Adenoviral transfer of human apo A-I in Balb/c mice induces a strong humoral immune response against the transgene product when expression is driven from the ubiquitously active CMVpromoter but induces no immune response when driven by the hepatocyte-specific 256–base pair apo A-I promoter. Here the hypothesis was tested, which is that the humoral immune response against the circulating transgene product correlates with its expression in antigen-presenting cells. No humoral immune response was observed after adenoviral transfer of vectors with human apo A-I expression driven by the hepatocyte-specific apo C-II or 1.5-kilobase (kb) humanα1-antitrypsin promoter, but antibodies were induced after transfer with vectors driven by the ubiquitously activeU1b promoter and the murine MHCII Eβpromoter. A strict correlation was observed between antigen expression in the spleen and the occurrence of an immune response. Coinjection of the 1.5-kb human α1-antitrypsin and the murine MHCII Eβ promoter–driven vectors resulted in a very short-lived humoral immune response against human apo A-I, suggesting that the time course of human apo A-I expression is a critical determinant of the development of tolerance for human apo A-I. High titers of antibodies against human apo A-I after subcutaneous gene transfer with the MHCII Eβ promoter–driven vector underscore the potential of this promoter for vaccination purposes. In conclusion, humoral immune response in mice against a circulating antigen induced by adenoviral transfer is strictly dependent on expression in antigen-presenting cells.
Introduction
Humoral immune responses are initiated with the cellular uptake and processing of foreign antigens by professional antigen-presenting cells. After migration to secondary lymphoid organs, interaction of antigen-presenting cells with naive T cells provides the T cell with all signals for their activation.1,2Activation and clonal B-cell expansion occur after primed antigen-specific CD4+ T cells engage with the antigen-specific B cells through T-cell receptor/major histocompatibility complex (MHC) II interaction.3-5
Previously, we observed a strong humoral immune response after adenoviral gene transfer of human apo A-I in Balb/c and Fvb mice when expression was driven by the ubiquitously activecytomegalovirus (CMV) promoter-driven construct, but not after transfer of a hepatocyte-specific minimal 256–base pair (bp) apolipoprotein (apo) A-I promoter-driven construct.6 Pastore et al7 showed that expression of human α1-antitrypsin (hAAT) induced by transfer with first generation adenoviral vectors resulted in an antibody response when its cDNA was expressed from the ubiquitous mousephosphoglycerate kinase promoter but not when expressed from the hepatocyte-specific mouse albumin promoter. In aggregate, these data suggest that use of a hepatocyte-specific promoter may preclude or mitigate antibody formation against immunogenic transgene products in mice. However, because circulating antigens can continuously be taken up and processed by antigen-presenting cells leading to MHC II presentation and CD4+ T-cell activation, the actual relationship between promoter and humoral immune response against the transgene product is unclear. To further investigate this relationship and to understand the underlying mechanism, 2 adenoviral constructs containing hepatocyte-specific promoters (the apo C-II and the 1.5-kilobase (kb) human α1-antitrypsin [hAAT] promoter) were compared with a construct containing the ubiquitously active murine U1b small nuclear RNA promoter (U1b) and a construct containing the murine MHC II Eβ promoter. Although α1-antitrypsin is expressed both in macrophages and hepatocytes, the 1.5-kb hAAT promoter does not contain the upstream sequences necessary to induce expression in macrophages8 and is strictly hepatocyte-specific. The murine MHC II Eβ promoter is active in macrophages, dendritic cells, and B cells, and has been shown to be significantly stronger than the Eα, Aα, and Aβpromoters.9 As little as 192 bp of theEβ proximal promoter was sufficient to direct constitutive and cytokine-inducible expression of Eβ in thymus, B cells, dendritic cells, and macrophages in vivo in mice with a nonexpressed endogenous Eβ gene.10 The underlying hypothesis of the present investigation was that, notwithstanding the fact that human apo A-I is a circulating antigen, humoral immune responses against human apo A-I would correlate with human apo A-I expression in antigen-presenting cells.
Materials and methods
Generation of E1-deleted recombinant adenoviral vectors
The construction and generation of the E1-deleted adenoviral vectors AdCMV, AdU, AdA4,AdC4, and AdAT4 has been described previously.11 12AdA4, AdC4, andAdAT4 contain the human apo A-I (A-I), the humanapo C-II (C-II), and the humanα1-antitrypsin promoter (hAAT),respectively, in front of the genomic human apo A-I sequence and 4 copies of the human apo E enhancer. AdCMVand AdU contain the ubiquitously activecytomegalovirus (CMV) and the ubiquitously active murineU1b small nuclear RNA (U1b) promoter, respectively, in front of the genomic human apo A-Isequence.
The MHCII Eβ promoter corresponding to the d allele was amplified from murine genomic Balb/c DNA using the forward primer 5′-CGGAATTCCGGAAGGGGAC CTGCAAACTGAAT-3′ and the reverse primer 5′-CGGGATCCCGCAGGA GTCAGAGGGGAAGGC-3′. The PCR product was ligated in pACpLpA,11 followed by insertion of the genomic apo A-I fragment not including the apo A-Ipromoter,11 generating the shuttle plasmid pACpLpA.Eβ.gA-I. The recombinant adenoviral vector AdEβwas generated by cotransfecting the rescue plasmid pJM1713 and the shuttle plasmid pACpLpA.Eß.gA-I as described before.14
Animal experiments
All experimental procedures in animals were performed in accordance with protocols approved by the Institutional Animal Care and Research Advisory Committee. Recombinant adenoviral vectors were administered by tail-vein injection into female Balb/c mice. Subcutaneous gene transfer was performed by injecting half of the viral dose in the footpath of each hindlimb. Experimental groups consisted of 5 to 10 animals for each recombinant vector and each experimental condition.
Human apo A-I ELISA
Human apo A-I levels were determined by sandwich enzyme-linked immunosorbent assay (ELISA) as described previously.6
Detection of anti–human apo A-I antibodies by ELISA
Human apo A-I antibodies were determined as described previously.11 Briefly, polystyrene microtiter plates (Elscolab, Kruibeke, Belgium) were coated with 0.14 μM human apo A-I (Biovalley, Paris, France) or 0.14 μM of human tissue-type plasminogen activator (t-PA), which was used as control antigen. Dilutions of plasma samples up to 1:256 000 were added to the wells for 2 hours. After washing of the plates, rabbit anti–mouse polyclonal antibodies conjugated with peroxidase (DAKO, Denmark) were diluted 1:10 000 and added to the wells for 2 hours. Peroxidase reaction was performed with H2O2 and orthophenylenediamine, and absorbance was measured at 492 nm. The titer of antibodies was defined as the plasma dilution for which the optical density of the antibody assay was higher than 0.100, which is significantly above background.
Detection of antiadenoviral antibodies by ELISA
Serial dilutions of plasma samples up to 1:640 000 were added overnight to 96-well polystyrene microtiter plates (Elscolab) coated with 100 μL control vector AdRR5 at a concentration of 2 × 107 plaque-forming units (pfu)/mL. After washing of the plates, rabbit anti–mouse polyclonal antibodies (DAKO) were diluted 1:10 000 and added to the wells for 2 hours. Peroxidase reaction was performed with H2O2 and orthophenylenediamine, and absorbance was measured at 492 nm. The titer of IgG antibodies against adenovirus serotype 5 was defined as the plasma dilution for which the optical density of the antibody assay was higher than 0.100, which is significantly above background.
Immunization with human apo A-I protein
Immunization was performed by injecting 25 μg human apo A-I formulated in complete Freund adjuvant in the footpad of each hindlimb.
Quantification of human apo A-I transgene DNA in the liver
Quantification of human apo A-I DNA was performed as described previously by real-time polymerase chain reaction (PCR).6 The human apo A-I DNA copy number was normalized to the copy number of the Prion Protein (PrP) gene.
Quantification of human apo A-I mRNA
Human apo A-I mRNA was quantified by real-time PCR after cDNA synthesis as described previously.6 The humanapo A-I cDNA copy number was normalized to the copy number of the glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) housekeeping gene.
Isolation of splenic dendritic cells, macrophages, B and T cells after transfer with AdEβ
Splenocytes were isolated 7 days after transfer with 5 × 108 pfu AdEβ. Because the absolute number of dendritic cells isolated from one spleen is too low to obtain sufficient mRNA, splenocytes isolated from 4 Balb/c were pooled, and human apo A-I mRNA of each cell type was quantified on 4 independent samples, each consisting of a pool of 4 mice. Ficoll Paque (Lymphoprep; Axis-Shield POC AS, Oslo, Norway) was used according to the instructions of the manufacturer to obtain mononuclear cells from the spleen. Subsequently, positive selection with CD11c+, CD11b+, and CD45R MACS Micro-beads (Miltenyi Biotec, Auburn, CA) was performed to isolate dendritic cells, macrophages, and B cells, respectively. All selections were performed on MiniMacs (MS) separation columns as instructed by the manufacturer (Miltenyi Biotec). The remaining eluent, containing T cells, was purified by negative Pan T-cell selection (Miltenyi Biotec). The purity of the enriched splenic dendritic cells, macrophages, and B and T cells was confirmed by flow cytometry using phycoerythrin-conjugated antibodies against CD11c+, CD11b+, CD19, and the Pan T cell Marker CD3ε (BD Pharmingen, Heidelberg, Germany), respectively. To isolate mRNA, the Quickprep micro mRNA purification kit (Amersham Pharmacia, Uppsala, Sweden) was used.
Statistical analysis
Human apo A-I data are expressed as mean ± standard error of the mean (SEM). Antibody titers are expressed as geometric mean ± standard error of the geometric mean. Comparison of antibody titers was performed on logarithmically transformed data by nonparametric Mann-Whitney test using the INSTAT V2.05a statistical program (Graph Pad Software, San Diego, CA). A 2-sided P value less than .05 was considered statistically significant.
Results
Humoral immune response against human apo A-I after transfer withCMV promoter and U1b promoter-driven constructs but not after transfer with apo C-II and hAATpromoter-driven constructs
We have previously demonstrated a strong humoral immune response against human apo A-I in Balb/c mice after transfer with aCMV promoter-driven construct (AdCMV), but follow-up was limited to 35 days.6 Human apo A-I expression after transfer with AdCMV was 6.2 ± 2.0 mg/dL at day 6 and below detection limit (1 mg/dL) within 14 days.6 To investigate whether high-titer antibodies against human apo A-I persisted after transfer with AdCMV, the titer of antibodies against human apo A-I was determined up to 12 months after transfer with 5 × 108 pfu AdCMV.Figure 1B illustrates that the geometric mean of the inverse of the titer of antibodies 12 months after transfer was 90 000 ± 16 000, and antibody titers had not declined at this time point.
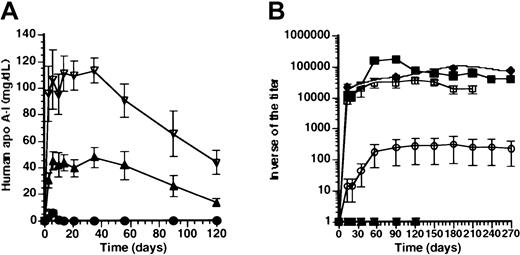
Effect of promoter on human apo A-I expression and anti-human apo A-I antibodies after adenoviral gene transfer.
(A) Human apo A-I expression after adenoviral gene transfer with 5 × 108pfu AdAT4 (▿), AdC4 (▴), AdU(●), and AdCMV (♦) in Balb/c mice. Human apo A-I levels were below detection limit after transfer with any dose ofAdEβ (not shown). (B) Inverse of the titer of antibodies against human apo A-I after transfer with 5 × 108 pfuAdAT4 (▿), AdC4 (▴), AdU (●),AdCMV (♦), and AdEβ (▪) and after transfer with 108 pfu AdEβ (■) in Balb/c mice.
Effect of promoter on human apo A-I expression and anti-human apo A-I antibodies after adenoviral gene transfer.
(A) Human apo A-I expression after adenoviral gene transfer with 5 × 108pfu AdAT4 (▿), AdC4 (▴), AdU(●), and AdCMV (♦) in Balb/c mice. Human apo A-I levels were below detection limit after transfer with any dose ofAdEβ (not shown). (B) Inverse of the titer of antibodies against human apo A-I after transfer with 5 × 108 pfuAdAT4 (▿), AdC4 (▴), AdU (●),AdCMV (♦), and AdEβ (▪) and after transfer with 108 pfu AdEβ (■) in Balb/c mice.
We have previously shown that after transfer with a construct driven by the hepatocyte-specific 256-bp minimal apo A-I promoter(AdA4), no humoral immune response against human apo A-I occurred in Balb/c mice. To corroborate a potential relationship between hepatocyte-specificity of promoters and absence of a humoral immune response against human apo A-I, human apo A-I expression (Figure1A), and antibodies against human apo A-I (Figure 1B) were compared after transfer with constructs driven by the hepatocyte-specificapo C-II and 1.5-kb hAAT promoters (AdC4 and AdAT4, respectively) and after transfer with the ubiquitously active U1b small nuclear RNA promoter (AdU).
Human apo A-I expression was sustained for the duration of the experiment (4 months) after transfer with AdC4 andAdAT4, and no antibodies against human apo A-I were observed. The decrease of expression levels after day 35 correlated with a 3.9-fold (P < .01) and a 3.3-fold (P < .01) decrease of the human apo A-Itransgene DNA copy number between day 35 and 4 months after transfer with AdC4 and AdAT4, respectively. After transfer with AdU, human apo A-I expression was below detection limit (1 mg/dL) within 10 days (Figure 1A), and antibodies against human apo A-I were observed. However, compared with AdCMV, the geometric mean of the inverse of the titer of antibodies after transfer with AdU was more than 300-fold lower.
Humoral immune response against human apo A-I after transfer with a murine MHCII Eβ promoter-driven construct
To investigate whether a humoral immune response against human apo A-I after transfer with ubiquitously active promoters was related to expression in antigen-presenting cells, titers were determined after transfer with a murine MHC II Eβpromoter-driven construct (AdEβ). Human apo A-I was below detection limit (1 mg/dL) at all time points after transfer withAdEβ. Figure 1B illustrates the inverse of the titer of antibodies against human apo A-I after transfer with 5 × 108 pfu and 108 pfu AdEβ. After transfer with 5 × 108 pfu, the geometric mean of the inverse of the titer of antibodies reached a peak of 180 000 ± 11 000 at 3 months after transfer, and high antibody titers persisted for 10 months. After transfer with 108 pfu, the geometric mean of the inverse of the titer of antibodies reached a peak of 37 000 ± at 4 months after transfer. No humoral immune response against human apo A-I was observed after transfer with 2 × 107 pfu of AdEβ (n = 5) nor in Balb/c mice treated with 5 × 108 pfu 10 days after splenectomy (n = 5) (data not shown).
To investigate whether humoral immune responses against human apo A-I correlate with human apo A-I expression in the spleen, human apo A-I mRNA in the spleen was quantified by real-time PCR after reverse transcription. Figure 2A illustrates that human apo A-I mRNA was detected in the spleen 7 days after transfer with 5 × 108 pfu ofAdCMV or AdEβ, whereas human apo A-ImRNA was significantly lower after transfer with AdU, and no human apo A-I mRNA was detected after transfer withAdA4, AdC4, or AdAT4. Spearman rank-order correlation coefficient between human apo A-ImRNA in the spleen at day 7 and the inverse of antibody titers at day 56 was 1.0 (P = .0028).
Human apo A-I mRNA levels.
(A) Human apo A-I mRNA level in the spleen at day 7 after transfer with 5 × 108 pfu different vectors. Human apo A-ImRNA in the spleen was below detection limit (* < .001) after transfer with 5 × 108 pfuAdA4, AdC4, and AdAT4. (B) Humanapo A-I mRNA level in dendritic cells, macrophages, B cells, and T cells isolated from spleens of mice injected with 5 × 108 pfu AdEβ. Human apo A-ImRNA in T lymphocytes was below detection limit (* < .001).
Human apo A-I mRNA levels.
(A) Human apo A-I mRNA level in the spleen at day 7 after transfer with 5 × 108 pfu different vectors. Human apo A-ImRNA in the spleen was below detection limit (* < .001) after transfer with 5 × 108 pfuAdA4, AdC4, and AdAT4. (B) Humanapo A-I mRNA level in dendritic cells, macrophages, B cells, and T cells isolated from spleens of mice injected with 5 × 108 pfu AdEβ. Human apo A-ImRNA in T lymphocytes was below detection limit (* < .001).
In transgenic mice, the Eβ promoter has been shown to be active in dendritic cells, macrophages, and B cells. Figure 2B illustrates human apo A-I expression in dendritic cells, macrophages, B cells, and T cells isolated 7 days after gene transfer with 5 × 108 pfu AdEβ. Human apo A-ImRNA level in dendritic cells was 12-fold higher than in B cells (P < .05) and 2.1-fold higher than in macrophages (P < .05). Human apo A-I mRNA level in T lymphocytes was below detection limit (< .001).
Time-course of human apo A-I expression may be a determinant of the humoral immune response against human apo A-I after adenoviral gene transfer
To investigate the mechanism of absence of a humoral immune response against human apo A-I, 5 × 108 pfuAdEβ and 5 × 108 pfu AdAT4 were coinjected in Balb/c mice. Human apo A-I expression after coinjection was very similar compared with transfer with AdAT4 (Figure3A). Figure 3B illustrates that high titers of antibodies against human apo A-I were observed at day 10 and day 14 after coinjection, but human apo A-I antibodies decreased rapidly and were undetectable at day 56 after transfer. Coinjection ofAdEβ and of AdAT4 induces human apo A-I expression in the spleen and the liver. Because the CMVpromoter is a ubiquitously active promoter and human apo A-I expression is induced both in the liver and the spleen (Figure 2B), human apo A-I expression (Figure 3A) and titers of antibodies against human apo A-I (Figure 3B) were determined after gene transfer with 2 × 109 pfu AdCMV. Human apo A-I levels at day 3 and day 6 were similar to those after coinjection ofAdEβ and AdAT4, but declined to 3.2 ± 1.3 mg/dL at day 14 and were below detection limit at day 21 and later. The titer of antibodies against human apo A-I at day 14 after transfer was not significantly different than after coinjection of AdEβand AdAT4, but in contrast to the coinjection experiment, titers persisted for several months after transfer. In aggregate, these data suggest that the time course of human apo A-I antigen levels may determine whether a sustained humoral immune response against human apo A-I or tolerance for human apo A-I develops. Expression of human apo A-I in the liver per se is insufficient for the induction of tolerance against the transgene product after adenoviral gene transfer. The presence of antibodies against human apo A-I in the first weeks after gene transfer in the coinjection experiment indicates that the initial absence of a humoral immune response against human apo A-I after transfer with AdAT4 may be due to immunologic ignorance and cannot be explained by anergy or deletion of immunoreactive cells at these early time points.
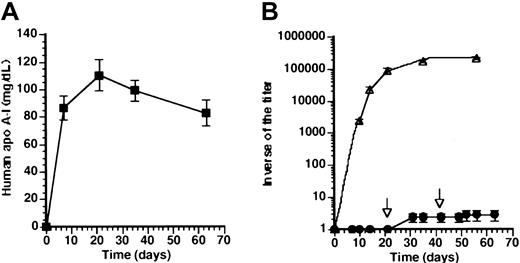
Effect of time-course of antigen levels on humoral immune response against human apo A-I.
(A) Human apo A-I expression after coinjection with 5 × 108 pfu AdAT4 and 5 × 108 pfu AdEβ (■) and after gene transfer with 2 × 109 pfu AdCMV (▴). (B) Inverse of the titer of antibodies against human apo A-I after coinjection with 5 × 108 pfu AdAT4 and 5 × 108 pfu of AdEβ (■) and after gene transfer with 2 × 109 pfu AdCMV(▴).
Effect of time-course of antigen levels on humoral immune response against human apo A-I.
(A) Human apo A-I expression after coinjection with 5 × 108 pfu AdAT4 and 5 × 108 pfu AdEβ (■) and after gene transfer with 2 × 109 pfu AdCMV (▴). (B) Inverse of the titer of antibodies against human apo A-I after coinjection with 5 × 108 pfu AdAT4 and 5 × 108 pfu of AdEβ (■) and after gene transfer with 2 × 109 pfu AdCMV(▴).
To evaluate whether the development of tolerance in the coinjection experiment was specific for human apo A-I, antibodies against adenovirus serotype 5 were determined. The inverse of the antibody titer was 200 000 ± 19 000 at 21 days and 190 000 ± 17 000 at 3 months, indicating the specificity of tolerance induction.
Gene transfer with AdAT4 induces tolerance for human apo A-I
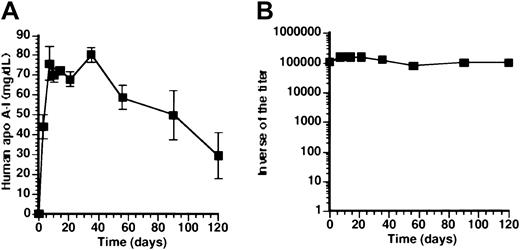
To investigate directly tolerance for human apo A-I at later time points after transfer with AdAT4, a subcutaneous immunization with human apo A-I protein was performed at 3 weeks (n = 8) after transfer with 5 × 108 pfuAdAT4. No immune response was observed in 7 of 8 mice injected at 3 weeks after transfer. A weak immune response was seen in one mouse (1-1000). A rechallenge subcutaneous immunization with human apo A-I protein was performed 6 weeks after gene transfer. No immune response was observed in the 7 mice without antibodies after the first immunization, whereas a slight increase of antibody titer occurred in the eighth mouse (1-4000). Human apo A-I level is shown in Figure4A and was not significantly different compared with mice without subcutaneous immunization (Figure 1A). The geometric mean of the inverse of the titer is shown in Figure 4B. As a positive control, 4 Balb/c mice not injected previously with an adenoviral vector were immunized by subcutaneous injection of human apo A-I and a strong humoral immune response was observed (Figure4B).
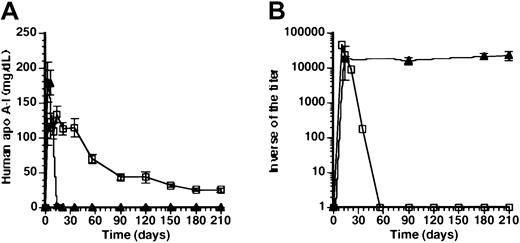
Tolerance for human apo A-I after transfer withAdAT4.
(A) Human apo A-I expression after gene transfer with 5 × 108 pfu AdAT4 (▪) in Balb/c mice, which were subcutaneously immunized with human apo A-I 3 weeks after gene transfer and rechallenged with subcutaneous human apo A-I immunization 6 weeks after gene transfer. (B) Inverse of the titer of antibodies against human apo A-I after subcutaneous immunization with human apo A-I protein in naı̈ve Balb/c mice (▵) and after gene transfer with 5 × 108 pfu AdAT4 in Balb/c mice, which were subcutaneously immunized with human apo A-I 3 weeks after gene transfer and rechallenged with subcutaneous human apo A-I immunization 6 weeks after gene transfer (●). Arrows indicate the time point of subcutaneous immunization in mice treated withAdAT4.
Tolerance for human apo A-I after transfer withAdAT4.
(A) Human apo A-I expression after gene transfer with 5 × 108 pfu AdAT4 (▪) in Balb/c mice, which were subcutaneously immunized with human apo A-I 3 weeks after gene transfer and rechallenged with subcutaneous human apo A-I immunization 6 weeks after gene transfer. (B) Inverse of the titer of antibodies against human apo A-I after subcutaneous immunization with human apo A-I protein in naı̈ve Balb/c mice (▵) and after gene transfer with 5 × 108 pfu AdAT4 in Balb/c mice, which were subcutaneously immunized with human apo A-I 3 weeks after gene transfer and rechallenged with subcutaneous human apo A-I immunization 6 weeks after gene transfer (●). Arrows indicate the time point of subcutaneous immunization in mice treated withAdAT4.
Antibodies against human apo A-I induced by subcutaneous immunization persist after subsequent gene transfer withAdAT4
To investigate human apo A-I expression and titers of antibodies against human apo A-I in mice preimmunized with human apo A-I protein, subcutaneous immunization with human apo A-I protein was performed 4 months before gene transfer. Figure5A illustrates that high human apo A-I protein levels were observed in preimmunized mice, notwithstanding persisting high titers of antibodies against human apo A-I (Figure 5B). Because the simultaneous presence of high human apo A-I antigen levels and anti–human apo A-I antibodies represents a paradox, 2 μg/mL of human apo A-I either as free protein or as human apo A-I present on high density lipoprotein (HDL) particles from mice injected withAdAT4 was added to 1:2000 dilutions of plasma samples obtained at day 56, 3 months, and 4 months after gene transfer withAdAT4 in preimmunized mice. The optical density in the antibody titer assay decreased 1.8-fold (P = .0005) after addition of human apo A-I protein, but decreased not at all (ratio = 1.0) after addition of human apo A-I containing HDL particles nor after addition of 2 μg/mL bovine serum albumin. This observation may be explained by the tertiary structure of human apo A-I in HDL particles15-17 leading to the shielding of epitopes.
Pre-existing humoral immune response against human apo A-I is not abrogated by AdAT4 gene transfer.
(A) Human apo A-I expression after gene transfer with 5 × 108 pfu ofAdAT4 (▪) in mice subcutaneously immunized with human apo A-I protein 4 months before gene transfer. (B) Inverse of the titer of antibodies against human apo A-I after gene transfer with 5 × 108 pfu AdAT4 in mice subcutaneously immunized with human apo A-I protein 4 months before gene transfer (▪). Day 0 is the time of gene transfer. Titers of antibodies were unchanged after gene transfer compared with titers in the preceding months, which are not shown.
Pre-existing humoral immune response against human apo A-I is not abrogated by AdAT4 gene transfer.
(A) Human apo A-I expression after gene transfer with 5 × 108 pfu ofAdAT4 (▪) in mice subcutaneously immunized with human apo A-I protein 4 months before gene transfer. (B) Inverse of the titer of antibodies against human apo A-I after gene transfer with 5 × 108 pfu AdAT4 in mice subcutaneously immunized with human apo A-I protein 4 months before gene transfer (▪). Day 0 is the time of gene transfer. Titers of antibodies were unchanged after gene transfer compared with titers in the preceding months, which are not shown.
Subcutaneous gene transfer with AdEβ induces a strong humoral immune response against human apo A-I
Eβ promoter–driven vectors may be used for vaccination purposes. For safety reasons, subcutaneous gene transfer is likely to be more suitable than intravenous gene transfer. Subcutaneous gene transfer performed by injecting 2.5 × 108 pfuAdEβ into the footpath of each hindlimb induced similar antibody titers than those induced after intravenous gene transfer with the same viral dose (data not shown).
Discussion
The main findings of the present study are that (1) gene transfer with hepatocyte-specific promoters is associated with absence of a humoral immune response against human apo A-I in Balb/c mice; (2) notwithstanding the fact that human apo A-I is a circulating antigen, a strict correlation was observed between antigen expression in antigen-presenting cells and a humoral immune response against human apo A-I after adenoviral gene transfer; and (3) the time course of human apo A-I expression may be a determinant of the development of tolerance for human apo A-I after adenoviral gene transfer.
Because the adenoviral backbone and the adenoviral capsid are identical in the different vectors, the presence or absence of an immune response against human apo A-I cannot be explained by differences in adjuvant effect related to cytokine release induced by adenoviral gene transfer.18 The absence of a humoral immune response against human apo A-I after adenoviral gene transfer with 1.5-kb human α1-antitrypsinpromoter-driven vectors has been confirmed in inbred C57BL/6, Fvb, 129, and C3H/HeJ mice and in outbred Swiss Webster mice (B.R.D., unpublished results, 2000).
Because the induction of IgG antibodies requires CD4-cell help19 and activation of CD4+ T cells is dependent on MHC II presentation of processed antigen by antigen-presenting cells, the question why endogenous expression of the antigen leads to more efficient MHC II presentation arises. MHC class II molecules are classically involved in the presentation of exogenous antigens but can also present endogenous antigens to CD4 T cells. MHC class II presentation of endogenous secreted hen egg lysozyme was much more efficient when compared with that of exogenous hen egg lysozyme and was not explained by reuptake of secreted antigen.20 Rowell et al21 demonstrated that class II presentation of the HIV-1 envelope protein by infected antigen-presenting cells may be mediated by rapid endocytosis from the cell surface, but also that class II MHC presentation occurred under conditions that prevented reuptake by endocytosis, indicating that an internal pathway for class II presentation exists. The in vivo data in the present investigation indicate that expression by antigen-presenting cells of secreted and circulating antigens potentiates humoral immune responses and is likely to be useful for efficient vaccination against such antigens. The potential of MHC class II promoters for vaccination purposes is underscored by the induction of high titers of antibodies against human apo A-I after subcutaneous gene transfer with AdEβ.
Interestingly, the lower human apo A-I mRNA signal in the spleen after transfer with AdU also resulted in significantly lower antibody titers. Lowering the dose ofAdEβ from 5 × 108 pfu to 108pfu also resulted in a significant decline of antibody titers, whereas after transfer with 2 × 107 pfu, no antibodies were observed. As expected, splenectomy abrogated an immune response against human apo A-I after transfer with AdEβ. In aggregate, these data demonstrate that intravenous gene transfer with relatively low doses of adenoviral vectors carrying a transgene under control of aMHC II promoter may induce potent humoral immune responses and that the spleen is playing a critical role in the initiation of the response.
The route of administration may play a role in the induction of a humoral immune response against a xenogenic transgene product.22 An antibody response against factor VIII was observed after intramuscular transfer of an AAV vector in C57BL/6 mice but not after intraportal vein administration, indicating that liver-directed gene transfer may result in mitigation of immune responses against the transgene product.22 Liver-derived dendritic cells have been shown to induce donor-specific hyporesponsiveness in transplantation studies23 and may also play a role in the induction of tolerance after adenoviral gene transfer, since adenoviral vectors in mice demonstrate a predominant hepatotropism and the production of antigen after transfer with hepatocyte-specific promoters is restricted to the liver. However, the present result suggests that the time course of human apo A-I expression and not human apo A-I expression in the liver per se determines development of tolerance for human apo A-I after adenoviral gene transfer. Coinjection of 5 × 108 pfuAdEβ and 5 × 108 pfu AdAT4 was associated with a strong early humoral immune response against human apo A-I, but tolerance was established at later time points. In contrast, gene transfer with 2 × 109 pfuAdCMV that, similar to the coinjection experiment, induces expression in liver and spleen, results in a persistent humoral immune response against human apo A-I. Because human apo A-I levels after gene transfer with 2 × 109 pfu AdCMVand after coinjection of AdEβ and AdAT4 were similar in the first week but differed thereafter, data on immune reactivity against human apo A-I in these experiments are consistent with the proposed role of dose and time of antigens in determining immune reactivity.24 Rapid decline of human apo A-I mRNA after transfer with AdCMV has previously been observed in Balb/c mice and was due both to promoter attenuation and to rapid transgene DNA decline.6 The continuous input of high amounts of antigen after gene transfer with AdEβ andAdAT4 may explain why the emerging humoral immune response was completely abrogated. Titers of antibodies against human apo A-I after transfer with 2 × 109 pfu AdCMV (Figure3B) were significantly lower than after transfer with 5 × 108 pfu (Figure 1B), indicating that the initial higher antigen concentrations after transfer with the higher dose ofAdCMV may have mitigated the response.
As expected, a pre-existing humoral immune response against human apo A-I induced by subcutaneous immunization with human apo A-I protein formulated in complete Freund adjuvant could not be abrogated by subsequent gene transfer with AdAT4. Notwithstanding persisting antibodies, high human apo A-I antigen levels were detected. We suggest that epitopes recognized by many of the anti–human apo A-I antibodies may be shielded in the specific tertiary structure of apo A-I on discoidal or spherical HDL particles.16,17 Higher reactivity of anti–apolipoprotein A-I antibodies against apo A-I and HDL particles than against denatured apo A-I also has been described in patients with systemic lupus erythematosus.15
In conclusion, a strict correlation was observed between antigen expression in professional antigen-presenting cells and a humoral immune response against human apo A-I after adenoviral gene transfer. This may be relevant both for inducing efficient humoral immune responses in vaccination studies and for avoiding such responses in gene transfer applications for correction of protein deficiencies.
Bart De Geest is a postdoctoral fellow of the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen. Sophie Van Linthout is a research assistant at the Instituut voor Wetenschappelijk en Technisch Onderzoek-Vlaanderen. We thank Jessy Hendrix and Zhiyong Zhang for superb technical assistance.
Prepublished online as Blood First Edition Paper, November 21, 2002; DOI 10.1182/blood- 2002-07-2146.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bart De Geest, Center for Molecular and Vascular Biology, Campus Gasthuisberg, Herestraat 49, 3000 Leuven, Belgium; e-mail: bart.degeest@med.kuleuven.ac.be.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal