Abstract
Fanconi anemia (FA) is a recessive genomic instability syndrome characterized by developmental defects, progressive bone marrow failure, and cancer. FA is genetically heterogeneous, however; the proteins encoded by different FA loci interact functionally with each other and with the BRCA1, BRCA2, and ATM gene products. Although patients with FA are highly predisposed to the development of myeloid leukemia and solid tumors, the alterations in biochemical pathways responsible for the progression of tumorigenesis in these patients remain unknown. FA cells are hypersensitive to a range of genotoxic and cellular stresses that activate signaling pathways mediating apoptosis. Here we show that ionizing radiation (IR) induces modestly elevated levels of p53 in cells from FA type C (Fancc) mutant mice and that inactivation of Trp53 rescues tumor necrosis factor α-induced apoptosis in myeloid cells from Fancc-/- mice. Further, whereas Fancc-/- mice failed to form hematopoietic or solid malignancies, mice mutant at both Fancc and Trp53 developed tumors more rapidly than mice mutant at Trp53 alone. This shortened latency was associated with the appearance of tumor types that are found in patients with FA but not in mice mutant at Trp53 only. Collectively, these data demonstrate that p53 and Fancc interact functionally to regulate apoptosis and tumorigenesis in Fancc-deficient cells. (Blood. 2003;102:4146-4152)
Introduction
Fanconi anemia (FA) is an autosomal recessive disorder characterized by a progressive bone marrow failure, developmental defects, and cancer. Individuals with FA have a 500- to 1000-fold increased risk of developing myeloid leukemia and a range of solid tumors that preferentially affect the head and neck, skin, and gastrointestinal and genitourinary systems.1-4 Recent studies have identified the existence of 7 FA gene products (denoted FANCA-FANCG) that appear to function, at least in part, in a complex of signaling proteins that modulate genomic stability (for a review, see Joenje and Patel5 ). In addition, one gene product (FANCC) appears to have a role in modulating apoptosis in addition to its role in the FANCA-FANCG complex.6-8 The ways the different FA proteins interact with each other and with other biochemical pathways to maintain genomic stability and prevent tumor formation is an area of active investigation.
Although the mechanisms underlying tumor formation in patients with FA are incompletely understood, epidemiologic and experimental observations raise a number of possibilities. First, the development of malignant cells from primary cells frequently requires alterations in apoptosis or cell cycle control. Because myeloid malignancies predominantly occur in the context of progressive apoptotic depletion of hematopoietic progenitors in patients with FA9 and in Fancc-/- mice,10 it has been hypothesized that leukemogenesis results from an accumulation of mutations that precipitates the emergence of apoptotic-resistant precursors. Second, p53 is a pivotal sensor of genotoxic and nongenotoxic stress and activates signaling pathways that result in cell cycle arrest or apoptosis. The apoptosis in FANCC-deficient lymphoblasts has previously been shown to be due to activation of protein kinase R (PKR).11 Because p53 is a downstream effector of PKR,12-14 inactivation or polymorphisms of p53 or proteins in the p53 pathway may be an important target for cellular transformation in FA-associated myeloid malignancies. Third, patients with FA are predisposed to solid tumors, including epithelial and squamous cell carcinomas, which have a high rate of p53 alteration in sporadic malignancies.15,16 The risk of these solid tumors in patients with FA goes up markedly after 20 years of age and increases to a cumulative risk of 90% by 40 years of age.2 Finally, recent studies have positioned the FA proteins in a biochemical pathway that includes BRCA1 and BRCA2.17,18 In fact, truncating, hypomorphic mutations of BRCA2 were observed in the FA-D1 complementation group.18 Further, studies using Brca1 and Brca2 conditional knockout mice support a role for p53 in tumor progression.19-21
Although epidemiologic and biochemical data suggest a role for p53 in mediating apoptosis and cancer in FA, a previous study found that a dominant-negative p53 did not affect apoptosis in FANCC-deficient, immortalized lymphoblasts exposed to genotoxic stress.22 However, the use of immortalized cell lines that may have altered p53 function23 is an inherent limitation in those studies. Therefore, to model endogenous stresses incurred by the organism in vivo, we performed a genetic intercross of Fancc and Trp53 mutant mice to characterize how these genes interact in mediating apoptosis and tumorigenesis.
Materials and methods
Cell culture
Primary murine embryo fibroblasts (MEFs) were obtained from day 12 to 14 mouse embryos derived from breeding of Fancc+/- mice. Embryos were obtained, and a small amount of tissue was used for polymerase chain reaction (PCR) analysis. Fibroblasts were derived by mincing embryos and culturing on tissue culture dishes in growth media consisting of Dulbecco modified Eagle/F12 media containing 10% fetal calf serum (FCS) supplemented with 2 mM l-glutamine and 100 U/mL penicillin and 100 U/mL streptomycin (Gibco BRL, Grand Island, NY). After 3 days of culture, plates were trypsinized and cells were expanded for experimental use or frozen. MEFs were maintained on the basis of a 3T3 protocol. All experiments using MEFs were performed with cells that were passage 4 to 6. For treating cells with ionizing radiation (IR), we used a Gammacell-40 exactor (Nordion, Ottawa, ON, Canada) containing a 137Cs source.
Mouse models
Mice containing a disruption of the Fancc gene were generously provided by Dr Manuel Buchwald (University of Toronto, Hospital for Sick Children).24 These mice were back-crossed for 10 generations into the C57BL/6J strain. Mice were genotyped using PCR to detect wild-type and mutant Fancc alleles as described.8 Mice harboring a disruption of the Trp53 gene have been previously described25 and were obtained in the C57BL/6J strain from Jackson Laboratories (Bar Harbor, ME). Trp53 genotype was determined using a PCR method and primer sequences as provided by the Jackson Laboratories. Fancc and Trp53 compound heterozygous mice were generated by mating Fancc+/- mice with Trp53+/- mice. The F2 generation was produced by crossing the compound heterozygotes derived from the F1 mice, and these mice were monitored long term for tumors and morbidity. Due to the low frequency of Fancc-/-Trp53-/- mice obtained, some mice of this genotype were generated by crossing Fancc+/- with Trp53-/- mice.
Histology and immunohistochemistry
Mice that were moribund were killed, and autopsies were performed by a veterinary pathologist. Tissues and tumors were fixed in 10% formalin, and paraffin sections were obtained. Immunohistochemistry was used to specifically identify the lineage of malignant cells in some tumors using Mac-2 (M3/38; Cedarlane Laboratories, Hornby, ON, Canada) antibody, which is a widely accepted marker of tissue macrophages/monocytes in myeloid malignancy.26 Immunohistochemistry was performed as recommended by the antibody supplier. Donkey antirat secondary antibody (Jackson Immunoresearch Labs, West Grove, PA) was detected using the streptavidin-horseradish peroxidase (HRP) LSAB2 system (Dako, Carpinteria, CA).
Immunoblotting
Whole-cell protein extracts were obtained from cells in lysis buffer (100 mM Tris-Cl [tris(hydroxymethyl)aminomethane], pH 7.4, 0.1% NP-40, 150 mM NaCl, 1 mM dithiothreitol [DTT]), and quantitated using the Bradford assay. Equivalent amounts of protein were electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, transferred to polyvinylidene difluoride (PVDF) Immobilon-P membranes (Millipore, Billerica, MA) and proteins were detected by Western blotting and the enhanced chemiluminescence (ECL) system (Amersham-Pharmacia, Piscataway, NJ). Polyclonal antibodies p21waf-cip1 (C-19) and actin (C-19) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and antibody to p53 (Ab1) was obtained from Oncogene Research Products (Boston, MA). All antibodies were used as specified by the manufacturer.
p53-binding assay
Binding of p53 to its consensus sequence was assessed from irradiated, whole cell extracts as previously described,27,28 using a modified procedure to precipitate oligonucleotide-bound p53 protein. The p53 consensus sequence TACAGAACATGTCTAAGCATGCTGGGGACT conjugated to agarose beads as well as beads containing a control p53 consensus sequence were obtained (Santa Cruz Biotechnology). Briefly, lymphocytes were obtained from the spleens of mice and cultured at a density of 1 × 106 cells/mL in 2.2 μg/mL concanavalin A for 48 hours. Cells were treated with IR, and whole-cell extracts were obtained in lysis buffer containing 0.5% NP-40, 150 mM NaCl, 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.9, 1 mM DTT, 10 μg/mL pepstatin and 10 μg/mL aprotonin, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Equivalent amounts of protein (100-500 μg) were incubated in the presence of the agarose bead/oligonucleotide conjugate. Incubation was carried out in binding buffer (10% glycerol, 2.5 mM MgCl2, 50 mM NaCl, 20 mM HEPES, pH 7.9, 0.5 mM DTT), followed by extensive washing of the beads in binding buffer. Electrophoresis of active p53 protein bound to the beads was carried out on 10% SDS-PAGE gels and was detected by Western blot with anti-p53 antibody.
Hematopoietic progenitor assays
Hematopoietic progenitors assays were performed by plating in triplicate 5 × 104 bone marrow low-density cells per milliliter. Cultures were established in 1% Iscove modified Dulbecco medium (IMDM) methylcellulose culture medium, with 30% FCS, 50 ng/mL recombinant murine Steel factor (Immunex, Seattle, WA), 1 U/mL recombinant human erythropoietin (Amgen, Thousand Oaks, CA), 5% vol/vol pokeweed mitogen spleen conditioned media (PWMSCM), and 0.1 mmol hemin (Sigma, St Louis, MO). Cells were incubated at 37°C, 5% CO2, and lowered (5%) O2. Bone marrow cells were cultured in methylcellulose progenitor assays with increasing concentrations of mitomycin C (Sigma), and recombinant murine tumor necrosis factor α (TNF-α; R&D Systems, Minneapolis, MN) to generate dose-response curves for Fancc-/- and Fancc+/+ hematopoietic progenitor cells. Granulocyte-macrophage colony-forming unit (CFU-GM) colonies were scored on day 7 of culture by an investigator who was blinded to the genotypes of the cultures.
Measurement of cell hypersensitivity and apoptosis assay
MEFs were treated with 50 ng/mL TNF-α and cultured for 72 hours. Viable cells were counted by trypan blue exclusion to assess viable cell numbers. To assess apoptosis, cells were fixed in paraformaldehyde after 48 hours of TNF-α treatment, and analyzed by terminal deoxynucleotidyl transferase-mediated deoxuridine triphosphate nick-end labeling (TUNEL) assay using the APO-direct system and annexin V (BD Biosciences, Mountain View, CA) as recommended by the manufacturer.
Results
The expression and function of p53 protein in primary murine Fancc-deficient cells
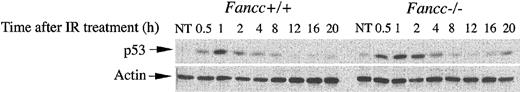
To determine whether p53 was appropriately activated in Fancc-deficient cells, we first used primary cells from inbred Fancc-/- mice to investigate p53 function following DNA damage. Levels of p53 were measured by Western blotting in primary, passage 4 MEFs that were exposed to IR (Figure 1). The course of p53 induction in wild-type cells was consistent with previous reports detailing the cyclic behavior of p53 induction following IR.29 The amount of p53 protein observed following IR damage was modestly increased in Fancc-/- cells, analogous to previous studies using mitomycin C.22,30 In addition, the basal p53 level in Fancc-/- MEFs was modestly elevated (mean of 3.2 ± 0.5 × 103 normalized density units in Fancc-/- versus 1.2 ± 0.1 × 103 density units in Fancc+/+, n = 3). In other experiments, we used a consensus p53-binding assay to verify that p53 protein derived from Fancc-/- lymphocytes appropriately bound DNA (data not shown). Collectively, these data indicate that p53 protein is induced in primary Fancc mutant cells and is functional.
Evaluation of p53 protein in Fancc-/- cells. Radiation-induced p53 protein in Fancc-deficient cells. MEFs were treated with 10 Gy IR and cultured for the indicated time periods, and protein extracts (50 μg) were analyzed by Western blotting using antibody specific to p53. A representative of 3 independent experiments is shown. Total protein levels are normalized using actin as a control, shown in the bottom panel.
Evaluation of p53 protein in Fancc-/- cells. Radiation-induced p53 protein in Fancc-deficient cells. MEFs were treated with 10 Gy IR and cultured for the indicated time periods, and protein extracts (50 μg) were analyzed by Western blotting using antibody specific to p53. A representative of 3 independent experiments is shown. Total protein levels are normalized using actin as a control, shown in the bottom panel.
The hypersensitivity of Fancc-deficient cells to apoptosis is significantly p53 dependent
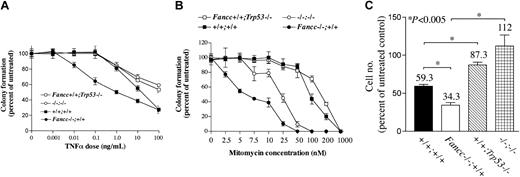
The role of p53 in executing apoptosis has been well characterized in response to stress, including inhibitory cytokines such as TNF-α and DNA damaging agents.12,31-33 Because Fancc-deficient cells are hypersensitive to cellular stresses that induce apoptosis, including TNF-α and interferon γ (IFN-γ),7,8,11,34,35 we investigated if functional p53 was required for the increased apoptosis observed in Fancc-/- cells. To test this hypothesis, we intercrossed Fancc and Trp53 mutant mice to generate F2 animals deficient at both loci (Fancc-/-Trp53-/-) and assessed the sensitivity of bone marrow-derived hematopoietic progenitor cells to TNF-α. Myeloid progenitor cells were initially used because these cells are hypersensitive to low concentrations of TNF-α and loss of this hypersensitivity is observed in myeloid malignancies in a FA experimental murine model10 and in patients with FA.9 Cultures were scored by an investigator who was blinded to the experimental genotypes. Consistent with previous studies,8 Fancc-deficient cells were hypersensitive to TNF-α (Figure 2A). Strikingly, this hypersensitivity was completely abrogated in cells lacking both Fancc and Trp53 (Figure 2A), indicating that the hypersensitivity of Fancc mutant cells to TNF-α is dependent on p53. We also tested whether the hypersensitivity of Fancc-/- myeloid progenitors to mitomycin C was p53 dependent. In contrast to progenitors deficient at Fancc only, the number of Fancc-/-Trp53-/- progenitors was rescued to wild-type levels at low concentrations of mitomycin C, indicating that this hypersensitivity was significantly p53 dependent (Figure 2B). The rescue of mitomycin C hypersensitivity was not maintained at higher mitomycin C doses, indicating that a p53-independent pathway of apoptosis also contributed to the hypersensitivity of Fancc-/- cells to mitomycin C.
The apoptotic hypersensitivity of Fancc-/- cells to TNF-α is p53 dependent. (A) Effect of p53 deficiency on the TNF-α-induced hypersensitivity of Fancc-/- progenitors. Hematopoietic progenitor colonies from bone marrow low density mononuclear cells were cultured in the presence of increasing amounts of TNF-α. Colonies were scored, and the data are plotted as percent of control CFU-GM colonies scored in the absence of TNF-α. Percent of control is shown on the y-axis, and the TNF-α dose is shown on the x-axis. The mean of each data point ± SD is shown. Each genotype is indicated by the corresponding symbol shown in the key. Statistics were assessed using the Student t test. The Fancc-/-Trp53+/+ genotypes were statistically different from all other groups (P < .001). A representative experiment conducted in triplicate cultures is shown. (B) Effect of p53 deficiency on the mitomycin C-induced hypersensitivity of Fancc-/- progenitors. Hematopoietic colonies were scored in the presence and absence of increasing doses of mitomycin C. The data are shown as percent of control (untreated) colony formation, and each genotype is indicated by the corresponding symbol shown in the legend. The mean of each data point ± SD is shown. The Fancc-/-Trp53-/- genotypes were statistically different from Fancc-/-Trp53+/+ progenitors from 2.5 to 50 nM mitomycin C concentrations (Student t test, P < .01). (C) The hypersensitivity of primary, Fancc-/- MEFs to TNF-α is dependent on p53. MEFs were cultured in the presence and absence of TNF-α (50 ng/mL), and were counted after 72 hours of culture. A representative experiment (n = 5) with similar results is shown. The change in cell number due to TNF-α treatment is expressed as percent of control, untreated cells for each genotype. Percent of control is shown on the y-axis, and the genotype is shown beneath each bar. Error bars indicate SD. Statistical analysis was performed using the Student t test. The differences between the Fancc+/+Trp53-/- and Fancc-/-Trp53-/- groups are not statistically significant.
The apoptotic hypersensitivity of Fancc-/- cells to TNF-α is p53 dependent. (A) Effect of p53 deficiency on the TNF-α-induced hypersensitivity of Fancc-/- progenitors. Hematopoietic progenitor colonies from bone marrow low density mononuclear cells were cultured in the presence of increasing amounts of TNF-α. Colonies were scored, and the data are plotted as percent of control CFU-GM colonies scored in the absence of TNF-α. Percent of control is shown on the y-axis, and the TNF-α dose is shown on the x-axis. The mean of each data point ± SD is shown. Each genotype is indicated by the corresponding symbol shown in the key. Statistics were assessed using the Student t test. The Fancc-/-Trp53+/+ genotypes were statistically different from all other groups (P < .001). A representative experiment conducted in triplicate cultures is shown. (B) Effect of p53 deficiency on the mitomycin C-induced hypersensitivity of Fancc-/- progenitors. Hematopoietic colonies were scored in the presence and absence of increasing doses of mitomycin C. The data are shown as percent of control (untreated) colony formation, and each genotype is indicated by the corresponding symbol shown in the legend. The mean of each data point ± SD is shown. The Fancc-/-Trp53-/- genotypes were statistically different from Fancc-/-Trp53+/+ progenitors from 2.5 to 50 nM mitomycin C concentrations (Student t test, P < .01). (C) The hypersensitivity of primary, Fancc-/- MEFs to TNF-α is dependent on p53. MEFs were cultured in the presence and absence of TNF-α (50 ng/mL), and were counted after 72 hours of culture. A representative experiment (n = 5) with similar results is shown. The change in cell number due to TNF-α treatment is expressed as percent of control, untreated cells for each genotype. Percent of control is shown on the y-axis, and the genotype is shown beneath each bar. Error bars indicate SD. Statistical analysis was performed using the Student t test. The differences between the Fancc+/+Trp53-/- and Fancc-/-Trp53-/- groups are not statistically significant.
We also measured TNF-α hypersensitivity to verify these observations in nonhematopoietic cells. MEFs of the same F2 genotypes were cultured in the presence and absence of TNF-α (Figure 2C). Like Fancc-deficient hematopoietic progenitors, the growth of Fancc-/- MEFs was abnormally sensitive to TNF-α. Genetic disruption of Trp53-/- abrogated this hypersensitivity phenotype in Fancc-/-Trp53-/- cells. In addition, consistent with previous studies in hematopoietic cells, the increased sensitivity of Fancc-/- MEFs to TNF-α was associated with increased apoptosis and this hypersensitivity was lost in cells deficient at both loci (data not shown). Taken together, these data show that the hypersensitivity of Fancc-deficient cells to cytokine-mediated apoptosis, and mitomycin C to an extent, is rescued by inactivation of Trp53.
Fancc and Trp53 cooperate in tumorigenesis
The increased p53 levels in Fancc-/- cells and ability of p53 to modulate apoptosis in Fancc-/- cells suggested that Fancc and Trp53 might cooperate in vivo. To test this hypothesis, compound Fancc and Trp53 heterozygotes were mated to produce offspring (F2) that were mutant at Trp53, Fancc, or both alleles. The expected mendelian frequency was obtained for all genotypes of the 399 mice generated from the intercross with the exception of mice that were nullizygous at both Fancc and Trp53. The actual number of viable animals with this genotype was only 1 of 70, compared to the expected frequency of 1 of 16 (P < .001 comparing number of viable embryos to predicted frequency), indicating a lethal developmental defect in approximately 75% of these embryos.
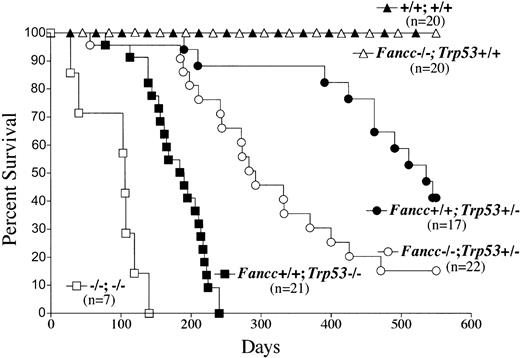
Although Fancc-/- mice do not spontaneously develop tumors, Trp53+/- and Trp53-/- mice have a high incidence of lymphoma and sarcoma. Mice from the Fancc and Trp53 genetic intercross were monitored weekly for tumor formation (Figure 3). A total of 107 mice of the 6 genotypes shown in the figure were monitored for 1.5 years. Consistent with previous studies, Fancc-/- mice did not develop tumors, and tumor formation and survival in Trp53+/- and Trp53-/- C57Bl/6 mice were comparable to previous studies using this mouse line.25 Trp53+/- and Trp53-/- mice had a median survival of 534 and 185 days, respectively. Importantly, absence of the Fancc gene significantly reduced the latency of tumor formation in both Fancc-/-Trp53+/- and Fancc-/-Trp53-/- mice with a median survival of 336 and 105 days, respectively (Figure 3).
Fancc and p53 cooperate in the progression of tumorigenesis in mice. Mice that were deficient for Fancc and Trp53 were monitored long term (1.5 years) for tumors (107 total mice). Mice were killed at the observance of tumors or significant morbidity. A Kaplan-Meier plot of percent survival (y-axis) as a function of time (x-axis) is shown. The genotypes are indicated next to each plot. Statistical significance of differences in survival between the groups was assessed by log rank analysis: P < .0001 comparing Fancc+/+Trp53-/- to Franc-/-Trp53-/-, and P < .005 comparing Fancc+/+Trp53+/- to Fancc-/-Trp53+/-.
Fancc and p53 cooperate in the progression of tumorigenesis in mice. Mice that were deficient for Fancc and Trp53 were monitored long term (1.5 years) for tumors (107 total mice). Mice were killed at the observance of tumors or significant morbidity. A Kaplan-Meier plot of percent survival (y-axis) as a function of time (x-axis) is shown. The genotypes are indicated next to each plot. Statistical significance of differences in survival between the groups was assessed by log rank analysis: P < .0001 comparing Fancc+/+Trp53-/- to Franc-/-Trp53-/-, and P < .005 comparing Fancc+/+Trp53+/- to Fancc-/-Trp53+/-.
Detailed histopathology was performed to determine the types of neoplasms in intercrossed mice (Table 1; Figure 4). The spectrum of tumors observed in Trp53+/- and Trp53-/- mice was similar to previous studies,25 which include osteosarcoma, lymphoma, and soft-tissue sarcomas. Although there was overlap in the spectrum of tumors observed in mice mutant at both Trp53 and Fancc compared to mice that are deficient at Trp53 only, several differences were observed. Consistent with prior studies, myeloid malignancies were not observed in either Trp53+/- or Trp53-/- mice that were wild-type at Fancc.25,36 One Fancc-/-Trp53+/- mouse had a myeloid malignancy, a histiocytic sarcoma with extensive myeloid invasion of the liver, spleen, and vertebrae (Figure 4A-F). Also, an undifferentiated spindle cell tumor of the neck (Figure 4G-H) and an ovarian tumor (Figure 4I) were observed in Fancc-/-Trp53+/- mice, but not Fancc+/+Trp53+/- mice. A majority of tumors derived from Fancc-/-Trp53+/- mice (4 of 5 analyzed) exhibited loss of heterozygosity of the normal p53 allele (data not shown). In addition, although the sample size of tumors was low in the Fancc-/-Trp53-/- group (due to the in utero demise of the majority of these animals), Fancc-/-Trp53-/- mice developed a medulloblastoma and an adenocarcinoma (Figure 4J-L), both of which have been observed in patients with FA37 but not in unmanipulated Trp53-/- mice.25,36
Location and type of tumors observed in mice from the FanccTrp53 intercross
Genotype (no. animals/group) . | Type of tumors observed . | No. of each tumor type observed . | % of each tumor type observed . |
|---|---|---|---|
| Fancc+/+Trp53+/+ (20) | No tumors | — | — |
| Fancc−/−Trp53+/+ (20) | No tumors | — | — |
| Fancc+/+Trp53+/− (17) | Osteosarcoma | 4 | 36 |
| Fancc+/+Trp53+/− | Sarcoma | 2 | 18 |
| Fancc+/+Trp53+/− | Lymphoma | 3 | 27 |
| Fancc+/+Trp53+/− | Hemangiosarcoma | 2 | 18 |
| Fancc+/+Trp53+/− | Total | 11 | 100 |
| Fancc−/−Trp53+/− (22) | Osteosarcoma | 4 | 31 |
| Fancc−/−Trp53+/− | Fibrosarcoma (muscle) | 1 | 8 |
| Fancc−/−Trp53+/− | Dermal spindle-cell tumor (neck) | 1 | 8 |
| Fancc−/−Trp53+/− | Lymphoma | 5 | 38 |
| Fancc−/−Trp53+/− | Myeloid malignancy (histiocytic) | 1 | 8 |
| Fancc−/−Trp53+/− | Ovarian tumor | 1 | 8 |
| Fancc−/−Trp53+/− | Total | 13 | 100 |
| Fancc+/+Trp53−/− (21) | Lymphoma (primarily thymic) | 11 | 73 |
| Fancc+/+Trp53−/− | Other anaplastic sarcoma | 4 | 27 |
| Fancc+/+Trp53−/− | Total | 15 | 100 |
| Fancc−/−Trp53−/− (7) | Adenocarcinoma (prostate) | 1 | 25 |
| Fancc−/−Trp53−/− | Lymphoma | 1 | 25 |
| Fancc−/−Trp53−/− | Anaplastic sarcoma (cardiac) | 1 | 25 |
| Fancc−/−Trp53−/− | Medulloblastoma | 1 | 25 |
| Fancc−/−Trp53−/− | Total | 4 | 100 |
Genotype (no. animals/group) . | Type of tumors observed . | No. of each tumor type observed . | % of each tumor type observed . |
|---|---|---|---|
| Fancc+/+Trp53+/+ (20) | No tumors | — | — |
| Fancc−/−Trp53+/+ (20) | No tumors | — | — |
| Fancc+/+Trp53+/− (17) | Osteosarcoma | 4 | 36 |
| Fancc+/+Trp53+/− | Sarcoma | 2 | 18 |
| Fancc+/+Trp53+/− | Lymphoma | 3 | 27 |
| Fancc+/+Trp53+/− | Hemangiosarcoma | 2 | 18 |
| Fancc+/+Trp53+/− | Total | 11 | 100 |
| Fancc−/−Trp53+/− (22) | Osteosarcoma | 4 | 31 |
| Fancc−/−Trp53+/− | Fibrosarcoma (muscle) | 1 | 8 |
| Fancc−/−Trp53+/− | Dermal spindle-cell tumor (neck) | 1 | 8 |
| Fancc−/−Trp53+/− | Lymphoma | 5 | 38 |
| Fancc−/−Trp53+/− | Myeloid malignancy (histiocytic) | 1 | 8 |
| Fancc−/−Trp53+/− | Ovarian tumor | 1 | 8 |
| Fancc−/−Trp53+/− | Total | 13 | 100 |
| Fancc+/+Trp53−/− (21) | Lymphoma (primarily thymic) | 11 | 73 |
| Fancc+/+Trp53−/− | Other anaplastic sarcoma | 4 | 27 |
| Fancc+/+Trp53−/− | Total | 15 | 100 |
| Fancc−/−Trp53−/− (7) | Adenocarcinoma (prostate) | 1 | 25 |
| Fancc−/−Trp53−/− | Lymphoma | 1 | 25 |
| Fancc−/−Trp53−/− | Anaplastic sarcoma (cardiac) | 1 | 25 |
| Fancc−/−Trp53−/− | Medulloblastoma | 1 | 25 |
| Fancc−/−Trp53−/− | Total | 4 | 100 |
Fifty-seven of the 107 mice shown in Figure 3 died during the course of the study, of which 44 were subjected to autopsy. Thirty-eight animals developed a total of 43 tumors, which were evaluated in detail by histologic examination. In some tumors, immunohistochemical analysis was also used to identify specific cell lineages. — indicates not applicable.
Histopathology of tumors in Fancc and Trp53 intercrossed mice. (A-F) Myeloid malignancy (histiocytic sarcoma) in a Fancc-/-Trp53+/- mouse. (A-B) Malignant histiocytes are observed in the red pulp of the spleen, indicated in panel A by the arrow. (C) Immunohistochemical characterization demonstrates that the malignant cells within the spleen are Mac-2+. (D-E) Malignant histiocytes are also observed in the liver as indicated by the arrow. (F) The malignant cells are shown to be Mac-2+ by immunohistochemical staining. (G-H) Dermal spindle cell tumor from the neck of a Fancc-/-Trp53+/- mouse shows a high mitotic index and undifferentiated cells with cigar-shaped nuclei. (I) Ovarian tumor from a Fancc-/-Trp53+/- mouse showing nests of round, tumor cells with rounded, pale nuclei. (J-K) Medulloblastoma of the cerebellum from a Fancc-/-Trp53-/- animal. Characteristic neoplastic cells with carrot-shaped nuclei, hyperchromatic coarse chromatin, and almost nonvisible cytoplasm, with frequent mitotic figures (indicated by arrows) are arranged in sheets with pseudo-rosette formations visible. (L) Adenocarcinoma of the prostate of a Fancc-/-Trp53-/- mouse shows malignant cells arranged in irregularly shaped glandular structures invading surrounding smooth muscle. Original magnifications: × 100 (G); × 200 (A,D,H,J); × 400 (B-C,E-F,I,K-L); and × 600 (H inset).
Histopathology of tumors in Fancc and Trp53 intercrossed mice. (A-F) Myeloid malignancy (histiocytic sarcoma) in a Fancc-/-Trp53+/- mouse. (A-B) Malignant histiocytes are observed in the red pulp of the spleen, indicated in panel A by the arrow. (C) Immunohistochemical characterization demonstrates that the malignant cells within the spleen are Mac-2+. (D-E) Malignant histiocytes are also observed in the liver as indicated by the arrow. (F) The malignant cells are shown to be Mac-2+ by immunohistochemical staining. (G-H) Dermal spindle cell tumor from the neck of a Fancc-/-Trp53+/- mouse shows a high mitotic index and undifferentiated cells with cigar-shaped nuclei. (I) Ovarian tumor from a Fancc-/-Trp53+/- mouse showing nests of round, tumor cells with rounded, pale nuclei. (J-K) Medulloblastoma of the cerebellum from a Fancc-/-Trp53-/- animal. Characteristic neoplastic cells with carrot-shaped nuclei, hyperchromatic coarse chromatin, and almost nonvisible cytoplasm, with frequent mitotic figures (indicated by arrows) are arranged in sheets with pseudo-rosette formations visible. (L) Adenocarcinoma of the prostate of a Fancc-/-Trp53-/- mouse shows malignant cells arranged in irregularly shaped glandular structures invading surrounding smooth muscle. Original magnifications: × 100 (G); × 200 (A,D,H,J); × 400 (B-C,E-F,I,K-L); and × 600 (H inset).
Discussion
p53 is a critical sensor of cell stress and modulator of apoptosis to a broad range of stimuli. Recent data have demonstrated that the apoptotic function of p53 is critical in protection from tumorigenesis.38,39 Our genetic evidence indicates that the increased stress-induced apoptosis observed in Fancc-deficient cells is, at least in part, p53 dependent. Apoptosis in hematopoietic progenitors was abrogated in the absence of p53 at all doses of the cytotoxic cytokine TNF-α, which does not induce DNA damage. Disruption of Trp53 in Fancc-/- cells reduced apoptosis to that of Fancc+/+ cells at low but not high doses of mitomycin C, indicating that apoptosis induced by cross-linking agents in Fancc mutant cells was only partially p53 dependent. DNA cross-linking agents, including mitomycin C, induce breaks and lesions that signal the activation of p53,40 and induce apoptosis largely by p53-dependent mechanisms.41 However, in our studies there was a significant p53-independent mechanism of apoptosis mediating the hypersensitivity of Fancc-deficient cells to mitomycin C, as was also observed previously in other FANCC-deficient cells.22 A potential candidate to consider in future studies that may mediate apoptosis in Fancc-deficient cells is the p53-related protein, p73. p73 is able to activate the transcription of p53-dependent promoters.42 In addition, others have shown that p73 functions in the absence of p53 in mediating apoptosis induced by cross-linking and other proapoptotic agents.43,44 Therefore, functional p73 may be able to induce apoptosis in the absence of p53 in Fancc-/-Trp53-/- cells as the dose of mitomycin C is increased.
FA proteins have been implicated in a range of “caretaker” functions including DNA repair, cell cycle control, apoptosis, and redox modulation that collectively function to maintain genomic integrity (for a review, see Joenje and Patel5 ). There are at least 3 potential mechanisms to explain the cooperation between Trp53 and Fancc. First, abnormalities in FA functions can lead to basal and genotoxic-induced chromosome instability. In this model p53 is activated following stress to limit cell growth or to initiate apoptosis of oncogenic cells as a mechanism of tumor avoidance. Thus, we speculate that the modestly increased p53 protein levels observed in Fancc-deficient cells in the current report (Figure 1) following genotoxic or nongenotoxic stress could be a result of intrinsic genomic instability present in these cells, which is detected by the signaling network that activates p53. An important priority in future studies will be to examine the role of p53 in suppressing genetic mutations in Fancc-deficient mice using a marker of genetic instability such as hypoxanthine-guanine phosphoribosyltransferase.
A second possibility is suggested by studies linking the function of the FA protein members with the BRCA1-dependent mono-ubiquitination of FANCD2, and the identification of BRCA2 mutations in the FA-D1 complementation group. BRCA1 was reported to physically interact with p53.45,46 Further, mammary cells from Brca1 knockout mice undergo increased apoptosis that is alleviated in the absence of Trp53 and acquire mammary cell malignancies on loss of p53 function.19 Interestingly, like Fancc-/- mice, basal p53 protein was increased in Brca1 conditional mutant mice, compared to wild-type mice.21 In contrast, whereas Fancc-/- cells have slightly increased p53 activity following DNA damage, Brca1-/- cells have reduced levels of induced p53 protein compared to wild-type controls. Therefore, although FA proteins functionally interact with BRCA1,17 based on the current and previously published21 murine models, the specific cooperation that p53 has with Fancc and Brca1, respectively, is distinct. Studies have also demonstrated cooperation between BRCA2 and p53. In one study, the activity and function of p53 in Brca2-deficient cells was elevated, analogous to our studies.47 Further, loss of p53 in conditional Brca2-deficient mice is associated with a decreased latency of tumorigenesis.20 The precise biochemical relationship between p53 and Brca2 may be due to an interaction of these proteins with the homologous recombination machinery, because a physical association between Brca2 and p53 has been demonstrated in a complex that also contains Rad51.48 These studies therefore raise the interesting possibility that Fancc also may functionally interact with p53 in a DNA repair or cell cycle pathway.
Lastly, Fancc may affect the p53 network by negatively regulating PKR. Fancc was recently shown to directly interact with the Hsp70 protein, and this interaction inhibits the function of PKR in executing apoptosis.35,49 Given that p53 is activated by PKR,13,14 these studies suggest the possibility that Fancc could function in a complex that is closely associated with regulating p53 function via PKR.
Although Fancc-/- mice with intact Trp53 do not develop tumors, genetic loss of both Fancc and Trp53 results in a markedly shortened latency of tumorigenesis in a Trp53 gene dose-dependent fashion. Further, the spectrum of tumors observed in the intercrossed mice overlaps with that observed in patients with FA. The data are consistent with the hypothesis that loss of p53 or its effectors has an important role in malignant progression in human FA tumors, particularly considering that patients with FA are highly predisposed to head and neck cancers and epithelial malignancies.3 Others have shown that inactivation of p53 occurs in the majority of these types of malignancies, usually by either mutation15 or inactivation due to papilloma virus infection.50-54 Data from this report support the rationale for a thorough evaluation of the p53 pathway in malignancies in patients with FA.
In summary, the present study in mice has identified p53 as an important cofactor with Fancc in suppressing tumorigenesis. Our findings establish that p53 is an important modulator of apoptosis in Fancc-deficient mice in vitro and is consistent with the observations that mice mutant at both loci acquire tumors at an accelerated rate in vivo. Finally, these mice provide a model to address fundamental aspects of FA and to test molecular therapeutic strategies.
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-03-0971.
Supported by National Institutes of Health grants RO1 HL 63219 (D.W.C.), NIDDK P30 DK49218 (D.W.C.), 1RO1 HL56416 (H.E.B.), RO1 HL67384 (H.E.B.), IT32 DK07519 (S.L.M.C.), NIH 1K08HL DK04071-01 (L.S.H.), and the Riley Children's Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Lee Ann Baldridge for excellent technical assistance with immunohistochemistry. We also thank Dr Kevin Shannon, Department of Pediatrics, University of California San Francisco, for reading the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal