Live attenuated measles virus (MV-Edm) has potent oncolytic activity against myeloma xenografts in mice. Therapy of multiple myeloma, a disseminated plasma cell malignancy, would require systemic administration of the virus. Thus, the virus should ideally be targeted to infect only myeloma cells to minimize collateral damage to normal tissues: viral binding to its natural receptors must be ablated and a new specificity domain that targets entry into myeloma cells be added. This study covers 2 critical steps toward generating such a retargeted virus: (1) a new specificity domain against the plasma cell marker CD38 was constructed in the form of a single-chain antibody (scFv) and (2) display of that scFv on the measles viral envelope glycoprotein successfully redirected virus entry through CD38 expressed on target cells devoid of the natural MV receptors. The anti-CD38 scFv was tethered to the C-terminus of the hemagglutinin (H) glycoprotein of MV-Edm through a Factor Xa protease cleavable linker. Immunoblot analysis demonstrated that the scFv was efficiently incorporated into recombinant viral particles. Replication of MV-αCD38 was not hindered by the scFv, reaching titers comparable to MV-Edm. Chinese hamster ovary (CHO) cells were resistant to infection by MV-Edm and MV-αCD38. In contrast, CHO cells expressing CD38 became susceptible to infection by MV-αCD38 but not MV-Edm. Removal of the displayed scFv rendered MV-αCD38 noninfectious on CHO-CD38 cells. Tumorigenicity of CHO-CD38 cells in immunocompromised mice was significantly attenuated by MV-αCD38, resulting in enhanced survival of these mice compared with the control group.
Introduction
Multiple myeloma, which is responsible for the deaths of 10 000 Americans annually, is a disseminated plasma cell malignancy, not curable with current therapy.1 Median survival is approximately 4 years, and new approaches to therapy are required. The disease responds initially to alkylating agents and corticosteroids but eventually becomes refractory. High-dose melphalan therapy followed by autologous stem cell transplantation leads to better remissions but does not greatly prolong survival.2-4
Viruses that replicate selectively in neoplastic cells hold considerable promise as novel therapeutic agents for the treatment of malignancy and several are being tested in clinical trials.5 We recently reported that the live attenuated Edmonston B vaccine strain of measles virus (MV-Edm) has potent and selective oncolytic activity against CD138 sorted plasma cells from patients with multiple myeloma and against myeloma xenografts.6 MV-Edm caused extensive cytopathic damage through cell-cell fusion and the formation of multinucleated giant cells (syncytia) in myeloma cells but not in phytohemagglutinin (PHA)–stimulated peripheral blood lymphocytes from healthy volunteers.6 Single or multiple doses of virus injected intratumorally or intravenously caused growth inhibition or total regression of subcutaneous human myeloma xenografts grown in immunocompromised mice.6 We have also generated trackable viruses that encode an inert soluble marker peptide in the viral genome such that viral gene expression can easily be monitored by following peptide levels in the cell culture media or body fluids.7The engineered virus enhanced the survival of mice bearing intraperitoneal ovarian cancer tumors by more than 250%.8Encouraged by these promising results, we are in the process of obtaining approval to proceed with a phase 1 dose escalation clinical trial for patients with advanced or recurrent ovarian cancer.
Application of MV-Edm for virotherapy of multiple myeloma would require systemic administration of the virus because of the disseminated nature of the disease. Thus, to prevent collateral damage to normal tissues, the oncolytic activity of the virus should be targeted exclusively to myeloma cells. We reported that MV-Edm was intrinsically oncolytic for tumor cells and caused minimal cytopathic damage on normal nontransformed cells.6,8 To further enhance specificity, virus entry and receptor dependent cell-cell fusion can potentially be targeted specifically to myeloma cells.9,10 The need for targeting entry and infection is readily apparent in biodistribution studies after administration of MV-Edm in MV-susceptible mice. We found that MV-Edm efficiently infects macrophages in the spleen, lymph nodes, and peritoneal cavity11 (K.-W.P. et al, unpublished observations, 2002). Thus, for systemic therapy of myeloma, we need to generate a retargeted virus, devoid of binding to its natural receptors, that will infect and fuse myeloma cells exclusively through a novel receptor. There are 4 steps to the development of such a retargeted virus: (1) identification of a receptor that is overexpressed in the myeloma cells to allow targeted binding of the virus to this receptor, (2) construction of the ligand (eg, single-chain antibody [scFv]) that will bind to the chosen receptor, (3) display of the ligand as a functional entity on the virus to redirect virus entry through the targeted receptor, and (4) ablation of the original tropism of the virus for its natural receptors. Measles virus entry into target cells is dependent on attachment of the envelope hemagglutinin (H) glycoprotein to cellular receptors and subsequent fusion of viral-cell membranes via the envelope fusion (F) glycoprotein.12 Two cellular receptors for MV entry have been identified, CD46, a ubiquitous cellular receptor found on all nucleated cells, and CDw150 or SLAM (signaling lymphocyte activation molecule), which is present on activated B cells, T cells, and monocytes.13-17 Mutations in the H glycoprotein that inhibit fusion through the CD46 receptor have been reported, and mutagenesis studies are currently in progress in our laboratories to identify mutations in H that ablate SLAM binding.
In the current study, we have taken major strides toward generating a fully retargeted measles virus for myeloma therapy. We first generated a scFv against CD38, a plasma cell marker densely expressed on myeloma cells, and then demonstrated that the anti-CD38 scFv could be tethered to the C-terminus of the measles virus H envelope glycoprotein. The scFv was incorporated into functional recombinant MV-Edm virions, and display of the anti-CD38 scFv mediated virus entry into CD38 receptor-positive rodent cells that otherwise are not permissive to infection by the unmodified virus. The targeted virus was oncolytic only for CD38 receptor-positive cells and caused extensive cell-to-cell fusion and cytopathic effects in these cells. In vivo experiments demonstrated that infection of 1% of the CD38+ cells by the recombinant virus was sufficient to significantly inhibit the growth of the xenografts and enhance the survival of mice.
Materials and methods
Cell culture
Cell lines were maintained in Dulbecco modified Eagle medium (DMEM) or RPMI-1640 (Gibco-BRL, Grand Island, NY) supplemented with heat inactivated fetal bovine serum (FBS) at 37°C in a 5% CO2 humidified incubator. The African green monkey kidney Vero cells were purchased from American Type Culture Collection (ATCC, Rockville, MD) and were grown in DMEM supplemented with 5% FBS. Peripheral blood lymphocytes (PBLs) were obtained from healthy volunteers and stimulated with 2.5 μg/mL PHA for 24 hours before use in infection assays. The Chinese hamster ovary (CHO) and HT1080 human fibrosarcoma cells were grown in 10% FBS-DMEM. CD38-expressing CHO cells (CHO-CD38) or HT1080-CD38 cells were generated by stable transfection of parental cells with a CD38 expression plasmid. The human CD38 cDNA was generated by reverse transcriptase–polymerase chain reaction (RT-PCR) of RNA isolated from a CD38-expressing myeloma cell line. The resultant cDNA was sequenced and compared with a previously published sequence for the CD38 antigen for homology.18 Clones of CHO or HT1080 cells stably expressing this antigen were selected using 1 mg/mL G418 (Gibco-BRL). Clones that expressed high levels of CD38 were identified by flow cytometry using a fluorescein isothiocyanate (FITC)–conjugated anti-CD38 antibody (Caltech, Burlingame, CA).
Generation of scFv against CD38
The cDNA for a scFv against human CD38 was generated by RT-PCR amplification and assembly of immunoglobulin genes from an anti-CD38 hybridoma, THB7, obtained from American Type Culture Collection (ATCC HB-136). This was performed using the recombinant phage antibody system from Pharmacia (Piscataway, NJ) according to manufacturer's instructions. The VH and VL genes were fused using PCR overlap extension techniques to generate a 750-bpSfiI-VH-linker-VL-NotI fragment. Flow cytometric analysis demonstrated binding of the scFv protein to CD38-expressing myeloma cells but not to CD38− cells.
Generation of scFv displaying recombinant MV-Edm
The pCGH plasmid19 encodes MV-H glycoprotein and was modified to include an IEGR Factor Xa protease cleavage site (single amino acid code), followed by SfiI andNotI cloning sites. The scFv cDNA was PCR amplified asSfiI/NotI fragments and inserted as in-frame fusions linked to the C-terminal codon of the H glycoprotein to generate pCGHX α-CD38. The genes for the recombinant H glycoproteins were then cloned as PacI/SpeI fragments into the full-length infectious clone p(+) MVNSe plasmid.20Recombinant viruses were rescued using the standard MV rescue system21 and were characterized by immunoblotting and infection assays.
Characterization of recombinant MV-Edm
Immunoblotting.
To facilitate detection of virions in immunoblots, a Flag tag (DYKDDDDK) was inserted after the start codon of the H glycoprotein of MV-Edm and MV-αCD38 using the Stratagene (La Jolla, CA) quick-change system according to the manufacturer's instructions.10 Conditioned media were harvested from infected Vero cells and centrifuged briefly to remove cellular debris, and viral particles were concentrated and purified through a 20% to 60% sucrose gradient dissolved in TNE buffer (10 mM Tris (tris(hydroxymethyl)aminomethane) pH 7.8, 100 mM NaCl, 1 mM EDTA (ethylenediaminetetraacetic acid)) by centrifugation at 100 000g (Discovery 100S; Kendro, Newton, CT) for 90 minutes at 4°C. The virus band was collected, diluted in TNE buffer, and centrifuged again at 28 000 rpm for 90 minutes. The resultant virus pellet was dissolved in 100 μL Opti-MEM, and virus titer was determined on Vero cells. Viral proteins (106 pfu virus) were then separated on a 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel, electrotransfered to nitrocellulose membrane (Hybond; Amersham, Piscataway, NJ), and immunoblotted using an anti-Flag antibody conjugated to horseradish peroxidase (HRP) at 1:2000 dilution (Sigma Chemical, St Louis, MO). The proteins were detected using a chemiluminescence kit (Pierce, Rockford, IL). To determine the recombinant nature of the H proteins incorporated in MV-αCD38, an aliquot of viral particles was pretreated with 20 μg/mL Factor Xa protease (FXa) for 90 minutes at room temperature before gel electrophoresis.
Virus titrations and infection assays.
To compare the growth characteristics of the recombinant virus with MV-Edm, Vero cells were infected with the respective virus at a multiplicity of infection (MOI) of 3.0 for 2 hours. The inoculum was then removed, standard media replaced, and the cells were maintained at 32°C for virus propagation. At 24, 36, 48, and 72 hours after infection, conditioned media were harvested and cleared of cell debris by centrifugation. Cells were scraped into 1 mL Opti-MEM (Gibco BRL, Rockville, MD), and the cell-associated viruses were released by 2 freeze-thaw cycles. Viral titers were determined by 50% end point dilution assays (median tissue-culture infectious dose [TCID50]) on Vero cells.6 For the specificity assays, CHO and CHO-CD38 cells were plated overnight in 6-well plates (2 × 105 cells/well), washed with phosphate-buffered saline (PBS) the next day, and infected with the viruses at various MOI (10−1 to 10−4) for 2 hours at 37°C. Thirty-six hours after infection, the cells were fixed in 0.5% glutaraldehyde and stained with 0.1% crystal violet, and the number of infectious centers (syncytia with > 20 nuclei) were counted. The cells were also infected with viruses that had been pretreated with FXa protease to cleave off the displayed scFv. MV-Edm or MV-αCD38 were incubated without or with 10 μg/mL FXa protease (New England Biolabs) for 2 hours at room temperature and then used to infect the target cells for 2 hours at 37°C. To determine if display of the scFv enhanced or inhibited viral infection in CD38+CD46+ cells, infection assays were also performed on CD46+ HT1080 and HT1080-CD38 cells using FXa treated or untreated virions. To determine if the tumor selective cytotoxicity of measles virus was altered by the displayed domain, a panel of myeloma cell lines and PHA-stimulated PBLs were infected by MV-Edm and MV-αCD38, and cell viability was determined using the MTS Cell Proliferation Assay (Promega, Madison, WI).
In vivo experiments
All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee. CHO-CD38 cells (5 × 106 cell/100 μL per site) were mixed with MV-Edm or MV-αCD38 at 4°C and implanted immediately subcutaneously into the right flank of 5-week-old female CB.17 severe combined immunodeficient (SCID) mice (Harlan Sprague Dawley, Indianapolis, IN). It is estimated that approximately 1% of the CHO-CD38 cells will be infected by the MV-αCD38 virus but not by MV-Edm. The mice were observed twice a week for tumor growth, and, when tumors were palpable, tumor sizes were measured using calipers in 2 dimensions. Tumor volumes were calculated as a2 × b × 0.5, where a is the shorter diameter and b is the longer diameter. Animals were killed if tumor volumes reached 10% of body weight, if the tumors ulcerated, if there was more than 10% of weight loss, if the animals were unable to obtain food and water, or if they were in distress.
Statistical analysis
The cumulative survival between the treatment and control groups were compared using repeated analysis of measurements on the SAS program.
Results
Generation of recombinant MV-αCD38
We constructed a recombinant MV-Edm displaying an anti-CD38 scFv on the C-terminus of the H envelope glycoprotein (Figure1A). The scFv was linked to the H glycoprotein via a linker sequence encoding an IEGR (single amino acid code) FXa cleavage signal, allowing removal of the scFv when the virions were treated with FXa. Recombinant viruses were rescued from 293-3-46 producer cells and were amplified in Vero cells.18 Correct expression of the scFv domain was investigated by immunoblotting using an anti-Flag antibody that recognizes the Flag-tag inserted at the N-terminus of the H envelope glycoprotein of purified MV-Edm and MV-αCD38 virions. As shown in Figure 1B, the H glycoprotein of recombinant MV-αCD38 had a higher apparent molecular weight than that of MV-Edm. After treatment with FXa, the anti-CD38 scFv was cleaved off from the hybrid protein, yielding an H glycoprotein with the same molecular weight as that of unmodified MV-Edm. Treatment of MV-Edm virions with FXa had no effect on the size of the unmodified H glycoprotein.
MV-Edm targeted to the CD38 receptor via αCD38.
(A) Schematic representation of the full-length infectious clone of recombinant MV-Edm targeted to the CD38 receptor via a single-chain antibody (αCD38). The single-chain antibody is displayed as a C-terminal extension on the H glycoprotein. * indicates stop codons. (B) Immunoblot of purified MV-αCD38 and MV-Edm virions. The recombinant H glycoprotein of MV-αCD38 (lane 1) has a higher molecular weight compared with that of MV-Edm (lane 3). After treatment with Factor Xa protease (20 μg/mL), the single-chain antibody was cleaved off, leaving a H glycoprotein (lane 2) that has the same molecular size as MV-Edm (lane 4).
MV-Edm targeted to the CD38 receptor via αCD38.
(A) Schematic representation of the full-length infectious clone of recombinant MV-Edm targeted to the CD38 receptor via a single-chain antibody (αCD38). The single-chain antibody is displayed as a C-terminal extension on the H glycoprotein. * indicates stop codons. (B) Immunoblot of purified MV-αCD38 and MV-Edm virions. The recombinant H glycoprotein of MV-αCD38 (lane 1) has a higher molecular weight compared with that of MV-Edm (lane 3). After treatment with Factor Xa protease (20 μg/mL), the single-chain antibody was cleaved off, leaving a H glycoprotein (lane 2) that has the same molecular size as MV-Edm (lane 4).
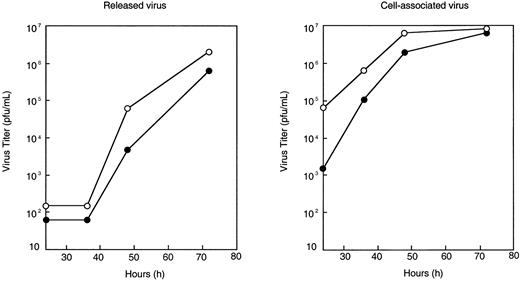
Efficient replication of the recombinant virus with a displayed scFv
The growth kinetics of the recombinant virus was compared with that of unmodified MV-Edm by performing a one-step growth curve in which Vero cells were infected with either virus at a high multiplicity of infection (MOI = 3.0). At 24, 36, 48, and 72 hours after infection, virus yield was determined by TCID50 titrations on Vero cells. The growth kinetics of MV-Edm and MV-αCD38 were similar (Figure 2). Display of the scFv did not severely hinder replication of MV-αCD38, although the maximal titers reached by MV-αCD38 are slightly (2- to 3-fold) less than that of MV-Edm (Figure 2).
One-step growth curve of MV-Edm and MV-αCD38.
Vero cells were infected with MV-Edm (○) or MV-αCD38 (●) at a multiplicity of infection of 3.0 for 2 hours at 37°C, after which the cells were incubated at 32°C for virus propagation. Media was collected, and cell lysates were harvested at 24, 36, 48, and 72 hours after infection to determine amount of (A) released or (B) cell-associated virus by TCID50 titration on Vero cells (plaque-forming unit per milliliter).
One-step growth curve of MV-Edm and MV-αCD38.
Vero cells were infected with MV-Edm (○) or MV-αCD38 (●) at a multiplicity of infection of 3.0 for 2 hours at 37°C, after which the cells were incubated at 32°C for virus propagation. Media was collected, and cell lysates were harvested at 24, 36, 48, and 72 hours after infection to determine amount of (A) released or (B) cell-associated virus by TCID50 titration on Vero cells (plaque-forming unit per milliliter).
MV-αCD38 infected and caused extensive cytopathic damage in CD38-expressing CHO cells
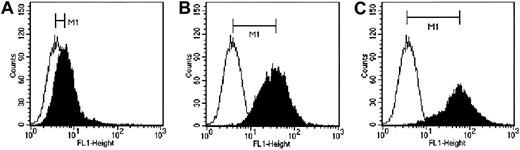
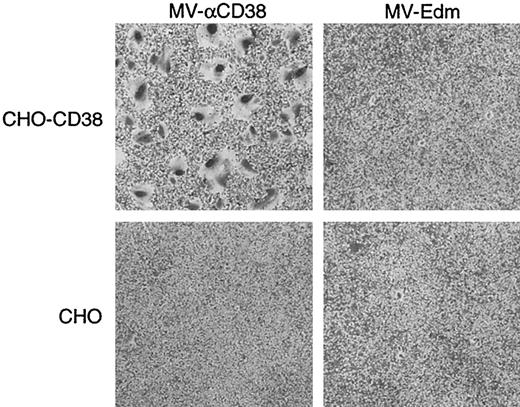
We next sought to determine whether the displayed anti-CD38 scFv could redirect virus attachment and entry to the targeted receptor, CD38. Rodent cells, including CHO cells, are not susceptible to infection by MV-Edm. CHO cells were, therefore, transfected with a CD38 expression plasmid, and the clone expressing the highest level of CD38 was identified by flow cytometry (Figure3). MV-Edm and MV-αCD38 viruses were titrated on CHO-CD38 and CHO cells by counting the number of infectious centers, defined as multinucleated syncytia containing more than 20 nuclei at 36 hours after infection. MV-αCD38 readily infected the CD38-expressing CHO cells while remaining noninfectious on the parental CHO cells (Figure 4). The unmodified MV-Edm did not form syncytia on the CHO cells, irrespective of CD38 status (Figure 4).
Three clones of CHO cells expressing 3 levels of human CD38 as analyzed by flow cytometry using an anti-CD38 antibody conjugated to FITC.
The clones express (A) low, (B) medium, and (C) high levels of human CD38. Empty histograms indicate isotype control; filled histograms, cells stained with a CD46-FITC antibody. M1 indicates difference in median fluorescence.
Three clones of CHO cells expressing 3 levels of human CD38 as analyzed by flow cytometry using an anti-CD38 antibody conjugated to FITC.
The clones express (A) low, (B) medium, and (C) high levels of human CD38. Empty histograms indicate isotype control; filled histograms, cells stained with a CD46-FITC antibody. M1 indicates difference in median fluorescence.
Display of a scFV against CD38 conferred MV-αCD38 with targeted entry and fusion in receptor-positive cells.
Parental CHO cells are not susceptible to infection by MV-Edm and MV-αCD38. CHO cells stably expressing CD38 receptor enable the recombinant virus, but not MV-Edm, to enter and spread within the culture, resulting in 5.5 × 106 syncytial-forming units/mL virus. Cells were stained with 0.1% crystal violet. Original magnification × 40.
Display of a scFV against CD38 conferred MV-αCD38 with targeted entry and fusion in receptor-positive cells.
Parental CHO cells are not susceptible to infection by MV-Edm and MV-αCD38. CHO cells stably expressing CD38 receptor enable the recombinant virus, but not MV-Edm, to enter and spread within the culture, resulting in 5.5 × 106 syncytial-forming units/mL virus. Cells were stained with 0.1% crystal violet. Original magnification × 40.
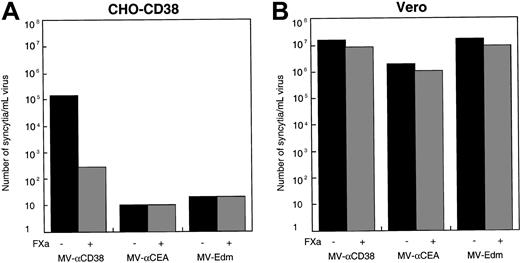
To further verify that infection of the CD38-expressing CHO cells by MV-αCD38 was mediated by its displayed scFv, MV-αCD38 was treated with Factor Xa protease to cleave the scFv from the surface of the viral particles. Protease cleavage ablated the infectivity of the MV-αCD38 on CD38-expressing CHO cells (Figure5A). The cleaved virus could still infect CD46-expressing Vero cells, indicating that the functional integrity of the underlying H glycoprotein was not compromised by exposure to Factor Xa protease (Figure 5B). Recombinant MV-Edm displaying a scFv against the carcinoembryonic antigen (CEA)10 or parental MV-Edm were also able to infect CD46-expressing Vero cells (Figure 5B) but were noninfectious on the CHO-CD38 cells (Figure 4A). None of the viruses were infectious on parental CHO cells (data not shown).
Targeted entry into CHO-CD38 cells.
(A) Only the MV-αCD38 virus and not MV-αCEA or MV-Edm was able to enter and infect CHO-CD38 cells. Infection was reduced if the MV-αCD38 virions were treated with FXa protease to remove the scFv. (B) All 3 viruses were fully infectious on Vero cells, and infectivities were not significantly affected by protease treatment.
Targeted entry into CHO-CD38 cells.
(A) Only the MV-αCD38 virus and not MV-αCEA or MV-Edm was able to enter and infect CHO-CD38 cells. Infection was reduced if the MV-αCD38 virions were treated with FXa protease to remove the scFv. (B) All 3 viruses were fully infectious on Vero cells, and infectivities were not significantly affected by protease treatment.
Display of scFv did not alter virus infection in CD38+CD46+ human cells
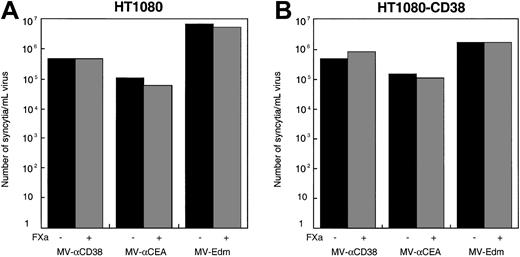
Human fibrosarcoma HT1080 cells were transfected with a CD38 expression plasmid, and clones expressing CD38 were selected. The cells were infected with MV-αCD38 or MV-Edm, and the number of syncytia-forming units per milliliter of virus was determined. Infection of CD38+ HT1080 cells and parental HT1080 cells by the recombinant MV-αCD38 virus were comparable, indicating that display of the scFv did not enhance or inhibit viral entry and infection in cells displaying the targeted receptor (Figure6). Additional studies of infectivity on a panel of myeloma cell lines expressing different levels of CD38 indicated that MV-Edm and MV-αCD38 infected these cells equally well (Table 1). In addition, the recombinant virus, like the unmodified MV-Edm, was potently cytotoxic for transformed myeloma cells and caused minimal cell death in nontransformed PHA-stimulated PBLs (Table 1).
Infection of recombinant MV-αCD38 virus in CD46+ human HT1080 cells expressing CD38 was not enhanced by the second receptor.
Infection of unmodified MV-Edm and the recombinant viruses displaying scFv against carcinoembryonic antigen or CD38 in HT1080 (A) or HT1080-CD38 (B) cells were comparable.
Infection of recombinant MV-αCD38 virus in CD46+ human HT1080 cells expressing CD38 was not enhanced by the second receptor.
Infection of unmodified MV-Edm and the recombinant viruses displaying scFv against carcinoembryonic antigen or CD38 in HT1080 (A) or HT1080-CD38 (B) cells were comparable.
Unmodified MV-Edm and the recombinant MV-αCD38 virus were equally cytotoxic for myeloma cell lines and caused minimal cell death in nontransformed PHA-stimulated PBLs
| Cells . | CD38 status . | Cytotoxicity, % cell death . | |
|---|---|---|---|
| MV-Edm . | MV-αCD38 . | ||
| ARH-77 | ++ | 87.6 | 88.2 |
| JJN-3 | + | 68.7 | 49.8 |
| RPMI 8226 | + | 71.2 | 70.5 |
| MM-1 | ++ | 75.6 | 85.9 |
| KAS 6/1 | + | 30.0 | 31.4 |
| PBLs* | + | <1.0 | <1.0 |
| Cells . | CD38 status . | Cytotoxicity, % cell death . | |
|---|---|---|---|
| MV-Edm . | MV-αCD38 . | ||
| ARH-77 | ++ | 87.6 | 88.2 |
| JJN-3 | + | 68.7 | 49.8 |
| RPMI 8226 | + | 71.2 | 70.5 |
| MM-1 | ++ | 75.6 | 85.9 |
| KAS 6/1 | + | 30.0 | 31.4 |
| PBLs* | + | <1.0 | <1.0 |
Cells were infected with either viruses at MOI of 1.0 and cell death was determined at 6 days after infection. Results are average of 2 different experiments. Cell death was expressed as a percentage of the respective uninfected controls.
MOI = 3.0.
In vivo antitumorigenic activity of the recombinant virus
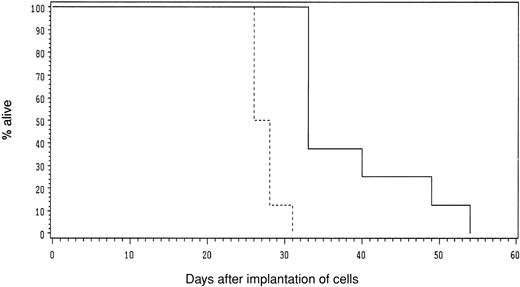
To determine if the recombinant virus could mediate CD38-targeted oncolysis in a model system, we premixed CHO-CD38 cells with MV-αCD38 or MV-Edm at 4°C and immediately implanted the cells subcutaneously into the flanks of SCID mice. In these experiments, about 1% of the cells would be infected by the recombinant MV-αCD38 virus but not by MV-Edm. As shown in Figure 7, the cumulative survival of mice in the test group was significantly longer (P < .001) than that of mice in the control group.
MV-αCD38, but not MV-Edm, significantly attenuated the tumorigenicity of CHO-CD38 cells in vivo.
Mice received CHO-CD38 premixed with MV-αCD38 (solid line) or MV-Edm (dotted line). Cumulative survival of mice was plotted. The survival curves are statistically significant (P < .001).
MV-αCD38, but not MV-Edm, significantly attenuated the tumorigenicity of CHO-CD38 cells in vivo.
Mice received CHO-CD38 premixed with MV-αCD38 (solid line) or MV-Edm (dotted line). Cumulative survival of mice was plotted. The survival curves are statistically significant (P < .001).
Discussion
Our long-term goal is to develop and use live attenuated MV-Edm as a novel virotherapy agent for the treatment of multiple myeloma. The Edmonston B strain of measles virus was derived from a clinical isolate in 1954 and was subsequently passaged in a variety of cells in tissue culture, resulting in attenuation of the virus and loss of pathogenicity.22 This virus forms the basis for the vaccine strains used in vaccination programs today and has significantly reduced the incidence of measles.23 We recently demonstrated that MV-Edm was potently cytopathic for CD138-sorted primary cells from patients with myeloma and that the virus showed significant antitumor activity against subcutaneous human myeloma xenografts in immunocompromised mice.6 In the first 2 critical steps toward generating a fully retargeted measles virus for myeloma therapy, we demonstrate in this report that (1) generation of a scFv against CD38, a receptor densely expressed on myeloma cells, and (2) successful targeted entry of MV-Edm into CD38+ rodent cells through display of the anti-CD38 scFv on the C-terminus of the H glycoprotein of MV-Edm.
We previously reported that MV-Edm is selectively oncolytic for transformed myeloma cells but causes minimal cytopathic damage in nontransformed PHA-stimulated PBLs. The intrinsic tumor selective cytotoxicity is an attractive feature of this agent when considering systemic virotherapy for multiple myeloma. However, we have also noted that administration of MV-Edm into MV-susceptible transgenic mice expressing the human CD46 receptor (with human-tissue specificity) resulted in infection of macrophages in the spleen, lymph nodes, and peritoneal cavity11 (K.-W.P. et al, unpublished observation, 2002). Clearly, it will be desirable to restrict the tropism of the virus. MV-Edm binds to at least 2 receptors: CD46, which is expressed on all nucleated cells, and SLAM, a receptor found on activated B cells, T cells, and monocytes.13-17 To obtain a fully targeted virus, measles binding to these cellular receptors will have to be ablated, and virus entry will have to be redirected through a myeloma cell surface marker. In the first 2 critical steps toward such a retargeted virus, we generated an anti-CD38 scFv and demonstrated that display of the scFv redirected virus binding and entry into CD38 receptor-positive cells that were devoid of natural measles receptors.
We previously reported that MV-Edm displaying an anti-CEA scFv could enter rodent cells through the tumor-associated CEA.10 The current study provides a second example of MV-Edm targeting through scFv display. Because our disease focus is multiple myeloma, we chose to target virus entry via CD38, a plasma cell marker. Human CD38 is a type II transmembrane protein (45 kDa) with multiple functions: it is a bifunctional enzyme capable of synthesizing cyclic adenosine diphosphate (ADP)–ribose from nicotinamide adenine dinucleotide (NAD) and hydrolyzing cADP-ribose to ADP-ribose and is also involved in adhesion and signaling of leukocytes.24Thus, besides growth factor receptors9 and proteins in the immunoglobulin family,10 we demonstrate in this study successful targeting of MV-Edm to a different class of cell surface receptor. CD38 is densely expressed on myeloma cells, and a high-affinity anti-CD38 monoclonal antibody has been tested in clinical trials for patients with myeloma.25 In the current study, we generated an anti-CD38 scFv from a hybridoma (THB7, ATCC HB-136) and displayed it as a C-terminal extension, tethered via a protease sensitive linker, on the extracellular domain of the MV-H envelope glycoprotein. The scFv was incorporated into recombinant virions and conferred the recombinant virus with a new tropism for CD38 receptor-positive CHO cells. Entry of the recombinant virus via CD38 was efficient and led to viral gene expression in infected CHO-CD38 cells, followed by cell-to-cell fusion and formation of large multinucleated syncytia. Removal of the anti-CD38 scFv by FXa treatment ablated infectivity of the recombinant virus on these CD38+CHO cells. A control recombinant virus displaying a scFv against CEA10 was not infectious on the CD38+ CHO cells, confirming that infection by MV-αCD38 was specifically mediated through interaction of CD38 and the anti-CD38 scFv. Most importantly, the scFv against CD38 was stably displayed and did not compromise replication of the recombinant virus on Vero cell monolayers or on human myeloma cells.
Whereas CD38 can substitute as an entry receptor for MV-αCD38, it does not further enhance virus entry in human cells expressing a natural MV-Edm receptor (CD46). Thus, the infectious titers of MV-αCD38 in CD46+ human fibrosarcoma HT1080 cells and HT1080-CD38 cells were comparable. Additional data on a panel of myeloma cells expressing different levels of CD38 indicated that the unmodified and recombinant viruses were equally cytotoxic to these cells. To generate a truly targeted virus that will infect human myeloma cells exclusively through the CD38 receptor, binding to the natural MV receptors needs to be ablated. Mutations that reduce or ablate CD46 interactions26-29 have been already reported, and studies are under way to identify additional H mutations that ablate SLAM binding. The recent ablation of Coxsackie-adenoviral receptor (CAR) and integrin binding sites in the adenovirus coat has greatly facilitated the development of fully retargeted adenoviral vectors.30-32 We anticipate that ablation of CD46 and SLAM binding sites on measles virus H glycoprotein will prevent infection of macrophages in the spleen and lymph nodes after systemic administration of the virus. In addition, ablating virus binding to SLAM might interfere with the immunosuppressive properties of the virus, thereby further reducing the dose-limiting toxicity for clinical studies.15-26
We thank Gabriella Rosales (Mayo Clinic, Department of Biostatistics) for help with the statistical analysis of the data.
Prepublished online as Blood First Edition Paper, November 14, 2002; DOI 10.1182/blood- 2002-07-2195.
Supported by grants from the Mayo Foundation, George M. Eisenberg Foundation, and Harold W. Siebens Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kah-Whye Peng, Molecular Medicine Program, Guggenheim 18, Mayo Foundation, 200 First St SW, Rochester, MN 55905; e-mail: peng.kah@mayo.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal