Abstract
In recent years, synthetic tyrosine kinase inhibitors have made a rapid transition from basic research to therapeutic application. These compounds represent a major clinical advance in the approach to cancer in their relative specificity of action and decreased toxicity. We report here the effects of a novel tyrosine kinase inhibitor CR4 that interferes with growth-promoting pathways to markedly inhibit the growth and survival of both Philadelphia-positive and -negative acute lymphoblastic leukemia (ALL) as well as acute myeloid leukemia (AML). While efficiently ablating leukemic cell growth, normal cell growth and differentiation remain unaffected by CR4. CR4 demonstrates an ability to inhibit the function of multiple growth-critical kinases and yet exhibits a low level of cytotoxicity. These findings suggest that CR4 may prove to be highly effective as a therapeutic agent. (Blood. 2003;102:4153-4158)
Introduction
Tyrosine phosphorylation is often central to the development of unregulated proliferation. Mutation, hyperactivation, translocation, and overexpression of tyrosine kinases are all associated with tumorigenesis, and aberrant kinases such as the Bcr-Abl fusion product have been specifically identified and can play a driving role in unregulated growth.1-6 The essential nature of Bcr-Abl and other tyrosine kinases to the growth process of transformed cells renders them ideal targets in attempting to prevent unregulated growth.
A small number of compounds with structures based upon mimicry of either the adenosine triphosphate (ATP) moiety or tyrosine residues have been shown to be relatively selective inhibitors of tyrosine kinases.7,8 As a result of their ability to inhibit tyrosine phosphorylation, these compounds can alter cell responses to growth factors or other processes driven by tyrosine kinase activity, including uncontrolled growth. The concept that tyrosine analogs act as anticancer agents was established by us and others using an inhibitor selective for the Jak kinases (AG-490) to kill by apoptosis human acute lymphoblastic leukemia (ALL) cells in vitro and in vivo.9,10
Recent years have seen the rapid transition of tyrosine kinase inhibitors from the laboratory bench to therapeutic application, and there is currently much interest in the development of small synthetic molecules that inhibit tyrosine kinases for anticancer therapy.7,8,11 The most noted success has been the development of the abl/kit kinase inhibitor STI571 (Gleevec; Novartis Pharmaceuticals, East Hanover, NJ),12-17 which is highly effective in chronic myelogenous leukemia (CML) and possibly other malignancies.11-20
However, the development of resistance to ST1571 has been reported recently in cultured cells21,22 and, more importantly, in a number of patients treated with STI571.23-25 Resistance to ST1571 appears to occur primarily through either increased expression of Bcr-Abl as the result of gene amplification21 or the acquisition of Bcr-Abl mutations,26 such that ST1571 binding is inhibited. These observations suggest that multitarget treatment or, alternatively, targeting of one kinase with multiple compounds demonstrating different binding properties may be a preferable approach.
We report here the inhibitory effect of a novel hydroxystyryl-acrylonitrile compound on the growth of both Philadelphia-positive (Ph+) and -negative acute lymphoblastic leukemias. This compound, CR4, appears to affect multiple tyrosine kinase targets, including the Bcr-Abl fusion protein and the Jak kinase family, both central to the growth of hematopoietic malignancies.
Materials and methods
Cells and antibodies
Ph+ ALL cell lines Z119, Z181, and Z33,27,28 acute myeloid leukemia (AML) (OCI-AML2 and 3), and Ph- ALL (ALL-C1, A1) were maintained in standard RPM1 or α modified essential medium (αMEM) supplemented with 10% fetal calf serum (FCS). The styryl-acrylonitrile compounds were synthesized in house and purified by repeated crystallizations (T.G. et al, submitted manuscript).
Treatment of leukemia cells with CR4
ALL and AML cell lines and primary cells were cultured in semisolid media containing 0.8% (vol/vol) methylcellulose to allow the assessment of individual colony formation. Primary ALL patient cells were prepared as previously described.29 Briefly, nonadherent cells and T cells were depleted from bone marrow (BM) or peripheral blood samples and plated in Iscove modified Dulbecco medium (IMDM) supplemented with 10% FCS and PHA T-cell-conditioned medium (PHA-TCM). Primary AML cells were prepared similarly, but plated in medium supplemented with 10% FCS, 15 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF), 40 U/mL interleukin-3 (IL-3), and 50 ng/mL macrophage growth factor (MGF). Cell lines were grown in standard medium with FCS. CR4 was added to the mediums at the indicated concentrations and colonies were scored after 7 (ALL) or 9 (AML) days in duplicate dishes. Each experiment was repeated at least 4 times.
Bone marrow colony assays. Colony-forming unit granulocyte-erythrocyte-megakaryocyte-macrophage (CFU-GEMM) assay was performed as previously described30,31 with some variations. In brief, heparinized bone marrow cells were layered over Percoll and centrifuged at 400g at 4°C for 10 minutes to remove neutrophils and red blood cells (RBCs). The fractionated BM cells at 2 × 105 cells/mL were cultured in IMDM containing 0.9% (vol/vol) methylcellulose supplemented with 30% FCS or normal human plasma, a cocktail of cytokines containing G-CSF (10 ng/mL), IL-3 (40 U/mL), MGF (50 ng/mL), erythropoietin (2 U/mL), or thrombopoietin (TPO; 10 ng/mL), and 5 × 10-5 M β-2-mercaptoethanol. CR4 was added to the mediums at escalating concentrations as indicated in “Results.” All cultures were evaluated at 14 days for the number of blast-forming unit-erythroid (BFU-E) colonies (defined as aggregates of more than 500 hemoglobinized cells, or 3 or more erythroid subcolonies), colony-forming unit-cell (CFU-C) colonies (defined as granulocyte or monocyte-macrophage cells, or both), and CFU-GEMM colonies (a mixed population composed of all elements). To prepare hematopoietic stem cells, normal bone marrow cells were isolated as above, and CD34+ cells were positively selected with anti-CD34 magnetic beads using the magnetic-activated cell sorter (MACS) system (Miltenyi Biotec, Auburn, CA). Normal bone marrow was voluntarily donated by bone marrow transplant donors, and fresh leukemic cells by patients with relapsed leukemia. Approval from the institutional review board (IRB) of the Hospital for Sick Children was obtained for both cell sources. At least 5 independent marrows were tested for sensitivity to CR4.
Phosphorylation and kinase assays. Immunoprecipitation, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and Western blotting were performed as previously described.32 For kinase assays, immunoprecipitates were washed in kinase buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.6], 10 mM MgCl2, and 10 mM MnCl2). Reactions were initiated by addition of γ33P-ATP. After 15 minutes at room temperature reactions were stopped by addition of SDS sample buffer; the samples were resolved by SDS-PAGE, transferred to nitrocellulose membrane, and exposed to film.
Treatment of Philadelphia-positive ALL in NOD-SCID mice. Nonobese diabetic-severe combined immunodeficiency (NOD-SCID) mice were sublethally irradiated (3.5 Gy) and injected with 5 × 106 Philadelphia-positive Z119 acute lymphoblastic leukemia cells. Then, 24 hours later Alzet 2001 micro-osmotic pumps (Alza, Palo Alto, CA) were subcutaneously implanted. Pumps contained either 20 mM CR4 in 50% dimethyl sulfoxide (DMSO)/medium or 50% DMSO/medium alone. Alzet 2001 pumps hold a total volume of 200 μL and release 1 μL per hour over 7 to 10 days, approximately equivalent to a dosage of 250 μg/kg per hour (25 g for a mouse). Pumps were replaced every 7 days. After 14 and 21 days, mice were killed and bone marrow extracted from the fore and hind limbs. To detect the presence of Z119 cells, single cell suspensions were prepared and the samples stained with antihuman CD19 (and antihuman HLA-DR; not shown) antibodies for flow cytometry analysis. These antibodies did not crossreact with murine cells. Bone marrow cell cultures were performed at day 14 to assess the viability of infiltrating Z119 cells. BM cells (5 × 104) were cultured in IMDM containing 0.9% (vol/vol) methylcellulose supplemented with 30% serum (consisting of a 1:1 mixture of FCS and normal human cord blood plasma). No cytokines were added. There was no growth of murine cells under these conditions. The number of ALL colonies was evaluated after 9 days.
Histochemical analysis
Tissue-touch preparations from mouse organs were fixed with methanol on glass slides and stained with Wright-Giemsa stain using a Hematek II slide stainer.
Results
CR4 prevents the growth of ALL and AML cells in culture
We systematically synthesized novel hydroxyl-containing styryl-acrylonitrile compounds possessing structural characteristics conducive to inhibition of tyrosine kinase activities. Leukemic and normal bone marrow cells were grown in the presence of the styryl-acrylonitrile compounds, and the number of colonies was counted after 7 (AML), 9 (ALL), or 14 (bone marrow) days. The morphology and health of individual colonies was also evaluated. Compounds were selected only if they displayed a minimal 40-fold difference in the concentration inducing leukemia cell death and that affecting normal cell growth and development.
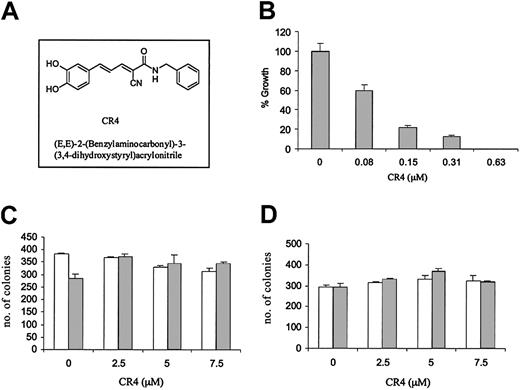
One compound in particular, (E, E)-2-(benzylaminocarbonyl)-3-(3,4-dihydroxystyryl)acrylonitrile (CR4) (Figure 1A), was highly effective against both Philadelphia-positive and -negative acute lymphoblastic leukemia, while demonstrating little evidence of nonspecific cytotoxicity. Philadelphia-positive ALL growth in vitro was significantly inhibited by exposure to levels of CR4 as low as 80 nM (Figure 1B), while in contrast there was no deleterious effect upon normal bone marrow differentiation or proliferation (Figure 1C). Normal human bone marrow cultured in CFU-GEMM colony-forming assays in the presence of CR4 (0-7.5 μM) differentiated into normal erythroid, mixed, and granulocyte colonies. Similar results were obtained with purified hematopoietic stem cells. CD34+ hematopoietic stem cells were isolated from normal bone marrow with anti-CD34 magnetic beads and cultured in the presence of increasing doses of CR4. Again, proliferation and differentiation were not detrimentally affected (Figure 1D). A number of established Ph+ ALL cell lines were assessed in vitro with CR4 and all were similarly inhibited (not shown). Thus, CR4 would appear to target growth pathways essential to Ph+ ALL while simultaneously permitting normal differentiation and proliferation.
CR4 inhibits Ph+ ALL cell growth but not normal bone marrow differentiation. (A) Structure of (E, E)-2-(benzylaminocarbonyl)-3-(3,4-dihydroxystyryl)acrylonitrite—CR4. (B) Ph+ ALL cells (Z119) were cultured in colony-forming assays in the presence of increasing concentrations of CR4, as indicated. ALL colony numbers were counted after 9 days. The solvent for CR4 (DMSO/Iscove) alone did not affect cell growth (not shown). Similar results were obtained with the Ph+ cell lines Z181 and Z33. (C) Single-cell suspensions of normal human bone marrow were prepared by Percoll gradient centrifugation. BM cells were plated in CFU-GEMM semisolid media cultures in the presence of CR4 as indicated. Colony type and numbers were assessed after 14 days of culture at 37°C, 5% CO2. BM cells differentiated into normal erythroid (BFU-E; □) and granulocyte/monocyte/macrophage (CFU-C; ▦) colonies. Mixed colony numbers (erythroid/granulocyte/monocyte/macrophage) were also normal (not shown). (D) CD34+ hematopoietic stem cells were isolated from bone marrow with anti-CD34 magnetic beads (Miltenyi Biotec) and cultured as for complete bone marrow (shown in panel C). □ indicates BFU-E colonies; and ▦, CFU-C colonies. In each case the average of 2 independent experiments is shown with standard errors.
CR4 inhibits Ph+ ALL cell growth but not normal bone marrow differentiation. (A) Structure of (E, E)-2-(benzylaminocarbonyl)-3-(3,4-dihydroxystyryl)acrylonitrite—CR4. (B) Ph+ ALL cells (Z119) were cultured in colony-forming assays in the presence of increasing concentrations of CR4, as indicated. ALL colony numbers were counted after 9 days. The solvent for CR4 (DMSO/Iscove) alone did not affect cell growth (not shown). Similar results were obtained with the Ph+ cell lines Z181 and Z33. (C) Single-cell suspensions of normal human bone marrow were prepared by Percoll gradient centrifugation. BM cells were plated in CFU-GEMM semisolid media cultures in the presence of CR4 as indicated. Colony type and numbers were assessed after 14 days of culture at 37°C, 5% CO2. BM cells differentiated into normal erythroid (BFU-E; □) and granulocyte/monocyte/macrophage (CFU-C; ▦) colonies. Mixed colony numbers (erythroid/granulocyte/monocyte/macrophage) were also normal (not shown). (D) CD34+ hematopoietic stem cells were isolated from bone marrow with anti-CD34 magnetic beads (Miltenyi Biotec) and cultured as for complete bone marrow (shown in panel C). □ indicates BFU-E colonies; and ▦, CFU-C colonies. In each case the average of 2 independent experiments is shown with standard errors.
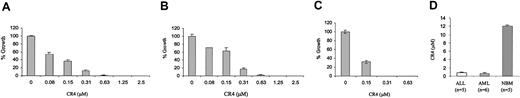
CR4 was found to be highly active not only against Ph+ ALL cells, but also against Philadelphia-negative (Ph-) ALL lines (Figure 2A). This suggested that CR4 was not a selective inhibitor of the Bcr-Abl oncogene. ALL proliferation is not necessarily driven by acquisition of the Philadelphia chromosome and may also result from abnormal cytokine responsiveness or similar growth-promoting changes. Similarly, the growth of AML cell lines was also determined to be highly sensitive to inhibition by CR4 (Figure 2B). Thus, CR4 appeared to either inhibit a pathway critical to all leukemic cell growth or, alternatively, to interfere with multiple pathways.
CR4 inhibits Ph- ALL and AML cell growth. (A) Ph- ALL cells, (B) AML cells, and (C) primary AML patient cells were cultured in colony-forming assays in the presence of increasing concentrations of CR4, as indicated. Colony numbers were assessed after 9 to 14 days. The average of 2 independent experiments is shown in each case. Primary AML cells were prepared by Percoll gradient centrifugation of patient samples. Each condition was assayed in duplicate, and one representative experiment of 3 is shown with standard deviations. The solvent for CR4 (DMSO/Iscove) alone did not affect cell growth (not shown). (D) Median inhibitory concentrations (IC50s) (± standard error) for primary ALL (n = 5) and AML (n = 6) and normal bone marrow (n = 5) were obtained by culturing each sample in the presence of varying concentrations of CR4 (0-20 μM).
CR4 inhibits Ph- ALL and AML cell growth. (A) Ph- ALL cells, (B) AML cells, and (C) primary AML patient cells were cultured in colony-forming assays in the presence of increasing concentrations of CR4, as indicated. Colony numbers were assessed after 9 to 14 days. The average of 2 independent experiments is shown in each case. Primary AML cells were prepared by Percoll gradient centrifugation of patient samples. Each condition was assayed in duplicate, and one representative experiment of 3 is shown with standard deviations. The solvent for CR4 (DMSO/Iscove) alone did not affect cell growth (not shown). (D) Median inhibitory concentrations (IC50s) (± standard error) for primary ALL (n = 5) and AML (n = 6) and normal bone marrow (n = 5) were obtained by culturing each sample in the presence of varying concentrations of CR4 (0-20 μM).
In addition to inhibiting the growth of established ALL and AML cell lines, CR4 efficiently killed highly aggressive primary leukemia cells obtained from patients suffering relapse of AML or ALL (Figure 2C-D). Once again, leukemic cell death was induced at CR4 concentrations well below those detrimentally affecting normal bone marrow proliferation and differentiation.
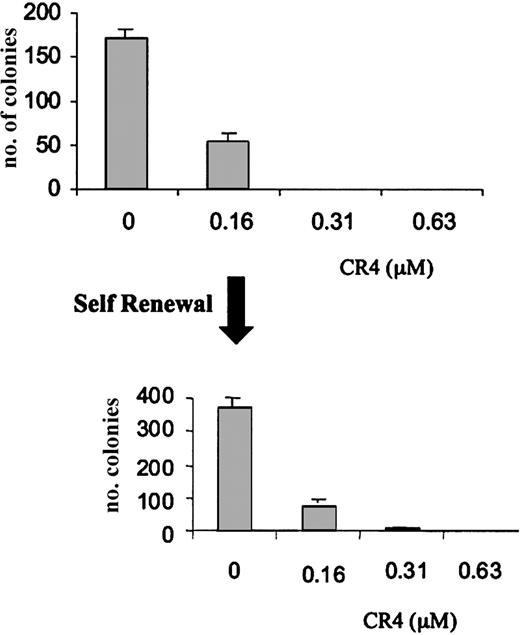
The inadvertent selection of drug resistance is a common phenomenon upon exposure of transformed cells to sublethal doses of an antiproliferative drug. We therefore performed a self-renewal assay in order to determine whether pre-exposure to CR4 could result in the selection of resistant cells. AML cells were cultured in a concentration of CR4 resulting in an approximately 70% ablation of growth (160 nM CR4). After 7 days, the surviving cells were rescued from culture and replated in fresh medium. The rescued cells were viable, growing normally in the absence of CR4. However, there was no evidence of decreased sensitivity to CR4. AML cells were efficiently killed upon re-exposure (Figure 3) and resupplementation of cultures with CR4 after 7 days. Under similar experimental conditions, CR4 was equally effective in killing previously exposed ALL cells (not shown). Interestingly, the addition of growth-promoting cytokines such as GM-CSF to the cultures could not prevent CR4-induced leukemia cell death (not shown).
Absence of CR4 resistance in AML self-renewal assay. AML cells were cultured in the presence of varying concentrations of CR4 for 7 days. Cells surviving culture with 160 nM CR4 were rescued and replated in fresh medium containing CR4 as indicated. Cells from several of the original cultures were pooled for the second phase. After a further 7 days of incubation, colony numbers were assessed. Normal cell growth is unaffected at these concentrations of CR4. Shown is one representative experiment of 2 with standard deviations.
Absence of CR4 resistance in AML self-renewal assay. AML cells were cultured in the presence of varying concentrations of CR4 for 7 days. Cells surviving culture with 160 nM CR4 were rescued and replated in fresh medium containing CR4 as indicated. Cells from several of the original cultures were pooled for the second phase. After a further 7 days of incubation, colony numbers were assessed. Normal cell growth is unaffected at these concentrations of CR4. Shown is one representative experiment of 2 with standard deviations.
Inhibition of tyrosine kinase activity by CR4
The sensitivity of PH+ ALL cell growth to inhibition by CR4 promoted the Bcr-Abl protein as a possible target kinase. However, the effectiveness of CR4 against Ph- ALL and AML cells suggested that it could inhibit other kinases as well. While Bcr-Abl activity is essential to the growth of Philadelphia-positive cells, Jak kinase activity would appear essential to non-Philadelphia leukemias (ALL and AML). Leukemia cells typically produce growth-promoting cytokines that contribute to their unregulated proliferation, and the Jak tyrosine kinases (Janus kinases) are central to signal transduction through the cytokine receptors.33-36 In vitro kinase assay analysis of the ability of CR4 to inhibit the catalytic activity of a variety of purified tyrosine kinases revealed that the JAK2 and Bcr-Abl kinases did appear to exhibit greater sensitivity to CR4 than members of a number of other protein tyrosine kinase families (Table 1). Most notably, the widely expressed Src-family kinases appeared to be unaffected by CR4. CR4 would therefore appear to target at least 2 different kinases. However, it is not unusual for a synthetic inhibitor to affect multiple kinases, as even “selective” inhibitors are not truly specific for a single target, most inhibiting subsets of kinases (eg, STI571 inhibits Bcr-Abl, c-kit, and platelet-derived growth factor receptor [PDGFR] activities).12,16,17 However, the low level of “nonspecific” toxicity observed in vitro suggests that CR4 is likely to be a relatively selective inhibitor, unlike compounds such as genestein, which is a nonspecific kinase blocker and consequently highly toxic.
Tyrosine kinase sensitivity to CR4 inhibition
Kinase . | IC50, μM . |
|---|---|
| Btk | > 5 |
| Bcr-Abl | 0.5-0.7 |
| Jak2 | 0.1-0.6 |
| Lck | > 5 |
| Lyn | > 50 |
| Src | > 25 |
| Syk | > 5 |
| Zap-70 | > 5 |
Kinase . | IC50, μM . |
|---|---|
| Btk | > 5 |
| Bcr-Abl | 0.5-0.7 |
| Jak2 | 0.1-0.6 |
| Lck | > 5 |
| Lyn | > 50 |
| Src | > 25 |
| Syk | > 5 |
| Zap-70 | > 5 |
Results represent multiple independent experiments with each protein tyrosine kinase. In particular, Jak-2 and Bcr-Abl kinase assays were performed repeatedly (>6 each) on enzymes extracted from various cell lines including Ph+ and Ph− ALL and AML. To reflect most accurately the large number of experiments, the range of IC50 for Jak-2 and Bcr-Abl is given.
CR4 prevents Philadelphia-positive ALL growth in vivo
The ability of CR4 to eliminate cancer growth in vivo was examined using a common murine model of tumor growth. NOD-SCID mice were injected intravenously with aggressive human Ph+ ALL cells. Engraftment is rapid and infiltration of multiple tissues occurs within 14 days. Under these conditions the Ph+ leukemia is lethal in 3 to 4 weeks. CR4 was delivered to 22 mice by a subcutaneously implanted osmotic pump (Alzet 2001), with a resultant theoretical dosage of 6 mg/kg per day. Control animals (n = 24) were treated with CR4 solvent alone.
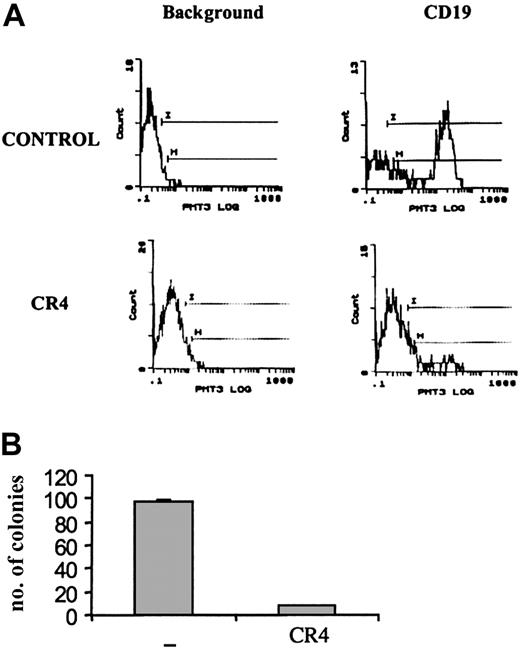
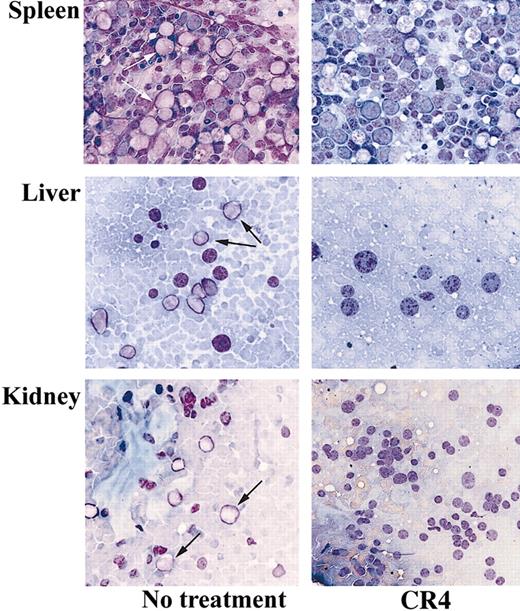
A substantial reduction in tumor load was evident after just 14 days of treatment with CR4. Flow cytometry analysis of bone marrow from treated animals revealed a more than 90% reduction in the number of infiltrating CD19+ human ALL cells compared with control mice (Figure 4A). Similar findings were observed upon analysis after 21 days of treatment (not shown). Bone marrow isolates from mice treated for 14 days were also cultured in colony-forming assays in vitro under conditions promoting ALL cell growth. While large numbers of ALL colonies arose from samples taken from control mice, few viable ALL cells remained in CR4-treated animals (Figure 4B). Histochemical staining of bone marrow, kidney, liver, and spleen sections revealed significant infiltration of Ph+ ALL blast cells in tissues from control mice after 3 weeks. In contrast, organs from CR4-treated animals were essentially normal and devoid of blast cells (Figure 5). Importantly, the tissues from mice treated with CR4 did not display signs of nonspecific drug-associated toxicity. Thus, the styryl-acrylonitrile compound CR4 would appear to be sufficiently stable and retained in sufficient quantities to act as an effective anticancer agent in vivo, in addition to its abilities in the test tube.
Ph+ ALL growth in vivo is inhibited by CR4. NOD-SCID mice were injected with Ph+ ALL (Z119) cells as described in “Materials and methods” and treated with CR4 or solvent (DMSO/PBS) delivered by osmotic pump. (A) After 14 days of treatment, mice were killed and single-cell suspensions prepared from bone marrow. After red cell lysis, the samples were stained with human-specific anti-CD19 (and isotype control) to detect infiltrating ALL cells. Anti-CD19 does not cross-react with murine cells. Similar results were obtained by staining with anti-HLA-DR. (B) To assess the viability of infiltrating Z119 cells, bone marrow cells were also cultured in colony-forming assays. BM cells were cultured in semisolid medium supplemented with 30% serum (1:1, bovine FCS-human plasma), but without addition of exogenous cytokines. There is no growth of murine cells under these conditions. The number of ALL colonies was evaluated after 9 days of culture. Shown is one of 2 experiments with standard deviations.
Ph+ ALL growth in vivo is inhibited by CR4. NOD-SCID mice were injected with Ph+ ALL (Z119) cells as described in “Materials and methods” and treated with CR4 or solvent (DMSO/PBS) delivered by osmotic pump. (A) After 14 days of treatment, mice were killed and single-cell suspensions prepared from bone marrow. After red cell lysis, the samples were stained with human-specific anti-CD19 (and isotype control) to detect infiltrating ALL cells. Anti-CD19 does not cross-react with murine cells. Similar results were obtained by staining with anti-HLA-DR. (B) To assess the viability of infiltrating Z119 cells, bone marrow cells were also cultured in colony-forming assays. BM cells were cultured in semisolid medium supplemented with 30% serum (1:1, bovine FCS-human plasma), but without addition of exogenous cytokines. There is no growth of murine cells under these conditions. The number of ALL colonies was evaluated after 9 days of culture. Shown is one of 2 experiments with standard deviations.
CR4 inhibits tissue infiltration of Ph+ ALL cells in vivo. Tissue-touch preparations were prepared from the organs of CR4-treated and control mice after 3 weeks and stained with Wright-Giemsa stain. The spleen, liver, and kidney of control mice demonstrate significant infiltration with ALL blast cells, whereas CR4-treated organs are essentially normal and devoid of ALL cells. Original magnification, × 400. Arrows indicate blasts.
CR4 inhibits tissue infiltration of Ph+ ALL cells in vivo. Tissue-touch preparations were prepared from the organs of CR4-treated and control mice after 3 weeks and stained with Wright-Giemsa stain. The spleen, liver, and kidney of control mice demonstrate significant infiltration with ALL blast cells, whereas CR4-treated organs are essentially normal and devoid of ALL cells. Original magnification, × 400. Arrows indicate blasts.
Discussion
We have described here a novel synthetic compound that selectively inhibits the growth of a variety of leukemia cells, including established cell lines and primary patient samples. CR4 is highly effective against acute lymphoblastic leukemias, both Philadelphia-positive and -negative, and acute myeloid leukemia, while demonstrating minimal toxicity to normal bone marrow cells. The ability to inhibit Ph+ ALL growth in vitro was seen to transfer into an ablation of ALL proliferation and tissue infiltration in vivo.
CR4 appears to inhibit the activity, or function, of a number of kinases, including the growth-promoting Bcr-Abl and Jak-2 kinases. The precise role that inhibition of these enzymes may play in CR4-induced Ph+ leukemia cell death is unclear, as the relative contributions of the Bcr-Abl and Jak kinases are uncertain. A more straightforward case can be made for the importance of autocrine cytokine signaling, or increased Jak activity, in Ph- ALL and AML,33-37 and inhibition of Jak activity is likely to underlie the ability of CR4 to inhibit AML and Ph- ALL. In a similar manner, enhanced proliferation due to autocrine growth factors has emerged as an important mechanism underlying apparent growth factor independence in Ph+ CML.38 Analysis of CML progenitors has demonstrated activation of IL-3 and G-CSF production in association with apparently growth factor-independent proliferation,39 and similar autocrine growth mechanisms may exist in Philadelphia-positive ALL.
CR4 appears to kill leukemia cells through the rapid induction of apoptosis, as significant annexin-V binding can be observed (T.G. and N.S., unpublished observation, February 2002). The induction of cell death in clonogenic assays is probably similarly accomplished and, in both cases, may be equivalent to growth factor withdrawal. In Ph+ cells, the removal of Bcr-Abl antiapoptic activity is also likely to contribute significantly to the induction of apoptosis.
It has been reported that the effectiveness of STI571 against Ph+ cells in culture is significantly decreased upon the addition of growth-promoting cytokines.40-42 This is presumably due to its inability to inhibit molecules other than Bcr-Abl that promote cell proliferation. In contrast, addition of growth-promoting cytokines did not significantly decrease the effectiveness of CR4, in agreement with its ability to inhibit Jak kinase activity. Cytokine stimulation could not enhance the survival of either Ph+ or Ph- cells in the presence of CR4. Thus, the ability to inhibit both Jak catalytic activity and Bcr-Abl function would appear to significantly widen the scope of CR4 action. This may also prove to include hematopoietic malignancies where chromosomal translocation results in the fusion of Jak2 with the transcription factor TEL43 creating an oncogenic form of the kinase, TEL-JAK2, which drives unregulated proliferation.44
While STI571 and AG-490 have been shown to target Bcr-Abl12-15,45 and the Jak kinases,9,46-48 respectively, their potential therapeutic use may be limited by this selective targeting. This has been highlighted by the recent studies describing development of resistance to STI571. The mutation T315I (threonine to isoleucine) in Bcr-Abl prevents binding of STI571 and has been found in a number of patients treated with STI571.13,26 Consequently, combinatorial treatment may be desirable, with several compounds directed against Bcr-Abl activity, or the development of multipathway inhibitors. The development of resistance to CR4, in Philadelphia-positive leukemias in particular, might be expected to be minimal due to its apparent ability to target more than one critical pathway.
While CR4 efficiently inhibited leukemia proliferation and survival, it did not deleteriously affect bone marrow differentiation and proliferation in culture. Similarly, human peripheral blood T-cell proliferation was normal in response to a variety of mitogens in the presence of CR4 concentrations resulting in ALL cell death (not shown). Presumably, the level of kinase inhibition achieved in both cases was not sufficient to block normal processes. Furthermore, normal hematopoietic cell development and proliferation did not appear to be inhibited in animals treated with CR4. In summary, the ability to inhibit the function of more than one growth-critical kinase, combined with its low level of cytotoxicity, suggests that CR4 may prove to be a significant contributor to the treatment of hematopoietic cancers.
Prepublished online as Blood First Edition Paper, July 24, 2003; DOI 10.1182/blood-2003-03-0860.
Supported by the Canadian National Cancer Institute, the Canadian Institute of Health Research, Lymphosign, and the John and Lotte Hecht Foundation. C.M.R. is a holder of the Donald and Audrey Campbell Chair in Immunology.
C.M.R. has declared a financial interest in a company whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ana Toro and Cher Rashotte for technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal