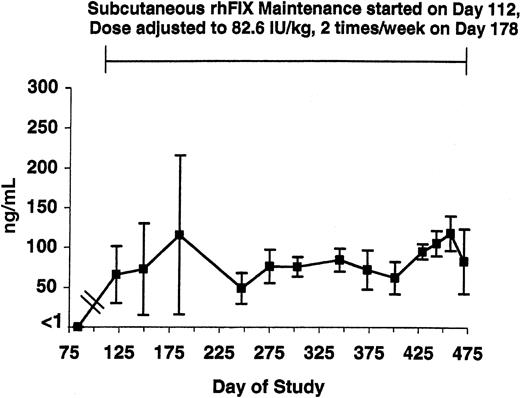
WBCT and APTT assays were performed at baseline and over the next 3.5 years in the 5 tolerized dogs (Figures 1-2, respectively). Both the WBCT and APTT were shortened compared both with nontreated hemophilia B dogs and 3 of the tolerized dogs in whom samples could be obtained after a 2-month pause in therapy. The degree of correction appears greater for the WBCT than for APTT because the WBCT is more sensitive to trace amounts of FIX.12 Both the WBCT and APTT increased during short time periods associated with the washouts described in Table 1 (data not shown). The level of rhFIX antigen varied depending on the sampling time as described for the APTT and the WBCT but was approximately 1% of normal (ie, approximately 50 ng/mL) while the dogs were receiving 82.6 IU/kg subcutaneously twice per week (Figure 3).
WBCT in tolerized hemophilia B dogs. The average WBCT values obtained quarterly from the 5 tolerized dogs are listed. Four dogs survived for 3.5 years, and the fifth died during year 2. At baseline (first value shown) and after 3.5 years of subcutaneous prophylaxis, the average value was markedly prolonged. The * denotes a sample drawn during a rhFIX-free washout period. The horizontal lines mark the WBCT range for healthy dogs (8-12 minutes) and for untreated hemophilia B dogs (> 60 minutes). Maintenance with subcutaneous rhFIX was started at day 112 as described in Table 1 and adjusted to 82.6 IU/kg twice weekly at day 178. This treatment markedly shortened the WBCT. All samples were drawn just prior to the next dose. Error bars indicate SD.
WBCT in tolerized hemophilia B dogs. The average WBCT values obtained quarterly from the 5 tolerized dogs are listed. Four dogs survived for 3.5 years, and the fifth died during year 2. At baseline (first value shown) and after 3.5 years of subcutaneous prophylaxis, the average value was markedly prolonged. The * denotes a sample drawn during a rhFIX-free washout period. The horizontal lines mark the WBCT range for healthy dogs (8-12 minutes) and for untreated hemophilia B dogs (> 60 minutes). Maintenance with subcutaneous rhFIX was started at day 112 as described in Table 1 and adjusted to 82.6 IU/kg twice weekly at day 178. This treatment markedly shortened the WBCT. All samples were drawn just prior to the next dose. Error bars indicate SD.
APTT in tolerized hemophilia B dogs. The APTT values from the 5 tolerized dogs are listed as described in Figure 1 and show some shortening while on subcutaneous rhFIX prophylaxis. Horizontal lines mark the APTT range for healthy dogs (22-30 seconds) and for untreated hemophilia B dogs (70-90 seconds). Maintenance with subcutaneous rhFIX was started at day 112 as described in Table 1 and adjusted to 82.6 IU/kg twice weekly at day 178. * denotes a sample drawn during a rhFIX-free washout period. The APTT is less sensitive than the WBCT to low levels of plasma FIX. All samples were drawn just prior to the next dose. Error bars indicate SD.
APTT in tolerized hemophilia B dogs. The APTT values from the 5 tolerized dogs are listed as described in Figure 1 and show some shortening while on subcutaneous rhFIX prophylaxis. Horizontal lines mark the APTT range for healthy dogs (22-30 seconds) and for untreated hemophilia B dogs (70-90 seconds). Maintenance with subcutaneous rhFIX was started at day 112 as described in Table 1 and adjusted to 82.6 IU/kg twice weekly at day 178. * denotes a sample drawn during a rhFIX-free washout period. The APTT is less sensitive than the WBCT to low levels of plasma FIX. All samples were drawn just prior to the next dose. Error bars indicate SD.
rhFIX antigen level in tolerized hemophilia B dogs. The level of rhFIX antigen is shown from just before initiation of maintenance through day 475 in the 5 tolerized hemophilia B dogs. Maintenance with subcutaneous rhFIX was started at day 112 as described in Table 1 and adjusted to 82.6 IU/kg twice weekly at day 178. All samples were drawn just prior to the next dose. Error bars indicate SD.
rhFIX antigen level in tolerized hemophilia B dogs. The level of rhFIX antigen is shown from just before initiation of maintenance through day 475 in the 5 tolerized hemophilia B dogs. Maintenance with subcutaneous rhFIX was started at day 112 as described in Table 1 and adjusted to 82.6 IU/kg twice weekly at day 178. All samples were drawn just prior to the next dose. Error bars indicate SD.
The average number of bleeding episodes experienced by the 5 tolerized hemophilia B dogs receiving subcutaneous rhFIX was 1.8 ± 0.5 as opposed to 5.5 ± 3.3 in the 10 nontolerized hemophilia B dogs in the Chapel Hill colony during the first year of life for both groups, a 69% reduction (P = .0007; Table 2). Between years 1 and 3.5, 6 nontolerized and 4 tolerized dogs were available for comparison. Four of the original 10 nontolerized dogs either died of bleeding or were entered into other protocols. One of the original 5 tolerized dogs died of bleeding. The average frequency of bleeds per year between years 1 and 3.5 was 49% lower in the tolerized dogs than that detected in the nontolerized dogs, 2.1 ± 1.0 versus 4.1 ± 3.6, respectively. Although the trend toward reduced bleeding was still evident between years 1 and 3.5, the difference was not significant in this small sample size.
Frequency of bleeding events per year in nontolerized hemophilia B dogs treated on demand and in tolerized hemophilia B dogs on chronic subcutaneous prophylactic rhFIX replacement therapy
. | Bleeding events per year . | . | |
|---|---|---|---|
| Hemophilia B dogs . | 0-1 y* . | 1-3.5 y† . | |
| Nontolerized (n) | 5.5 ± 3.3 (10) | 4.1 ± 3.6 (6) | |
| Tolerized (n) | 1.8 ± 0.5 (5) | 2.1 ± 1.0 (4) | |
| Reduction, % | 69 | 49 | |
| P‡ | .0007 | .44 | |
. | Bleeding events per year . | . | |
|---|---|---|---|
| Hemophilia B dogs . | 0-1 y* . | 1-3.5 y† . | |
| Nontolerized (n) | 5.5 ± 3.3 (10) | 4.1 ± 3.6 (6) | |
| Tolerized (n) | 1.8 ± 0.5 (5) | 2.1 ± 1.0 (4) | |
| Reduction, % | 69 | 49 | |
| P‡ | .0007 | .44 | |
Average number of bleeding events per year in the nontolerized and tolerized hemophilia B dogs during the first year of life.
Average number of bleeds per year in the tolerized hemophilia B dogs between the end of year 1 and the end of the study at 3.5 years.
P was determined by exact Wilcoxon rank sum test.
Two tolerized hemophilia B female dogs had successful gestation and parturition during this study.19 These pregnancies were managed with intravenous rhFIX peripartum (50 IU/kg per day, starting several days before delivery and continuing until vaginal bleeding stopped postpartum). Neither dog required blood transfusions peripartum. In addition, several surgical procedures, including dental care, spaying, and repairs of traumatized tissues, were managed with additional intravenous rhFIX during the course of this study. None of the procedures had bleeding complications. All of the tolerized hemophilia B dogs were restarted on subcutaneous 82.6 IU/kg when judged safe by the attending veterinarian.
Clearance of intravenous rhFIX in tolerized hemophilia B dogs
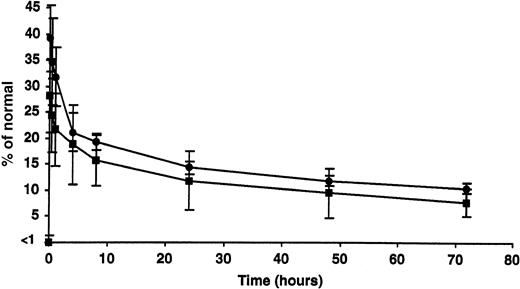
The percentage of recovery and half-life of infused rhFIX (50 IU/kg) were estimated while the tolerized dogs were on subcutaneous therapy and after 2 months without rhFIX (Figure 4). Respectively, the percentage of recovery (approximately 28.6% ± 6.9% and 34.8% ± 7.4%) and half-life (between 18 and 20 hours in both cases) appeared comparable under these 2 conditions and similar to what has been reported in the nontolerized hemophilia B dogs.10
Clearance of intravenous rhFIX with or without concurrent subcutaneous rhFIX prophylaxis. The peak recovery and half-life of infused rhFIX (50 IU/kg) were estimated while the tolerized dogs were on subcutaneous rhFIX therapy (▪) and after 2 months without rhFIX (•). Both appeared comparable under these 2 conditions at this dose. Error bars indicate SD.
Clearance of intravenous rhFIX with or without concurrent subcutaneous rhFIX prophylaxis. The peak recovery and half-life of infused rhFIX (50 IU/kg) were estimated while the tolerized dogs were on subcutaneous rhFIX therapy (▪) and after 2 months without rhFIX (•). Both appeared comparable under these 2 conditions at this dose. Error bars indicate SD.
Discussion
Our data show that sustained prophylactic administration of rhFIX subcutaneously to tolerized hemophilia B dogs significantly reduced the incidence of bleeding events during the first year of treatment consistent with an improvement in phenotype. This study became feasible with the development of reproducible methods for inducing tolerance to rhFIX in approximately 60% of the neonatal animals treated (ie, 7 of 12, including 2 of 4 from study 1 and 5 of 8 from study 3). This tolerance constituted a significant reduction in Bethesda inhibitor formation relative to historical controls (5 of 12 versus 20 of 20, P < .001).10 Remarkably, these hemophilic dogs maintained tolerance to rhFIX for more than 3.5 years, including relatively short interruptions in therapy (ie, up to 2 months), intravenous challenges, gestation and parturition, and surgical procedures. In addition, the subcutaneous route and dose used consistently shortened the WBCT and APTT and yielded FIX antigen levels of approximately 1%. This level of correction is generally associated with moderately severe hemophilia. Indeed, the significant reduction in bleeding events observed in the tolerized dogs on sustained subcutaneous prophylaxis is consistent with a proportional or dose responsive improvement in phenotype. These findings in a relevant animal model of hemophilia B reinforce the rationale for administering prophylactic factor replacement in humans when feasible and for considering a subcutaneous route of administration with a target trough FIX level of more than 1%.
The choice of administering rhFIX subcutaneously in this study was a practical one on the basis of the ease of administering medications by this route. The advantage of the subcutaneous route was particularly evident in the 1- to 3-day-old neonatal puppies in which venous access was limited. The high concentration of rhFIX used in these studies (690-14 500 IU/mL) was also critical to the success of the subcutaneous route. These formulations were 7 to 150 times higher concentrations of FIX than is found in commercial products. This high concentration allowed very low volumes of injection throughout the study period. The subcutaneous route was well tolerated, circumvented the challenges associated with venipuncture, and partially corrected the hemophilic coagulopathy. Moreover, these data underscore the advantages of prophylactic therapy for decreasing hemorrhages and associated complications in an animal model.
A potential pitfall of extravascular administration of hFIX could be increased immunogenicity of the therapeutic protein. Although the incidence varies among reports, an estimated 7.5% of patients with hemophilia B develop measurable Bethesda inhibitors at some point during their lifetime.20-22 Clearly, monitoring any change in the expected incidence of inhibitors is an essential component of any clinical trial in hemophilia treatment. A series of studies that administer the FIX protein subcutaneously have been reported in animals and humans with dosing that ranges from 15 to 200 IU/kg.23-25 All these studies detected measurable FIX in the recipient's plasma following subcutaneous administration. In the study by Liles et al,25 a single adult patient with hemophilia B (cross-reacting material negative, CRM-) given a high-purity plasma-derived hFIX subcutaneously developed a transient, low titer inhibitor (1-2 Bethesda units) months after the clinical study while being treated for a traumatic bleed.25 Currently, even after rechallenge, he has no detectable inhibitor. This patient had been extensively treated in the past and had not shown evidence of developing an inhibitor, although he had not been as rigorously monitored as he was in the clinical protocol.
These results raise the issue of whether or not extravascular administration of FIX protein could result in an immune response with a greater frequency than intravascular administration. This important issue cannot be fully addressed with data from the one patient who received plasma-derived FIX given that host response can vary considerably. However, at least 2 points can be raised. First, many patients probably receive some FIX protein in the extravascular space when intravenous lines infiltrate, a common occurrence in any clinical setting. Whether patients with hemophilia are more likely to develop an inhibitor with extravasation of FIX protein is unknown. Second, FIX has been shown to have binding sites in the extravascular space and on endothelium. Cheung et al26 built on the studies of Heimark et al27 and Stern et al28 to document that FIX binds to type IV collagen via lysine at position 5 or valine at position 10, identifying the mechanism of extravascular and endothelial binding of hFIX.26,29,30 The published data23-25 and this report support the hypothesis that FIX can traverse not only from the extravascular space to the systemic circulation but also from the systemic circulation to the extravascular space. Whether or not FIX in the extravascular space has a function beyond preventing bleeding into joints and soft tissues so characteristic of patients with hemophilia B is unknown. However, to test the relative immunogenicity of FIX given subcutaneously versus intravenously in a rigorous fashion would require additional studies such as administering species-specific FIX to hemophilic dogs and mice.
The extravascular distribution of FIX and its binding to collagen IV and endothelium suggest that the clearance of FIX from plasma would be altered by this complex compartmentalization. Remarkably, the half-life of infused rhFIX did not appear to be altered by the dose used in our subcutaneous protocol. This observation suggests that the clearance of rhFIX from plasma does not change when the extravascular space is occupied by FIX to the extent achieved at this dose.
Our data support considering subcutaneous administration of rhFIX as an alternative to IV infusions if proven safe and efficacious in clinical trials in human patients with hemophilia B. Twice weekly injections of 82.6 IU/kg as used in our study would require approximately $86 000 per year for each 10 kg of body weight, assuming a cost of approximately $1 per unit of rhFIX. The total cost would then be $258 000 over the first 3 years of life for a hemophilic child with an average weight of 10 kg. Alternatively, our dose simulation estimates8 suggest that the combination of an 86-IU/kg intravenous loading dose and a 15-IU/kg rhFIX subcutaneous daily prophylactic dose would maintain rhFIX activity above 2% at the lowest cost. A target trough level of 2% would be higher than that achieved in our study in hemophilic dogs and, therefore, might produce a greater reduction in the frequency of bleeding. Again, assuming a cost of approximately $1 per unit of rhFIX, daily injections of 15 IU/kg would cost approximately $54 600 per year for each 10 kg of body weight. This cost would translate to approximately $164 000 over the first 3 years of life for a hemophilic child with an average weight of 10 kg. Although the costs in both cases are prohibitive in adult patients with easily accessible veins, pediatric patients with difficult or absent vascular access sites might have the greatest potential benefit. Such a clinical trial would require careful monitoring of the immune response to rhFIX.
Prepublished online as Blood First Edition Paper, August 21, 2003; DOI 10.1182/blood-2003-05-1498.
Supported in part by funds from Genetics Institute and Wyeth Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms P. McElveen and Ms M. Fleishman and their staff for the outstanding care given to these hemophilic dogs. We thank Ms Kate Leitermann for skillful technical assistance. We thank Nicholas Warne, Chandra Webb, and Elizabeth Bartlett for their preparation of the high concentration rhFIX formulations. We thank S. P. Khor for the dose simulation estimates.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal