As measured by the long-term repopulating cell (LTRC) assay, only a few hematopoietic stem cells (HSCs) or perhaps a single HSC are required to totally repopulate the lymphohematopoietic tissues of lethally irradiated mice, cats, and humans, raising the question as to why large mammals require more marrow cells to either rescue them from lethal irradiation or establish a long-term hematopoietic graft than do small mammals. An explanation might be that HSC marrow frequency across species is not constant, but decreases as species body weight increases. This hypothesis was tested by comparing the LTRC marrow concentration of mice to that of rats. Specifically, histocompatible AKR/J Thy 1.1 marrow was transferred to 7-Gy irradiated C3H/HeN, Thy 1.2 mice, and histocompatible Norway Black marrow (NBr), RT 7.2 marrow was transferred to 7-Gy irradiated RT 7.1 Lewis rats. The recipients were scored for successful grafts 6 to 20 weeks later. By limiting dilution analysis, a value of 1 LTRC/47 700 marrow cells was calculated for mice, but only 1 LTRC/502 000 marrow cells was calculated for rats. Viewed in the context of marrow grafting in larger mammals, these results suggest that species with greater body mass have lower marrow HSC frequency.
Introduction
Lethal dose (LD) irradiation is usually characterized as an LD50/30 that by definition is the amount of whole body irradiation resulting in 50% mortality within 30 days following irradiation. The mechanism underlying an LD50/30 is believed to be reduction of the hematopoietic stem cell (HSC) compartment below a critical level necessary to maintain hematopoiesis in 50% of irradiated recipients. For the mouse, this critical level appears to be a single HSC per animal. Using the endogenous long-term repopulating cell assay (e-LTRC) for murine HSC,1 it was found that an average of 1.67 HSCs from a total cell population (N) of 10 300 HSCs survive a murine LD50/30 of 7.2 Gy. If surviving HSCs were Poisson distributed throughout the irradiated group, then in 19% of the mice no HSCs would have survived and these mice would have died. A murine radiation LD50/30, therefore, closely correlates with HSC survival.
Although HSC radiation sensitivity (Do) is similar across species, the sizes of HSC populations in species other than the mouse are not known. This makes it difficult to correlate LD50/30 with HSC survival in large mammals. For the human the problem is this: if HSC numbers are proportional to body weight, then a human being 2800 times larger than a mouse might be expected to have an HSC compartment of approximately 28 million cells. For a cell population of this size, it would be expected that more than 600 000 HSCs would survive an estimated human LD50/30 of approximately 0.3 Gy.2 (Because primitive hematopoietic cells have little or no repair capacity following ionizing radiation,3 numbers [n] of HSCs surviving LD50/30 irradiation are estimated by: n = Ne−D/Do where D is set to LD50/30.) This is several hundred times more cells than the hundred or so HSCs necessary to maintain normal hematopoiesis in humans,4 and several hundred thousand times more than the 4 or 5 clones that can establish a successful human marrow graft.5 If true, postirradiation marrow failure in an LD50/30-exposed human would have to be attributed to something other than reduction of HSC numbers below levels needed to either maintain normal hematopoiesis or establish a marrow graft.
An alternative explanation might be that HSC numbers are not present in direct proportion to species body weight,6,7 but are conserved across species. If all mammals have approximately 10 000 to 11 000 HSCs, as was found for the mouse, then simple calculations show that for large animals, about one HSC per several hundred grams body weight would be needed to survive a species LD50/30. It is interesting that Abkowitz et al8 recently reported that cats have nearly the same size HSC compartment as mice, and by extrapolation a human might also be expected to have a similar size HSC compartment.
A good test of this hypothesis would be a direct rat/mouse comparison using the classically defined long-term repopulating cell (LTRC) assay for HSCs. A rat having 10 times the marrow volume of a mouse9,10 should have one tenth the marrow LTRC concentration of a mouse. The advantage of such a comparison is that in both species, the LTRC assay can be undertaken under nearly identical irradiation and marrow transfer protocols. Also in both species the older but less accurate HSC assay,11 that being the spleen colony-forming cell assay (CFU-s) and its seeding efficiency,f, is well characterized.
In this study, histocompatible RT 7.2 Norway Black rat (NBr) marrow was grafted into 7-Gy irradiated RT 7.1 Lewis rats, and histocompatible Thy 1.1 AKR/J mice marrow was grafted into 7-Gy irradiated Thy 1.2 C3H/HeN mice. Successful grafts were scored by immunofluorescence cell staining for either the RT 7.2 or Thy 1.1 antigen. Using limiting dilution technique, the concentration of LTRCs in the NBr rat marrow was found to be 1 cell/502 000 ± 70 800 (± SE) marrow cells. This is significantly less than that for AKR/J marrow, which is 1 LTRC/47 700 ± 4600 cells.
Materials and methods
Animals
Lewis and NBr male rats were obtained from Charles River Laboratories (Kingston, RI) at 4 weeks of age and used at 8 to 14 weeks of age. AKR/J male mice from The Jackson Laboratory (Bar Harbor, ME) and C3H/HeN male mice from National Cancer Institute (Bethesda, MD) were obtained at 4 weeks of age and used at 8 to 14 weeks of age. All animals were kept in a facility approved by the American Association for Accreditation of Laboratory Animal Care. Rats and mice were killed by CO2 gas inhalation. The Institutional Animal Care and Use Committee approved all animal protocols.
Irradiation
Rats and mice received total body irradiation (TBI) in a bilateral γ radiation field as described previously.1During irradiation the animals were restrained in Plexiglas cages. The midline tissue doses ranged from 6 to 9.5 Gy and were delivered at a dose rate of 0.4 Gy/min. Before the irradiation, the dose rate was established using acrylic rat and mice phantoms. A 0.5-cc tissue equivalent ionization chamber (calibration factor traceable to the National Institute of Standards and Technology) was used for in-phantom dosimetry. The tissue-to-air ratio was 0.94 and 0.96 for rat and mouse phantoms, respectively. The radiation fields were uniform to within ± 5%. Dosimetric measurements were made according to the American Association of Physicists in Medicine protocol for the determination of the absorbed dose from high-energy photon and electron beams.12
Radiation chimeras
Sublethally irradiated recipient RT 7.1 Lewis rats (7 Gy TBI, 0.4 Gy/min) were injected intravenously 2 hours after irradiation with histocompatible RT 7.2 NBr rat marrow cells. For mice, Thy 1.1 AKR/J mouse marrow was injected into sublethally irradiated histocompatible Thy 1.2 C3H/HeN recipients. The reverse, or C3H/HeN into AKR/J, was also used. At times specified in “Results,” the percentage of cells of donor origin was measured by selective immunofluorescence staining.
Fluorescence antibody labeling
Labeling of cells has been previously described.13Rat cells were labeled with the fluorescein isothiocyanate (FITC)–HIS41 monoclonal antibody purchased from Pharmigen (San Diego, CA). For mice, thymocytes were labeled with anti–Thy 1.1 antibody phycoerythrin (PE)–OX7Fab or FITC-anti–Thy 1.2 antibody (Caltag, Burlingame, CA). The percentage of positive cells was measured with a fluorescence-activated cell sorter (FACS) II instrument.
LD50/30 assay
Various numbers of NBr marrow cells were injected intravenously into irradiated Lewis recipient rats (9.5 Gy TBI, 0.4 Gy/min). For mice, either AKR/J marrow cells were injected into C3H/HeN recipients (9.5 Gy TBI, 0.4 Gy/min) or vice versa. The recipient rats and mice were maintained on drinking water containing 1.0 mg/mL tetracycline. Rats and mice were scored for survival at 30 days.
Limiting dilution assay
Lewis recipient rats received 7 Gy TBI, 0.4 Gy/min, and 2 hours later were injected intravenously with various numbers of NBr marrow cells. The same irradiation protocol was used for the transfer of AKR/J marrow into C3H/HeN recipients and the reverse. Donor origin cells were detected 6, 12, and 20 weeks later by immunofluorescence technique. Because the results were similar using the various time points, the results were pooled for limiting dilution assay. Mice were scored at 6 weeks only. The logarithm of the fraction negative animals (ln Po) was plotted as a function of the cells transferred. Regression lines were fitted using weighted least squares. Frequency of LTRCs was calculated for the slope (F) of the plot, ln Po = −FC, where C is the number of cells transferred.
One SD of the slope is estimated by the SE of the slope, which is given as sβ1 = s/SQRT(SSxx) where s is the estimated SE of the regression model ors = SQRT(SSE/n − 2). The SE of F was calculated by the method of propagation of error. It is the SE of the slope divided by the square of the slope.
Cumulative distribution graphs
Cutoff values for scoring animals as negative or positive were determined using cumulative distribution graphs. Groups of grafted rats or mice were sorted in ascending order according to their percent donor thymocytes (y-value), after which the ranking of each animal was divided by total number of animals to give fraction cumulative distribution (x-value). Individual animals were plotted according to their respective x- and y-values. The cutoff value for scoring LTRC grafting was set at the sharp break in the curve.
Results
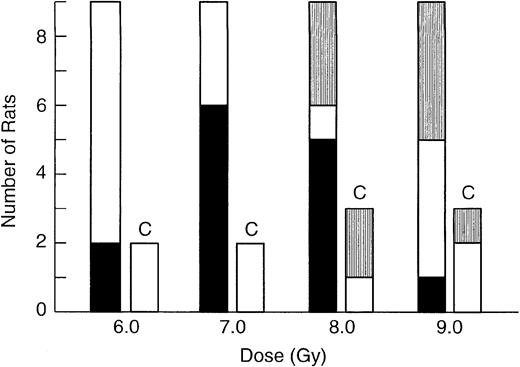
As shown in Figure 1, grafting of NBr LTRC into Lewis rats was detected at 6-Gy TBI, but optimal detection of grafting required a 7-Gy dose. At 8 Gy, 33% of the recipient rats died. In an attempt to improve survival, 9-Gy (Figure 1) recipients were given host marrow 24 hours following donor marrow transfer,14 but 44% of the recipients died. Therefore, the 7-Gy sublethal protocol was found to be optimal and it was used for limiting dilution analysis. For LD50/30 studies, the radiation dose was increased to 9.5 Gy resulting in 100% lethality in nongrafted control rats. Although these same irradiation protocols were used for the murine LTRC limiting dilution assays and LD50/30 studies, the C3H/HeN and AKR/J mice were more sensitive to ionizing radiation than the Lewis rat, and approximately 20% of recipient mice died when the 7-Gy LTRC irradiation protocol was used.
Effect of conditioning radiation dose on NBr marrow grafting in Lewis rats.
Four groups of 9 Lewis rats received 2 × 105 NBr marrow cells 2 hours following irradiation. Groups of 2 to 3 Lewis rats that were irradiated, but not grafted, are labeled control (C). TBI60Co doses are listed on the x-axis. Rats were assayed 12 weeks after grafting. Solid bar (▪) indicates positive recipients; open bar (■), negative recipients; and striped bar (▥), rats not surviving.
Effect of conditioning radiation dose on NBr marrow grafting in Lewis rats.
Four groups of 9 Lewis rats received 2 × 105 NBr marrow cells 2 hours following irradiation. Groups of 2 to 3 Lewis rats that were irradiated, but not grafted, are labeled control (C). TBI60Co doses are listed on the x-axis. Rats were assayed 12 weeks after grafting. Solid bar (▪) indicates positive recipients; open bar (■), negative recipients; and striped bar (▥), rats not surviving.
Successful grafting in the rat was detected using the HIS41 monoclonal antibody,15 which binds leukocyte common antigen on all hematopoietic cells of rats with the RT 7.2 allotype (NBr), but not the RT 7.1 allotype (Lewis). Background staining with this FITC-antibody was minimal for both thymus and lymph node cells, notably higher for the spleen cells, and presented a special case for the marrow. In the marrow, erythrocytes did not stain with HIS41, lymphocytes stained weakly, and granulocytes, as characterized by both high forward and perpendicular light scatter, stained brightly. The only marrow cells that could be dependably scored as being of either host or donor origin were granulocytes. However, granulocytes have high levels of autofluorescence and, because it is difficult to correct for both autofluorescence and background staining, all data are presented uncorrected for both.
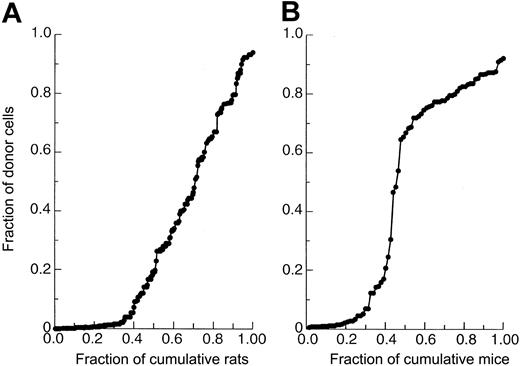
Although all cell lineages were considered in scoring recipients as being either positive or negative, measurements of thymocytes were considered the most accurate because of their low background staining. For rats, false positives were rejected using a cutoff value of less than 4% HIS41+ thymocytes as determined using a cumulative distribution graph (Figure 2A). With the 4% cutoff value, more than 95% of recipients scoring positive for donor thymocytes also contained substantial numbers of donor cells within the other cell lineages. In rare cases (Figure 4A), when the thymocyte value for scoring rats was not clear, other cell lineages were used to make the determination. For mice, chimerism was measured only in the thymus using either anti–Thy 1.1 or anti–Thy 1.2 antibody. For mice to be scored as negative, less than 4% donor origin thymocytes was also used as a cutoff value (Figure 2B). As with rats, it has been reported that scoring of only thymocytes is sufficient for the detection of donor LTRC grafting in mice.16
Cumulative distribution of the fraction of total recipients whose fraction of donor thymocytes is equal to or less than that listed on y-axes.
(A) Lewis recipients receiving NBr marrow. (B) C3H/HeN recipients receiving AKR/J marrow. For both species, the cutoff value was set at 4% or greater for positive animals, whereas less than 4% was used to score animals as being negative.
Cumulative distribution of the fraction of total recipients whose fraction of donor thymocytes is equal to or less than that listed on y-axes.
(A) Lewis recipients receiving NBr marrow. (B) C3H/HeN recipients receiving AKR/J marrow. For both species, the cutoff value was set at 4% or greater for positive animals, whereas less than 4% was used to score animals as being negative.
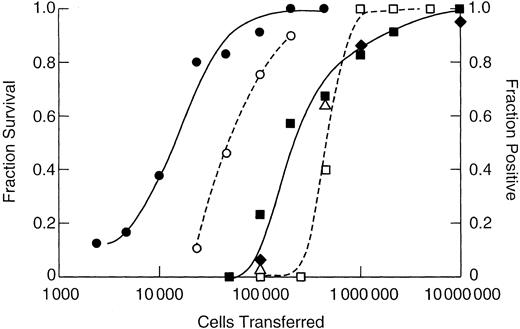
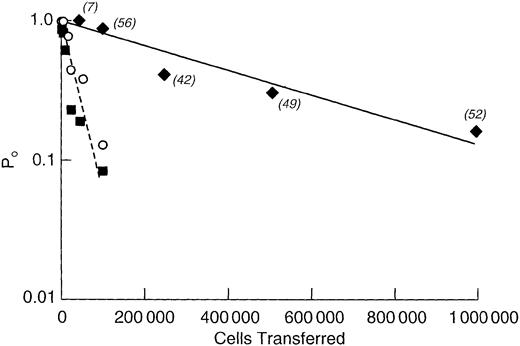
As shown in Figure 3, 10 times more rat marrow cells were required for both protection against lethal irradiation and for the generation of a chimera than that required for the mouse. For either animal, fewer cells were required to establish a chimera than were required to protect the animal from lethal irradiation. A semilog plot of the fraction of negative animals versus cells transferred is shown in Figure 4. From the slope of the weighted least squares fitted line, the concentration of LTRCs in NBr rat marrow was calculated to be 1 cell/502 000 ± 70 800 (± SE) marrow cells. For the AKR/J mouse, it was 1 LTRC/47 700 ± 4600 cells, and for the C3H/HeN mouse, 1 LTRC/41 800 ± 8700 cells.
Number of marrow cells required to establish a chimera or protect against lethal irradiation.
On the left y-axis is shown the 30-day fraction survival of lethally 9.5-Gy irradiated Lewis recipients receiving NBr marrow (■) and C3H/HeN recipients receiving AKR/J marrow (○). The right y-axis shows the fraction of sublethally 7-Gy irradiated recipient Lewis rats positive for donor NBr marrow when determinations were made at 6 weeks (▪), 12 weeks (♦), and 20 weeks (▵) after grafting, and C3H/HeN recipients receiving AKR/J marrow assayed at 6 weeks only (●). The x-axis indicates cells transferred by intravenous tail injection.
Number of marrow cells required to establish a chimera or protect against lethal irradiation.
On the left y-axis is shown the 30-day fraction survival of lethally 9.5-Gy irradiated Lewis recipients receiving NBr marrow (■) and C3H/HeN recipients receiving AKR/J marrow (○). The right y-axis shows the fraction of sublethally 7-Gy irradiated recipient Lewis rats positive for donor NBr marrow when determinations were made at 6 weeks (▪), 12 weeks (♦), and 20 weeks (▵) after grafting, and C3H/HeN recipients receiving AKR/J marrow assayed at 6 weeks only (●). The x-axis indicates cells transferred by intravenous tail injection.
Limiting dilution analysis of LTRC marrow concentration with frequency estimated as the negative inverse of the least squares derived slope.
For NBr marrow the results of assays performed at 6, 12, and 20 weeks were pooled and plotted as a single point (♦, solid line); frequency was 1 LTRC/502 000 ± 70 800 (± SE) marrow cells withr2 = 0.91 and y-intercept of 0.91 (0.79 − 1.05 ± SE range). AKR/J marrow (▪, dotted line), 1/ 47 700 ± 4600, r2 = 0.96, y-intercept of 0.98 (0.89-1.08). C3H/HeN mice (○, line not shown); 1/41 800 ± 8700, r2 = 0.85, y-intercept of 0.71 (0.56-0.90). Numbers of rats per point are in parentheses. For mice, depending on survival, 6 to 12 mice were used per point.
Limiting dilution analysis of LTRC marrow concentration with frequency estimated as the negative inverse of the least squares derived slope.
For NBr marrow the results of assays performed at 6, 12, and 20 weeks were pooled and plotted as a single point (♦, solid line); frequency was 1 LTRC/502 000 ± 70 800 (± SE) marrow cells withr2 = 0.91 and y-intercept of 0.91 (0.79 − 1.05 ± SE range). AKR/J marrow (▪, dotted line), 1/ 47 700 ± 4600, r2 = 0.96, y-intercept of 0.98 (0.89-1.08). C3H/HeN mice (○, line not shown); 1/41 800 ± 8700, r2 = 0.85, y-intercept of 0.71 (0.56-0.90). Numbers of rats per point are in parentheses. For mice, depending on survival, 6 to 12 mice were used per point.
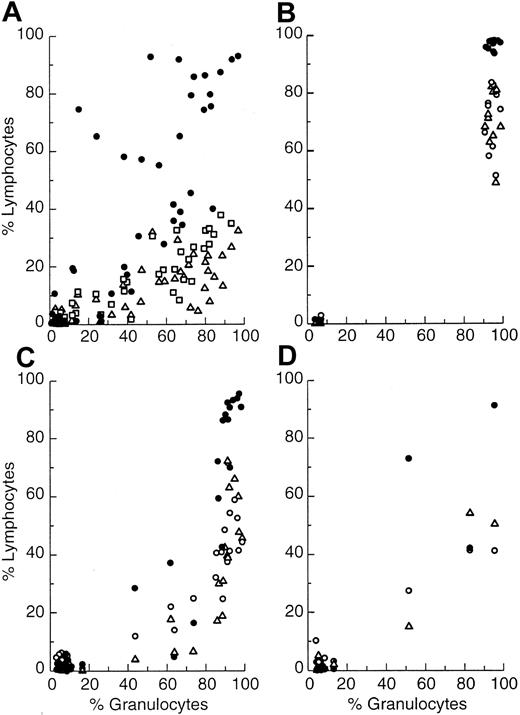
Shown in panels Figure 5A-B are plots of fraction HIS41+ cells/total cells for thymus, spleen, and lymph nodes as a function of fraction HIS41+ marrow granulocytes in recipients, as measured at 6 and 20 weeks after grafting. Repopulation of the host marrow granulocytic compartment by donor cells required less time than that required for the thymus, spleen, or lymph nodes. However, if given sufficient time, the extent of donor thymic repopulation was similar to that of the marrow granulocyte compartment. In contrast, neither the spleen nor lymph nodes ever repopulated to the extent seen for thymus or marrow (Figure5B). At 20 weeks after grafting, all positive recipients had nearly 100% donor cells in both the marrow granulocytic compartment and thymus, whereas only 50% to 75% of cells in the lymph nodes or spleen were of donor origin.
Fraction of NBr donor HIS41+ cells in thymus, spleen, and lymph nodes as correlated with fraction positive marrow granulocytes.
Thymus (●), spleen (▵), and lymph node cells (○), except for plot A where spleen is (■) and lymph node is (▵), in Lewis recipients plotted as a function of fraction positive donor marrow granulocytes from individual recipients. (A) Primary recipients at 6 weeks. (B) Primary recipients at 20 weeks. (C) Secondary recipients at 6 weeks when marrow was transferred from primary recipients at 6 weeks. (D) Tertiary recipients at 70 weeks when marrow was transferred from secondary recipients at 20 weeks and from primary recipients at 6 weeks.
Fraction of NBr donor HIS41+ cells in thymus, spleen, and lymph nodes as correlated with fraction positive marrow granulocytes.
Thymus (●), spleen (▵), and lymph node cells (○), except for plot A where spleen is (■) and lymph node is (▵), in Lewis recipients plotted as a function of fraction positive donor marrow granulocytes from individual recipients. (A) Primary recipients at 6 weeks. (B) Primary recipients at 20 weeks. (C) Secondary recipients at 6 weeks when marrow was transferred from primary recipients at 6 weeks. (D) Tertiary recipients at 70 weeks when marrow was transferred from secondary recipients at 20 weeks and from primary recipients at 6 weeks.
Donor marrow was serially passed from primary to secondary and then to tertiary recipients. To estimate the optimum time of assay, a wide range of time spans between the marrow transfers was used. Marrow was transferred at 6 weeks and 20 weeks after engraftment from primary recipients to secondary recipients. The 2 groups of secondary recipients were then assayed at either 6, 20, or 70 weeks after engraftment. Following marrow transfer from primary recipients at 6 weeks and secondary recipients at 20 weeks, tertiary recipients were assayed at 70 weeks only. Regardless of the time periods used, no differences in fraction positive recipients within the various groups were noted, indicating that the assay was not scoring short-term repopulating clones. Therefore, data within the primary, secondary, and tertiary subgroups were combined and analyzed by limiting dilution technique (Table 1). From the tertiary experiments and the estimate of 4.0 × 109total hematopoietic cells for the rat,9 it was calculated that the rat LTRCs could self-renew through at least 10 cell doublings.
Transfer of rat marrow to primary, secondary, and tertiary recipients
| Cells transferred to primary recipient . | Cells transferred to secondary recipient . | Cells transferred to tertiary recipient . | Fraction positive recipients . | LTRC frequency* ± SE . |
|---|---|---|---|---|
| 108 | — | — | 8/9 | 5.0 × 105 ± 7.1 × 104† |
| 107 | — | — | 9/9 | |
| 106 | — | — | 9/9 | |
| 105 | — | — | 1/8 | |
| 108 | 107 | — | 12/13 | 3.7 × 106 ± 1.2 × 106‡ |
| 106 | — | 10/13 | ||
| 105 | — | 1/12 | ||
| 107 | 107 | — | 9/10 | 4.2 × 106 ± 5.0 × 105 |
| 106 | — | 6/13 | ||
| 105 | — | 0/8 | ||
| 106 | 107 | — | 10/12 | 5.6 × 106 ± 2.1 × 105 |
| 106 | — | 1/12 | ||
| 105 | — | 0/12 | ||
| 105 | 107 | — | 1/10 | 9.6 × 107 ± 6.8 × 106 |
| 106 | — | 0/12 | ||
| 105 | — | 0/8 | ||
| 108 | 107 | 107 | 3/4 | 7.3 × 106 ± 7.3 × 105 |
| 106 | 0/2 | |||
| 107 | 107 | 107 | No Survivors | |
| 106 | No Survivors | |||
| 106 | 107 | 107 | 0/5 | |
| 106 | 0/2 |
| Cells transferred to primary recipient . | Cells transferred to secondary recipient . | Cells transferred to tertiary recipient . | Fraction positive recipients . | LTRC frequency* ± SE . |
|---|---|---|---|---|
| 108 | — | — | 8/9 | 5.0 × 105 ± 7.1 × 104† |
| 107 | — | — | 9/9 | |
| 106 | — | — | 9/9 | |
| 105 | — | — | 1/8 | |
| 108 | 107 | — | 12/13 | 3.7 × 106 ± 1.2 × 106‡ |
| 106 | — | 10/13 | ||
| 105 | — | 1/12 | ||
| 107 | 107 | — | 9/10 | 4.2 × 106 ± 5.0 × 105 |
| 106 | — | 6/13 | ||
| 105 | — | 0/8 | ||
| 106 | 107 | — | 10/12 | 5.6 × 106 ± 2.1 × 105 |
| 106 | — | 1/12 | ||
| 105 | — | 0/12 | ||
| 105 | 107 | — | 1/10 | 9.6 × 107 ± 6.8 × 106 |
| 106 | — | 0/12 | ||
| 105 | — | 0/8 | ||
| 108 | 107 | 107 | 3/4 | 7.3 × 106 ± 7.3 × 105 |
| 106 | 0/2 | |||
| 107 | 107 | 107 | No Survivors | |
| 106 | No Survivors | |||
| 106 | 107 | 107 | 0/5 | |
| 106 | 0/2 |
— represents not applicable.
Expressed as the negative reciprocal of the slope.
Results taken from Figure 4.
Line fit constrained so that a C = 0, Po = 1.
The pattern of aggressive repopulation of marrow and thymus, and the weaker repopulation of the spleen and lymph nodes by donor marrow, did not change as the marrow was serially passed from primary to secondary to tertiary recipients (Figure 5C-D). If serial passage of the marrow weakens its proliferative capacity,17 this diminished capacity was not expressed through any one cell lineage, but was distributed throughout all cell lineages, as would be expected for the grafting of a multipotent HSC.
Discussion
By limiting dilution technique, the concentration of NBr rat marrow LTRC was found to be 1 cell/502 000 ± 70 800 (± SE) marrow cells. For AKR/J mice, a value of 1 LTRC/47 700 ± 4600 marrow cells was calculated, and for C3H/HeN mice, the concentration was 1 LTRC/41 800 ± 8700 cells. In both species, the transfer of donor cells was to histocompatible, allogeneic recipients conditioned with sublethal 7-Gy 60Co TBI. The present value of 2 to 3 LTRC/105 marrow cells for mice agrees with that reported by Micklem et al18 and Spangrude et al,14 both of whom used lethally irradiated syngeneic or congenic recipients. However, their estimates are lower than the 11 LTRC/105cells reported by Boggs et al19 and higher than the 0.7 LTRC/105 cells reported by Harrison et al.20Boggs et al used nonirradiated genetically defective F1 mice as recipients, whereas Harrison et al measured donor LTRCs at a longer time period using lethally irradiated syngeneic recipients. Strain differences, expressions of latent radiation damage to the microenvironment, or dominant clones appearing at the later stages of grafting may account for these discrepancies.
The difference in mouse/rat LTRC concentration is in agreement with 12-day CFU-s data.7 In the mouse, these cells are present at a concentration of 200 to 400 cells/106 marrow cells, but for rats only 25 to 50 12-day CFU-s/106 marrow cells are found.
Because allogeneic NBr marrow was grafted into histocompatible Lewis rats, rather than congenic or syngeneic recipients, there was the possibility of graft rejection across minor histocompatible barriers. However, radiation doses of 6 to 7.5 Gy are sufficient to eradicate this type of rejection in mice,21 dogs,22 and humans.23 For the rat, 7 Gy was also found to be satisfactory. Further evidence that rejection did not take place across minor histocompatible barriers was that: (1) allogeneic NBr marrow was found more effective for protection of lethally irradiated Lewis rats than syngeneic Lewis marrow cells,13 (2) NBr marrow usually generates 1.5 times greater numbers of 12-day CFU-s in Lewis recipients than does Lewis marrow (data not shown), and (3) NBr marrow can be serially transplanted, at near limiting dilution, through sublethally irradiated recipients with 10 or more cell doublings, which is similar to that observed in mice using retrovirally tagged cells.24 Taken as a whole, there is no evidence for rejection of NBr marrow by histocompatible Lewis recipients conditioned with radiation doses of 7 Gy. Also, there was no indication of graft-versus-host disease in chimeric rats maintained as long as 70 weeks after grafting.
The finding of numerous host cells in the lymph nodes and spleen after grafting is consistent with several reports of split chimerism following allogeneic marrow transplantation. At 20 weeks after grafting, split chimerism was primarily limited to the peripheral lymph organs and was minimal in the thymus and marrow granulocytic compartment. This might indicate that peripheral host lymphocytes were not being continually generated from host stem cells, but were derived from a pool of either radioresistant long-lived cells25 or were generated fairly soon after irradiation from radioresistant lymphocytic stem cells.
A 10-fold difference between rat and mouse marrow LTRC concentration was observed. Is the marrow concentration of LTRCs further reduced in larger animals? Nash et al26 measured human marrow LTRC concentration using binomial statistics. Following allogeneic transfer of 3.3 × 108 buffy coat marrow cells per kilogram body weight, an average of 116 hematopoietic stem clones of donor origin was observed. Based on the standard 70-kg human, the observed concentration of human marrow LTRCs would be approximately 1 LTRC/200 million marrow cells. For several reasons, the authors emphasized that this value probably represented a low estimate. More recently, Lapidot et al27 showed multipotent engraftment of human cells in severe combined immunodeficient mice following transfer of 20 to 40 million human marrow cells. Abkowitz et al28 transferred limiting numbers of autologous marrow cells into lethally irradiated cats and detected 7 to 11 active clones in recipients receiving 10 to 20 million marrow buffy coat cells per kilogram body weight. The total body mass of the cats was within the range of 2 to 4 kg. Cats receiving 7.5 × 106 marrow cells did not graft. By stochastic analysis,8 the frequency of LTRCs in cat marrow was estimated at 1 LTRC/1.7 million marrow cells. Thus, it is suggested that the marrow HSC frequency across species is inversely proportional to species body mass.
An alternate hypothesis is that the seeding efficiency, f, of stem cells, rather than stem cell numbers, varies inversely with host size. The size of successful grafts, therefore, would decrease with host size. However, for CFU-s there is no species difference between rat and mouse f values when measured in either spleen (f ≈ 0.10-0.20) or bone marrow (f ≈ 0.50).29-31 Further, assaying rat CFU-s numbers in irradiated mice gives the same numbers of 12-day CFU-s as occur when irradiated rats are used as recipients.32 This shows body mass is not an important consideration in calculating seeding efficiencies. Only when f was determined by the extrapolation method33 were differences between mice and rats observed.
In both rats and mice, the seeding efficiency of colony-forming cells to total hematopoietic tissue is the sum of the marrow and splenicf values, which is approximately 0.60 to 0.70. Based on a murine LTRC concentration of 2 to 3 cells/100 000 marrow cells, it can be calculated from e-LTRC data1 that the assay efficiency of murine LTRCs is between 0.47 and 0.96. Given the similarity of rat/mouse CFU-s seeding efficiencies, it is highly unlikely that significant differences in rat/mouse LTRC-assay efficiencies would exist. It would be expected then, that rat LTRC-assay efficiency would also be within this range. This does not appear to be something unique to the order Rodentia because both mice and cats, as measured by transfer assay,8 have the same number of HSCs. Not only are HSC numbers conserved across species, but their nearly unit homing to radiation-depleted hematopoietic tissue also appears to be conserved.
I thank Mr William Jackson for help in statistical analysis of data, Mr Philip Craw for excellent technical assistance, and Mrs Emmeline McCarthy for proofreading this manuscript.
The author is not affiliated with an institution.
Submitted October 3, 2002; accepted November 27, 2002. Prepublished online as Blood First Edition Paper, January 9, 2003; DOI 10.1182/blood-2002-10-3026.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth F. McCarthy, 83 Fort Walker Dr, Hilton Head Island, SC 29928; e-mail: mcneth@aol.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal