Abstract
Graft-versus-host disease (GVHD) represents one of the major complications of allogeneic hematopoietic stem cell transplantation. Techniques to prevent GVHD have included ex vivo T-cell depletion of the graft or prolonged in vivo immunosuppression. Both reduce the frequency and severity of GVHD but also reduce T-cell-mediated graft-versus-malignancy effect, and increase the risk of infection. A major goal in transplantation is to prevent alloreactivity while preserving activity against tumors and infectious agents. We have used activation of the Notch pathway to try to generate T cells able to specifically regulate alloantigen responses. We used allogeneic Epstein-Barr virus lymphoblastoid B cells (EBV-LCLs) as stimulator cells. Such LCLs are excellent (allo) antigen-presenting cells and can be obtained in large numbers even from donors who have received extensive chemo/radiotherapy. We overexpressed a Notch ligand, Jagged-1, in these cells by adenoviral vector transduction. Stimulation of CD45RA+ naive T cells by Jagged-1 EBV-LCL reduces production of interferon-γ, interleukin-2, and interleukin-5, but up-regulates transforming growth factor-β1 synthesis, consistent with induction of a regulatory T-cell phenotype. Transfer of these T cells to fresh lymphocyte cultures inhibits proliferative and cytotoxic immune responses to the priming alloantigens while sparing responses to third-party stimulator cells. Notch activation in the presence of alloantigen-presenting cells may therefore be a means of inducing specific regulatory T cells while preserving other T-cell functionality. (Blood. 2003;102:3815-3821)
Introduction
Graft-versus-host disease (GVHD) remains a major concern after allogeneic stem cell transplantation and is a particular problem where donor and recipient are HLA mismatched.1 The use of donor T-cell depletion undoubtedly reduces the incidence and severity of the complication, but at the cost of increasing the risks of relapse (where transplantation is used to treat malignant disease), viral/fungal infection, and rejection.2 While posttransplantation donor leukocyte infusion can help address some of these concerns, such cells are themselves capable of inducing the GVHD that the T-cell depletion was intended to avoid. Consequently there has been considerable interest in developing selective T-cell depletion and inactivation techniques that specifically target only alloreactive T cells and spare those lymphocytes with antitumor or antiviral activity.
Reports have been published in which there is selective removal of T cells expressing activation-dependent markers during a donor-recipient mixed lymphocyte reaction (MLR),3,4 or in which an inhibitory “second signal” is delivered to donor T cells reactive against the host.5 We have explored whether it is also possible to generate regulatory T cells capable of actively inhibiting the alloantigen response by stimulation of the Notch pathway in T cells. Members of the Notch family are an important decision point factor in the development of many cell types. The Notch receptors (Notch 1-4) have a series of ligands classified into 2 groups based on the prototypes Serrate and Delta ligands first identified in Drosophila. In mammals, 2 Delta-like molecules (Delta-1, -3) and 2 Serrate-like molecules (Jagged-1, -2) have been identified, but little is known about the specificity of these various Notch ligands for each receptor.6,7 A cell in which Notch has been activated by one or more of its several ligands will have a different fate to a daughter cell in which Notch activation was absent. The choices between T- and B-cell development,8 between production of CD4 and CD8 T cells,9 and between αβ or γδ receptor expression10 are all determined by whether Notch has been activated at the time the decision point is reached. More recently, Hoyne et al have observed that overexpression of Serrate-1 (the mouse homologue of the human Notch ligand, Jagged-1) by murine dendritic cells (DCs) induces antigen-specific regulatory T cells that could transfer tolerance to naive recipient mice.11
Since induction and maintenance of allospecific T-cell tolerance is a major challenge in transplantation to prevent GVHD and for long-term graft survival,12 we looked at whether activation of the Notch pathway was able to induce CD45RA+ naive T cells to become regulatory T cells able to inhibit the allogeneic MLR. Even though the response to alloantigens in vitro does not predict with absolute reliability the development of GVHD, several clinical trials are using crude peripheral blood mononuclear cell (PBMC) populations to achieve a selective depletion of alloreactive T cells, using an anti-CD25 immunotoxin, with reasonable success.4 As (allo) antigen-presenting cells (APCs) we used Epstein-Barr virus (EBV)-transformed B cells because they are excellent antigen-presenting cells, expressing high levels of class I and II HLA and a range of costimulator molecules, and are readily obtained and expanded even from cancer patients who have received extensive prior chemo/radiotherapy.13,14 Moreover, EBV-lymphoblastoid B cells (LCLs), should be free of contaminating tumor cells, and unlike professional APCs, should not express any tumor-specific antigens15,16 although they may express B-lineage-restricted tumor-associated antigens. This is an important requirement if regulatory T cells that inhibit responses to such tumor antigens are not to be induced at the same time as regulators of the alloresponse. To activate the Notch pathway, we overexpressed the ligand Jagged-1 in the alloantigen-presenting LCL (LCL-J1). We find that LCL-J1 effectively induces regulatory T cells from the CD45RA+ population that inhibits alloantigen-induced proliferative and cytotoxic responses. The regulatory T cells appear specific for the inducing antigens and block the responses of fresh T cells that have themselves never been exposed to the Notch ligand.
Materials and methods
Cell and cell lines
Buffy-coats from healthy adults were obtained from blood products destined for transfusion and the mononuclear cells (MNCs) isolated by Ficoll density gradient centrifugation. For isolation of CD45RA+ T-cell subsets, PBMCs were first depleted by incubation with a cocktail of microbead-coupled antibodies (anti-CD14, anti-CD19, anti-CD56, and anti-CD45RO) using the gradient MidiMacs magnetic separation column (Multisort kit; Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. CD4/CD8 T-cell subsets were then separated from the CD45RA+ naive T-cell population using the same magnetic procedure. The purity of each population evaluated by fluorescent-activated cell sorter (FACS) analysis (FACScalibur; Becton Dickinson, San Jose, CA) was always higher than 98% and the viability more than 95%.
EBV-LCL were obtained by Epstein-Barr virus (B95-8) immortalization of mature B cells of healthy donors. Bone marrow (BM) stromal cells that express high levels of Jagged-1 were used as a control for Jagged-1 protein expression.17 To establish the BM stromal cell lines, a suspension of whole BM was obtained using institutional review board (IRB)-approved protocols, and 5 × 107 cells seeded in a T75 culture flask (Nunc, Oslo, Norway) and cultured in complete medium (below). After a week, nonadherent cells were removed by washing and fresh medium was added. When the BM stromal cell line was established, a portion of the culture was harvested and lysates were prepared for Western blotting.
All cultures used complete medium prepared with RPMI 1640 (Hyclone, Logan, UT) supplemented with 10% fetal calf serum (FCS; Hyclone), antibiotics, and l-glutamine and maintained at 37°C in 5% CO2.
Adenoviral vector
EBV-LCL lines were infected using a chimeric adenovirus Ad5/F35. As previously described by Shayakhmetov et al, this is an adenovirus serotype 5 vector in which the fiber gene has been substituted by the fiber of an adenovirus serotype 35.18 The complementary DNA (cDNA) for the full-length Jagged-1 (kindly provided by Prof Margaret Dallman, Imperial College, London, United Kingdom) or enhanced green fluorescent protein (EGFP; Clontech, Palo Alto, CA) was cloned into the shuttle plasmid pShuttle-X (Clontech). The entire region containing the cytomegalovirus (CMV) promoter, Jagged-1, or EGFP, followed by an SV40 polyadenylation site was excised by I-CeuI and pI-SceI digest and then transferred to pAd5/F35, cleaved using the same restriction enzymes to form pAd5/F35-Jagged-1 or pAd5/F35-EGFP. Both Ad5/F35 vectors were produced by Lipofectamine (Life Technologies, Gaithersburg, MD) transfection of a human embryonic kidney (HEK293) cell line. A single purified plaque was amplified in HEK293 cells, and virus was extracted by 3 consecutive freeze/thaw cycles. The vectors were concentrated and purified by double cesium-chloride gradient ultracentrifugation and desalted on a dialysis cassette (Pierce, Rockford, IL). The vector titers were established by plaque assay using HEK293 (for infectious units [IU]) and by measuring optical density at 260 nm (for viral particles, vp). The vp/IU ratios were 160 and 200 for Ad5/F35-Jagged-1 and Ad5/F35-EGFP, respectively. This is a higher ratio than usually found with Ad5, but is typical for vectors based on this chimeric virus.19
EBV-LCL cell lines were transduced with either Ad5/F35-Jagged-1 or Ad5/F35-EGFP (controls) at 37°C with 200 IU per cell for both viruses in RPMI 1640, with 2% FCS. After 12 hours, transduced cells were washed twice in phosphate-buffered saline, resuspended in fresh complete medium for 2 days, and then processed for Western blotting and real-time quantitative polymerase chain reaction (RQ-PCR), or used for the generation of Notch-activated T cells as described in “Cell cultures.”
Western blotting
To detect Jagged-1 protein expressed in EBV-LCLs and in the stromal cell line, cell extracts were prepared from pelleted cells by lysing them in Laemmli sample buffer, and boiling them for 10 minutes, followed by centrifugation to remove insoluble material. Under reducing conditions, the protein components of cell lysates were separated using a 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Hybond-P; Amersham, Uppsala, Sweden). The membranes were probed with antibodies to Jagged-1 (H-114; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour at room temperature and then visualized with horseradish peroxidase-coupled anti-immunoglobulin (705-0350147; Jackson Immuno Research Laboratories, West Grove, PA) for 30 minutes at room temperature. We used the enhanced chemiluminescence system for detection (Amersham). To compare loading between samples, the membrane was reprobed with antivinculin antibody (Sigma-Aldrich, St Louis, MO).
RNA extraction and complementary DNA synthesis
Total RNA was extracted from transduced, nontransduced, and control cell lines with the Rneasy Mini Kit (Qiagen, Valencia, CA). RNA was eluted in 40 μL RNAse-free water and stored at -80°C until use. Contaminating DNA was removed by DNAse I digestion (Gibco Invitrogen, Carlsbad, CA). RNA was then reverse transcribed to cDNA using random hexamers and the SuperScript II reverse-transcriptase system (SuperScript First-Strand Synthesis System for RT-PCR; Gibco Invitrogen).
Real-time quantitative PCR (TaqMan) assay
A real-time PCR assay was developed, using TaqMan technology (PE Applied Biosystems, Foster City, CA), for detection and quantification of Jagged-1, Deltex, and HES-1 transcripts. Primers and probe spanning the junction between exon 22 and exon 23 of the Jagged-1 gene (GenBank sequence database accession number U73936) were designed by means of the Primer Express software (PE Applied Biosystems). Sequences for the primers and probe were as follows: forward primer (exon 22), 5′-ACC TGC CAG TGC CTG AAT G-3′; reverse primer (exon 23), 5′-AGG CAA GGT CGA GGG CC-3′; probe (junction between exon 22 and 23), 5′-FAM-ACG GAT CGC CTG CTC AAA GGT CTG-TAMRA-3′, Deltex primers and probe, forward primer (exon 5), 5′-TGG TTC GAA GAT ACA TGC AGA AG-3′; reverse primer (exon 6), 5′-ACC AGT CGC TCC ATG CAG AT-3′; probe (junction between exon 5 and 6), 5′-FAM-TGA AAA ACC CAC CTG ATG AGG ACT GCA C-TAMRA-3′, and HES-1 primers and probe, forward primer (exon 6), 5′-TGG GTG CCA AGC ACT GC-3′; reverse primer (exon 7), 5′-TCG TGA CCA CCT TGT TTT TCT G-3′; probe (junction between exon 6 and 7), 5′-FAM-AAG GAA GTG GTC GAA GCT CAC GTG GA-TAMRA-3′, with FAM and TAMRA representing the reporter and quencher dye, respectively. As a positive control and calibration for quantification, samples were analyzed with the TaqMan Ribosomal RNA Control Reagents (PE Applied Biosystems) according to the manufacturer's recommended protocol. Furthermore, RNA without reverse transcription was run in parallel for each sample. PCR amplification was performed with 2 × TaqMan Universal Master Mix (PE Applied Biosystems) with 300 nM primers and 200 nM probe, 10 mL DNA and nuclease-free water (Promega, Madison, WI).20 Amplification was performed using the ABI PRISM 7700 Sequence Detection System (SDS; PE Applied Biosystems) and consisted of 2 minutes at 50°C (inactivation of possible carry-over contamination by uracil N′-glycosylase [UNG]), and 40 2-step cycles of 15 seconds at 95°C and 60 seconds at 60°C. Real-time fluorescence measurements were taken, and the threshold (CT) cycles of amplification, where the plots crossed a defined baseline, were determined for each sample. For relative quantification, the expression of Jagged-1 in samples was normalized by comparison with the ribosomal RNA (rRNA) amount using the ΔΔCT method.21
Cell cultures
To generate Notch-activated T cells, a primary one-way mixed MLC was performed by coculturing 1 × 106 purified CD45RA+ T cells from the responder with 2.5 × 104 5000 cGy γ-irradiated stimulator EBV-LCL cells transduced by Ad5/F35-Jagged-1 (Notch ligand) or Ad5/F35-EGFP control vector (40:1 final ratio) in a 48-well plate (Nunc) in 1 mL complete medium. For cytokine measurements, 400 μL of day -2, -3 culture supernatants was harvested and then stored at -20°C for future assays. After 7 days, viable cells were recovered, washed, rested in fresh medium for 2 days, and then assessed phenotypically by fluorescence flow cytometry, and functionally by measuring cytotoxicity and proliferation in a secondary MLC.
The ability of Notch-activated T cells to regulate the alloantigenic-specific response of untreated T cells was examined in a secondary MLC. As stimulator cells we used the original EBV-LCLs or completely HLA-mismatched third-party EBV-LCLs. Responder cells (1 × 106) consisting of newly isolated T cells plus the indicated number of 3000 cGy γ-irradiated primed T cells22,23 from the primary MLC and 2.5 × 104 5000 cGy γ-irradiated nontransduced stimulator EBV-LCL cells were cultured for 7 days. The effects of the Notch-activated T cells, on proliferation and CTL activity, in these secondary cultures were assessed and compared with the activity of the cells newly stimulated by nontransduced LCLs.
Measurement of proliferation
T cells (5 × 104 cells/well) were cultured in 200-μL 96-U-bottomed-well plate (Nunc) with γ-irradiated (4000 cGy) Jagged-1 or control-transduced stimulator EBV-LCLs at a 40:1 ratio and the indicated number of primed T cells for 5 days. Proliferation was analyzed by 3H-thymidine (3H-Thy) incorporation and 1 μCi (0.037 MBq) 3H-Thy was added 18 hours before terminating the cultures. 3H-Thy uptake was measured in a TriCarb liquid scintillation counter (Packard Instruments, Downers Grove, IL) and expressed as mean counts per minute and SD of triplicate measurements.
Flow cytometric analysis
Freshly purified and MLC-cultured cells were washed, stained for 20 minutes at 4°C with optimal dilution for each antibody or its isotype control, and analyzed by flow cytometry (FACScalibur and CellQuest software; Becton Dickinson). A total of 10 000 events were analyzed for each determination. Evidence of activation was assessed by forward scatter (FSC) profiles and via the coexpression on T-cell population of the activation marker interleukin-2 receptor (IL-2R) α-chain (CD25). Cells were stained with fluorescein isothiocyanate, phycoerythrin, or peridinin chlorophyll alpha protein (PerCP) monoclonal antibodies (mAbs) to CD4 (Clone L200), CD8 (RPA-T8), CD25 (M-A251), CD40 (5C3), CD80 (L307.4), CD86 (FUN-1), CD45RA (5H9), CD45RO (9UCHL-1), HLA-A, B, C (G46-2.6), HLA-DR (L243), CD62L (Dreg 56), and interferon γ (IFN-γ)/IL-4 FastImmune (BD-Biosciences, San Jose, CA). Annexin V staining was used to detect apoptotic cells, following the manufacturer's instructions (BD Pharmingen, San Diego, CA).
For intracellular staining of IFN-γ and IL-4, cells were first incubated for 2 hours with Brefeldin-A (Sigma-Aldrich) in the presence of LCLs used for the primary MLC. After 2 washes, the cells were stained with PerCP-conjugated anti-CD8 then fixed and permeabilized with FACSLysing and FACSPermeabilizing solutions, respectively (BD-Biosciences). After one wash, fluorescent-conjugated mAbs to IFN-γ and IL-4 were added to the cells according to the manufacturer's instructions. Stained cells were gated on the CD4+/CD8+ populations, and IFN-γ/IL-4 content was analyzed using CellQuest software.
Cytotoxicity measured by chromium 51 release assay
The cytotoxic activity of primed T cells was determined in a 4-hour 51chromium (51Cr) release assay using EBV-LCLs (B cells) and phytohemagglutinin (PHA) blasts (T cells) both derived from the donor of the MLC stimulator cells, as well as the K562 cell line, as the target cells. Target cells (1.5 × 106) were labeled with 100 mCi (3.7 mBq) 51Cr (Amersham) and used at 5000 cells per well. Multiple effector-target (E/T) ratios were tested in triplicate and cytotoxic activity measured as percent specific lysis calculated as follows: [(cpm released experimental - cpm spontaneous)/(cpm total lysis - cpm spontaneous)] × 100.
Measurement of cytokine production
Supernatants, previously frozen at -20°C, were analyzed for their cytokine content using the Cytometric Bead Array kit (CBA; Pharmingen/BD-Biosciences, San Diego, CA). Day-3 X-VIVO-15 culture supernatants were assessed for transforming growth factor β1 (TGF-β1) content by enzyme-linked immunosorbent assay (ELISA) (Quantikine ELISA kit; R&D Systems, Minneapolis, MN) following the manufacturer's instructions.
After 7 days of culture, intracellular IL-4 and IFN-γ were also assayed by flow cytometry according to the manufacturer's protocol (BD-Biosciences).
Statistical analysis
Means ± SD of independent experiments were analyzed using the SigmaStat 2.03 software (SigmaStat, Chicago, IL). For intergroup comparison, paired t testing was used and P values less than .05 was considered statistically significant.
Results
Transduction of EBV-LCLs by Ad5/F35-Jagged-1
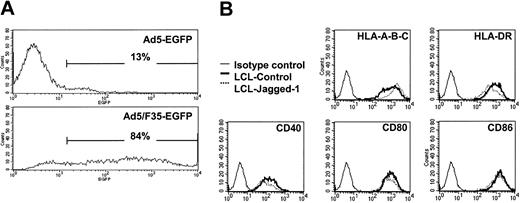
The Ad5/F35-EGFP vector we used efficiently transduces LCLs, so that at a multiplicity of infection (MOI) of 200 plaque-forming units (pfu) per cell, more than 80% of LCLs are strongly positive for the fluorescent marker, compared with less than 15% using conventional Ad5-EGFP at an MOI of 1500 pfu per cell (Figure 1A).
Comparison of Ad5 and Ad5/F35 infectivity in EBV-LCL cells. EBV-LCL cells were cocultured for 18 hours with 1500 pfu per cell of Ad5-EGFP or 200 pfu per cell of Ad5/F35-EGFP, and then washed. At 48 hours after infection, the cells were analyzed for EGFP or surface molecule expression by flow cytometry. (A) The results are expressed as the percentage of EGFP-expressing cells. (B) EBV-LCLs transduced by Ad5/F35-Jagged-1 showed the same profile of surface expression as control, MHC class I and II, and costimulatory ligands (ie, HLA-A, -B, -C; HLA-DR; CD40; CD80; and CD86). For each assay, 1 representative experiment of 4 is shown.
Comparison of Ad5 and Ad5/F35 infectivity in EBV-LCL cells. EBV-LCL cells were cocultured for 18 hours with 1500 pfu per cell of Ad5-EGFP or 200 pfu per cell of Ad5/F35-EGFP, and then washed. At 48 hours after infection, the cells were analyzed for EGFP or surface molecule expression by flow cytometry. (A) The results are expressed as the percentage of EGFP-expressing cells. (B) EBV-LCLs transduced by Ad5/F35-Jagged-1 showed the same profile of surface expression as control, MHC class I and II, and costimulatory ligands (ie, HLA-A, -B, -C; HLA-DR; CD40; CD80; and CD86). For each assay, 1 representative experiment of 4 is shown.
Surface immunofluorescence staining showed that neither transduction by control Ad vector nor overexpression of Jagged-1 had any effect on LCL expression of major histocompatibility complex (MHC) molecules (HLA-A, -B, -C, -DR, -DQ) or of the costimulator molecules CD40, CD80, and CD86 (Figure 1B).
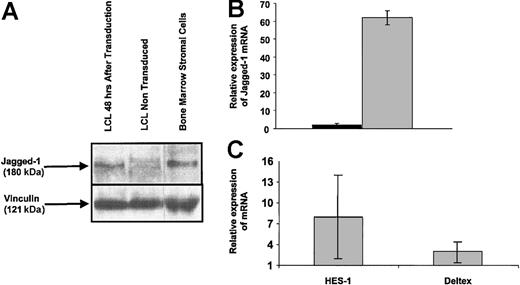
To confirm that LCLs were efficiently transduced by the Ad5/F35-Jagged-1 vector, we measured expression of the transgene at the protein and transcript levels. At 48 hours after transduction, Western blotting detected the 180-kDa Jagged-1 protein in Ad5/F35-Jagged-1-transduced EBV-LCLs but not in control LCLs (Figure 2A).
Jagged-1 expression in Ad5/F35-Jagged-1-transduced EBV-LCL cells. Transduction efficiency of EBV-LCLs using Ad5/F35-Jagged-1 was monitored after 48 hours by Western blotting and RQ-PCR. (A) Jagged-1 protein (≈180 kDa) was detected in EBV-LCLs 48 hours after transduction by Ad5/F35-Jagged-1 vector. Human bone marrow stromal cells were used as the positive control for Jagged-1 protein expression. (B) RQ-PCR assay showed a mean 62-fold higher quantity of Jagged-1 mRNA in EBV-LCLs after transduction by Ad5/F35-Jagged-1 vector (gray bar), compared with control-transduced EBV-LCLs (black bar) (mean ± SD of 3 experiments). (C) To confirm the functionality of Jagged-1 expressed on transduced LCLs, T-cell populations were analyzed for HES-1 and Deltex mRNA content after 12 hours of activation in an MLC (mean ±SD of 3 experiments).
Jagged-1 expression in Ad5/F35-Jagged-1-transduced EBV-LCL cells. Transduction efficiency of EBV-LCLs using Ad5/F35-Jagged-1 was monitored after 48 hours by Western blotting and RQ-PCR. (A) Jagged-1 protein (≈180 kDa) was detected in EBV-LCLs 48 hours after transduction by Ad5/F35-Jagged-1 vector. Human bone marrow stromal cells were used as the positive control for Jagged-1 protein expression. (B) RQ-PCR assay showed a mean 62-fold higher quantity of Jagged-1 mRNA in EBV-LCLs after transduction by Ad5/F35-Jagged-1 vector (gray bar), compared with control-transduced EBV-LCLs (black bar) (mean ± SD of 3 experiments). (C) To confirm the functionality of Jagged-1 expressed on transduced LCLs, T-cell populations were analyzed for HES-1 and Deltex mRNA content after 12 hours of activation in an MLC (mean ±SD of 3 experiments).
At the same time point, real-time quantitative PCR (RQ-PCR) showed a mean 62-fold higher level of Jagged-1 mRNA in Ad5/F35-Jagged-1-transduced than in control-transduced cells (Figure 2B). Control-transduced and Jagged-1-transduced LCLs were analyzed for rRNA content as a positive control and to calibrate the quantification. As a measure of functionality, Jagged-1 LCL-stimulated T cells showed an 8-fold up-regulation of HES-1 and a 3-fold up-regulation of Deltex 12 hours after activation, in comparison with control LCL-stimulated T cells (Figure 2C). Since HES-1 and Deltex are downstream transcriptional regulators induced by Notch receptor activation, this confirms that the expressed Jagged-1 protein is biologically active.24,25
Stimulation by Jagged-1 modulates proliferative and cytotoxic primary immune responses
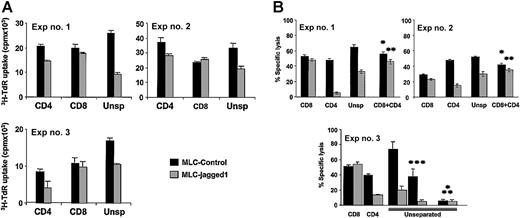
To measure the effects of Jagged-1 expression on responses to alloantigens, we cocultured CD45RA (naive) T cells with control or Jagged-1-transduced allogeneic LCLs. In 12 experiments there was a consistent decrease of 3H-Thy uptake (mean 36%) compared with control LCL-stimulated cultures (P = .0011). As illustrated in Figure 3A, separation of the responder T cells into CD4+ and CD8+ T-cell subsets showed the primary effect to be on CD4+ cells whose 3H-Thy uptake was reduced by a mean of 30% (P < .01), while the 3H-Thy uptake of the CD8+ population was unafffected (P = .557).
Priming CD45RA+T-cell subset in the presence of Jagged-1 induces alloantigen hyporesponsiveness. Primary MLR cultures consisted of CD45RA+ CD4+ and/or CD45RA+ CD8+ T-cell responders and control or Jagged-1-transduced EBV-LCL stimulator cells. (A) The proliferative responses were determined by 3H incorporation at day 5 of the cultures. Results show the means ±SD of triplicates studied in each experiment for a P value for the effect of Jagged-1 of P < .001 for CD4, P = .557 for CD8, and P = .001 for unseparated T cells. A further 9 experiments revealed an identical pattern of results for an overall P value of .00114 for unseparated cells. (B) Specific lysis of target cells after a day-7 MLC, against which the responder cells were generated, is shown. Moreover, when CD4+ T cells either from the control culture (*) or from the Jagged-1 culture (**) were added to CD8+ T cells primed with control LCLs at a 1:1 ratio, no cytotoxic effect was observed. These experiments used PHA-blasts (***) from the same donor as additional target cells as well as EBV-LCLs from a fully mismatched third party (pyramid-stacked *). These cells acted as specific killing controls.
Priming CD45RA+T-cell subset in the presence of Jagged-1 induces alloantigen hyporesponsiveness. Primary MLR cultures consisted of CD45RA+ CD4+ and/or CD45RA+ CD8+ T-cell responders and control or Jagged-1-transduced EBV-LCL stimulator cells. (A) The proliferative responses were determined by 3H incorporation at day 5 of the cultures. Results show the means ±SD of triplicates studied in each experiment for a P value for the effect of Jagged-1 of P < .001 for CD4, P = .557 for CD8, and P = .001 for unseparated T cells. A further 9 experiments revealed an identical pattern of results for an overall P value of .00114 for unseparated cells. (B) Specific lysis of target cells after a day-7 MLC, against which the responder cells were generated, is shown. Moreover, when CD4+ T cells either from the control culture (*) or from the Jagged-1 culture (**) were added to CD8+ T cells primed with control LCLs at a 1:1 ratio, no cytotoxic effect was observed. These experiments used PHA-blasts (***) from the same donor as additional target cells as well as EBV-LCLs from a fully mismatched third party (pyramid-stacked *). These cells acted as specific killing controls.
These results imply that Jagged-1 stimulation modifies CD45RA+ CD4+ cell behavior, but that the ligand has no direct inhibitory effect on CD45RA+ CD8+ cells. Similarly, there was no inhibition when allogeneic LCL-J1s were added to CD45RO+ “memory” CD4+ and CD8+ T cells (not shown).
To determine whether CD4+ T cells modified by Jagged-1 exposure can in turn affect the function of CD8+ cells, we measured the cytotoxic activity of each population. Following mixed lymphocyte cultures of T cells with MHC-mismatched LCL stimulator cells, cytotoxic activity is normally generated against PHA blasts (T-cell derived) and LCLs (B-cell derived) prepared from the donor of the stimulator cells. This activity has been shown to be mediated by cytotoxic T cells and to be unaffected by depletion of CD56 cells.11,12 As shown in Figure 3B (Exp no. 3), the development of cytotoxic effector cells directed against these T- and B-cell targets is markedly inhibited if the stimulator LCLs were expressing Jagged-1. Cytotoxic activity against stimulator-donor-derived PHA blasts was reduced by more than 90% (P < .01), while killing of LCLs was reduced by 75% in the LCL-J1-compared with the LCL-stimulated cultures (P < .01). Killing of third-party LCLs was low and was not affected whether stimulation had been by LCLs or LCL-J1s. As with the proliferative response, exposure to LCL-J1s had no direct effect on the cytotoxic activity of the CD8+ T cells when these were cultured alone (Figure 3B). Instead CD4+ cells were required in the primary cultures for LCL-J1-mediated inhibition of CD8 function to be observed. In addition, when Jagged-1 primed CD4+ T cells were added to CD8 T cells primed with control LCLs (at a 1:1 ratio), before the cytotoxicity assays, we observed no inhibition (Figure 3B, Exp nos. 1 and 2). The above effects occurred in populations depleted of CD56 natural killer (NK) cells, providing additional evidence that the ultimate targets of LCL-J1 inhibition are cytotoxic T lymphocytes. Finally, addition of mAb to MHC class 1 molecules (W6/32) inhibited killing by a mean of 42%, consistent with cytotoxic effector function mediated by MHC class I antigen-restricted CD8+ T cells.
Phenotypic analysis of Jagged-1-stimulated T cells
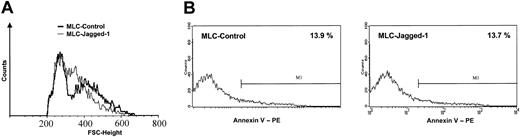
To analyze the effects of Jagged-1 stimulation on T-cell phenotype, we harvested the T cells from control LCL or LCL-J1 cultures after 7 days and analyzed them using flow cytometry. In the LCL-J1 cultures, allostimulated T cells displayed the features of an activated phenotype but with distinct forward scatter (FSC) characteristics compared with LCL-stimulated controls (Figure 4A).
Phenotypic characteristics of tolerized T cells. After a 7-day MLC in the absence or presence of Jagged-1, the responder cells were harvested, allowed to rest for 2 days, and analyzed by flow cytometry. (A) The blastlike (ie, large-sized) T cells emerged with distinct forward scatter (FSC) characteristics in the Jagged-1 MLC. (B) Annexin V staining showed a comparable level of apoptotic cells between control and Jagged-1 MLC (1 representative experiment of 3).
Phenotypic characteristics of tolerized T cells. After a 7-day MLC in the absence or presence of Jagged-1, the responder cells were harvested, allowed to rest for 2 days, and analyzed by flow cytometry. (A) The blastlike (ie, large-sized) T cells emerged with distinct forward scatter (FSC) characteristics in the Jagged-1 MLC. (B) Annexin V staining showed a comparable level of apoptotic cells between control and Jagged-1 MLC (1 representative experiment of 3).
Table 1 shows the significant decrease in mean value for expression of CD25 (interleukin-2 receptor alpha chain) (P = .001) and the mean fluorescence intensity (MFI) (P = .035) in 6 separate experiments.
Effects of Jagged-1 on alloactivated naive T cells
Parameter . | Control, % . | Jagged-1, % . | P . |
|---|---|---|---|
| CD25 | 45 ± 15 | 35 ± 14 | .001 |
| MFI | 438 ± 207 | 264 ± 146 | .035 |
Parameter . | Control, % . | Jagged-1, % . | P . |
|---|---|---|---|
| CD25 | 45 ± 15 | 35 ± 14 | .001 |
| MFI | 438 ± 207 | 264 ± 146 | .035 |
Percentage of CD25+ cells and MFI in the Jagged-1 MLC compared with controls. Mean ± SD of 6 separate experiments.
These observations are consistent with Notch pathway activation delivering a signal that inhibits activation/differentiation of naive T cells.
Of note, the proliferative and cytotoxic hyporesponsiveness we observed following exposure to Jagged-1 cannot be attributed to increased induction of apoptosis since Annexin V staining showed less than 15% Annexin V-positive cells in the Jagged-1-stimulated T-cell population, a figure identical to that observed in the controls (Figure 4B).
Cytokine profile of Jagged-1-stimulated T cells
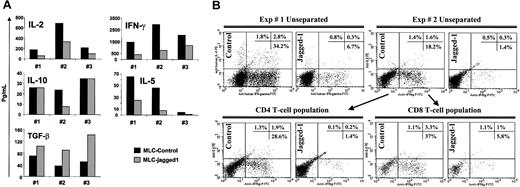
We next investigated changes in cytokine production relating to Jagged-1 stimulation. LCL-J1 stimulation produced a consistent shift in the pattern of cytokines produced compared with control LCL stimulation. IFN-γ (80% reduction), IL-2 (55% reduction), and IL-5 (73% reduction) were all diminished, while TGF-β1 release was increased (Figure 5A).
Cytokine profile of Jagged-1 primed naive T cells. (A) Cytokine profiles of unseparated (CD4 and CD8) naive T cells were analyzed in day-3 culture supernatants by CBA assay and ELISA for Th1/Th2 cytokines and TGF-β content, respectively. Results from 3 experiments are shown with a P value for the effect of Jagged-1 of P = .0135 for IL-2, P = .049 for IFN-γ, P = .57 for IL-10, P = .0144 for IL-5, and P = .0071 for TGF-β. (B) Intracellular staining for IL-4 and IFN-γ on day 7 of the unseparated cultures, and with gating on CD4+ or CD8+ T-cell populations.
Cytokine profile of Jagged-1 primed naive T cells. (A) Cytokine profiles of unseparated (CD4 and CD8) naive T cells were analyzed in day-3 culture supernatants by CBA assay and ELISA for Th1/Th2 cytokines and TGF-β content, respectively. Results from 3 experiments are shown with a P value for the effect of Jagged-1 of P = .0135 for IL-2, P = .049 for IFN-γ, P = .57 for IL-10, P = .0144 for IL-5, and P = .0071 for TGF-β. (B) Intracellular staining for IL-4 and IFN-γ on day 7 of the unseparated cultures, and with gating on CD4+ or CD8+ T-cell populations.
No significant differences were noted in IL-10 production between the control and the Jagged-1-stimulated cultures. In addition, intracellular staining for IFN-γ and IL-4 in T cells showed an 80% reduction of the number of cells producing these cytokines in Jagged-1 LCL-stimulated cultures compared with controls for both CD4+ and CD8+ T-cell populations (Figure 5B). Overall, these results are consistent with induction of Th3-type regulatory T cells in the allogeneic cultures.
Regulatory T cells induced by activated Notch can specifically transfer inhibition of the immune response in a secondary MLC
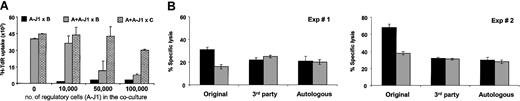
Addition of LCL-J1-stimulated T cells (A-J1) from responder A to fresh naive T cells from the same donor in a new MLC incorporating the same LCL as stimulator (B), (A + A-J1 × B), produced consistent and significant (P < .01) inhibition of responsiveness. Inhibition was seen at ratios of Jagged-1 activated T cells to fresh responder T cells of 1:5, while at a 1:1 ratio, inhibition was more than 70%. The capacity to respond to third-party (C) alloreactive stimulator cells (A + A-J1 × C) was unimpaired by addition of a low ratio of regulatory cells, although at a ratio of 2 Jagged-1-stimulated T cells to 1 fresh T-responder cell, some nonspecific third-party inhibition was seen (Figure 6A).
Allospecific regulatory T cells suppress naive alloreactive T cells and do not confer bystander suppression. Regulatory T cells were generated in a Jagged-1 MLC. (A) Irradiated regulatory T cells (1 to 10 × 104) were added to an MLC containing 5 × 104 responder naive T cells and 1.25 × 103 irradiated stimulator EBV-LCLs. The proliferative responses were determined from 3H incorporation at day 5 of culture. (B) Specific inhibition of target cell lysis was evaluated at day 7 of secondary MLC using a 2:1 ratio between naive and regulatory T cells (gray bar) and compared with control cultures (containing irradiated T cells cultured with nontransduced LCLs) (black bar). The stimulator cells were either the original stimulator EBV-LCLs (used in the primary MLC), third-party EBV-LCLs (completely HLA-mismatched with the original cell stimulator), or autologous EBV-LCLs. Results are expressed as mean ± SD of triplicate measurements.
Allospecific regulatory T cells suppress naive alloreactive T cells and do not confer bystander suppression. Regulatory T cells were generated in a Jagged-1 MLC. (A) Irradiated regulatory T cells (1 to 10 × 104) were added to an MLC containing 5 × 104 responder naive T cells and 1.25 × 103 irradiated stimulator EBV-LCLs. The proliferative responses were determined from 3H incorporation at day 5 of culture. (B) Specific inhibition of target cell lysis was evaluated at day 7 of secondary MLC using a 2:1 ratio between naive and regulatory T cells (gray bar) and compared with control cultures (containing irradiated T cells cultured with nontransduced LCLs) (black bar). The stimulator cells were either the original stimulator EBV-LCLs (used in the primary MLC), third-party EBV-LCLs (completely HLA-mismatched with the original cell stimulator), or autologous EBV-LCLs. Results are expressed as mean ± SD of triplicate measurements.
The ability of Jagged-1-stimulated T cells to inhibit fresh cocultures of responder cells also extended to activity on cytotoxic effector function. Figure 6B shows the effect of adding Jagged-1 (A-J1) or control LCL-stimulated T cells to fresh cultures of responder T cells that were stimulated either with the original stimulator LCLs (A + A-J1 × B) or third-party LCLs (A + A-J1 × C). The Jagged-1-stimulated T cells inhibited cytotoxicity against the original LCLs (A × B) by more than 40% at a 1:2 ratio between J1-stimulated T cells and fresh responder T cells, while having no discernible effect against the third-party LCLs (A × C). Notably, responsiveness to autologous LCLs was unimpaired—an important consideration if the approach is to be adopted for allogeneic stem cell transplantation in which EBV lymphoproliferative disease affecting donor B cells is a significant cause of morbidity and mortality. In all cultures the experiments were done after CD56+ cell depletion so that NK activity, evaluated by K562 killing, was consistently less than 10%.
Discussion
Our results indicate that overexpression of the Notch ligand, Jagged-1, in alloantigen-presenting cells induces alloantigen-specific regulatory T cells to develop from a CD45RA+ (CD4+) naive population. We demonstrated hyporesponsiveness, mostly driven by a Jagged-1-activated CD4 T-cell subset, in CD4 and CD8 proliferative and cytotoxic responses to alloantigens expressed on both T cells (PHA blasts) and B cells (LCLs). This hyporesponsiveness can be transferred to fresh lymphocyte cultures that have not been exposed to Jagged-1. Reactivity to third-party target cells is unimpaired.
The molecular pathways involved in the development of regulatory T (Treg) cells remain poorly understood. The Notch pathway, defined by the Notch family of receptors and its ligands, which belong to the Jagged and Delta molecular families in humans, may play a role in the differentiation of Treg cells responsible for tolerance. Hoyne et al have demonstrated in mice that antigen presented by dendritic cells overexpressing Notch-1 ligand results in the differentiation of antigen-specific CD4 T cells into regulatory cells that can transfer tolerance to naive mice.11 Other indirect arguments suggest a role for Notch and its ligands in the development or maintenance of Treg cells. One is the demonstration in human cells that Deltex, a positive regulator of the Notch pathway, is highly up-regulated in CD4+CD25+ cells, which are naturally occurring T regulatory cells.26 Following stimulation with anti-CD3 and anti-CD28, the transcription of Notch-4 and Delta-1 dramatically increased only in these cells.26 Using the same system, we demonstrated that Jagged-1 LCL-stimulated T cells showed up-regulation of both HES-1 and Deltex, compared with control LCL-stimulated T cells. Since HES-1 and Deltex are downstream transcriptional regulators induced by Notch receptor activation, this confirms the functionality of the expressed Jagged-1 protein.24,25 Jagged-1 is not normally expressed on B-lineage cells including LCLs,27 but has been well documented in human thymic epithelium,28,29 which appears to play a key role in the generation of Treg cells.30,31 Our results support the concept that the Notch pathway contributes to the differentiation of human T regulatory cells. In our experimental model, we suggest that overexpression of Jagged-1 in the context of alloantigen presentation represents the critical signal for induction of specific regulatory T cells. The first signal is given to the T cell by the allogeneic MHC molecules expressed by the lymphoblastoid cell lines, which ensure the specificity of the T-cell response. The subsequent signals are given by CD28-B7 and CD40-CD40 ligand interactions between the alloantigen B-cell and the antigen-stimulated T cell. Concomitant ligation of Jagged-1 by a Notch receptor modulates the consequences of these signals so that progression to a Th1 or cytotoxic effector T cell is instead redirected to the formation of regulatory T cells. All the above effects could also be directly linked to Jagged-1 overexpression on LCLs if they were abrogated by blocking the Notch pathway, which can be achieved by using γ-secretase inhibitors. Unfortunately, we found that available γ-secretase inhibitors have a high level of nonspecific toxicity for primary T cells, making it impossible to discern their effects in assays requiring analysis of T-cell function, such as those required to show regulatory activity.
At present, human regulatory T cells are poorly characterized, and most work has focused on naturally occurring regulatory cells circulating in peripheral blood, which are CD4+CD25+.22 Induced suppressor cells, by contrast, have a more diverse phenotype, and have been associated with multiple combinations of CD4, CD8, or CD25 antigen expression.26,32-34 In rodents, 2 functional classes of suppressor cells are described, Tr1 and Th3, of which the Th3 class appears to most closely resemble the regulatory T cells generated in this human study. Th3 cells have been best characterized in murine autoimmune models in which oral administration of autoantigens has been shown to induce tolerance.35 CD4+ T-cell clones isolated from mesenteric lymph nodes in orally tolerized animals produce TGF-β1 and down-regulate Th1-cell responses. In humans, Th3 cells have been described to down-regulate both Th1 and Th2 responses,35 matching the pattern of regulatory activity we see here. The production of inhibitory cytokines such as TGF-β1 may not contribute substantially to the effects of Th3 regulatory T cells, since most studies suggest that regulation itself occurs independently of such cytokines and is instead critically dependent on cell-to-cell contact.36
A potential clinical application for regulatory cells induced by the Jagged-1 stimulation would be to induce and sustain tolerance after stem cell transplantation. To date, strategies to produce long-term graft survival in humans have used immunosuppressive drugs, T-cell depletion, or blockade of costimulatory signals.37,38 One major disadvantage of these strategies is the lack of specificity for alloantigens. This lack of specificity increases the risk of infectious events and may lead to reduction of a graft-versus-tumor effect. Regulatory T cells induced by the Jagged-1/Notch pathway may be more effective since they appear to be specific for the tolerizing set of alloantigens presented in the culture. Not only is activity against third-party alloantigens retained, but so too is activity against autologous EBV-LCLs. This indicates that there is retention of activity against viral antigens presented by donor HLA molecules even when the T cells have been exposed to the same viral antigens presented on allogeneic recipient EBV-LCLs. Such a result is of specific interest in stem cell transplantation since it suggests that responsiveness against donor EBV-LCLs will be retained in vivo, and will thereby reduce the risk of posttransplantation lymphoproliferative disease. More generally, the observation further supports the concept that the inhibitory effects resulting from Notch activation will spare responsiveness against other viral and malignancy associated antigens, not present at the tolerizing event.
In conclusion, we have shown that overexpression of Jagged-1 on EBV-LCLs may induce the differentiation of antigen-specific regulatory T cells from CD45RA+ T lymphocytes and thereby reduce the alloreactivity of human T lymphocytes. These Jagged-1-stimulated cells can transfer tolerance in ex vivo cultures and may therefore be able to complement other approaches3-5,30 aimed at reducing the alloreactivity of MHC-mismatched donor cells while sparing reactivity against clinically relevant antigens.
Prepublished online as Blood First Edition Paper, July 3, 2003; DOI 10.1182/blood-2002-12-3826.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal