Chronic myeloid leukemia (CML) is characterized by expression of the BCR-ABL fusion gene that encodes a 210-kDa protein, which is a constitutively active tyrosine kinase. At least 70% of the oncoprotein is localized to the cytoskeleton, and several of the most prominent tyrosine kinase substrates for p210BCR-ABLare cytoskeletal proteins. Dendritic cells (DCs) are bone marrow–derived antigen-presenting cells responsible for the initiation of immune responses. In CML patients, up to 98% of myeloid DCs generated from peripheral blood mononuclear cells areBCR-ABL positive. In this study we have compared the morphology and behavior of myeloid DCs derived from CML patients with control DCs from healthy individuals. We show that the actin cytoskeleton and shape of CML-DCs of myeloid origin adherent to fibronectin differ significantly from those of normal DCs. CML-DCs are also defective in processing and presentation of exogenous antigens such as tetanous toxoid. The antigen-processing defect may be a consequence of the reduced capacity of CML-DCs to capture antigen via macropinocytosis or via mannose receptors when compared with DCs generated from healthy individuals. Furthermore, chemokine-induced migration of CML-DCs in vitro was significantly reduced. These observations cannot be explained by a difference in the maturation status of CML and normal DCs, because phenotypic analysis by flow cytometry showed a similar surface expression of maturation makers. Taken together, these results suggest that the defects in antigen processing and migration we have observed in CML-DCs may be related to underlying cytoskeletal changes induced by the p210BCR-ABLfusion protein.
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative disorder arising from the clonal expansion of an altered pluripotent hematopoietic stem cell. The disease is characterized by a t(9;22) chromosomal translocation that generates the Philadelphia (Ph) chromosome. This contains a BCR-ABL fusion gene, created by the juxtaposition of the ABL proto-oncogene with the BCR gene, and encodes a 210-kDA (p210BCR-ABL) fusion protein. p210BCR-ABL is a constitutively active tyrosine kinase and probably initiates the neoplastic process.1 As a result of the malignant transformation, CML cells display abnormal adhesion properties to stromal cells and extracellular matrix. In addition, cytoskeletal abnormalities have been described in BCR-ABL–transformed murine hematopoietic cells and CD34+ cells derived from the peripheral blood of CML patients.2 These include an increase in spontaneous cell motility, membrane ruffling, formation of long actin extensions (filopodia), and an increase in the rate of protrusion and retraction of pseudopodia on fibronectin-coated surfaces.
Dendritic cells (DCs) are bone marrow–derived antigen-presenting cells (APCs) that play a central role in the development of both innate and adaptive immune responses.3,4 DCs are heterogeneous and can be divided into 2 major populations based on their origin, expression of surface markers, and function (myeloid and plasmacytoid DCs).5 The state of activation of DCs is an important determinant of function, and this can be modified by local cytokine production as well as by direct interaction with pathogens. Immature DCs reside in peripheral tissues where they capture and process antigens.6 Macropinocytosis has emerged as a key mechanism of antigen capture by DCs, allowing substantial volumes of the extracellular milieu to be engulfed and processed.7 8Macropinosomes are believed to arise from deformations of the plasma membrane, a process driven by constant remodeling of the actin cytoskeleton.
DC maturation is triggered by a number of signals, and this process is characterized by a series of coordinated events. These include loss of endocytic/phagocytic activity; up-regulation of costimulatory molecules such as CD80, CD86, and CD40; expression of CD83; loss of adhesive properties; changes in cytoskeletal organization that lead to acquisition of high cellular motility; and changes in major histocompatibility complex (MHC) class II–containing intracellular compartments. During maturation, DCs acquire the capacity to migrate to the secondary lymphoid organs and prime naive T cells.6
In CML patients, between 73% to 100% of monocyte-derived DCs are positive for the chimeric BCR-ABLgene.9-11 A number of groups have demonstrated that DCs derived from both healthy subjects and patients with CML differentiated and matured in culture in a similar way.11,12 However, there are conflicting data regarding the ability of CML-DCs to stimulate T cells compared with those from healthy individuals.11 12
In this paper we describe differences in the organization of the actin cytoskeleton in DCs generated from CML patients compared with normal DCs. This observation led us to investigate whether these changes were associated with functional abnormalities in CML-DCs in vitro. We demonstrate that DCs generated from CML patients have a reduced capacity to capture and process antigen when compared with DCs from healthy controls. In addition, the capacity of CML-DCs to migrate is also impaired.
The relevance of these findings to spontaneous and induced immunity to CML antigens is discussed.
Patients, materials, and methods
Patients
Peripheral blood samples were collected either by phlebotomy or by leukapheresis from patients with CML in first chronic phase (CP1) at Hammersmith Hospital, London. All patients were Ph-positive, and no other cytogenetic abnormalities were detected. The leukapheresis products were used either immediately or following cryopreservation in liquid nitrogen. Details of the patients analyzed in this study are shown in Table 1. Heparinized peripheral blood samples were also taken from healthy volunteers or derived from buffy coats to serve as appropriate controls and used either immediately or after cryopreservation in liquid nitrogen. Cord blood was used as a source of CD4+CD45RA+ T cells.
Details of CML patients used for the generation of DCs
| Patient no. . | Age, y . | DRB1** . | Prior treatment† . | Antigen capture‡ . | Antigen process/present‡ . | Migration‡ . | FACS‡ . | MLR‡ . |
|---|---|---|---|---|---|---|---|---|
| 1 | 29 | 0701, 1302 | NO | 4 | 12 | 3 | 3 | — |
| 2 | 14 | 7/17 | NO | 4 | 10 | 3 | 3 | — |
| 3 | 42 | 0404/9 | NO | — | — | 2 | 1 | 4 |
| 4 | 39 | 15/17 | NO | — | 1 | 1 | 1 | 2 |
| 5 | 32 | 0401/9 | NO | — | — | 3 | 1 | 2 |
| 6 | 55 | 15 | NO | 1 | 1 | 1 | 1 | 1 |
| 7 | 17 | 4/7 | NO | 2 | 5 | — | 2 | 1 |
| 8 | 33 | 0405/0701 | HU | 1 | 5 | — | 2 | 1 |
| 9 | 31 | 17/13 | HU | — | — | — | 1 | — |
| 10 | 14 | 7/11 | NO | 1 | — | — | — | — |
| 11 | 37 | 0701/1104 | HU | 1 | — | — | — | 1 |
| 12 | 29 | 0402/1303 | HU | 1 | — | — | — | 1 |
| 13 | 60 | Not done | HU | 1 | — | — | — | — |
| 14 | 39 | Not done | HU | 1 | — | — | — | — |
| 15 | 42 | 15/15 | NO | 1 | 2 | — | — | — |
| Patient no. . | Age, y . | DRB1** . | Prior treatment† . | Antigen capture‡ . | Antigen process/present‡ . | Migration‡ . | FACS‡ . | MLR‡ . |
|---|---|---|---|---|---|---|---|---|
| 1 | 29 | 0701, 1302 | NO | 4 | 12 | 3 | 3 | — |
| 2 | 14 | 7/17 | NO | 4 | 10 | 3 | 3 | — |
| 3 | 42 | 0404/9 | NO | — | — | 2 | 1 | 4 |
| 4 | 39 | 15/17 | NO | — | 1 | 1 | 1 | 2 |
| 5 | 32 | 0401/9 | NO | — | — | 3 | 1 | 2 |
| 6 | 55 | 15 | NO | 1 | 1 | 1 | 1 | 1 |
| 7 | 17 | 4/7 | NO | 2 | 5 | — | 2 | 1 |
| 8 | 33 | 0405/0701 | HU | 1 | 5 | — | 2 | 1 |
| 9 | 31 | 17/13 | HU | — | — | — | 1 | — |
| 10 | 14 | 7/11 | NO | 1 | — | — | — | — |
| 11 | 37 | 0701/1104 | HU | 1 | — | — | — | 1 |
| 12 | 29 | 0402/1303 | HU | 1 | — | — | — | 1 |
| 13 | 60 | Not done | HU | 1 | — | — | — | — |
| 14 | 39 | Not done | HU | 1 | — | — | — | — |
| 15 | 42 | 15/15 | NO | 1 | 2 | — | — | — |
— indicates not done; NO, nothing; HU, hydroxyurea.
HLA-DR typing.
Treatment prior to taking of the sample was NO or HU.
Number of functional experiments performed with each preparation of DCs.
Generation of dendritic cells
Peripheral blood mononuclear cells (PBMCs) were isolated by Lymphoprep (Nycomed Pharma, Oslo, Norway) density gradient centrifugation. To generate DCs, mononuclear cells were resuspended in serum-free X-VIVO 20 medium (BioWhittaker, Walkersville, MD) at 5 × 106/mL and cultured in 3 mL per well in 6-well plates (Nunc, Glasgow, United Kingdom). After 2 hours of incubation, the nonadherent population was removed and each well was washed 3 times with phosphate-buffered saline (PBS). RPMI 1640 medium containing 50 IU/mL penicillin, 50 μg/mL streptomycin, and 2 mMl-glutamine (Invitrogen, Paisley, Scotland) and supplemented with 10% human serum (RPMI/10%HS) was added to make up a final volume of 3 mL per well. Recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF, kindly donated by Dr S. Brett, Glaxo-Wellcome, Stevenage, United Kingdom) and interleukin-4 (First Link, West Midlands, United Kingdom) were added at final concentrations of 20 ng/mL and 10 ng/mL, respectively. Fresh cytokine-supplemented medium was added every 2 days. Immature DCs (iDCs) were used after 4 or 5 days of culture. DCs were rendered mature (mDCs) after 4 days of culture by adding 20 ng/mL each of tumor necrosis factor-α (TNF-α) (First Link) and interleukin-1 (IL-1) (First Link) for 2 additional days.
Generation of DCs from CD34+ cells
CD34+ cells derived from PBMCs of healthy individuals or CML patients were isolated by positive selection using an indirect immunomagnetic separation system (MACS CD34 Isolation Kit, Milteny Biotec, Sunnyvale, CA). The efficiency of the procedure was assessed by fluorescence-activated cell sorter (FACS) analysis, and the median purity of CD34+ cells after selection was higher than 90%, with cell viability more than 95% in all cases.
CD34+ cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 50 ng/mL GM-CSF, 30 ng/mL stem cell factor (SCF) (First Link), 10 ng/mL TNF-α, and 10 ng/mL IL-4 at 2.5 × 104/mL. At weekly intervals, half the medium was replaced by fresh medium with growth factors. After 16 days, CD34-derived DCs were used for functional experiments.
Fluorescence in situ hybridization (FISH)
The percentage of BCR-ABL fusion gene-positive DCs was determined by fluorescence in situ hybridization (FISH) using a dual-color DNA probe (Oncor, Gaithersburg, MD) containing aBCR-specific probe labeled with green fluorochrome and anABL-specific probe labeled with red fluorochrome in accordance with the manufacturer's protocol. A total of 300 nuclei were counted for the reciprocal translocation between chromosome 9q34 and 22q11.
Immunocytochemistry
DCs were allowed to adhere to glass coverslips coated with 100 ng/mL fibronectin (Sigma, St Louis, MO) for 4 hours. The cells were then fixed with 4% buffered paraformaldehyde (Sigma) at 4°C. After 20 minutes, the slides were washed 3 times with PBS and the cells stained with 1 ng/mL tetramethyl rhodamine B isothiocyanate (TRITC)–conjugated phalloidin (Sigma) for 30 minutes at 37°C. The slides were washed 3 times with PBS, air dried, and mounted in mounting solution (DAKO, Cambridgeshire, United Kingdom).
Wide field fluorescence microscopy
Specimens were visualized with a Coolview 12 cooled CCD camera (Photonic Science, Newbury, United Kingdom) mounted over an Axiophot microscope (Zeiss, Welwyn Garden City, United Kingdom). A × 20 NA 0.6 objective and a standard epi-illuminating rhodamine fluorescence filter cube were used, and the 12-bit image data sets were generated as described elsewhere.13
Confocal microscopy
Specimens were visualized with an LSM 510 confocal microscope using a × 40 NA 1.3 Neofluar objective (Zeiss) aligned as described previously.14 The specimens were illuminated with nonsaturating levels of the 543 nm line from a HeNe laser. The fluorescent emissions were filtered out with a 560 nm long-pass filter where the 12-bit gray scale values in the final image data sets were proportional to the number of photons that emanated from the specimen.15 Each image data set was composed of a matrix of 1024 × 1024 pixels and represented the average of 16 passes of the illumination collected at 0.267 Hz.
Phenotypic analysis of DCs by FACS analysis
For cell surface marker analysis of in vitro–cultured DCs, the following monoclonal antibodies (mAbs), conjugated with either fluorescein isothiocyanate (FITC) or phycoerythrin (PE), were used: CD11 (DAKO), CD14 (Sigma), CD83 (Caltag, CA), CD86 (Caltag), HLA-DR (Sigma), and CCR1 (R&D Systems, Oxon, United Kingdom). Isotype controls were included. Cells were stained at a concentration of 1 × 105 in 100 μL. Samples were incubated for 30 minutes at 4°C with the conjugated antibody and then washed twice with PBS. The samples were analyzed using a FACSCalibur flow cytometer and CellQuest software (Becton Dickinson, Mountain View, CA).
Evaluation of mannose receptor–mediated endocytosis and macropinocytosis
DCs (1 × 105 per tube) were suspended in serum-free RPMI 1640 media with either FITC-conjugated dextran (FITC-DX, Sigma) or Lucifer Yellow (LY, Sigma) at a final concentration of 1 mg/mL and incubated for 10 to 60 minutes. The cells were washed 3 times with cold PBS before FACS analysis. The background (cells pulsed at 0°C) was subtracted.8
T-cell clones
Tetanus toxoid (TT)–specific T-cell clones (TT1, CG1, and NIJ27) were derived from 3 individuals expressing DRB1*0701 by culturing PBMCs with 8 × 10−2 U/mL TT vaccine (EVANS Vaccine Limited, Liverpool, United Kingdom) in RPMI/10%HS. TT-specific T-cell lines were cloned by limiting dilution in U-bottomed 96-well plates (Nunc) in the presence of irradiated PBMCs previously pulsed with 10 μg/mL phytohemagglutinin (PHA) (Murex Biotech, Dartford, United Kingdom) and recombinant IL-2 (rIL-2) (10 U/mL) (Roche, Mannheim, Germany). HLA-DRB1*0701–restricted human T-cell clones (7P24 and 7P61) specific for influenza virus hemagglutinin peptide, residues 307-319 (HA 307-319) were generated as previously described.16 T-cell clones were stimulated every 7 to 14 days with irradiated PBMCs previously pulsed with the relevant cognate antigen in RPMI/10%HS and rIL-2. T-cell clones were used for proliferative tests 1 or 2 weeks from their last stimulation.
T-cell proliferation assays
Immature DCs were pulsed overnight with TT, HA307-319, or TT947-967 at the doses indicated in “Results.” The following day, T-cell clones (1 × 104 per well) were cocultured with serial dilutions of antigen-prepulsed irradiated DCs in 96 U-bottomed well plates in a total volume per well of 200 μL. After 48 hours' incubation, tritiated thymidine (3HTdR) was added at 1 μCi (0.037 MBq) per well (Amersham Pharmacia, Bucks, United Kingdom). Thymidine incorporation was measured after a further 20 hours' incubation by a liquid-scintillation counter (Wallac).
Mixed lymphocyte reactions (MLRs) were carried out in 96 U-bottomed well plates with a total volume per well of 200 μL. Sequential (1:3) dilutions of either irradiated iDCs or irradiated mDCs (1 × 103 to 1 × 105 per well) were performed in RPMI/10%HS. Purified CD4+CD45RA+ T cells (5 × 104 per well) were used as responders. After 5 days of culture, wells were pulsed with 1 μCi (0.037 MBq) per well 3HTdR.3HTdR incorporation was measured after 20 hours by a liquid-scintillation counter.
Assay for DC migration
The migration of DCs was assessed in vitro in a 24-transwell cell culture plate (Costar, New York). DCs (2 × 105) were added to the 8-μm pore filter (Becton Dickinson). The lower chamber contained 1 mL RPMI/10%HS. MIP-1α (10 ng/mL) was added to the lower chamber, and the number of DCs migrating to the lower chamber after 2, 5, and 24 hours was assessed.17
Results
CML-DCs show altered morphology and F-actin distribution
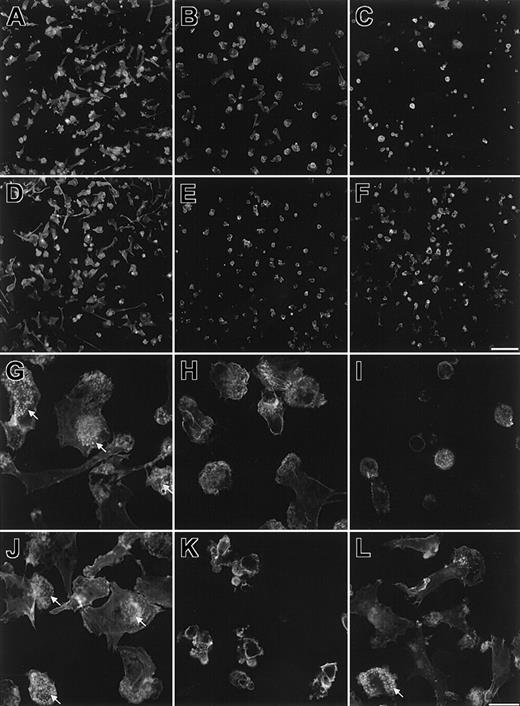
It is well established that the p210BCR-ABLoncoprotein binds to actin, and a number of studies have demonstrated associated cytoskeletal changes.2 Several of the most prominent tyrosine kinase substrates for p210BCR-ABL are cytoskeletal proteins (tensin, talin, vinculin, paxillin, and p125FAK), and the colocalization of ABL with F-actin, paxillin, and vinculin has been described.18,19 In CML patients, between 73% to 100% of monocyte-derived DCs are positive for the chimeric BCR-ABL gene.9-11 To investigate the effects of the BCR-ABL gene on cytoskeletal organisation in DCs, we have compared the distribution of F-actin in myeloid DCs generated from CML patients and healthy volunteers. DCs were generated as described in “Patients, materials, and methods.” The purity of monocyte (CD14+ cells) in the adherent populations was more than 98% in all DC preparations from healthy individuals and CML patients (data not shown). DCs were allowed to adhere to fibronectin-coated coverslips and incubated for 4 hours. The morphology of CML-DCs was very different from that of DCs generated from healthy volunteers (Figure 1). Most normal DCs assumed a polarized morphology (Figure 1A,D) and developed multiple small F-actin foci representing podosomes (Figure 1G,J). Podosomes are sites of adhesion to the extracellular matrix and have previously been described in osteoclasts, DCs, macrophages, and some oncogenically transformed fibroblasts.20-22 In contrast, CML-DCs remained more rounded, and the percentage of cells with a similar morphology to normal DCs was dramatically reduced (2% to 30%) (Figure 1B,C,E,F,H,I,K,L). Clusters of podosomes were localized primarily to one region in most (more than 90%) normal DCs, consistent with their role at the leading edge of migrating cells, whereas in CML-DCs this clustering was either absent or present in a reduced number of cells (less than 30%). In particular, DCs from 2 CML patients, patient 2 (Figure 1C,I) and patient 3 (Figure 1E,K), failed to spread significantly on fibronectin and lacked podosomes. Similar differences in morphology and F-actin distribution of normal DCs and CML-DCs were observed after adherence of DCs to gelatin-coated coverslips and lipopolysaccharide (LPS) stimulation (data not shown). We postulated that at least some of the cells with normal DC morphology in samples from patients were likely to be those that lack the BCR-ABL translocation. The same DC preparations were also analyzed for the presence of the BCR-ABL gene using FISH. The percentage of BCR-ABL–positive cells in each sample was 80% for patient 1 (Figure 1B,H), 92% for patient 2 (Figure1C,I), 89% for patient 3 (Figure 1E,K), and 79% for patient 6 (Figure 1F,L).
CML-DCs have altered morphology and F-actin distribution.
DCs were allowed to adhere to fibronectin-coated glass coverslips for 4 hours and were then fixed and stained with tetramethyl B rhodamine isothiocyanate (TRITC)–labeled phalloidin to show F-actin. Images are shown of 2 independent preparations of normal DCs (A,D,G, and J) and for CML-DCs from patient 1 (B,H), patient 2 (C,I), patient 3 (E,K), and patient 6 (F,L). Low-magnification images (A-F) were collected with a cooled charge-coupled device (CCD) camera (scale bar in panel F represents 100 μm) and high-magnification images (G-L) with a confocal laser scanning microscope (scale bar in panel L represents 20 μm). Arrows indicate regions within cells that contain clusters of podosomes.
CML-DCs have altered morphology and F-actin distribution.
DCs were allowed to adhere to fibronectin-coated glass coverslips for 4 hours and were then fixed and stained with tetramethyl B rhodamine isothiocyanate (TRITC)–labeled phalloidin to show F-actin. Images are shown of 2 independent preparations of normal DCs (A,D,G, and J) and for CML-DCs from patient 1 (B,H), patient 2 (C,I), patient 3 (E,K), and patient 6 (F,L). Low-magnification images (A-F) were collected with a cooled charge-coupled device (CCD) camera (scale bar in panel F represents 100 μm) and high-magnification images (G-L) with a confocal laser scanning microscope (scale bar in panel L represents 20 μm). Arrows indicate regions within cells that contain clusters of podosomes.
DCs from CML patients are defective in antigen processing
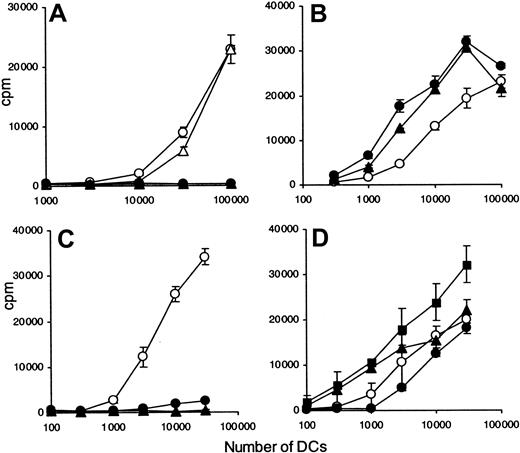
We hypothesized that BCR-ABL–induced alterations in DC morphology could interfere with the endocytic capacity of CML-DCs. Therefore, antigen processing was assessed.
Immature DCs generated from DRB1*0701 CML patients and DRB1*0701 healthy volunteers were pulsed overnight with an optimal concentration of intact TT (0.08 IU/mL) and their capacity to stimulate 3 different DRB1*0701-restricted, TT-specific T-cell clones compared (Figure 2A-B, TT1; Figure 2B, CG2; Figure 2C, JNI27). Identical results were obtained at all doses of TT tested (data not shown). CML-DCs were incompetent at inducing proliferation of T-cell clones compared with DCs derived from healthy controls. These data were confirmed on cells from 4 additional CML patients (data not shown). The lack of antigen-processing capacity was independent of the morphology of the CML-DC preparation. To determine whether these results reflected a defect in antigen presentation or antigen processing, T cells specific for a defined peptide of hemagglutinin (HA) also restricted by DR7 were used. The same DC preparations were pulsed with an optimal concentration (10 μg/mL) of either HA307-319 or TT947-967 peptide, and the response of DRB1*0701-restricted T-cell clones was evaluated. In contrast to the T-cell responses to the intact antigen TT, CML-DCs derived from patient 1 were as effective as normal DCs in presenting an optimal concentration of peptide to HA307-319–specific T-cell clones (Figure 2D). The comparable capacity to present HA peptide was confirmed with DCs derived from 4 additional CML patients (data not shown). Furthermore, the proliferation of the T-cell clone JNI27, specific for peptide TT947-967, was similar in response to peptide-pulsed DCs derived from healthy volunteers and from CML patient 7 (Figure 2F). However, a reduction in T-cell response was seen when DCs derived from patient 2, for which the morphology was grossly abnormal (Figure 1C), were compared with normal DCs (Figure 2E).
CML-DCs are defective in antigen processing.
DCs were generated from healthy individuals (○) and from CML patients (●): patient 1 (A,D), patient 2 (B,E), and patient 7 (C,F). Immature HLA-DR7–expressing DCs were pulsed overnight with either whole TT (0.008 IU/mL) (A-C) and HA307-319 peptide (10 μg/mL) (D-E) or TT947-967 peptide (10 μg/mL) (F). Different numbers of irradiated and prepulsed DCs were cultured in the presence of either TT-specific T-cell clones ([A] clone TT1; [B] clone TT1, solid lines, and clone CG1, dotted lines; [C] clone JNI27) or HA307-319–specific T-cell clone ([D-E] clone 7P61) or TT947-967–specific T-cell clone ([F] clone JNI27). After 2 days, 3HTdR was added and thymidine incorporation was measured after 20 hours. The data are expressed as counts per minute (cpm) ± standard deviations (SD). Results are representative of 15 experiments obtained with DCs derived from different patients as indicated in Table 1.
CML-DCs are defective in antigen processing.
DCs were generated from healthy individuals (○) and from CML patients (●): patient 1 (A,D), patient 2 (B,E), and patient 7 (C,F). Immature HLA-DR7–expressing DCs were pulsed overnight with either whole TT (0.008 IU/mL) (A-C) and HA307-319 peptide (10 μg/mL) (D-E) or TT947-967 peptide (10 μg/mL) (F). Different numbers of irradiated and prepulsed DCs were cultured in the presence of either TT-specific T-cell clones ([A] clone TT1; [B] clone TT1, solid lines, and clone CG1, dotted lines; [C] clone JNI27) or HA307-319–specific T-cell clone ([D-E] clone 7P61) or TT947-967–specific T-cell clone ([F] clone JNI27). After 2 days, 3HTdR was added and thymidine incorporation was measured after 20 hours. The data are expressed as counts per minute (cpm) ± standard deviations (SD). Results are representative of 15 experiments obtained with DCs derived from different patients as indicated in Table 1.
To further analyze this antigen-processing defect, DCs were generated from CD34+ cells and compared with monocyte-derived DCs. CD34+ cells from patients 1, 7, and 8 were positively selected, and their capacity to present TT to T-cell clone TT1 was evaluated (Figure 3). CML-DCs derived from both the adherent population and CD34+ cells were unable to present TT (Figure 3A,C). In contrast, DCs from either origin from healthy individuals were very effective in processing and presenting intact TT. As shown previously for monocyte-derived DCs, the capacity to present the optimal concentration of peptide HA307-319 to clone 7P24 was maintained in CD34+ CML-DCs and was similar to the capacity of DCs derived from a healthy individual (Figure3B,D).
CML-DCs derived from CD34+ cells are defective in antigen processing.
DCs were generated from healthy individuals (open symbols) and from CML patients (filled symbols). DCs were obtained from the adherent population of PBMCs (circles) or from CD34+ cells (squares and triangles): patient 1 (A-B); patients 7 and 8 (C-D). HLA-DR7–expressing DCs were pulsed overnight with whole TT (0.008 IU/mL) (A,C) and HA307-319 peptide (10 μg/mL) (B,D). Different numbers of irradiated and prepulsed DCs were cultured in the presence of either TT-specific (A,C) or HA307-319–specific T-cell clones (B,D). After 2 days, 3HTdR was added and thymidine incorporation was measured after 20 hours. The data are expressed as counts per minute (cpm) ± standard deviations (SD). The results are representative of 3 experiments.
CML-DCs derived from CD34+ cells are defective in antigen processing.
DCs were generated from healthy individuals (open symbols) and from CML patients (filled symbols). DCs were obtained from the adherent population of PBMCs (circles) or from CD34+ cells (squares and triangles): patient 1 (A-B); patients 7 and 8 (C-D). HLA-DR7–expressing DCs were pulsed overnight with whole TT (0.008 IU/mL) (A,C) and HA307-319 peptide (10 μg/mL) (B,D). Different numbers of irradiated and prepulsed DCs were cultured in the presence of either TT-specific (A,C) or HA307-319–specific T-cell clones (B,D). After 2 days, 3HTdR was added and thymidine incorporation was measured after 20 hours. The data are expressed as counts per minute (cpm) ± standard deviations (SD). The results are representative of 3 experiments.
DCs from CML patients are defective in presentation of limited amount of peptide
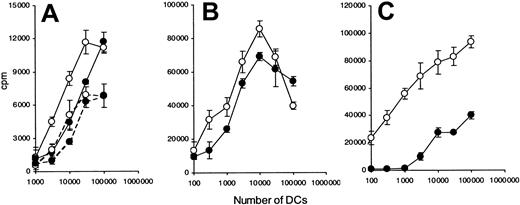
The decreased ability of CML-DCs to present peptide was further investigated using DCs pulsed with varying doses of peptide. No significant difference in the capacity of DCs generated from patient 1 and normal healthy volunteers to present peptide was observed at the concentration range analyzed (1 to 0.01 μg/mL) (Figure 4A-C).
Of the patients tested, DCs derived from patient 1 showed the most similar morphology to normal DCs (Figure 1B,H). In contrast, DCs generated from patient 2 were morphologically very different from normal DCs (Figure 1C), and a reduced capacity to present peptide was observed at concentration 1 μg/mL or less (Figure 4D-F).
The heterogeneity of CML-DCs in their capacity to present peptide was further investigated using DCs as stimulators and allogeneic naive T cells as responders in a mixed lymphocyte reaction (MLR) (Figure5). Mature CML-DCs derived from patients 5 and 3 and immature CML-DCs from patient 5 were compared with normal DCs, and they were equally effective at inducing a T-cell response at all DC numbers analyzed (1 × 103 to 3 × 104) (Figure 5A-B). In contrast, mature DCs derived from patient 4, which showed a very similar morphology to those from patient 2 (Figure 1C and data not shown), were less efficient in stimulating allogeneic T cells compared with normal DCs (Figure5C).
CML-DCs are defective in presentation at suboptimal dose of peptide.
DCs were generated from healthy individuals (○) and from CML patients (●): patient 1 (A-C) and patient 2 (D-F). Immature HLA-DR7–expressing DCs were pulsed overnight with 3 different doses of peptide HA307-319 (1 μg/mL [A,D]; 0.1 μg/mL [B,E]; 0.01 μg/mL [C,F]). Different numbers of irradiated and prepulsed DCs were cultured in the presence of HA307-319–specific T-cell clone (7P24). The data are expressed as counts per minute (cpm) ± standard deviations (SD). Data are representative of 5 experiments.
CML-DCs are defective in presentation at suboptimal dose of peptide.
DCs were generated from healthy individuals (○) and from CML patients (●): patient 1 (A-C) and patient 2 (D-F). Immature HLA-DR7–expressing DCs were pulsed overnight with 3 different doses of peptide HA307-319 (1 μg/mL [A,D]; 0.1 μg/mL [B,E]; 0.01 μg/mL [C,F]). Different numbers of irradiated and prepulsed DCs were cultured in the presence of HA307-319–specific T-cell clone (7P24). The data are expressed as counts per minute (cpm) ± standard deviations (SD). Data are representative of 5 experiments.
The responses of allogeneic naive CD4+ T cells to DCs derived from CML patients are heterogeneous.
DCs were generated from healthy individuals (○) and from CML patients (●): patient 5 (A), patient 3 (B), and patient 4 (C). Different numbers of immature (dotted lines) or mature (solid lines) DCs were cultured with naive CD4+ T cells purified from cord blood. After 5 days, 3HTdR was added and thymidine incorporation was measured after 20 hours. The data are expressed as counts per minute (cpm) ± standard deviations (SD). The results are representative of 10 experiments obtained with DCs derived from different patients as indicated in Table 1.
The responses of allogeneic naive CD4+ T cells to DCs derived from CML patients are heterogeneous.
DCs were generated from healthy individuals (○) and from CML patients (●): patient 5 (A), patient 3 (B), and patient 4 (C). Different numbers of immature (dotted lines) or mature (solid lines) DCs were cultured with naive CD4+ T cells purified from cord blood. After 5 days, 3HTdR was added and thymidine incorporation was measured after 20 hours. The data are expressed as counts per minute (cpm) ± standard deviations (SD). The results are representative of 10 experiments obtained with DCs derived from different patients as indicated in Table 1.
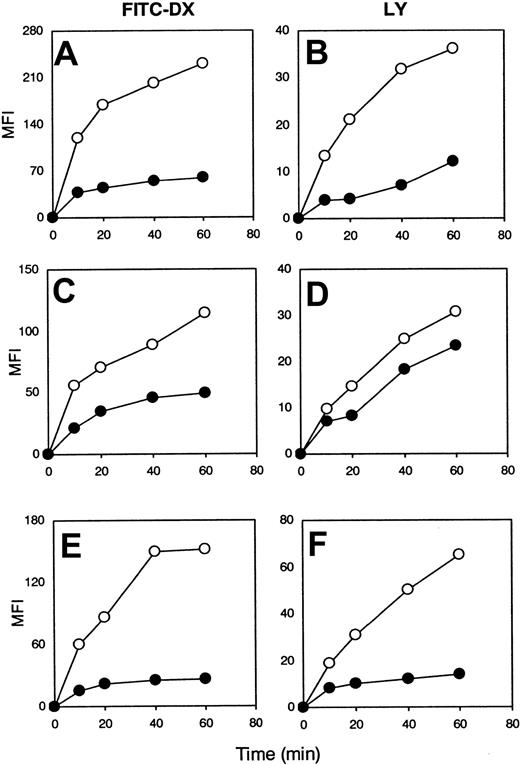
CML-DCs are defective in mannose receptor–mediated endocytosis and macropinocytosis
To dissect the mechanisms involved in the antigen-processing defect, we compared the capacity of CML-DCs and DCs generated from healthy individuals to capture antigen via mannose receptors or macropinocytosis by coincubating DCs with FITC-DX or LY, respectively.8 At each time point analyzed the mean fluoresence intensity was lower than that observed with DCs generated from healthy individuals (Figure 6). Thus, the capacity of DCs generated from CML patients to capture antigen assessed by these methods was reduced compared with DCs from healthy controls, and this defect was independent of the phenotype of the CML-DCs described in Figure 1. However, DCs derived from 2 of the 15 CML patients analyzed were equally effective at capturing antigen when compared with normal DCs. In contrast, the capacity of DCs to process antigen was analyzed in 1 of these 2 patients (patient 7), and this was impaired (Figure 2C). This result suggests that a defect in antigen capture is not the only mechanism underlying the functional abnormalities observed.
CML-DCs are defective in antigen capture.
DCs were generated from healthy individuals (○) and from CML patients (●): patient 6 (A-B), patient 1 (C-D), and patient 15 (E-F). Immature DCs were incubated with either FITC-DX (A,C,E) or with LY (B,D,F), and the mean fluorescence intensity (MFI) was evaluated at different time points, as indicated. The results are representative of 15 experiments obtained with DCs derived from different patients as indicated in Table 1.
CML-DCs are defective in antigen capture.
DCs were generated from healthy individuals (○) and from CML patients (●): patient 6 (A-B), patient 1 (C-D), and patient 15 (E-F). Immature DCs were incubated with either FITC-DX (A,C,E) or with LY (B,D,F), and the mean fluorescence intensity (MFI) was evaluated at different time points, as indicated. The results are representative of 15 experiments obtained with DCs derived from different patients as indicated in Table 1.
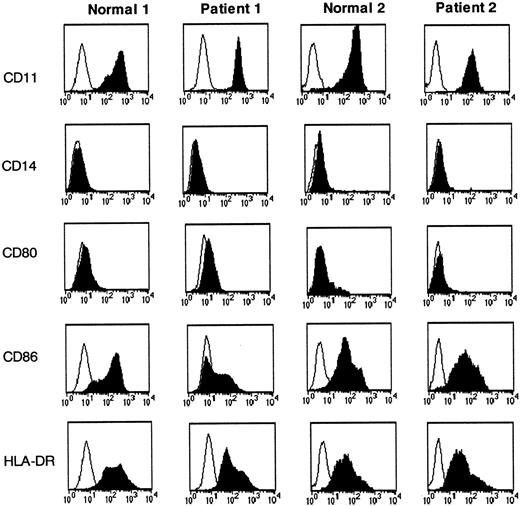
DCs from CML patients and from healthy volunteers have comparable expression of maturation markers
The capacity to capture antigen effectively is a characteristic of immature DCs, and thus we investigated the possibility that DCs from CML patients exhibited diverse differentiation characteristics when generated from monocyte precursors compared with DCs generated from healthy individuals. CML and normal DCs were incubated with different antibodies, and the expression of different markers was analyzed by flow cytometry. As expected, both DC preparations were CD11c+ and CD14−. The 2 preparations of DCs were similar in their maturation state as evaluated by the expression of CD80, CD86, and HLA-DR (Figure 7).
Comparable expression of maturation markers between CML-DCs and DCs derived from healthy volunteers.
Immature DCs derived from healthy individuals (normals 1 and 2) and from CML patients (patients 1 and 2) were incubated with mAbs specific for the molecules indicated (black histograms) and with isotype-matched mAbs (white histograms). Cell surface expression was measured by flow cytometry. The results are representative of 10 experiments obtained with DCs derived from different patients as indicated in Table1.
Comparable expression of maturation markers between CML-DCs and DCs derived from healthy volunteers.
Immature DCs derived from healthy individuals (normals 1 and 2) and from CML patients (patients 1 and 2) were incubated with mAbs specific for the molecules indicated (black histograms) and with isotype-matched mAbs (white histograms). Cell surface expression was measured by flow cytometry. The results are representative of 10 experiments obtained with DCs derived from different patients as indicated in Table1.
All these markers were up-regulated following DC maturation (data not shown).
The migratory ability of CML-DCs to a chemokine gradient is reduced compared with normal DCs
The rounded morphology and altered actin distribution of CML-DCs suggested that they were less migratory than normal DCs (Figure 1). We therefore investigated the ability of CML-DCs to migrate to a chemokine gradient. The capacity of CML-DCs to migrate in the presence of MIP-1α was dramatically reduced when compared with normal DCs (Figure 8A-B). The difference in the migratory capacity of CML-DCs compared with DCs from healthy individuals was not due to a differential expression of CCR1, which binds to MIP-1α (Figure 8C-D).
Reduced capacity of CML-DCs to migrate.
DCs were generated from healthy individuals (■ [A-B]; dotted lines [C-D]) and from CML patients (▪ [A-B]; solid lines [C-D]): patient 1 (A,C) and patient 6 (B,D). (A-B) Immature DCs were added to the 8 μm pore size filter in a transwell cell culture chamber. In the lower chamber MIP-1α was added. After 2, 5, and 24 hours, the DCs were counted in the lower chamber. (C-D) The expression of CCR1 on CML-DCs (solid lines) and DCs derived from healthy volunteers (dotted lines) was evaluated by staining with an anti-CCR1 mAb (thick lines) and isotype-matched mAb (thin lines). The results are representative of 11 experiments obtained with DCs derived from different patients as indicated in Table 1.
Reduced capacity of CML-DCs to migrate.
DCs were generated from healthy individuals (■ [A-B]; dotted lines [C-D]) and from CML patients (▪ [A-B]; solid lines [C-D]): patient 1 (A,C) and patient 6 (B,D). (A-B) Immature DCs were added to the 8 μm pore size filter in a transwell cell culture chamber. In the lower chamber MIP-1α was added. After 2, 5, and 24 hours, the DCs were counted in the lower chamber. (C-D) The expression of CCR1 on CML-DCs (solid lines) and DCs derived from healthy volunteers (dotted lines) was evaluated by staining with an anti-CCR1 mAb (thick lines) and isotype-matched mAb (thin lines). The results are representative of 11 experiments obtained with DCs derived from different patients as indicated in Table 1.
A significant statistical difference between the transmigration capacity of DCs derived from CML patients and healthy individuals was observed at each time point analyzed. Using multiple linear regression analysis, it was shown that significantly fewer CML-DCs transmigrated over time than DCs derived from healthy individuals (P < .001).
Discussion
The results presented in this study show that DCs generated from CML patients have a decreased ability to spread and polarize and have fewer podosomes compared with normal DCs. This correlates with the impaired capacity of CML-DCs to migrate, capture, and process antigen. DCs derived from all the patients presented in Table 1 demonstrated similar behavior in the functional experiments. A summary of the experiments performed using DCs derived from each patient is shown in Table 1.
Ph chromosome–positive CML originates in a single pluripotent hematopoietic stem cell, which serves as a precursor for granulocytic, erythroid, megakaryocytic, and monocyte/macrophage lineages.1 DCs are known to develop from CD34+progenitors, monocytes, or lymphoid precursors. FISH analysis of monocyte-derived DCs generated from the peripheral blood of patients with CML has displayed the presence of the BCR-ABL fusion gene in 73% to 100% of DCs.9-11 This observation is consistent with the view that most myeloid DCs are part of the CML clone while a proportion are of normal origin. A broader range ofBCR-ABL positivity (3% to 100%) has been reported in CML-DCs generated from bone marrow CD34+cells.12 23
The morphology and distribution of F-actin in DCs generated from CML patients was very similar to that seen in DCs generated from patients lacking Wiskott-Aldrich syndrome protein (WASp), in which the formation of filopodia and podosomes is severely affected.20 The role of podosomes in cell spreading and migration and the mechanism of their formation or dissolution are not yet clearly understood, although they are generally localized to the front of migrating cells and may play a role in modifying the extracellular matrix to enhance migration.
Altered cytoskeletal organization is one of the biologic consequences of BCR-ABL transformation, and yet studies have yielded equivocal results. Many reports using BCR-ABL–transformed cells or bone marrow from CML patients have documented a diminished capacity to adhere to stromal layers or to fibronectin and its proteolytic fragments.24-26 Futhermore, CML cells have been reported to have increased adhesion to laminin and collagen type IV.26 However, other studies have shown that the capacity of CML cells to adhere to stromal surfaces was time dependent.24 Short-term adhesion to fibronectin was increased by BCR-ABL transformation, whereas longer-term adhesion was decreased.24 More recently, Wertheim et al have used a cell detachment device to measure adhesive changes and reported that P210BCR-ABL expression increased adhesion to fibronectin nearly 2-fold following transfection of the myeloblastic cell line, 32D, compared with control vector.27 In addition, Salgia et al described an increase in the level of spontaneous motility in both BCR-ABL–transformed cells and primary progenitor cells from patients with CML. Specifically, they found an increased staining for F-actin and an enhanced rate of formation and retraction of actin-containing protrusions such as pseudopodia and filopodia.2 The active motility and pseudopodia formation of BCR-ABL–transformed cells was observed on fibronectin-coated as well as uncoated surfaces. Altogether, these data suggest that the accumulation of the p210BCR-ABL oncoprotein in the cytoskeleton may affect a variety of important cell functions. This conclusion led us to study 2 of the major functions of DCs—namely, their ability to migrate and their capacity to capture and process antigen.
DC maturation is intimately linked with their migration from the peripheral tissues to the draining lymphoid organs where DCs present antigen and initiate the adaptive phase of the immune response. In this study, the chemotactic migratory properties of CML-DCs and normal DCs were compared. The proportion of DCs that migrated was small, and a significant and consistent difference was observed between CML-DCs and normal DCs. In addition, the comparable levels of CCR1 expression measured in the 2 DC preparations suggests that the defect in the ability of CML-DCs to migrate was not due to a reduced sensitivity to chemokine. This finding appears to contradict observations of an increased level of spontaneous migration inBCR-ABL–transformed cell lines.2 However, Salgia et al2 did not investigate migration of CML-DCs, which may respond differently to BCR-ABL transformation compared with other cell types. In addition, we have measured migration through transwell filters, whereas Salgia et al2 used time-lapse videomicroscopy to monitor cell behavior. In agreement with our data are reports from a number of groups in which the function of polymorphonuclear leukocytes (PMNLs) from CML patients has been analyzed. Abnormalities in chemotaxis, fluid phase pinocytosis, phagocytosis, and degranulation in response to the chemotactic peptide formyl-methionyl-leucyl-phenylanine (fMLP) in PMNLs derived from CML patients have been described.28-30
One of the major findings of this study is that CML-DCs are defective in antigen processing. The use of 3 different T-cell clones specific for TT and derived from 3 individuals may suggest that the processing defect is general rather than specific for a particular T-cell epitope. This idea is further supported by the results demonstrating a decreased capacity to capture antigen via mannose receptors or macropinocytosis measured using FITC-dextran and Lucifer Yellow, respectively, in 13 of 15 patients tested. The results observed in one patient in whom the presentation of intact TT was impaired (patient 7) were not accompanied by a reduced ability to capture antigen, suggesting that the defect in antigen capture is not the only mechanism underlying the functional abnormalities described. Three previously published papers have shown that CML-DCs are able to capture, process, and present antigen. However, the sensitivity to detect antigen processing defects may have been lower in those reports because responder cells were purified polyclonal resting T lymphocytes rather than a homogeneous population of established antigen-specific T-cell clones.11,12,31 In addition, Chen et al do not provide a comparison between CML-DCs and DCs derived from healthy individuals.31
The role of cytoskeletal proteins in antigen capture and processing is not yet completely understood. Macropinocytosis has emerged as a key mechanism of antigen capture by DCs and is believed to arise from deformations of the plasma membrane known as ruffles driven by constant remodeling of the actin cytoskeleton.7,8 To distinguish between antigen processing and antigen presentation, T-cell responses to well-defined peptides were studied. T-cell proliferation to the presentation of an optimal concentration of either HA307-319 peptide or TT947-967 was similar between CML-DCs and normal DCs. In contrast, for some of the CML patients T-cell responses to limiting doses of peptide presented by CML-DCs were impaired. These results were particularly evident with CML-DCs that had a significantly abnormal morphology, and they were further confirmed using the same DC preparations as stimulators in MLRs. Altogether, these results are consistent with the observation that DCs actively polarize F-actin and fascin upon clustering with T cells and suggest an important role for the DC cytoskeleton in the establishment of the immunologic synapse.32
An increase in the incidence of infection has not been reported in patients with CML, and a number of reasons may underlie this. Estimates of blood DC numbers range from 0.1% of mononuclear cells (MNCs) to 1% of MNCs.33-35 Recently, Mohty et al reported a tremendous expansion (1.36% to 41%) of myeloid DCs in 59% of patients with AML. DC numbers have not been recorded in patients with CML, but in light of the expansion of myeloid and monocytic lineages, an increase in the number of myeloid DCs would be expected. Such an increase in vivo may serve to compensate for the functional impairment observed in vitro in DCs generated from patients with CML.36 Furthermore, plasmacytoid DCs have been shown to play an important role in the host defense against viral infection. Plasmacytoid DCs are of lymphoid origin,6 and lymphocytes in CML patients are rarely derived from a Ph+ progenitor.37
Altogether, these data raise questions concerning the suitability of CML-DCs for vaccination. Much depends on whether or not their ability to present intracellular proteins, such as BCR-ABL, with MHC class II molecules is intact. The data of Choudhury et al38 suggest that this may be the case; however, much of their work was focused on CD8+ T-cell responses. This issue is the subject of further investigation. If a defect in the presentation of intracellular proteins does exist, this may be reversible by in vitro treatment with interferon-α (IFN-α) or tyrosine kinase inhibitors.
We thank Ms Nicola Foot and Emma Walker for their help with the FISH analysis and separation of CD34+ cells, respectively.
Prepublished online as Blood First Edition Paper, December 27, 2002; DOI 10.1182/blood-2002-06-1841.
Supported by an MRC Component Grant. K.C. is the holder of an MRC CSC Training Fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Giovanna Lombardi, Department of Immunology, Faculty of Medicine, Imperial College at Hammersmith Hospital, DuCane Road, London, W12 ONN, United Kingdom; e-mail:g.lombardi@ic.ac.uk.

![Fig. 2. CML-DCs are defective in antigen processing. / DCs were generated from healthy individuals (○) and from CML patients (●): patient 1 (A,D), patient 2 (B,E), and patient 7 (C,F). Immature HLA-DR7–expressing DCs were pulsed overnight with either whole TT (0.008 IU/mL) (A-C) and HA307-319 peptide (10 μg/mL) (D-E) or TT947-967 peptide (10 μg/mL) (F). Different numbers of irradiated and prepulsed DCs were cultured in the presence of either TT-specific T-cell clones ([A] clone TT1; [B] clone TT1, solid lines, and clone CG1, dotted lines; [C] clone JNI27) or HA307-319–specific T-cell clone ([D-E] clone 7P61) or TT947-967–specific T-cell clone ([F] clone JNI27). After 2 days, 3HTdR was added and thymidine incorporation was measured after 20 hours. The data are expressed as counts per minute (cpm) ± standard deviations (SD). Results are representative of 15 experiments obtained with DCs derived from different patients as indicated in Table 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/9/10.1182_blood-2002-06-1841/4/m_h80934217002.jpeg?Expires=1769081363&Signature=BwREvLu2dmRWQtObCzXR5M7tLVCte7E7fuCylK73Sj5aX2OxIPmP9DxPYPBXqPOK~tpj2L3H1SroiWJeWSWYQQX9~0p~ZnWNHORUuPTRfU3vDGGzdwybMuzKPhO4RxIBPXVFdmV1NKuoJCMlAuTQhUqekEmG58Zvcsh4u18~36~qlCVkoZSezmRxNWl-Ho9Dzw-YJIUoOmYXrQA1seoxmQvmpyv-p~En0MeIxsiYFncQ05Ovv03RMudhpEZ9gL4pAuFOrFlydkrg17a2pRVleQYv1RV5CxysKIjNpgpQO8n~i16b-hLa-hynZYOqq4yNILvI7cFL6RRFeSY3loTlGQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. CML-DCs are defective in presentation at suboptimal dose of peptide. / DCs were generated from healthy individuals (○) and from CML patients (●): patient 1 (A-C) and patient 2 (D-F). Immature HLA-DR7–expressing DCs were pulsed overnight with 3 different doses of peptide HA307-319 (1 μg/mL [A,D]; 0.1 μg/mL [B,E]; 0.01 μg/mL [C,F]). Different numbers of irradiated and prepulsed DCs were cultured in the presence of HA307-319–specific T-cell clone (7P24). The data are expressed as counts per minute (cpm) ± standard deviations (SD). Data are representative of 5 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/9/10.1182_blood-2002-06-1841/4/m_h80934217004.jpeg?Expires=1769081363&Signature=MQHKwmUJudhaMmIMNr~sOPniG3f2vq18HC~zWioumQZr7zvRa3FPCwBqmZfyfUvf2h0~IW-N7XokcaxOTGot-RLmo1iOxxIvU38KrsGrI-iDTpR74vuk7rcldOQwbH4270VmzQYsB4jAwQSB1f34PCXDWBG4Qw6VFWJUJuBoqIEI-CtyPkJs4FJ9CDq-fdP-3uQnLIi3MYUdo~y27SYNGeXW0~0H2hSW~JAQ0NcqJOKFEKuTws0IOzKEzQF6opR4~mmRkYRcPUIRMUPbothmh2Gk-C5U0qOJZPrH5Cq~oYTD55t2wyS3XSnoChvsM~b7hzHggOAR5fDuMX~U7p6tnQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Reduced capacity of CML-DCs to migrate. / DCs were generated from healthy individuals (■ [A-B]; dotted lines [C-D]) and from CML patients (▪ [A-B]; solid lines [C-D]): patient 1 (A,C) and patient 6 (B,D). (A-B) Immature DCs were added to the 8 μm pore size filter in a transwell cell culture chamber. In the lower chamber MIP-1α was added. After 2, 5, and 24 hours, the DCs were counted in the lower chamber. (C-D) The expression of CCR1 on CML-DCs (solid lines) and DCs derived from healthy volunteers (dotted lines) was evaluated by staining with an anti-CCR1 mAb (thick lines) and isotype-matched mAb (thin lines). The results are representative of 11 experiments obtained with DCs derived from different patients as indicated in Table 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/9/10.1182_blood-2002-06-1841/4/m_h80934217008.jpeg?Expires=1769081363&Signature=Ar3b0RgVJC9qFmw8Kdd0owXOaOEAKsjDG3pZFrIu57GjbwzfNw9hT91JP5GR8fjplmQ7tDENoN-9DraWfpYmmS3CjiJYP2oCdXBUhiSnf3eQhAaNRT6HrZaNpfRZQNViuKEyyWygoG7jnva~~PiI6HBm9jBPY2cS2gDuefXl9ajaTfOZvFQOqjVDjGVHfnxBLO04zkpitIzthwsH3GZmfIjtWWkXcxffMD4HGLP1n-N9XG90d5rD3P8Qw--xXvbfvdY5x9dm0W0huIRO18nMl0Cy8VLJ0CgPDznTiTY~~dPw0i0S2USijTmMpgrIM2u2iBiO97OvMtLEHWoTCZihmQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal