Abstract
Dendritic cells (DCs) have a remarkable functional plasticity in response to conidia and hyphae of the fungus Aspergillus fumigatus. In the present study we sought to assess the capacity of DCs activated by live fungi or fungal RNA to generate antifungal immunity in vivo. We found that both human and murine DCs pulsed with live fungi or transfected with fungal RNA underwent functional maturation, as revealed by the up-regulated expression of histocompatibility class II antigen and costimulatory molecules and the production of interleukin 12 (IL-12) in response to conidia or conidial RNA and of IL-4/IL-10 in response to hyphae or hyphal RNA. DCs pulsed with conidia or transfected with conidial RNA activated antigen-specific, interferon γ (IFN-γ)-producing T lymphocytes in vitro and in vivo on adoptive transfer in mice otherwise susceptible to aspergillosis. TH1-dependent antifungal resistance could also be induced in mice receiving allogeneic bone marrow transplants and was associated with an accelerated recovery of myeloid and lymphoid cells. Because the efficacy of the infusion of DCs was superior to that obtained on the adoptive transfer of Aspergillus-specific T cells, these results indicate the vaccinating potential of DCs pulsed with Aspergillus conidia or conidial RNA in hematopoietic transplantation. (Blood. 2003;102:3807-3814)
Introduction
Invasive aspergillosis (IA) is the leading cause of both nosocomial pneumonia and death in recipients of allogeneic hematopoietic stem cell (HSC) transplants given myeloablative1,2 or nonmyeloablative3 conditioning. Despite advances in early diagnosis4 and new antifungal agents,2 the majority of cases of IA remain undiagnosed and untreated at the individual's death. The most important risk factor for IA has historically been neutropenia. However, recent studies on the epidemiology of IA in recipients of an HSC transplant indicate a reduced neutropenia-related infection and an increased “late-onset” infection, in concomitance with the occurrence of graft-versus-host disease.5,6 These findings, together with the occurrence in nonneutropenic patients,2,7 attest to the importance of specific defects in both the innate and adaptive immune effector mechanisms in the pathogenesis of the disease.8-13 The recent evidence that, in healthy individuals and in patients surviving IA, a significant antigen-specific proliferation of interferon γ (IFN-γ)-producing T cells occurred14 confirms the crucial role of a T helper (TH)1 reactivity in the control of infection.9,10,15
Dendritic cells (DCs) orchestrate the overall antifungal immune resistance in the lungs.10,16,17 A dense network of DCs has been described in the respiratory tracts.18 The evidence that pulmonary DCs, through production of interleukin 10 (IL-10), mediate unresponsiveness to respiratory antigens,19 suggests that the ability of DCs to instruct the appropriate T-cell responses to the invading pathogens may be affected by local immunoregulatory events. In the case of Aspergillus, by using distinct pattern recognition receptors, including Toll-like receptors (TLRs), murine pulmonary DCs were able to finely discriminate between conidia and hyphae of Aspergillus in terms of induction of adaptive TH responses.16,17 A protective TH1-mediated resistance was induced on vaccination with Aspergillus antigens and the TLR-9 ligand CpG oligodeoxynucleotide as adjuvant.17 These results suggest that the proper manipulation of DC functioning in vivo may translate into beneficial effects in fungal infections.
Recent evidence suggests the utility of DCs pulsed with Candida albicans in adoptive transfer experiments. The ability of Candida-pulsed DCs to prime for TH1- and TH2-cell activation correlated with the occurrence of resistance and susceptibility to the fungus.20 In addition, transfecting DCs with fungal RNA was also an effective way to induce antifungal protective immunity in vivo.21 In the current study we assessed the utility of Aspergillus-pulsed DCs in conferring antifungal resistance in vivo. Ex vivo DCs were pulsed with conidia, hyphae, or fungal RNA and adoptively transferred into mice that were recipients of allogeneic HSC transplants.22 Parameters of infection and immunity were then evaluated on the infection with the fungus.
Materials and methods
Mice
Female, 8- to 10-week-old inbred BALB/c, C57BL6, and C3H/HeJ mice were obtained from Charles River Breeding Laboratories (Calco, Italy). Mice were bred under specific pathogen-free conditions at the breeding facilities of the University of Perugia (Perugia, Italy). Mice receiving HSC transplants were kept in small sterile cages (5 animals in each cage) and fed with sterile food and water. Procedures involving animals and their care were conducted in conformity with national laws and policies.
Aspergillis fumigatus strain, culture conditions, and isolation of fungal RNA
The origin, characteristics, and culture conditions of the strain of A fumigatus used in this study have already been described.16,17 For generation of hyphae, resting or swollen conidia were allowed to germinate (> 98% germination) by incubation in Sabouraud broth (∼ 20 and 6 hours, respectively). Total RNA was isolated from actively growing conidia and hyphae as described.21 Total RNA was quantitated by measuring optical density (OD) at 260 and 280 nm. OD 260/280 ratios were typically 1.65 to 2.0. RNA was pelleted, washed with ethanol, air dried, and dissolved in water.
Allogeneic HSCT model
Bone marrow cells from donor BALB/c mice were prepared by differential agglutination with soybean agglutinin, as described.22 T-depleted cells (containing < 1% of contaminating T cells on fluorescence-activated cell sorter [FACS] analysis) were injected intravenously at the concentration of 4 × 106/mL or more into recipient C3H/HeJ mice exposed to a lethal dose of 9 Gy as described.22 Without HSC transplantation (HSCT), mice died within 14 days. According to previous studies,22 more than 95% of the mice survived, showing a stable, donor-type hematopoietic chimerism, as revealed by donor-type major histocompatibility complex (MHC) class I antigen expression on cells from spleens. Neutrophil depletion was achieved by a single intraperitoneal administration of 1 mg of the antibody (RB6-8C5) against the neutrophil marker Gr-1 the day of the infection. Immunophenotypic analysis performed on lung cells (at 4 days after treatment) revealed that the percentage of Gr-1+ cells decreased from 31% (control group) to less than 2% (anti-Gr-1-treated group). The treatment did not affect the number of CD4+ and CD8+ T cells in the lungs.
Purification, pulsing, and culture of DCs
Murine DCs were purified from spleens by magnetic cell sorting with MicroBeads (MiniMACs, Miltenyi Biotec, Bologna, Italy) conjugated to hamster antimouse CD11c monoclonal antibodies (mAbs; clone N-418), as described.16,20,21 Blood CD11c+ immature human DCs (imDCs) were generated from adherent mononuclear cells by magnetic cell sorting.23-25 CD14+ monocytes were cultured for 5 days in Iscove modified medium, containing 10% filtered human serum, 50 μM 2-mercaptoethanol, sodium pyruvate (1 mM), 2 mM l-glutamine, HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; 10 mM), and 50 μg/mL gentamycin in the presence of 50 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF; Schering-Plough, Milano, Italy) and 200 U/mL rhIL-4 (Peprotech, Inalco, Milano, Italy) to obtain imDC1 or of 10 ng/mL IL-3 (R&D Systems, Space Import-Export, Milano, Italy) to obtain imDC2. The imDCs were cultured for 24 hours with 1000 ng/mL trimeric human CD40 ligand-leucine-zipper fusion protein (Immunex, Seattle, WA) to obtain mature DC1 and DC2 (mDC1 and mDC2). FACS analysis revealed that imDCs and mDCs were CD1a+, CD11c+, CD11b+, CD4+, CD14low, and CD8-, the only major difference between DC1 and DC2 being the increased CD123 expression on DC2. For phagocytosis and cytokine production, 2 × 106 DCs were exposed to live conidia or hyphae in Iscove medium without serum and with polymyxin B (at DC/fungi ratio of 1:1 and 5:1, respectively) for 2 hours before addition of 2.5 μg/mL amphotericin B (Sigma, St Louis, MO) to prevent Aspergillus overgrowth.16 Transfection with RNA was performed as described.21 The cationic lipid, N-[1-(2,3-dioleoyloxypropyl]-N, N, N,-trimethylammonium methylsulfate (DOTAP, Boehringer Mannheim, Mannhein, Germany) was used to deliver RNA into cells.26 The complex of RNA and DOTAP was incubated with DCs at 37°C in a water bath with occasional agitation for 2 hours before extensive washings. After pulsing or transfection, the cells were left for an additional 22 hours in culture before cytometric analysis, assessment of cytokine production, and adoptive transfer. Lipopolysaccharide (LPS)-matured DCs were obtained on exposure of DCs to 10 μg/mL LPS (Salmonella Minnesota Re 595, Sigma). Percentage of internalization was calculated as described.16,20 Photographs were taken using a high-resolution microscopy color camera AxioCam Colour, using the AuxioVision Software Rel. 3.0 (Carl Zeiss, Milano, Italy).
Adoptive transfer, fungal challenge, and assessment of protection
DCs (5 × 105/each injection) were injected subcutaneously in 20 μL phosphate-buffered saline (PBS) twice, at the first and seventh day after HSCT or the second and first week before the infection in mice that did not receive transplants, as previously described.21 For infection, Aspergillus conidia were given either intravenously (5 × 105/0.5 mL saline) or intratracheally (2 × 108/80 μL saline), as described.9,16 Mice were anesthetized by intraperitoneally injection of 2.5% avertin (Sigma). For disseminated candidiasis, mice were injected intravenously with 5 × 105/0.5 mL C albicans. The origin and characteristics of the C albicans strain used in this study has been described.20,21 Infections were done a week after the last DC administration. Resistance to infection was assessed in terms of survival, colony-forming units (CFUs; mean ± SE) per organ, and production of cytokines by enzyme-linked immunosorbent assay (ELISA) on culture supernatants of antigen-activated CD4+ splenocytes, by enzyme-linked immunospot (ELISPOT) assay on freshly harvested CD4+ T cells,16 and by real-time polymerase chain reaction (PCR) on unfractionated splenocytes (“Quantitation of cytokine transcripts by real-time RT-PCR”).
Adoptive transfer of T cells
CD4+ T cells were purified from spleens of naive or vaccinated BALB/c mice, as described.10 Briefly, mice were injected with crude culture filtrate antigens (CCFAs) of Aspergillus a number of days before the intratracheal injection of 2 × 108 conidia. Fourteen days after the infection, CD4+ T cells were restimulated in vitro with splenic DCs pulsed with CCFAs for 3 days before cell collection and transfer into HSC transplant recipients. Different numbers of cells were injected intravenously a day after the intratracheal infection performed a week after transplantation.
T-lymphocyte stimulation
A graded number of irradiated (1500 rad) DCs from BALB/c mice were cultured with 106 allogeneic CD4+ T cells from C57BL/6 mice and the proliferative response was measured on day 5 with the fluorescent dye carboxifluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) as described.17 Murine DCs either were left untreated or cultured for 40 hours with 10 μg/mL LPS (Sigma). DCs were transfected with conidial or hyphal RNA as described (“Purification, pulsing, and culture of DCs”). CD4+ T cells (> 99% pure on FACS analysis) were purified from spleens as described.20 For stimulation of human T cells, 106 CFSE-labeled T cells, purified from the peripheral blood as described,27 were cocultured with 105 autologous imDC1 or imDC2 for 6 days. imDCs were either unpulsed or pulsed with conidia or hyphae as described (“Purification, pulsing, and culture of DCs”).
Flow cytometry
Fluorescein isothiocyanate (FITC)-conjugated rat IgG directed to murine I-Adb (clone 34-5-3), CD80 (clone 1G10), CD86 (clone GL1), and CD40 (clone 3/23) and phycoerythrin (PE)-conjugated rat IgG2b (clone RB6-8C5) directed to murine Gr-1 were from PharMingen (Palo Alto, CA). Staining for CD69 and CD25 was done on gated CD4+ cells. Cells were analyzed by FACScan flow cytometry (Becton Dickinson, Mountain View, CA). FcR blocking was performed by incubating cells with FcR-blocking reagents (Miltenyi Biotec). Histograms are representative of one of 4 independent experiments.
Quantitation of cytokine transcripts by real-time RT-PCR
Real-time reverse transcription PCR (RT-PCR) was performed as described.28 Total RNA (5 μg), extracted from spleens using RNeasy Mini Kit (Qiagen, Milan, Italy), was reverse transcribed with Sensiscript Reverse Transcriptase (Qiagen) according to the manufacturer's directions. PCR primers were obtained from Applied Biosystems (Foster City, CA). Samples were subjected to 40 cycles of amplification at 95°C for 15 seconds followed by 60°C for 1 minute using an ABI PRISM 7000 Sequence Detection System (Applied Biosystems). PCR amplification of the housekeeping eukaryotic 18S rRNA gene was performed for each sample to control for sample loading and to allow normalization between samples as per the manufacturer's instructions (Applied Biosystems). Water controls were included to ensure specificity. Each data point was examined for integrity by analysis of the amplification plot. The eukaryotic18S rRNA-normalized data were expressed as relative cytokine mRNA (ΔΔCt) in experimental groups compared to that of naive mice.
Cytokine and ELISPOT assays
The levels of cytokines in culture supernatants were determined by kit ELISAs (Endogen Human Elisa and R&D Systems, Space Import-Export, Milano, Italy). The detection limits (pg/mL) of the assays were less than 3 for IL-12 p70 and IL-10 and less than 19 for IFN-γ (human) and less than 10 for IFN-γ and less than 3 for IL-4 (murine). For enumeration of cytokine-producing CD4+ T cells, ELISPOT assay was used on purified splenic or lung CD4+ T cells, as described.16,20 Results are expressed as the mean number of cytokine-producing cells (± SE) per 105 cells, calculated using replicates of serial 2-fold dilutions of cells.
Statistical analysis
Survival data were analyzed using the Mann-Whitney U test. The Student t test was used to determine the significance of values in experimental groups (significance was defined as P < .05). In vivo groups consisted of 6 to 8 animals. The data reported were pooled from 3 to 5 experiments, unless otherwise specified.
Results
Activation of murine DCs on transfection with fungal RNA
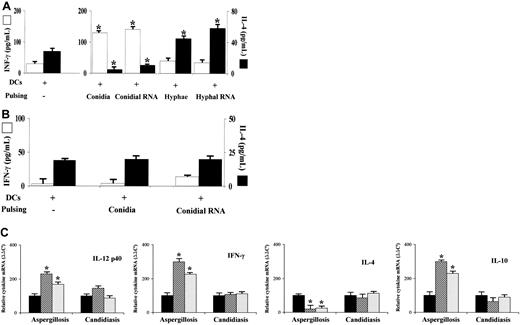
Murine DCs are known to internalize conidia and hyphae of Aspergillus, undergo functional maturation, and produce IL-12 in response to conidia and IL-4/IL-10 in response to hyphae.16 Here we show that RNA from conidia and hyphae activated DCs, as indicated by the increased expression of MHC class II antigen and costimulatory molecules on RNA transfection, an effect similar, although to a lesser extent, to that observed on LPS stimulation (Figure 1A). In line with previous findings,21 treatment of RNA with RNase-free DNase I or proteinase did not abolish the induction of costimulatory molecules (data not shown), a finding suggesting that sensitization of DCs is mediated by RNA. On assessing DC activation in terms of frequency of cytokine-producing cells, we found that the frequency of DCs producing IL-12 was higher on transfection with RNA from conidia than from hyphae, whereas the opposite was true for the frequency of IL-4-producing DCs. The results paralleled those obtained on exposure to live conidia or hyphae. Interestingly, the frequency of IL-10-producing DCs increased on transfection with conidial RNA, although it was further increased on the exposure to hyphae or hyphal RNA (Figure 1B).
Activation of murine dendritic cells by RNA fromA fumigatusconidia or hyphae. (A) Expression of MHC class II antigen and costimulatory molecule on splenic dendritic cells (DCs) transfected with RNA from conidia or hyphae of Aspergillus for 2 hours at 37°C before wash. FACS analysis was done 22 hours later. Black histograms indicate control cells stained with an unrelated mAb. For comparison, LPS-matured DCs were also assessed. None indicates untransfected DCs. (B) Frequency of cytokine-producing cells on DCs either transfected with fungal RNA or pulsed with viable conidia or hyphae. DCs were exposed to viable fungi for 2 hours at 37°C, before the addition of amphotericin B to prevent the fungal overgrowth, and left for an additional 22 hours before ELISPOT assay. None indicates unpulsed cells. *P < .05 (pulsed versus unpulsed DCs). (C) Proliferative response of allogeneic CD4+ splenic T cells after 5 days of coculture at 37°C with graded numbers of DCs transfected with conidial or fungal RNA. CFSE staining was done as described in “Materials and methods.” Shown are the results from one representative experiment of 3. The number refers to the median fluorescence intensity of T cells unstimulated (black histograms) or stimulated with 102 (red lines), 103 (blue lines), 104 (yellow lines), and 105 (green lines) DCs.
Activation of murine dendritic cells by RNA fromA fumigatusconidia or hyphae. (A) Expression of MHC class II antigen and costimulatory molecule on splenic dendritic cells (DCs) transfected with RNA from conidia or hyphae of Aspergillus for 2 hours at 37°C before wash. FACS analysis was done 22 hours later. Black histograms indicate control cells stained with an unrelated mAb. For comparison, LPS-matured DCs were also assessed. None indicates untransfected DCs. (B) Frequency of cytokine-producing cells on DCs either transfected with fungal RNA or pulsed with viable conidia or hyphae. DCs were exposed to viable fungi for 2 hours at 37°C, before the addition of amphotericin B to prevent the fungal overgrowth, and left for an additional 22 hours before ELISPOT assay. None indicates unpulsed cells. *P < .05 (pulsed versus unpulsed DCs). (C) Proliferative response of allogeneic CD4+ splenic T cells after 5 days of coculture at 37°C with graded numbers of DCs transfected with conidial or fungal RNA. CFSE staining was done as described in “Materials and methods.” Shown are the results from one representative experiment of 3. The number refers to the median fluorescence intensity of T cells unstimulated (black histograms) or stimulated with 102 (red lines), 103 (blue lines), 104 (yellow lines), and 105 (green lines) DCs.
Consistent with the effect on DC maturation and cytokine production, transfection with fungal RNA significantly increased the capacity of DCs to stimulate a proliferative response by allogeneic CD4+ T cells, even though LPS as a positive control exerted a more robust increase (Figure 1C). These results indicate that, similar to conidia and hyphae, conidial and hyphal RNA activate DCs for a distinct pattern of cytokine production, although both stimuli are comparable in their ability to up-regulate costimulation and activate alloreactive T cells.
Activation of human DCs on exposure to Aspergillus conidia and hyphae
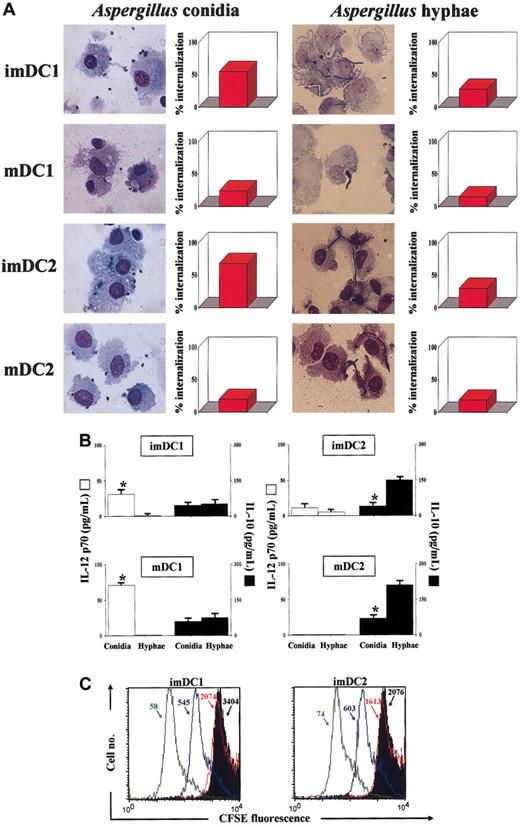
Human DCs have already been shown to internalize Aspergillus conidia; to up-regulate HLA class II antigen, CD80, and CD86 costimulatory molecules; and to produce IL-12.29 To find out whether human DCs also discriminate between the fungal morphotypes in terms of cytokine production, we exposed different subsets of human DCs to unopsonized conidia and hyphae. Both immature and mature DC subsets phagocytosed conidia and, to a lesser extent, hyphae, with the imDCs more active than mDCs (Figure 2A).
Activation of human dendritic cells byA fumigatus. Immature (im) and mature (m) dendritic cells (DCs) DC1 and DC2 were obtained from CD11c+ blood mononuclear cells. (A) DCs were exposed to unopsonized conidia or hyphae for the assessment of phagocytosis after 60 minutes. After a Diff Quik staining, aliquots of cells were spun down on slides on a cytocentrifuge and examined for conidia internalization by light microscopy. For each experiment, at least 5 fields in each slide were counted, and at least 200 DCs were analyzed in each well. All conditions were tested in triplicate. The data are the means of 3 independent experiments and expressed as percent internalization. (B) Cytokine production by DCs pulsed with conidia or hyphae. DCs were exposed to the different stimuli for 2 hours at 37°C before the addition of amphotericin B to prevent fungal overgrowth, and left for an additional 22 hours. The levels of cytokines were determined in the culture supernatants by cytokine-specific ELISA. *P < .05 (cytokine production by conidia-pulsed versus hypha-pulsed DCs). Cytokine levels in unexposed cells were below the detection limits of the assays. Error bars indicate SE. (C) Proliferative response of peripheral blood T cells unstimulated (black histograms) or stimulated by autologous imDC1 and imDC2, either unpulsed (red lines) or pulsed with conidia (green lines) or hyphae (blue lines), for 6 days at 37°C. CFSE staining was done as described in “Materials and methods.” Shown are the results from one donor. Similar results were obtained with 5 different donors. Numbers with arrows to the median fluorescence intensity.
Activation of human dendritic cells byA fumigatus. Immature (im) and mature (m) dendritic cells (DCs) DC1 and DC2 were obtained from CD11c+ blood mononuclear cells. (A) DCs were exposed to unopsonized conidia or hyphae for the assessment of phagocytosis after 60 minutes. After a Diff Quik staining, aliquots of cells were spun down on slides on a cytocentrifuge and examined for conidia internalization by light microscopy. For each experiment, at least 5 fields in each slide were counted, and at least 200 DCs were analyzed in each well. All conditions were tested in triplicate. The data are the means of 3 independent experiments and expressed as percent internalization. (B) Cytokine production by DCs pulsed with conidia or hyphae. DCs were exposed to the different stimuli for 2 hours at 37°C before the addition of amphotericin B to prevent fungal overgrowth, and left for an additional 22 hours. The levels of cytokines were determined in the culture supernatants by cytokine-specific ELISA. *P < .05 (cytokine production by conidia-pulsed versus hypha-pulsed DCs). Cytokine levels in unexposed cells were below the detection limits of the assays. Error bars indicate SE. (C) Proliferative response of peripheral blood T cells unstimulated (black histograms) or stimulated by autologous imDC1 and imDC2, either unpulsed (red lines) or pulsed with conidia (green lines) or hyphae (blue lines), for 6 days at 37°C. CFSE staining was done as described in “Materials and methods.” Shown are the results from one donor. Similar results were obtained with 5 different donors. Numbers with arrows to the median fluorescence intensity.
The exposure to both conidia and hyphae was followed by the increase in the expression of HLA class antigen and CD80, CD86, and CD40 costimulatory molecules on both the imDC subsets (data not shown). In terms of cytokine production, mainly DC1 produced IL-12 in response to conidia and mainly DC2 produced IL-10 in response to hyphae (Figure 2B).
Consistent with the effect on DC maturation, pulsing with either conidia or hyphae increased the capacity of DCs to stimulate a proliferative response by autologous T (mainly CD4+) cells (Figure 2C). Interestingly, the production of IFN-γ was significantly higher in culture supernatants of T cells activated by imDC1 pulsed with conidia than hyphae (from 149 to 849 pg/mL with unpulsed DCs or 10 μg/mL phytohemagglutinin [PHA], to 425 or 218 pg/mL with conidia-pulsed or hypha-pulsed DCs, respectively). Therefore, similar to the murine counterpart, human DCs are able to discriminate between conidia and hyphae of the fungus in terms of cytokine production and activation of IFN-γ-producing T cells.
TH priming in vivo on adoptive transfer of fungus-pulsed or RNA-transfected DCs
To assess whether DCs pulsed with live fungi or transfected with RNA would induce TH priming to the fungus in vivo, ex vivo DCs were exposed in vitro to conidia, hyphae, and RNA from both and injected into naive recipients. Mice were subsequently infected intravenously with A fumigatus and assessed for microbiologic and immunologic parameters of infection. Previous work has demonstrated the dependency of the efficacy of DC delivery in vivo on the subcutaneous route of administration.21 We have found that DCs delivered subcutaneously migrated to the lungs, spleen, thoracic lymph nodes, and, interestingly, thymus of mice, as indicated by the presence of CFSE-labeled cells in these organs (data not shown). The adoptive transfer of conidia-pulsed or conidial RNA-transfected DCs significantly altered the course and outcome of the Aspergillus infection, as indicated by the 95% to 100% survival (> 60 days) after the infection and the reduced fungal growth in the target organs, as opposed to mice not receiving DCs or receiving unpulsed DCs, which all died of infection with extensive fungal growth. No protection was observed on the adoptive transfer of hypha-pulsed or hyphal RNA-transfected DCs, with survival and fungal growth in vivo similar to those of controls (Figure 3A).
Effect of adoptively transferredAspergillus-pulsed DCs on resistance to aspergillosis or candidiasis. Splenic DCs were pulsed with either viable Aspergillus conidia or hyphae for 2 hours at 37°C before the addition of amphotericin B to prevent fungal overgrowth or transfected with fungal RNA for 2 hours at 37°C before wash. DCs (5 × 105) were administered into recipient mice subcutaneously, twice, 2 weeks and 1 week before the intravenous injection of 5 × 105A fumigatus conidia (A) or C albicans (B). Resistance to infection was assessed in terms of median survival time (MST; days) and CFUs, mean ± SE. *P < .05 (mice receiving pulsed DCs versus mice not receiving DCs or receiving unpulsed DCs). In parentheses are the number of dead animals over total injected.
Effect of adoptively transferredAspergillus-pulsed DCs on resistance to aspergillosis or candidiasis. Splenic DCs were pulsed with either viable Aspergillus conidia or hyphae for 2 hours at 37°C before the addition of amphotericin B to prevent fungal overgrowth or transfected with fungal RNA for 2 hours at 37°C before wash. DCs (5 × 105) were administered into recipient mice subcutaneously, twice, 2 weeks and 1 week before the intravenous injection of 5 × 105A fumigatus conidia (A) or C albicans (B). Resistance to infection was assessed in terms of median survival time (MST; days) and CFUs, mean ± SE. *P < .05 (mice receiving pulsed DCs versus mice not receiving DCs or receiving unpulsed DCs). In parentheses are the number of dead animals over total injected.
To evaluate the specificity of the adoptively transferred DCs, mice given Aspergillus-pulsed DCs were infected with C albicans and assessed for resistance to the infection. It was found that conidia-pulsed or conidial RNA-transfected DCs failed to confer protection against invasive candidiasis. The survival and the fungal growth in the kidneys of infected mice were comparable with and without the infusion of Aspergillus-pulsed DCs (Figure 3B).
To correlate these findings with pattern of TH priming, the production of IFN-γ and IL-4 was evaluated in culture supernatants of splenic CD4+ T cells from mice with aspergillosis or candidiasis. The results showed that the production of IFN-γ was higher and that of IL-4 lower in Aspergillus-infected mice that had received conidia-pulsed or conidial RNA-transfected DCs, as opposed to the pattern observed in mice receiving DCs pulsed with hyphae or transfected with hyphal RNA (Figure 4A). In contrast, the production of IFN-γ did not increase or decrease that of IL-4 in mice with candidiasis on receiving conidia-pulsed or conidial RNA-transfected DCs (Figure 4B). Quantification of cytokine gene transcripts by real-time PCR confirmed that mRNAs for IL-12 p40 and IFN-γ were significantly enhanced and those of IL-4 significantly decreased in the spleens of mice with aspergillosis, but not with candidiasis, on receiving conidia-pulsed or RNA-transfected DCs. Interestingly, mRNA for IL-10 also increased in mice with aspergillosis (Figure 4C).
Effect of adoptively transferredAspergillus-pulsed DCs on the induction of antifungal THimmunity. Splenic DCs were pulsed and administered into mice subsequently infected intravenously with A fumigatus or C albicans as described in Figure 3. Antifungal TH immunity was assessed in terms of cytokine production (ELISA) by antigen-stimulated CD4+ T splenocytes from mice with aspergillosis (A) or candidiasis (B) or cytokine gene expression by real-time PCR (C). ELISA and real-time PCR were done 6 days after the infection. *P < .05 (mice receiving pulsed DCs versus mice receiving unpulsed DCs). ▪, unpulsed DCs; ▨, conidia-pulsed DCs; ▦, conidial RNA-transfected DCs. Error bars indicate SE.
Effect of adoptively transferredAspergillus-pulsed DCs on the induction of antifungal THimmunity. Splenic DCs were pulsed and administered into mice subsequently infected intravenously with A fumigatus or C albicans as described in Figure 3. Antifungal TH immunity was assessed in terms of cytokine production (ELISA) by antigen-stimulated CD4+ T splenocytes from mice with aspergillosis (A) or candidiasis (B) or cytokine gene expression by real-time PCR (C). ELISA and real-time PCR were done 6 days after the infection. *P < .05 (mice receiving pulsed DCs versus mice receiving unpulsed DCs). ▪, unpulsed DCs; ▨, conidia-pulsed DCs; ▦, conidial RNA-transfected DCs. Error bars indicate SE.
Fungus-pulsed or RNA-transfected DCs accelerate functional recovery of antifungal TH1 immunity in mice given HSC transplants
In a mouse model of allogeneic T cell-depleted HSCT, susceptibility or resistance to fungal infections correlates with the temporal occurrence of TH2- and TH1-cell responses, with TH2 reactivity accounting for susceptibility to the infection in the early engraftment period.22
To assess whether the infusion of Aspergillus-pulsed DCs would accelerate TH1-cell recovery and antifungal resistance in mice given transplants, recipient mice received donor DCs in vitro pulsed with conidia or conidial RNA. One week after the last DC administration, mice were intratracheally infected with Aspergillus conidia and monitored for resistance to infection, myeloid and lymphoid cell recovery, and parameters of TH-dependent immunity, such as the frequency of IFN-γ-, IL-4-, and IL-10-producing CD4+ lung T lymphocytes. In selected experiments, mice receiving fungus-pulsed DCs were depleted of neutrophils by the administration of the anti-Gr-1 antibody, known to deplete myeloid lineage cells for up to 5 days.9,13 The results showed that the infusion of conidia-pulsed or conidial RNA-transfected DCs dramatically increased resistance to the infection, as indicated by the ability of mice to survive the infection, restrict the local fungal growth, and prevent dissemination. The infusion of hypha-pulsed DCs, though not showing beneficial effects, actually exacerbated the infection (Figure 5A). The posttransplantation administration of anti-Gr-1 antibody abrogated the protection afforded by conidial RNA-transfected DCs, resulting in a significantly decreased survival and increased fungal growth (Figure 5A).
Adoptive transfer ofAspergillus-pulsed DCs protects mice with bone marrow transplants from aspergillosis. Lethally irradiated C3H/HeJ mice each received a transplant with at least 2 × 106 T cell-depleted allogeneic bone marrow cells from BALB/c mice 2 weeks before the intratracheal infection with 2 × 108Aspergillus conidia. One and 7 days after transplantation, mice received 5 × 105 pulsed DCs subcutaneously. Treatment with the anti-Gr-1 antibody (1 mg intraperitoneally) was done the day of the infection. (A) MST (days) and CFUs of infected mice on adoptive transfer of DCs pulsed with different stimuli or unpulsed (none). In parentheses are the number of dead animals over total injected. (B) Cell recovery into the lungs of infected mice, as determined by FACS analysis. Staining for CD69 and CD25 was done on gated CD4+ cells. (C) Frequency of cytokine-producing CD4+ lung T lymphocytes as determined by ELISPOT assay. CFU, FACS analysis, and ELISPOT assay were performed at 6 days after the infection. *P < .05 (mice receiving pulsed DCs versus mice receiving unpulsed DCs). ▪, unpulsed DCs; ▧, conidia-pulsed DCs; ▦, conidial RNA-transfected DCs.
Adoptive transfer ofAspergillus-pulsed DCs protects mice with bone marrow transplants from aspergillosis. Lethally irradiated C3H/HeJ mice each received a transplant with at least 2 × 106 T cell-depleted allogeneic bone marrow cells from BALB/c mice 2 weeks before the intratracheal infection with 2 × 108Aspergillus conidia. One and 7 days after transplantation, mice received 5 × 105 pulsed DCs subcutaneously. Treatment with the anti-Gr-1 antibody (1 mg intraperitoneally) was done the day of the infection. (A) MST (days) and CFUs of infected mice on adoptive transfer of DCs pulsed with different stimuli or unpulsed (none). In parentheses are the number of dead animals over total injected. (B) Cell recovery into the lungs of infected mice, as determined by FACS analysis. Staining for CD69 and CD25 was done on gated CD4+ cells. (C) Frequency of cytokine-producing CD4+ lung T lymphocytes as determined by ELISPOT assay. CFU, FACS analysis, and ELISPOT assay were performed at 6 days after the infection. *P < .05 (mice receiving pulsed DCs versus mice receiving unpulsed DCs). ▪, unpulsed DCs; ▧, conidia-pulsed DCs; ▦, conidial RNA-transfected DCs.
Cytofluorimetric analysis of lung cells revealed that the numbers of CD4+ were significantly higher in mice that had received conidia-pulsed or RNA-transfected DCs as compared with mice not receiving DCs. CD4+ T cells also stained positive for the CD69 and CD25 activation surface markers (Figure 5B). CD8+ cells significantly increased in mice receiving conidia-pulsed DCs (data not shown). The number of Gr-1+ cells also significantly increased in the lungs of mice receiving conidia-pulsed or conidial RNA-transfected DCs, as compared with mice receiving unpulsed DCs (Figure 5B). In vitro, neutrophils purified from mice receiving conidia-pulsed or conidial RNA-transfected DCs showed a heightened antifungal activity against Aspergillus conidia (data not shown).
On assaying the pattern of cytokine production, the frequency of IFN-γ-producing cells was increased, and that of IL-4 decreased, in CD4+ T cells from mice receiving either conidia-pulsed or RNA-transfected DCs as compared with recipients not receiving DCs. Interestingly, the number of IL-10-producing lymphocytes also appeared to increase, particularly in mice receiving RNA-transfected DCs (Figure 5C).
Adoptive transfer of Aspergillus -specific CD4 + T cells in mice receiving HSC transplants
We have previously shown that the adoptive transfer of Aspergillus-specific TH1 cells confers protection in mice with IA that did not receive transplants.10 To assess whether, similar to DCs, the adoptive transfer of Aspergillus-specific TH1 cells could increase antifungal resistance in HSCT, mice were intratracheally infected with Aspergillus conidia a week after HSCT and infused with Aspergillus-specific CD4+ T cells a day after the infection. Mice were monitored for survival after the infection. It was found that the infusion of antigen-specific T cells significantly increased the survival of mice compared with mice not infused or infused with cells from naive donors. Eventually, however, all the mice died of the infection with signs of extensive fungal growth in the lungs. Resistance to the infection was not increased by the infusion of higher numbers of T cells (Table 1).
Adoptive transfer of Aspergillus-specific CD4+ T cells in mice with IA undergoing HSCT
. | . | A fumigatus infection . | . | |
|---|---|---|---|---|
| Type of cells . | No. of cells . | MST, d . | D/T . | |
| — | — | 5.5 | 8/8 | |
| Unstimulated | 5 × 105 | 6 | 8/8 | |
| Aspergillus specific | 5 × 105 | 18* | 8/8 | |
| Aspergillus specific | 5 × 106 | 16* | 8/8 | |
. | . | A fumigatus infection . | . | |
|---|---|---|---|---|
| Type of cells . | No. of cells . | MST, d . | D/T . | |
| — | — | 5.5 | 8/8 | |
| Unstimulated | 5 × 105 | 6 | 8/8 | |
| Aspergillus specific | 5 × 105 | 18* | 8/8 | |
| Aspergillus specific | 5 × 106 | 16* | 8/8 | |
Splenic CD4+ T cells were from BALB/c mice either untreated or treated with A fumigatus CCFAs before intranasal infection with Aspergillus conidia (“Materials and methods”). Two weeks later, cells were restimulated in vitro with CCFAs and purified spleen DCs for 3 days before adoptive transfer (intravenously) into mice receiving HSC transplants, a day after the infection. Mice received 2 × 108Aspergillus conidia intratracheally, a week after transplantation. MST indicates median survival time (days); D/T, dead animals over total injected. — indicates no cells transferred.
P<.01 (treated versus untreated).
Discussion
Both human29 and murine16 DCs were shown to internalize conidia of Aspergillus, undergo functional maturation, produce IL-12, and induce TH1-cell priming. The present study shows that murine DCs pulsed with Aspergillus conidia or transfected with conidial RNA are fully competent at inducing antifungal TH1 priming on adoptive transfer in vivo. This translated in the occurrence of protection to infection in mice with HSC transplants. Neither the induction of antifungal TH1 priming nor the occurrence of protection was observed on delivery of DCs pulsed with Aspergillus hyphae or transfected with hyphal RNA, a finding suggesting the importance of DC plasticity at the host/fungus interface. On adoptive transfer in vivo, conidial or hyphal RNA-transfected DCs migrated to different organs, including the lungs and spleens, where they activated Aspergillus-specific TH1 or TH2 cells, respectively, as revealed by the frequencies of TH1- and TH2-cytokine-producing CD4+ T cells. Associated with the occurrence of Aspergillus-specific TH1 reactivity was the induction of a state of antifungal resistance mediated by the accelerated recovery of Gr-1+ myeloid cells capable of efficiently restricting the fungal growth in vivo and in vitro. Interestingly, DCs also migrated to the thymus, an event that may have important consequences, should peripheral DC migration to the thymus contribute to the induction of peripheral tolerance, as suggested.30
Because the exploitation of DCs as nature's adjuvant of vaccines for infectious diseases is an intense area of investigation,31 it was important to define whether human DCs also show functional plasticity in response to the fungus. Here we show that the different subsets of human DCs internalized conidia and hyphae of the fungus and up-regulated the expression of HLA class II antigen and costimulatory molecules in response to both. However, mainly the DC1 subset produced IL-12 in response to conidia, whereas mainly the DC2 subset produced IL-10 in response to hyphae. At variance with murine DCs, IL-4 was inconsistently produced by DC2 in response to hyphae (data not shown). Fungus-pulsed DCs also stimulated the proliferation and IFN-γ production by T cells, a finding consistent with the ability of TH1 priming by IL-12-producing DCs.16,20 The finding that imDC2 also activated IFN-γ-producing TH1 cells suggests that the TH1-priming ability is retained in the presence of IL-10. Therefore, similar to the murine counterpart, human DCs are able to discriminate between conidia and hyphae of the fungus in terms of cytokine production and TH-cell activation.
Preliminary observations suggest that the phagocytosis of conidia and TH priming were both severely impaired in imDC1 and imDC2 raised from patients with haplotype-mismatched hematopoietic transplants in the early period after transplantation (< 7% conidia internalization by both DC subsets at 1, 2, or 3 months after transplantation). Should this be a general finding in HSCT, it will provide the rationale for the replacement therapy with fungus-pulsed donor DCs in transplantation.
In the case of Candida, we provided evidence for the expression of fungal antigens capable of inducing protective immunity on the surface of DCs on transfection with fungal RNA.21 The failure of DCs transfected with conidial RNA to confer antifungal immune protection against C albicans strongly argues for the occurrence of an antigen-specific T cell-mediated effect. At least 2 Aspergillus antigens have been described recently and used successfully to screen healthy individuals and patients for Aspergillus-specific responses.14 However, it is not known at the moment whether these antigens are involved in our system.
An interesting observation of the present study was that the infusion of Aspergillus-pulsed DCs accelerated the recovery of functional antifungal TH1 responses in our model of T cell-depleted allogeneic HSCT. Patients receiving allogeneic HSC transplants are unable to develop antigen-specific T-cell responses soon after transplantation.27 It has been demonstrated that T-cell depletion of allogeneic HSC transplants is associated with a slow recovery of CD4+ and CD8+ T cells.32 In one particular study, T-cell depletion was found to be the most powerful predictive factor associated with invasive aspergillosis in patients receiving transplants.33 However, functional recovery of the T-cell system after T cell-depleted allogeneic HSCT has been demonstrated,32,34 and both donor and recipient DCs may participate in the reconstitution of the T-cell repertoire in transplantation through distinct pathways of antigen presentation.35 We have recently demonstrated that an imbalanced production of TH1 and TH2 cytokines was responsible for the susceptibility to fungal infections in our HSCT model. However, readdressing the balance between TH1 and TH2 subsets, as by treatment with TH2 cytokine antagonists, accelerated the recovery of TH1-mediated antifungal resistance.22 Here we show that recovery of functional T cells producing IFN-γ could also be accelerated by the infusion of Aspergillus-pulsed DCs. Therefore, although conflicting data exist as to whether donor DCs are “friends” or “foes” in transplantation,31,35 our findings suggest that DCs may contribute to the educational program of T cells in HSCT during reconstitution, as already suggested.21,36,37
The observation that the occurrence of a protective TH1 reactivity coexisted with the detection of significant levels of IL-10 is intriguing. It is known that high levels of IL-10 are associated with tolerance to HLA-mismatched bone marrow stem cells38 and that IL-10 is required for the induction of regulatory T cells mediating tolerance to alloantigens in vivo39 and inhibiting graft-versus-host disease lethality.41 We have recently shown that regulatory T cells are generated in mice with candidiasis and are essential components of host-protective antifungal TH1 immunity.41 Whether IL-10 produced in mice receiving HSC transplants and infused with Aspergillus-pulsed DCs may serve to support the growth of regulatory T cells preventing donor TH1 alloreactivity remains a working hypothesis, although it may help to explain the long-term, disease-free survival of the mice.
It has been shown that DCs generated ex vivo and pulsed with Aspergillus conidia restored antifungal proliferative response in vitro in patients receiving HSC transplants.29 The present work suggests that DCs could act as effective vaccines against IA in mice that did not receive transplants and in mice that did receive an HSC transplant. We have previously shown that the adoptive transfer of Aspergillus-specific TH1 cells confers protection in mice with IA that did not receive transplants.10 Here we show that the infusion of fungus-specific T cells transiently increased resistance to the infection in mice given HSC transplants, a finding in line with that obtained in HLA haplotype-mismatched transplants, where the infusion of donor Aspergillus-specific CD4+ T-cell clones was effective at conferring antifungal resistance.42 Further studies are needed to definitively compare the efficacy of the DC vaccination approach with that of the adoptive transfer of Aspergillus-specific T cells in HSCT. Ultimately, we will focus on whichever strategy might be capable of overcoming the limitations and side effects of antigen-nonspecific therapy of infectious diseases in HSCT.
Prepublished online as Blood First Edition Paper, June 5, 2003; DOI 10.1182/blood-2003-03-0748.
Supported by the National Research Project on AIDS, contract 50D.27, “Opportunistic Infections and Tuberculosis,” and by the Project n. 1AF/F “Immunotherapeutic Approaches in Fungal Infections” from the Istituto Superiore Sanità, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Lara Bellocchio for dedicated editorial assistance and Carla Barabani of the animal facility at the University of Perugia for technical assistance.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal