Abstract
To measure the ability of human hematopoietic stem cells (HSCs), the SCID-repopulating cell (SRC) assay has been widely used. Conventionally, human HSCs are transplanted into a nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse via a tail vein. However, those cells must go through various obstacles until they reach the mouse marrow environment, which could explain the generally low homing efficiency in this system. Thus, the capability of HSCs may not be studied accurately by this intravenous transplantation method. In our attempt to reveal actual SRC potential, ie, self-renewal and multilineage differentiation in recipient bone marrow, we introduced cells into mouse marrow directly (intrabone marrow [iBM]) to minimize the effect of factors that may interfere with the homing of HSCs and compared the results obtained by intravenous and iBM methods. When cord blood CD34+CD38− cells were transplanted in NOD/SCID mice by iBM, a 15-fold higher frequency of SRC, 1 in 44 CD34+CD38− cells, was achieved compared with 1 in 660 by the intravenous method. Furthermore, the iBM transplant showed high levels of engraftment in the secondary transplantation. Pretreatment of CD34+ cells with antibodies that block either very late antigen 4 (VLA-4) or VLA-5 reduced engraftment partially, whereas blockage of both molecules resulted in complete inhibition of engraftment, which suggests that VLA-4 and VLA-5 are involved in different processes in engraftment or have complementary roles. Our results indicate that the iBM injection strategy is a more sensitive and direct way to measure the capability of human SRCs and is useful to investigate the interaction of HSCs and marrow environment in vivo.
Introduction
Measurement of human hematopoietic stem cell (HSC) activity has been greatly facilitated by the development of xenotransplantation assay. Especially, nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice have proven to be a reliable recipient for detecting human hematopoietic-repopulating cells that differentiate into multilineage mature cells and self-renew in mice.1-3 Human hematopoietic-repopulating cells identified in this assay, operationally defined as SCID-repopulating cells (SRCs), have been shown to be enriched among an extremely rare CD34+CD38− subfraction of lineage-depleted (Lin−/low) cells, and the frequency of SRCs was 1 in 617 Lin−/lowCD34+CD38− cord blood (CB) cells.4
This assay, however, quite possibly underestimates human hematopoietic-repopulating cell frequencies. When candidate human stem cells are transferred by intravenous injection, cells travel into the right atrium, ventricle, and lungs in which most of the cells are trapped, then to the systemic circulation and lodge in organs according to organ blood flow; therefore, only a small fraction of injected cells can lodge in bone marrow (BM).5-8 The marrow seeding efficiency of murine repopulating stem cells was reported around 20%,9-11 and that of human stem cells in the sublethally irradiated NOD/SCID mouse was even lower than in the syngeneic murine situation. van Hennik et al12demonstrated that the seeding efficiencies of human CB CD34+ cells in NOD/SCID mice were 4.4% by week 6 cobblestone area–forming cell (CFAC) assay and 2.3% by flow cytometry analysis. Cashman and Eaves13 reported that the proportion of total injected human CB competitive repopulating units (CRUs) in the marrow of mice was 7%, as determined by limiting-dilution assays in NOD/SCID mice.
To exclude stem cell homing interference (eg, stem cell trapping in lung and/or liver, transendothelial migration step) and focus on the phases of the stem cell and BM stromal cell interaction, we carried out direct injection of human hematopoietic cells into mouse BM (intra-BM [iBM] injection), as previously reported by Kushida et al,14 and compared it with the SRC assay conducted by intravenous injection. IBM injection is revealed to be sensitive and adequate means to measure human HSC capability and enabled us to investigate the interaction of HSCs and marrow environment in vivo directly.
Materials and methods
Collection and purification of human CB CD34+cells
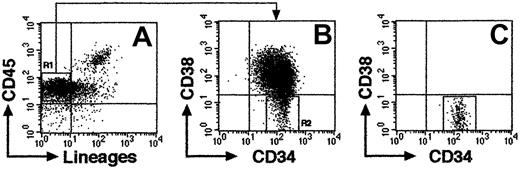
CB samples were obtained from full-term deliveries according to the institutional guidelines approved by Tokai University Committee on Clinical Investigation. Mononuclear cells (MNCs) were isolated by Ficoll-Hypaque (Lymphoprep, 1.077 ± 0.001 g/mL; Nycomed, Oslo, Norway) density gradient centrifugation. Cells were washed and suspended in phosphate-buffered saline (PBS) containing 0.1% of human serum albumin (HSA; Sigma, St Louis, MO). CD34+ cell fractions were prepared from Ficoll-separated MNCs using the CD34 Progenitor Cell Isolation Kit (Miltenyi Biotec, Sunnyvale, CA) according to the manufacturer's directions. For isolation of Lin−/lowCD34+CD38− and CD34+CD38+ cells, CD34+-enriched cells were stained with fluorescein isothiocyanate (FITC)–conjugated antilineage-specific antigens; CD3 (UCHT1), CD41 (P2), glycophorin A (11E4B-7-6) (all Coulter/Immunotech, Marseille Cedex, France), CD4 (SK3), CD7 (4H9), CD14 (MφP9), CD16 (3G8), CD19 (SJ25C1) CD20 (2H7), CD33 (WM53), and CD56 (NCAM16.2) (all BD Biosciences, San Jose, CA), and phycoerythrin (PE)–conjugated anti-CD38 (HB7; BD Biosciences), phycoerythrin–Texas Red (ECD)–conjugated anti-CD34 (581; Coulter/Immunotech), and allophycocyanin (APC)–conjugated anti-CD45 (J.33; Coulter/Immunotech) monoclonal antibodies (mAbs). Cells were sorted using FACSVantage flow cytometer (BD Biosciences) equipped with HeNe and argon lasers. CD38− region was defined as below isotype control. The expression of CD34 and CD38 cell surface antigens on a representative Lin−/low population is shown in Figure 1A-B. Lin−/lowCD34+CD38− cells, which comprise 5% to 8% of total CD34+ population, was isolated with 97% to 99% (n = 16) purity using FACSVantage (Figure1C).
Representative FACS profile of sorted CB Lin−/lowCD34+CD38− cells.
(A) Column-enriched CD34+ cells were stained with anti-CD45 mAb and antilineage-specific mAb cocktail and were gated on lineage marker–negative and/or low expression region (R1). (B) R1 cells were further gated on CD34+CD38− region (R2). (C) Re-analysis of isolated CD34+CD38− cells from R2 (99.8% purity).
Representative FACS profile of sorted CB Lin−/lowCD34+CD38− cells.
(A) Column-enriched CD34+ cells were stained with anti-CD45 mAb and antilineage-specific mAb cocktail and were gated on lineage marker–negative and/or low expression region (R1). (B) R1 cells were further gated on CD34+CD38− region (R2). (C) Re-analysis of isolated CD34+CD38− cells from R2 (99.8% purity).
NOD/SCID mice
Male or female NOD/Shi-scid (NOD/SCID) mice were obtained from CLEA JAPAN (Tokyo, Japan) and were maintained until use in the animal facility of Tokai University School of Medicine in microisolator cages. Mice were fed ad libitum with autoclaved food and water. All experiments were approved by the animal care committee of Tokai University.
Intrabone marrow or intravenous injection of human hematopoietic cells
NOD/SCID mice (7-9 weeks old) were irradiated with 300 cGy X-rays and thereafter received acidified water containing 1.1 g/L neomycin sulfate and 131 mg/L polymyxin B sulfate (Sigma). Within a few hours after irradiation, the mice were injected intravenously or by iBM with human CB Lin−/lowCD34+CD38−cells along with 104 irradiated (15 Gy) CD34+CD38+ cells as carrier cells to support engraftment and expansion of immature CD34+CD38− cells.15,16 IBM injection was carried out as described previously with slight modifications.14 In brief, a 29-gauge needle was inserted into the joint surface of the right tibia of anesthetized mice, and human hematopoietic cells in 40-μL suspension were injected into the BM cavity. For the punctures and injections, 1-mL insulin syringe with fixed 29-gauge needle (Terumo, Tokyo, Japan) was used. The advantage of this needle is the absence of dead space at the connection between the needle and the syringe, which minimizes the loss of samples.
For in vivo blocking experiments, column-enriched whole CD34+ cells (> 95% purity, n = 10) were preincubated with 20 μg/mL antihuman-CXCR4 (12G5; BD Biosciences), antihuman–VLA-4 (HP2/1; Coulter/Immunotech), or antihuman–VLA-5 (SAM-1; Coulter/Immunotech) mAbs for 30 minutes on ice. After washing, 2 × 105 or 2 × 104 CD34+cells were injected into mice intravenously or by iBM, respectively. All blocking antibodies used in this study were nontoxic to human CD34+ cells as determined by colony-forming assays and our stromal cell–dependent culture system as described previously (data not shown).17 For cell surface analysis, column-enriched CD34+ cells were again stained with APC-conjugated antihuman CD34 mAb and with FITC-conjugated antihuman–VLA-4, –VLA-5 mAbs, or PE-conjugated antihuman CXCR4 mAb. Cells labeled with mouse isotype control antibodies were used as negative control. Fluorescence-activated cell sorter (FACS) analysis was performed using FACSCalibur (BD Biosciences).
Tracing of intrabone marrow–injected CD34+cells
Column-enriched CB CD34+ cells (> 95% purity, n = 16), washed once with PBS, were suspended in PKH diluent, and the PKH26 dye (Sigma) at 20 μM was added. Cells were incubated at room temperature for 3 minutes with gentle agitation. Fetal calf serum (FCS; 2 mL) was added to cell suspension to stop the reaction, and then cells were centrifuged and washed twice with alpha-minimal essential media containing 10% FCS. PKH26-stained cells, 106 cells per animal, were injected iBM or intravenously into irradiated NOD/SCID mice. Aliquots of cells were reserved for staining control. At 5 minutes and 20 hours after transplantation, BM samples were aspirated from the human cell noninjected side tibia through the knee joint, as previously described.18 19 Peripheral blood (PB) was aspirated from retroorbital sinus. After washing and hemolysis, samples were suspended in PBS containing propidium-iodide (PI) for flow cytometric analysis using FACSCalibur (BD Biosciences). Dead cells stained with PI were excluded from the analysis.
Flow cytometric analysis
Six to 9 weeks after transplantation, mice were killed, and BM, spleen, and PB were collected for analyzing the presence of human cells by flow cytometry. Human hematopoietic cells were distinguished from mouse cells by the expression of human CD45. BM cells were collected separately from tibia of human cell–injected side and noninjected side and suspended in PBS using a 27-gauge needle. Spleen was teased apart. PB was aspirated from retroorbital sinus. Samples were prepared as single cell suspensions in PBS containing 0.1% HSA and passed through a nylon filter to remove debris. Cells were stained with mAbs to human leukocyte differentiation antigens. FITC-conjugated antihuman CD34 (Coulter/Immunotech), CD14, CD19, CD33, CD56 (all BD Biosciences) mAbs, PE-conjugated antihuman CD38 (BD Biosciences), and APC-conjugated antihuman CD45 mAbs (Coulter/Immunotech) were used. Four-color flow cytometric analysis was conducted using FACSCalibur or FACSVantage (BD Biosciences). Quadrants were set to include at least 97% of the isotype-negative cells. Dead cells stained with PI were excluded from the analysis.
Analysis of human cell engraftment in mice receiving transplants by polymerase chain reaction (PCR)
Genomic DNA was isolated from the BM of mice receiving transplants by standard extraction protocols. DNA samples (100 ng) were subjected to PCR to detect a 1171-bp fragment of human chromosome 17-specific α-satellite using the primers: forward 5′-ACACTCTTTTTGCAGGATCTA-3′ and reverse 5′-AGCAATGTGAAACTCTGGGA-3′ under the following conditions: 94°C for 2 minutes (1 cycle); 94°C for 1 minute, 65°C for 1 minute, 72°C for 2 minutes (30 cycles); and 72°C for 7 minutes (1 cycle). PCR was performed using the RNA PCR kit (TAKARA SHUZO, Tokyo, Japan). PCR products were separated on 1.0% agarose gel and visualized by ethidium bromide staining. The level of human cell engraftment was determined by comparing the characteristic 1171-bp PCR product with that of human/mouse DNA mixture control (detection limit of 0.001% human DNA).
Analysis of the integration site of lentivirally marked CD34+ cells
Transduction of enhanced green fluorescent protein (EGFP) encoded gene to CD34+ cells by recombinant lentivirus infection was carried out as described previously.20Briefly, CB CD34+ cells were prestimulated by incubating in StemPro-34 medium (Invitrogen, Carlsbad, CA) containing cytokines at 37°C in 5% CO2 for 24 hours. Recombinant human thrombopoietin (TPO; 50 ng/mL; kindly donated from Kirin Brewery, Tokyo, Japan), stem cell factor (SCF; 50 ng/mL; donated from Kirin Brewery), and Flk-2/Flt-3 ligand (FL; 50 ng/mL; R&D Systems, Minneapolis, MN) were used. Prestimulated CD34+ cells were cultured for 12 hours under the same conditions in the presence of highly concentrated lentiviral supernatant at an MOI (multiplicity of infection) of 500. Lentiviral-infected CD34+ cells were transplanted into irradiated NOD/SCID mice by iBM injection. Genomic DNA was extracted from the injected side tibia, noninjected side tibia, and spleen of mice receiving transplants at 8 weeks after transplantation. DNA (10 μg) was digested with EcoRI, which recognizes a unique site in viral genome, and was electrophoresed on 0.7% agarose gel. After transferring to nylon membranes, DNA was hybridized with 32P-labeled random-primed EGFP probe.
Secondary transplantation
BM cells were obtained from tibiae and femurs of highly engrafted primary mice that received iBM transplants (23%-87% chimerism of human CD45+ cells, n = 10) at 6 weeks after transplantation, and cells were injected intravenously or by iBM into irradiated secondary NOD/SCID recipients. Six weeks after transplantation, presence of transplanted human cells in recipient tibiae was analyzed by flow cytometry, as was described for primary recipients.
Statistical analysis
Engraftment was determined positive when more than 0.01% human CD45+ cells in FACS or 0.01% human satellite PCR products in PCR were detected. For limiting dilution analysis, results of mice that received transplants scored positive by both methods were used. The data from several limiting dilution experiments were combined and used for analysis. The frequency of SRCs in the test BM sample was calculated by applying Poisson statistics to the proportion of negative recipients at different dilutions using L-Calc software (StemCell Technologies, Vancouver, BC, Canada). Data are represented as mean ± SD. The 2-sided P value was determined, testing the null hypothesis that the 2 population medians are equal.P < .05 was considered significant.
Results
Tracking intravenous or iBM transplanted human primitive hematopoietic cells
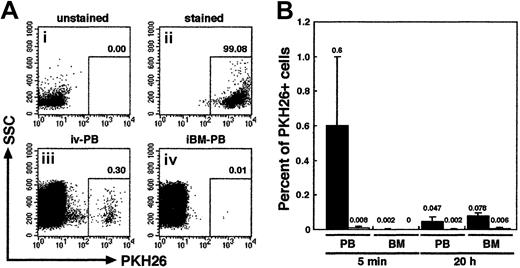
We first examined the distribution of infused human primitive hematopoietic cells. PKH26-stained CB CD34+ cells (106 cells per mouse) were transplanted to irradiated NOD/SCID mice by direct injection into a BM cavity (iBM) or conventional tail vein infusion (intravenous). PB from retroorbital sinus and BM cells from noninjected side tibia were aspirated from each animal at 5 minutes and 20 hours after transplantation. The donor cells were detected by PKH26 fluorescence using FACS analysis (Figure2A). As expected, PKH26+donor cells appeared in the blood stream of intravenously injected animals at 5 minutes (0.6% ± 0.39%) and, albeit fewer, at 20 hours after transplantation (0.047% ± 0.025%). However, in the BM, intravenously injected donor cells could be detected only at 20 hours after transplantation (0.078% ± 0.016%) (Figure 2B). Because one tibia contains approximately 5% of the total BM cellularity of a mouse,21 a calculated seeding efficiency of intravenously injected PKH26+ donor cells at 20 hours after transplantation is 1.56% ± 0.32% and is consistent with a previous report by van Hennik et al12 However, in mice receiving iBM transplants, very few donor cells were present in the blood stream at both time points analyzed: 5 minutes (0.008% ± 0.008%), 20 hours (0.002% ± 0.004%). In addition, none, at 5 minutes, or very few, 0.006% ± 0.005% at 20 hours, of donor cells were detected in the noninjected side tibia (Figure 2B). The levels of iBM-transplanted PKH26+ cells in PB and left (noninjected side) BM were significantly lower than those of intravenously transplanted cells on either time points (P < .01). The results confirmed that there was little, if any, leakage of the injected cells when iBM strategy was used.
Tracing of intravenously or iBM-injected PKH26-labeled CB CD34+ cells.
(A) Representative FACS analysis of PKH26-labeled cells. Column-enriched CB CD34+ cells (i) were stained with PKH26 (ii) and were transplanted into irradiated NOD/SCID mice by intravenous (iii) or iBM (iv) injection. Five minutes later, PKH26+cells circulating into peripheral blood stream were identified by flow cytometry. The number at each panel represents the percentage of PKH26 bright cells detected. (B) Comparison of the percentage of PKH26+ cells detected in PB and BM from intravenous (filled bars; ▪) or iBM (open bars; ■) injected NOD/SCID mice at 5 minutes and 20 hours after transplantation. BM cells were aspirated from left (noninjected side) tibia. Fifty thousand events were acquired to calculate the proportion of PKH26+ cells. Data shown are the mean ± SD values of 3 independent experiments (n = 5). The number above each bar represents the mean percentage of PKH26+ cells.
Tracing of intravenously or iBM-injected PKH26-labeled CB CD34+ cells.
(A) Representative FACS analysis of PKH26-labeled cells. Column-enriched CB CD34+ cells (i) were stained with PKH26 (ii) and were transplanted into irradiated NOD/SCID mice by intravenous (iii) or iBM (iv) injection. Five minutes later, PKH26+cells circulating into peripheral blood stream were identified by flow cytometry. The number at each panel represents the percentage of PKH26 bright cells detected. (B) Comparison of the percentage of PKH26+ cells detected in PB and BM from intravenous (filled bars; ▪) or iBM (open bars; ■) injected NOD/SCID mice at 5 minutes and 20 hours after transplantation. BM cells were aspirated from left (noninjected side) tibia. Fifty thousand events were acquired to calculate the proportion of PKH26+ cells. Data shown are the mean ± SD values of 3 independent experiments (n = 5). The number above each bar represents the mean percentage of PKH26+ cells.
IBM injection of CB Lin−/lowCD34+CD38− cells resulted in the higher frequency of engrafting human hematopoietic cells in NOD/SCID mice
No or very little leakage of injected cells in iBM method may result in more efficient engraftment than intravenous transplantation. To compare the frequency of CB Lin−/lowCD34+CD38− cells capable of engrafting NOD/SCID mice, cells were transplanted by iBM or intravenous injection for limiting dilution assay. In the human-specific PCR-based method, mice were scored positive for engraftment when more than 0.01% human DNA was detected in the murine BM (detection limit of human DNA is 0.001%) (Figure3C). In FACS analysis, more than 0.01% of human CD45+ dots was considered positive. Statistical analysis was performed using data from 84 recipients receiving transplants of pooled CB from multiple donors (Figure 3; Table1), and the frequency of human cells capable of engraftment was calculated as described.4,22 As determined by FACS and human DNA measurements, the frequency of CB Lin−/lowCD34+CD38− cells capable of engrafting NOD/SCID mice was 1 in 44 by iBM injection (95% confidence intervals, 1 in 27 to 1 in 70). In contrast, the frequency by intravenous injection (95% confidence intervals, 1 in 289 to 1 in 1510) was 1 in 660 Lin−/lowCD34+CD38− cells, which is consistent with the previous report by Bhatia et al.4By using the iBM transplantation method, more than 15-fold higher frequency of human SRCs could be detected in NOD/SCID mice.
Summary of human cell engraftment levels in the BM of NOD/SCID mice.
(A-B) NOD/SCID mice were given transplants of CB Lin−/lowCD34+CD38− cell fractions by iBM (●) (A) or intravenous (○) (B). Eight weeks after transplantation, BM cells obtained from NOD/SCID mice were analyzed by FACS and PCR. Numbers indicate the dose of Lin−/lowCD34+CD38− cells transplanted. Number “0” means mice were given transplants of 104 irradiated CD34+CD38+ carrier cells alone. Each dot represents 1 mouse, and bars indicate the average levels of engraftment. The horizontal lines indicate threshold of positive engraftment. (C) Representative PCR analysis of individual NOD/SCID mice given transplants of CB Lin−/lowCD34+CD38− cells by iBM (lanes 1-5 indicate 100 cells injected, lanes 6-10 indicate 10 cells injected). DNA was extracted from the BM cells 8 weeks after transplantations and amplified using a human chromosome 17 α-satellite specific primer. Representative PCR analysis of 5 independent experiments is shown. M, size marker; P, 100% human DNA; Mo, 100% mouse DNA; N, distilled water (DW).
Summary of human cell engraftment levels in the BM of NOD/SCID mice.
(A-B) NOD/SCID mice were given transplants of CB Lin−/lowCD34+CD38− cell fractions by iBM (●) (A) or intravenous (○) (B). Eight weeks after transplantation, BM cells obtained from NOD/SCID mice were analyzed by FACS and PCR. Numbers indicate the dose of Lin−/lowCD34+CD38− cells transplanted. Number “0” means mice were given transplants of 104 irradiated CD34+CD38+ carrier cells alone. Each dot represents 1 mouse, and bars indicate the average levels of engraftment. The horizontal lines indicate threshold of positive engraftment. (C) Representative PCR analysis of individual NOD/SCID mice given transplants of CB Lin−/lowCD34+CD38− cells by iBM (lanes 1-5 indicate 100 cells injected, lanes 6-10 indicate 10 cells injected). DNA was extracted from the BM cells 8 weeks after transplantations and amplified using a human chromosome 17 α-satellite specific primer. Representative PCR analysis of 5 independent experiments is shown. M, size marker; P, 100% human DNA; Mo, 100% mouse DNA; N, distilled water (DW).
Comparison of the frequency of SRCs by limiting dilution assay
| Route . | No. cells injected . | No. engrafted mice . | No. transplantations . |
|---|---|---|---|
| iBM | 10 000 | 2 | 2 |
| 1 000 | 6 | 6 | |
| 500 | 4 | 4 | |
| 250 | 5 | 5 | |
| 100 | 8 | 10 | |
| 50 | 7 | 12 | |
| 10 | 5 | 10 | |
| 5 | 2 | 9 | |
| Intravenous | 10 000 | 2 | 2 |
| 5 000 | 5 | 5 | |
| 2 000 | 3 | 4 | |
| 500 | 3 | 5 | |
| 100 | 2 | 5 | |
| 50 | 1 | 5 |
| Route . | No. cells injected . | No. engrafted mice . | No. transplantations . |
|---|---|---|---|
| iBM | 10 000 | 2 | 2 |
| 1 000 | 6 | 6 | |
| 500 | 4 | 4 | |
| 250 | 5 | 5 | |
| 100 | 8 | 10 | |
| 50 | 7 | 12 | |
| 10 | 5 | 10 | |
| 5 | 2 | 9 | |
| Intravenous | 10 000 | 2 | 2 |
| 5 000 | 5 | 5 | |
| 2 000 | 3 | 4 | |
| 500 | 3 | 5 | |
| 100 | 2 | 5 | |
| 50 | 1 | 5 |
NOD/SCID mice (n = 84) were transplanted with various numbers of Lin−/low CD34+CD38− CB cells by iBM or intravenous injection. Engraftment in murine BM was analyzed at 8 weeks. Mice were considered to be engrafted when they were determined positive by both FACS and PCR analysis. The frequency of engrafting cells in CB Lin−/lowCD34+CD38− cells transplanted by iBM and intravenously was found to be 1 in 44 Lin−/lowCD34+CD38− cells (95% confidence limits, 1 in 27 to 1 in 70) and 1 in 660 Lin−/lowCD34+CD38− cells (95% confidence limits, 1 in 289 to 1 in 1510), respectively. SRC frequencies were calculated using Poisson statistics and the method of maximum likelihood with the assistance of the L-Calc software (StemCell Technologies).
Higher levels of human hematopoietic cell engraftment by iBM injection
The level of hematopoietic repopulation is a critical parameter in stem cell transplantation. Therefore, we compared the engraftment levels of mice that received transplants in both injection strategies using the same number of Lin−/lowCD34+CD38− cells. Transplantation of CB Lin−/lowCD34+CD38− cells into NOD/SCID mice by iBM injection achieved higher levels of engraftment than by intravenous injection in NOD/SCID mice (Figure 3; injected cell number, 500 cells, P <.04; 100 cells,P < .03; 50 cells, P < .05). Furthermore, the engraftment level of mice injected with 1000 cells by iBM was significantly higher than those injected intravenously with 2000 or 5000 cells (2000 cells, P < .02; 5000 cells,P < .05).
Multilineage differentiation and distribution of human hematopoietic cells in NOD/SCID mice that received transplants by iBM injection
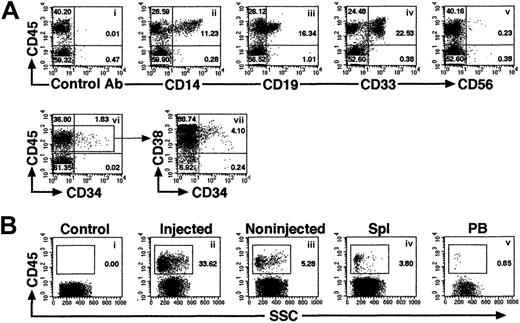
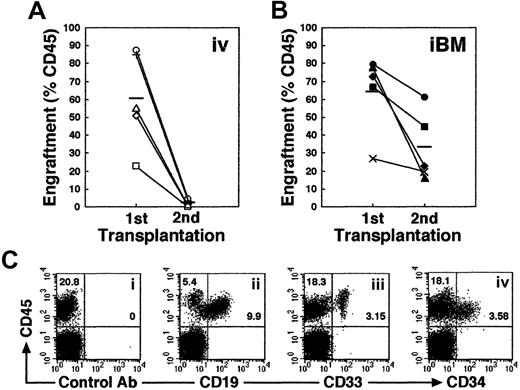
Multilineage differentiation of iBM-injected SRCs was observed in the injected side BM of NOD/SCID mice that received transplants, which included lymphoid CD45+CD19+ B cells, CD45+CD56+ natural killer (NK) cells (Figure 4Aiii,v), and myeloid CD45+CD14+, CD45+CD33+cells (Figure 4Aii,iv). In addition, presence of primitive Lin−/lowCD34+CD38− cells were evident in the marrow of these mice (Figure 4Avi,vii). To examine the distribution of reconstituted human hematopoietic cells in iBM or intravenously injected NOD/SCID mice, BM cells were collected separately from injected (right leg) and noninjected (left leg) side of tibia. Lymphoid, myeloid, and stem/progenitor cell lineages are defined as CD19+/CD45+ cells, CD33+/CD45+ cells, and CD34+/CD45+ cells, respectively. As shown in Table 2, human hematopoietic cells with the ability of lymphomyeloid differentiation were detected not only in the injected side tibia but also in the noninjected side tibia, spleen, and PB in all animals analyzed (Figure 4B; Tables2-3). Multilineage differentiation was also detected in mice with low chimerism of human cell (Table 2, mouse iBM-injected mice 1, 8, 9). In the spleen, lymphoid cells were 85.64% ± 6.31% (iBM) and 90.65% ± 5.91% (intravenous), and myeloid cells were 1.85% ± 1.13% (iBM) and 2.064% ± 1.59% (intravenous). In the PB, lymphoid cells were 71.19% ± 6.23% (iBM) and 74.84% ± 14.51% (intravenous), and myeloid cells were 4.16% ± 2.63% (iBM) and 8.21% ± 4.22% (intravenous) (data not shown). Although 10 times more CD34+ cells were transplanted by intravenous injection (2 × 105 cells), the levels of engraftment were the same as the cases of iBM injection (2 × 104 cells) (Table 2; iBM injected right tibia, 45.51% ± 26.96% versus intravenously injected BM, 27.98% ± 13.56% [mean percentage of sum of 2 legs],P = .14). Human hematopoietic cells were also detected in noninjected side tibia, spleen, and PB of Lin−/lowCD34+CD38− cells transferred to NOD/SCID mice (Table 3). As few as 5 Lin−/lowCD34+CD38− cells successfully engrafted in the noninjected side tibia (data not shown). These results suggested that the engrafted human hematopoietic cells in the injected side BM migrate to a noninjected BM by blood flow circulation and differentiate into multilineage cells even when the human hematopoietic cell chimerism showed low level.
Distribution of human hematopoietic cells in iBM or intravenously injected NOD/SCID mice
| Mouse . | Percentage of human CD45+cells . | Lymphoid . | Myeloid . | Stem/progenitor . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Right . | Left . | Spl . | PB . | Right . | Left . | Right . | Left . | Right . | Left . | |
| iBM | ||||||||||
| 1 | 44.21 | 0.60 | 2.46 | ND | 67.13 | 63.26 | 6.96 | 18.68 | 28.94 | 13.98 |
| 2 | 15.83 | 12.00 | 0.85 | ND | 82.82 | 82.79 | 3.64 | 9.54 | 19.62 | 6.09 |
| 3 | 34.85 | 77.90 | 34.65 | ND | 69.43 | 59.60 | 4.39 | 5.57 | 20.66 | 29.92 |
| 4 | 55.73 | 50.43 | 31.92 | ND | 62.21 | 68.69 | 7.22 | 8.96 | 19.86 | 22.83 |
| 5 | 17.57 | 4.76 | 2.42 | ND | 69.35 | 70.87 | 8.62 | 16.00 | 26.59 | 22.68 |
| 6 | 77.81 | 84.74 | 41.83 | 14.48 | 65.16 | 84.50 | 31.78 | 8.53 | 13.29 | 22.75 |
| 7 | 96.07 | 34.23 | 34.15 | 11.67 | 67.21 | 63.01 | 14.64 | 13.89 | 24.83 | 21.56 |
| 8 | 28.40 | 1.84 | 4.33 | 0.97 | 57.12 | 62.66 | 12.41 | 10.76 | 12.84 | 10.89 |
| 9 | 39.11 | 1.87 | 4.04 | 2.05 | 70.45 | 68.73 | 4.71 | 5.96 | 22.37 | 28.04 |
| Mean (± SD) | 45.51 (26.96) | 29.82 (33.79) | 17.41 (17.52) | 7.29 (6.79) | 67.88 (6.99) | 69.35 (8.88) | 10.49 (8.79) | 10.88 (4.47) | 21.00 (5.48) | 19.86 (7.91) |
| Intravenous | ||||||||||
| 1 | 49.10 | 52.46 | 39.18 | 8.19 | 74.02 | 75.04 | 14.40 | 11.50 | 12.71 | 19.36 |
| 2 | 32.29 | 21.77 | 3.64 | 1.97 | 86.51 | 85.86 | 4.08 | 5.07 | 6.00 | 9.03 |
| 3 | 42.37 | 39.48 | 18.35 | 5.52 | 69.54 | 71.22 | 6.99 | 9.32 | 11.59 | 14.02 |
| 4 | 22.69 | 16.63 | 9.20 | 2.01 | 86.32 | 73.07 | 10.38 | 12.67 | 19.31 | 21.79 |
| 5 | 28.16 | 22.76 | 21.16 | 4.85 | 80.36 | 79.76 | 13.65 | 15.07 | 16.88 | 14.22 |
| 6 | 19.67 | 22.54 | 7.55 | 1.36 | 68.41 | 65.59 | 7.98 | 11.65 | 19.28 | 16.71 |
| 7 | 12.90 | 8.92 | 6.50 | 1.74 | 62.05 | 95.64 | 30.65 | 3.18 | 11.18 | 9.29 |
| Mean (± SD) | 29.6 (12.77) | 26.37 (14.72) | 15.08 (12.41) | 3.66 (2.58) | 75.32 (9.40) | 78.03 (10.08) | 12.59 (8.76) | 9.78 (4.26) | 13.85 (4.89) | 14.92 (4.79) |
| Mouse . | Percentage of human CD45+cells . | Lymphoid . | Myeloid . | Stem/progenitor . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Right . | Left . | Spl . | PB . | Right . | Left . | Right . | Left . | Right . | Left . | |
| iBM | ||||||||||
| 1 | 44.21 | 0.60 | 2.46 | ND | 67.13 | 63.26 | 6.96 | 18.68 | 28.94 | 13.98 |
| 2 | 15.83 | 12.00 | 0.85 | ND | 82.82 | 82.79 | 3.64 | 9.54 | 19.62 | 6.09 |
| 3 | 34.85 | 77.90 | 34.65 | ND | 69.43 | 59.60 | 4.39 | 5.57 | 20.66 | 29.92 |
| 4 | 55.73 | 50.43 | 31.92 | ND | 62.21 | 68.69 | 7.22 | 8.96 | 19.86 | 22.83 |
| 5 | 17.57 | 4.76 | 2.42 | ND | 69.35 | 70.87 | 8.62 | 16.00 | 26.59 | 22.68 |
| 6 | 77.81 | 84.74 | 41.83 | 14.48 | 65.16 | 84.50 | 31.78 | 8.53 | 13.29 | 22.75 |
| 7 | 96.07 | 34.23 | 34.15 | 11.67 | 67.21 | 63.01 | 14.64 | 13.89 | 24.83 | 21.56 |
| 8 | 28.40 | 1.84 | 4.33 | 0.97 | 57.12 | 62.66 | 12.41 | 10.76 | 12.84 | 10.89 |
| 9 | 39.11 | 1.87 | 4.04 | 2.05 | 70.45 | 68.73 | 4.71 | 5.96 | 22.37 | 28.04 |
| Mean (± SD) | 45.51 (26.96) | 29.82 (33.79) | 17.41 (17.52) | 7.29 (6.79) | 67.88 (6.99) | 69.35 (8.88) | 10.49 (8.79) | 10.88 (4.47) | 21.00 (5.48) | 19.86 (7.91) |
| Intravenous | ||||||||||
| 1 | 49.10 | 52.46 | 39.18 | 8.19 | 74.02 | 75.04 | 14.40 | 11.50 | 12.71 | 19.36 |
| 2 | 32.29 | 21.77 | 3.64 | 1.97 | 86.51 | 85.86 | 4.08 | 5.07 | 6.00 | 9.03 |
| 3 | 42.37 | 39.48 | 18.35 | 5.52 | 69.54 | 71.22 | 6.99 | 9.32 | 11.59 | 14.02 |
| 4 | 22.69 | 16.63 | 9.20 | 2.01 | 86.32 | 73.07 | 10.38 | 12.67 | 19.31 | 21.79 |
| 5 | 28.16 | 22.76 | 21.16 | 4.85 | 80.36 | 79.76 | 13.65 | 15.07 | 16.88 | 14.22 |
| 6 | 19.67 | 22.54 | 7.55 | 1.36 | 68.41 | 65.59 | 7.98 | 11.65 | 19.28 | 16.71 |
| 7 | 12.90 | 8.92 | 6.50 | 1.74 | 62.05 | 95.64 | 30.65 | 3.18 | 11.18 | 9.29 |
| Mean (± SD) | 29.6 (12.77) | 26.37 (14.72) | 15.08 (12.41) | 3.66 (2.58) | 75.32 (9.40) | 78.03 (10.08) | 12.59 (8.76) | 9.78 (4.26) | 13.85 (4.89) | 14.92 (4.79) |
BM cells, spleen cells, and PB of NOD/SCID mice 6 to 9 weeks after iBM or intravenous transplantation of column-enriched but unsorted whole CD34+ cell populations (20 000 cell for iBM and 200 000 cells for intravenous) were stained with antihuman CD45 mAbs and analyzed. BM cells were collected separately from injected (right leg) and noninjected (left leg) side tibia. Lymphoid, myeloid, and stem/progenitor cell lineages are defined as CD19+/CD45+ cells, CD33+/CD45+ cells, and CD34+/CD45+ cells, respectively, and each number indicates the percentage of human CD45+ cells expressing respective surface marker. The proportion of each lineage was calculated from 50 000 to 100 000 events acquired using CELLQuest software.
ND indicates not determined.
Representative FACS profile of human multilineage engraftment in a NOD/SCID recipient given transplants of Lin−/lowCD34+CD38− cells by iBM.
(A) At 8 weeks after transplantation of 500 Lin−/lowCD34+CD38− human CB cells, BM cells were removed from iBM-injected side tibia of a NOD/SCID mouse and analyzed for the presence of human CD45+ cells (i). Human lineage–specific mAbs were used to detect lymphoid CD45+CD19+ (iii), CD45+CD56+ (v), myeloid CD45+CD14+ and CD45+CD33+ (ii,iv), and immature CD34+CD38−/low (vi,vii) progenitor cells in the marrow of engrafted NOD/SCID mice. (B) Distribution of human hematopoietic cells in iBM-injected NOD/SCID mice. Eight weeks after transplantation, BM cells (ii,iii), spleen cells (iv), and PB (v) of NOD/SCID mice were stained with antihuman CD45 mAbs and analyzed by flow cytometry. BM cells were collected separately from injected (ii) and noninjected (iii) tibiae. BM cells that were injected with irradiated CD34+CD38+ (used as carrier cells in these experiments) alone were used as negative control (i). The relative frequencies of each population are indicated. Representative FACS analysis of 5 independent experiments is shown.
Representative FACS profile of human multilineage engraftment in a NOD/SCID recipient given transplants of Lin−/lowCD34+CD38− cells by iBM.
(A) At 8 weeks after transplantation of 500 Lin−/lowCD34+CD38− human CB cells, BM cells were removed from iBM-injected side tibia of a NOD/SCID mouse and analyzed for the presence of human CD45+ cells (i). Human lineage–specific mAbs were used to detect lymphoid CD45+CD19+ (iii), CD45+CD56+ (v), myeloid CD45+CD14+ and CD45+CD33+ (ii,iv), and immature CD34+CD38−/low (vi,vii) progenitor cells in the marrow of engrafted NOD/SCID mice. (B) Distribution of human hematopoietic cells in iBM-injected NOD/SCID mice. Eight weeks after transplantation, BM cells (ii,iii), spleen cells (iv), and PB (v) of NOD/SCID mice were stained with antihuman CD45 mAbs and analyzed by flow cytometry. BM cells were collected separately from injected (ii) and noninjected (iii) tibiae. BM cells that were injected with irradiated CD34+CD38+ (used as carrier cells in these experiments) alone were used as negative control (i). The relative frequencies of each population are indicated. Representative FACS analysis of 5 independent experiments is shown.
Distribution of human hematopoietic cells (CD34+CD38−) in iBM-injected NOD/SCID mice
| No. cells . | Percentage of human CD45+ cells . | |||
|---|---|---|---|---|
| Injected tibia . | Noninjected tibia . | Spleen . | PB . | |
| 10 000 | 86.13 | 57.91 | 64.68 | 27.86 |
| 1 000 | 33.62 | 5.28 | 3.80 | 0.85 |
| 500 | 47.94 | 1.28 | 2.44 | ND |
| 250 | 5.00 | 0.10 | 0.10 | 0.04 |
| 100 | 5.58 | 0.12 | 0.35 | 0.02 |
| No. cells . | Percentage of human CD45+ cells . | |||
|---|---|---|---|---|
| Injected tibia . | Noninjected tibia . | Spleen . | PB . | |
| 10 000 | 86.13 | 57.91 | 64.68 | 27.86 |
| 1 000 | 33.62 | 5.28 | 3.80 | 0.85 |
| 500 | 47.94 | 1.28 | 2.44 | ND |
| 250 | 5.00 | 0.10 | 0.10 | 0.04 |
| 100 | 5.58 | 0.12 | 0.35 | 0.02 |
BM cells, spleen cells, and PB of NOD/SCID mice 8 weeks after transplantation of CB Lin−/lowCD34+CD38− cells by iBM were stained with antihuman CD45 mAbs and analyzed. BM cells were collected separately from tibiae of injected and noninjected side.
ND indicates not determined.
Clonal analysis of engrafted human hematopoietic cells transplanted by iBM injection
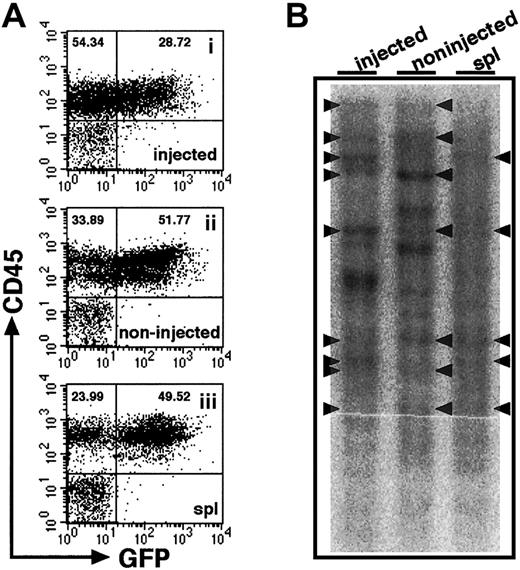
Relatively high frequencies of human hematopoietic cells found in noninjected BM might suggest that cells entering the blood stream because of the pressure applied in iBM procedure or release after engraftment in injected side tibia. Although we found little evidence of leakage (Figure 2), we proceeded in examining the clonalities of cells present in different hematopoietic organs. Retroviral gene marking provides the ideal tool for studies of clonal analysis because they randomly and permanently integrate into the genome of the host cell. Thus, each genomic integration site is a distinct clonal marker that can be used to trace the progeny of individual stem cells after transplantation.19 23-25 Therefore, we transduced theEGFP gene to CB CD34+ cells by lentiviral infection and transplanted them into NOD/SCID mice by iBM injection. Eight weeks after transplantation, BM and spleen were isolated and analyzed by flow cytometry. BM cells were isolated separately from tibiae of injected side and noninjected side. High frequencies of EGFP+ cells were demonstrated in the engrafted mice (61.16% ± 23.99%, n = 4) (Figure5A). As shown in Figure 5B, common clones were evident among the injected side BM and other hematopoietic organs. The results suggest that HSCs, directly injected into BM, engraft in the marrow environment and migrate to other hematopoietic organs by mobilization through a systemic circulation.
Clonal analysis of iBM-injected SRCs.
(A) The proportion of EGFP+ human cells was analyzed at 8 weeks after iBM transplantation. Samples were obtained from injected side tibia (i), noninjected side tibia (ii), and spleen (iii). Cells were stained with antihuman CD45 mAb and analyzed by flow cytometry. Representative FACS profiles are shown from 4 independent experiments. The relative frequencies of each population are indicated. (B) Southern blot analysis for lentivirus integration sites. Genomic DNA extracted from samples indicated earlier was digested with EcoRI, which recognizes a unique site in lentivirus vector, and was hybridized with an EGFP probe. Each band represents a unique lentiviral integration site, and it corresponds to each clone. Arrowheads indicate common integration sites. Representative Southern blot analysis of 2 independent experiments is shown.
Clonal analysis of iBM-injected SRCs.
(A) The proportion of EGFP+ human cells was analyzed at 8 weeks after iBM transplantation. Samples were obtained from injected side tibia (i), noninjected side tibia (ii), and spleen (iii). Cells were stained with antihuman CD45 mAb and analyzed by flow cytometry. Representative FACS profiles are shown from 4 independent experiments. The relative frequencies of each population are indicated. (B) Southern blot analysis for lentivirus integration sites. Genomic DNA extracted from samples indicated earlier was digested with EcoRI, which recognizes a unique site in lentivirus vector, and was hybridized with an EGFP probe. Each band represents a unique lentiviral integration site, and it corresponds to each clone. Arrowheads indicate common integration sites. Representative Southern blot analysis of 2 independent experiments is shown.
Higher engraftment in secondary NOD/SCID mice that received transplants by iBM injection
Self-renewal of hematopoietic stem cells can be assessed by serial transplantation in SRC assay. A theory behind this assay is that stem cells which give rise to multilineage hematopoiesis in primary recipients are also capable of repeating this process in recipients of secondary transplants. The proportion of human cells in the secondary mice, however, is usually more than 10 times lower than in the primary mice. Consistent with previous studies, secondary mice that received intravenous transplants showed low levels of engraftment (Figure 6A). In contrast, engraftment levels in secondary recipients were significantly higher when they received transplants by iBM (Figure 6B; the reduction rate in chimerism from first to second recipients is 0.031 ± 0.018 in intravenous versus 0.546 ± 0.268 in iBM; P < .01). Thus, human cells recovered from BM of primary NOD/SCID mice that received iBM transplants possessed sufficient ability for consecutive multilineage engraftment in secondary recipients (Figure 6C), suggesting that human HSCs transplanted by iBM injection can self-renew in murine BM.
Secondary transplantation.
(A-B) Whole human BM cells obtained from each primary recipient mouse given an iBM transplant (n = 10) were transplanted to a secondary recipient mouse by intravenous injection (n = 5) (A) or iBM injection (n = 5) (B). Secondary recipient mouse BM cells were analyzed for the expressions of human CD45 at 6 weeks after transplantation. Each symbol represents 1 mouse, and bars indicate the average engraftment level in 3 independent experiments. (C) Representative FACS analysis of a NOD/SCID mouse that received a secondary transplant. Human CD45+ cells in BM were analyzed for the expression of CD19 (ii), CD33 (iii), and CD34 (iv). The relative frequencies of each population are indicated.
Secondary transplantation.
(A-B) Whole human BM cells obtained from each primary recipient mouse given an iBM transplant (n = 10) were transplanted to a secondary recipient mouse by intravenous injection (n = 5) (A) or iBM injection (n = 5) (B). Secondary recipient mouse BM cells were analyzed for the expressions of human CD45 at 6 weeks after transplantation. Each symbol represents 1 mouse, and bars indicate the average engraftment level in 3 independent experiments. (C) Representative FACS analysis of a NOD/SCID mouse that received a secondary transplant. Human CD45+ cells in BM were analyzed for the expression of CD19 (ii), CD33 (iii), and CD34 (iv). The relative frequencies of each population are indicated.
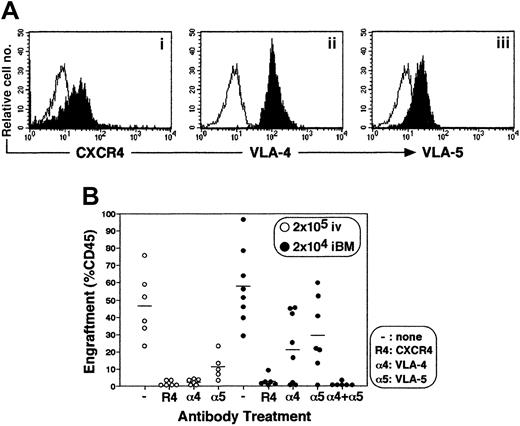
Engraftment of CD34+ cells by iBM injection is dependent on CXCR4, VLA-4, and VLA-5
Stem cell engraftment involves multistep processes, including activation of specific adhesion molecules. A number of studies have reported crucial roles of VLA-4, VLA-5, and other molecules in interaction of HSCs and microenvironment during homing and engraftment processes (reviewed in Prosper and Verfaillie26 and in Lapidot27). Up-regulation of these molecules through SDF-1–CXCR4 (stromal cell–derived factor 1 and CXC chemokine receptor 4) interaction is reported to increase homing and engraftment of primitive SRCs.27-29 Primitive human CB CD34+cells express a chemokine receptor CXCR4 and major β1 integrin VLA-4 and VLA-5 (Figure 7A). The iBM transplantation offers the opportunity to investigate the interaction between stem cells and BM stromal cells in vivo by eliminating the processes before entering marrow environment. To determine the in vivo role of CXCR4, VLA-4, and VLA-5 during the engraftment process of human SRCs, enriched CB CD34+ cells were pretreated with antibodies against the cell surface molecules mentioned and then transplanted. When CB CD34+ cells were transplanted intravenously, neutralizing antibodies to CXCR4, VLA-4, and VLA-5 completely blocked BM engraftment as described previously (Figure 7, indicated as open circles, P < .01),27-29whereas neutralizing antibodies to either VLA-4 or VLA-5 caused only partial inhibition of engraftment in iBM transplants (Figure 7, indicated as filled circles, P < .05). However, when CD34+ cells were pretreated with anti-CXCR4 or with both anti–VLA-4 and anti–VLA-5 antibodies, human SRCs engraftment was blocked completely (Figure 7, indicated as filled circles,P < .01).
Effect of antibodies to CXCR4 and β1 integrins on engraftment of cord blood CD34+ cells in NOD/SCID mice BM.
(A) Expression of CXCR4 (i), VLA-4 (ii), and VLA-5 (iii) on enriched CD34+ cells. Expression levels of CXCR4, VLA-4, and VLA-5 on gated CD34+ population are shown. A representative FACS analysis of 3 independent experiments is shown. The white histogram indicates negative control staining with isotype control antibody; the black histogram indicates CXCR4 (i), VLA-4 (ii), and VLA-5 (iii). (B) Percentage of engraftment in murine BM by CB CD34+ cells pretreated with antibodies to either CXCR4 (R4), VLA-4 (α4), VLA-5 (α5), or VLA-4 + VLA-5 (α4 + α5) was quantified at 6 weeks after transplantation by immunostaining with antihuman CD45 mAb. Open circles (○) represent the mouse that received a transplant of 2 × 105 of CD34+ cells intravenously. Filled circles (●) represent the mouse that received a transplant of 2 × 104 CD34+ cells by iBM. Each circle represents 1 mouse, and bars indicate the average of engraftment. Results were combined from 3 independent experiments.
Effect of antibodies to CXCR4 and β1 integrins on engraftment of cord blood CD34+ cells in NOD/SCID mice BM.
(A) Expression of CXCR4 (i), VLA-4 (ii), and VLA-5 (iii) on enriched CD34+ cells. Expression levels of CXCR4, VLA-4, and VLA-5 on gated CD34+ population are shown. A representative FACS analysis of 3 independent experiments is shown. The white histogram indicates negative control staining with isotype control antibody; the black histogram indicates CXCR4 (i), VLA-4 (ii), and VLA-5 (iii). (B) Percentage of engraftment in murine BM by CB CD34+ cells pretreated with antibodies to either CXCR4 (R4), VLA-4 (α4), VLA-5 (α5), or VLA-4 + VLA-5 (α4 + α5) was quantified at 6 weeks after transplantation by immunostaining with antihuman CD45 mAb. Open circles (○) represent the mouse that received a transplant of 2 × 105 of CD34+ cells intravenously. Filled circles (●) represent the mouse that received a transplant of 2 × 104 CD34+ cells by iBM. Each circle represents 1 mouse, and bars indicate the average of engraftment. Results were combined from 3 independent experiments.
Discussion
Primitive human hematopoietic cells can be assayed on the basis of their ability to repopulate immune-deficient NOD/SCID mice and have been termed SCID-repopulating cells (SRCs).1-3 By using this human-to-mouse xenogeneic transplantation model, studies reported substantially low recoveries of SRCs.12,13 These results imply that homing of primitive hematopoietic cells to the BM is nonselective and/or an inefficient process. A number of factors, such as entrapment in liver and/or lung, molecular incompatibility between human integrins and its ligands expressed on mouse, would interfere with homing and engraftment of HSCs. Therefore, it has been speculated that if one could eliminate such factors, SRC frequency should become markedly higher than that reported previously.4,22 30 Here we report that by introducing cells directly into the marrow environment with the use of the iBM method, the frequency of SRCs became more than 15-fold higher (1 in 44 Lin−/lowCD34+CD38− cells; 95% confidence intervals, 1 in 27 to 1 in 70) than intravenous injection (1 in 660 Lin−/lowCD34+CD38− cells; 95% confidence intervals, 1 in 289 to 1 in 1510) (Figure 3; Table 1). This finding is compatible with the previous speculation that the SRC frequency could become 10 to 20 times higher, assuming there is no interference. Furthermore, multilineage reconstitution (Figure 4; Table2) and self-renewal (Figure 6) were demonstrated by human SRCs injected by iBM method. One might argue that iBM injection created a hematoma in the injected site and reflected survival of human cells. However, the results clearly demonstrated maintenance of hematopoiesis as was seen in intravenous injection of human HSCs. Detection of no human cells in mice given transplants of 104 irradiated CD34+CD38+ carrier cells alone also eliminated the former possibility (Figure 3). Furthermore, a mouse given a transplant of 5 human CD34+CD38−cells showed 0.04% of chimerism in the injected tibia (Figure 3). On the basis of the number of cells recovered from the tibia (2 × 106), a calculated value of human cells was approximately 800, which could represent a 160-fold proliferation of injected cells.
In addition, human hematopoietic cells with the ability of multilineage differentiation were detected in the noninjected side tibia, spleen, and PB (Figure 4; Tables 2-3). In our iBM strategy, there was little, if any, leakage of the injected cells into the peripheral circulation (Figure 2). In much the same way, recently Zhong et al,31using the mouse-to-mouse intrafemur injection method, demonstrated that the donor murine cell in PB was undetectable at early points. At the late time points, transplanted donor cells in the noninjected femur were detected as the same level to the injected femur. In our experiments, however, engraftment in the noninjected BM tends to be low (Tables 2-3). The discrepancy may come from the lowering ability of human HSCs to home to the noninjected BM in xenoenvironment. Importantly, we confirmed the existence of common clones in the injected BM and other hematopoietic organs using a retroviral gene marking (Figure 5). We speculate that the human cells introduced directly into the murine marrow environment proliferate, migrate through a physiologic circulatory system, and engraft in other hematopoietic spaces. The level of chimerism may depend on the ability of proliferation and survival in each engrafted clone.19Independent clones were also present in the injected side and the noninjected side of legs (Figure 5). There may be 2 possibilities: leakage of HSCs during iBM injection and behavioral differences in SRC clones engrafted in the injected BM. The results of our tracing experiments (Figure 2) strongly support the later possibility. Also, Wright et al6 demonstrated a dynamic circulation of HSCs. HSCs rapidly and constitutively migrate from BM to blood stream, and this circulation plays a physiologic role in the functional re-engraftment of another place of BM. Using this iBM method, we might shed light on the mechanisms of human HSCs homing (noninjected side BM) and engraftment (injected side BM).
Self-renewal is a key characteristic of primitive stem cells and distinguishes them from short-lived progenitor cells. However, assessment of self-renewal in SRC assay requires transplantation into secondary recipients and has been difficult because of the lack of a sensitive and reliable method. Treatment of marrow cells derived from primary recipient mice with interleukin 6 (IL-6) and SCF up-regulated the surface expression of CXCR4 and resulted in higher engraftment levels than those of untreated cells when cells were transplanted in secondary recipients.28 29 Thus, the expression of CXCR4 appears essential for homing, and therefore engraftment, of human cells. In other words, reduced expression of CXCR4 and other homing-related molecules in primary-engrafted SRCs may be a reason for the diminished level of human cells in a secondary recipient. The iBM strategy we used in this study can disregard the effect of homing factors, and we successfully demonstrated high levels of engraftment and multilineage differentiation of HSCs in secondary recipient (Figure 6).
Homing and lodgement of transplanted HSCs to recipient BM are critical steps in engraftment and initiation of marrow reconstitution. In the first phase “homing,” transplanted cells must home to vascular sites and need to penetrate the basal lamina that is composed of extracellular matrix (ECM) proteins. In the second phase “lodgement,” HSCs must stay in the appropriate niches of microenvironment where these cells survive, proliferate, and differentiate to reconstitute hematopoiesis, that is, “engraftment.” Human HSC engraftment in NOD/SCID mice depends on the expression of the chemokine SDF-1 and its receptor CXCR4.28,29,32 The SDF-1 activates the integrins lymphocyte function antigen 1 (LFA-1), VLA-4, and VLA-5 on human CD34+ cells. HSCs polarize and migrate through the ECM toward local gradients of SDF-1, which are produced by specialized stromal cells, and orient themselves through the different elements of the BM microenvironment and settle in the stem cell niches.27,29 Although previous studies showed the inhibition of engraftment in Ab blocking experiments,28,29,33 34 whether each molecule contributes to either the homing or lodgement step or both has not been clarified, because this system could not differentiate the homing and lodgement. Our iBM strategy is useful to evaluate lodgement as it bypasses the homing step.
In line with the previous studies by Peled et al,28,29intravenous injection of human CD34+ cells pretreated with anti-CXCR4, –VLA-4, and –VLA-5 mAbs resulted in complete inhibition of engraftment in NOD/SCID mice (Figure 7). However, the same researchers demonstrated that pretreatment with anti-integrin mAbs reduced homing, but not engraftment, of human cells into BM by approximately 30% to 50%.32 From these results, we hypothesize that VLA-4 and VLA-5, expressed on HSCs, are involved in part in both homing and lodgement processes. With the use of the iBM strategy, blocking of either VLA-4 or VLA-5 affected engraftment only partially, whereas neutralization of CXCR4 or VLA-4 and VLA-5 together completely inhibited human HSC engraftment (Figure 7). Therefore, engraftment requires interaction between integrins expressed on BM microenvironment and each VLA-4 and VLA-5 expressed on human HSCs. A number of in vitro studies suggested the importance of integrin-mediated signaling pathways for localization of HSC in BM microenvironment35-39 as well as survival and proliferation of human HSCs.40-43 To our knowledge, the results of our iBM strategy are the first to demonstrate directly the important in vivo roles of integrins and chemokine receptor for not only homing but also lodgement of HSCs in BM microenvironments. Further studies will determine the molecular mechanisms concerning the HSCs lodgement by our iBM strategy. We are in the process of examining the molecules participating only in HSC lodgement.
Our study indicates that iBM transplantation is a method that can accurately evaluate the innate ability of human HSCs. By using this highly sensitive method, more primitive human hematopoietic stem cells, including cells that have lower capability for homing, such as Lin−CD34− cells,44,45 can be identified at a single cell level as previously demonstrated in the murine experiment.46 The iBM method is also suitable to analyze BM cells and mobilized PB cells that exhibit low engraftment capability compared with CB in the SRC assay.12,22 As shown by Kushida et al,14 47 the iBM strategy makes it possible to coadministrate hematopoietic and hematopoietic-supporting mesenchymal stromal cells into recipient BM, which in turn may facilitate the human hematopoietic cell engraftment in murine BM. These studies would further our understandings of the mechanisms of homing and engraftment of human HSCs and lead to possible applications in clinical transplantation medicine.
We thank Dr Hiroshi Kawada for useful discussion, Hideyuki Matsuzawa and Tomomi Takanashi for technical assistance, Tomoko Uno for secretarial works, members of the animal facility of Tokai University, especially Mitsugu Hirano and Mayumi Nakagawa, for meticulous care of experimental animals, and members of Tokai Cord Blood Bank for their assistance.
Prepublished online as Blood First Edition Paper, October 31, 2002; DOI 10.1182/blood-2002-07-1995.
Supported by a grant-in-aid for a Research Grant of the Science Frontier Program from the Ministry of Education, Science, Sports, and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kiyoshi Ando, Department of Hematology, Tokai University School of Medicine Bohseidai, Isehara, Kanagawa 259-1193, Japan; e-mail:andok@keyaki.cc.u-tokai.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal