A novel murine system was developed to study the in vivo localization of xenotransplanted human cells and assess their therapeutic effect in an authentic model of disease. The β-glucuronidase (GUSB) mutation of the mucopolysaccharidosis type VII (MPSVII) mouse was backcrossed onto the nonobese diabetic/severe combined immunodeficient (NOD/SCID) xenotransplantation strain. The resulting NOD/SCID/MPSVII mice displayed the characteristic features of lysosomal storage disease because of GUSB deficiency and were also capable of engrafting human cells. Human CD34+hematopoietic progenitor cells from healthy, GUSB+donors engrafted NOD/SCID/MPSVII mice in a manner similar to that of standard NOD/SCID mice. Six to 12 weeks following transplantation, 1% to 86% of the host bone marrow was positive for human CD45. By using a GUSB-specific histochemical assay, human engraftment was detected with single-cell sensitivity not only in well-characterized hematopoietic tissues like bone marrow, spleen, lymph node, and thymus, but also in other nonhematopoietic organs like liver, kidney, lung, heart, brain, and eye. Quantitative measurements of GUSB activity confirmed this expansive tissue distribution. The GUSB-specific assays were validated for their accuracy in identifying human cells through colocalization of human CD45 expression with GUSB activity in tissues of mice receiving transplants. An analysis of the therapeutic effects of engrafted human cells revealed a reduction of pathologic storage material in host organs, including the bone, spleen, and liver. Such xenotransplantation experiments in the NOD/SCID/MPSVII mouse represent a powerful approach to both study the in vivo biology of human cells and gather preclinical data regarding treatment approaches for a human disease.
Introduction
Xenotransplantation into immunodeficient animals like the nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse has produced valuable data on the biology of human cells.1 In the case of human hematopoietic progenitor cells, such in vivo assays provide a stringent test for stem cell properties through the production of both human lymphoid and myeloid cells over long periods of time.2,3 Human hematopoietic engraftment in these assays has been well characterized in tissues of NOD/SCID recipients like the bone marrow, spleen, and blood. However, tracking of human cells to other nonhematopoietic organs like the lung or liver has typically focused on the first few days after transplantation.4-6 At later time points, such information has been limited to flow cytometric analysis of a few major organs.7
In an effort to gather more detailed data on the in vivo localization of xenotransplanted human cells, a novel NOD/SCID mouse with a deficiency in β-glucuronidase (GUSB) activity was generated. GUSB is a lysosomal enzyme that is expressed in virtually all cell types. On transplantation into a GUSB-deficient host, cells from a healthy donor can be identified by virtue of their GUSB expression with a number of sensitive techniques. A simple histochemical assay specific for GUSB activity allows the precise in situ detection of individual positive donor cells.8,9 A biochemical assay specific for GUSB activity permits quantitation of donor enzyme in tissue homogenates.10 Finally, a fluorogenic substrate for GUSB enables the flow cytometric measurement of enzyme activity.11 In immunocompetent GUSB-deficient mice, these assays have been used to study the localization of transplanted murine and rat cell types such as T cells,12macrophages,13 neuronal progenitor cells,14amniotic epithelial cells,15 and highly purified hematopoietic stem cells.16 In the present study, a GUSB-deficient NOD/SCID mouse allowed similarly powerful studies with human CD34+ hematopoietic progenitor cells.
Xenotransplantation assays also provide an excellent opportunity to examine the therapeutic uses of human cells in vivo. In the experiments described here, GUSB deficiency results in the lysosomal storage disease mucopolysaccharidosis type VII (MPSVII).17 In the absence of GUSB activity, partially degraded glycosaminoglycans progressively accumulate in tissues throughout the body. The GUSB-deficient MPSVII mouse shares most of the clinical manifestations seen in diseased human patients, including reduced life span, skeletal dysplasia, mental retardation, and auditory and visual impairment.18-23
The MPSVII mouse has been a powerful tool for the study of the biology of the disease and the development of novel therapies for this disorder. Transplantation of syngeneic bone marrow from a normal mouse into MPSVII mice has been shown to reverse pathologic storage lesions in many tissues through a phenomenon known as “cross-correction.”24,25 In this process, lysosomal enzymes are released in small quantities from normal cells and subsequently taken up by deficient cells through either mannose receptor– or mannose 6-phosphate receptor–mediated salvage pathways.26-28 In MPSVII mice transplanted with normal syngeneic bone marrow cells, cross-correction allows the widely distributed GUSB+ cells to locally deliver the enzyme to tissues throughout the body. The resultant reduction of lysosomal storage material has been shown to yield measurable improvements in functional parameters, including longevity and visual and auditory perception.22-25 In the current study, a GUSB-deficient NOD/SCID mouse allowed a similar assessment of the therapeutic effects of transplanted human cells.
The experiments presented here introduce a novel GUSB-deficient MPSVII model congenic with the NOD/SCID xenotransplantation strain (NOD/SCID/MPSVII). Six to 12 weeks following transplantation of human CD34+ hematopoietic progenitor cells into these mice, large numbers of donor-derived, GUSB+ cells were identified in tissues throughout the body. In addition, a substantial therapeutic effect of these engrafted cells was evident through the reduction of storage material in several host organs. This study demonstrates the utility of the NOD/SCID/MPSVII mouse in exploring the in vivo biology of human cells as well as in directly evaluating therapeutic strategies for lysosomal storage disease involving human cell transplantation.
Materials and methods
Development of the NOD/SCID/MPSVII mouse
NOD/LtSz-scid (NOD/SCID)29 and B6.C-H-2bm1/ByBir-gusmps+(MPSVII)18 mice were purchased from the Jackson Laboratories (Bar Harbor, ME) and bred in a colony maintained by M.S.S. at Washington University School of Medicine (St Louis, MO). All mice were kept on high-fat feed (PicoLab Mouse Chow 20-5058; PMI, St Louis, MO). NOD/SCID mice were also given water supplemented with trimethoprim and sulfamethoxazole for 3 consecutive days a week.30 The mutant GUSB allele31 of the MPSVII mouse was backcrossed 10 generations from its original B6.C-H-2bm1strain onto the NOD/SCID mouse. For all backcrosses female mice heterozygous for the MPSVII mutation (MPSVII+/−) were identified by a specific polymerase chain reaction (PCR) assay32 and bred to NOD/SCID males. At the fifth backcross, the scid mutation was found to be homozygous by a specific PCR assay.33 At the 10th backcross generation, NOD/SCID/MPSVII+/− mice were interbred, and offspring homozygous for the GUSB mutation (NOD/SCID/MPSVII) were identified by a biochemical assay.10 Control NOD/SCID mice raised in a distinct breeding colony were used in the comparisons with NOD/SCID/MPSVII mice.
Human cell preparation
Fresh human cord blood and mobilized peripheral blood samples were obtained from healthy donors at Cardinal Glennon Hospital (St Louis, MO) and Barnes-Jewish Hospital (St Louis, MO), respectively, through informed consent and according to institutional procedures. Cord blood samples were diluted 1:3 in phosphate-buffered saline (PBS) with 2 mM EDTA (ethylenediaminetetraacetic acid) and separated on Hypaque (Sigma, St Louis, MO) prior to CD34+ cell isolation. Three to 4 cord blood samples were pooled per prep to reduce potential donor variability. A single mobilized peripheral blood pheresis product from an adult donor was used for CD34+ cell purification. In both cases, magnetic cell-sorting (MACS) CD34 Multi-Sort kits (Miltenyi, Auburn, CA) were used to yield cell populations that were more than 90% CD34+ as assessed by flow cytometry and more than 95% viable as determined by Trypan Blue exclusion.
Transplantation protocol
Fresh cord blood CD34+ cells in PBS were injected immediately following purification at a dose of 5 × 105per mouse. Mobilized peripheral blood CD34+ cells were frozen in autologous plasma with 10% dimethyl sulfoxide (DMSO) and stored in liquid-phase nitrogen until use. These cells were rapidly thawed at 37°C, diluted 1:1 in PBS, and then injected at a dose of 1 × 106 total cells per mouse. An average of 65% of these cells were viable after the thawing and dilution as assessed by Trypan Blue exclusion. All injections were performed through the lateral tail veins of mice 6 to 8 weeks of age that had been conditioned with 300 rad of γ-radiation from a 137Cs source 10 to 16 hours previously. Mice also received an intraperitoneal injection of 1 μg daniplestim (gift from Pharmacia, Peapack, NJ) at the time of irradiation, immediately following injection of cells, and then 3 times weekly throughout the duration of the study. Daniplestim is an agonist of the human interleukin 3 (IL-3) receptor.34 Mice were analyzed with the assays described at 6 and 12 weeks after transplantation.
Flow cytometric analysis
Single-cell suspensions of peripheral blood leukocytes, bone marrow, and splenocytes were collected from animals at the time of analysis. Red blood cells were lysed in 154 mM ammonium chloride, 10 mM potassium bicarbonate, and 0.1 mM EDTA at 37°C, and samples were subsequently stored on ice in PBS containing 0.5% bovine serum albumin (BSA) and 2 mM EDTA (fluorescence-activated cell sorter [FACS] buffer). Cells at a concentration of 5 × 106/mL in FACS buffer were blocked with human immunoglobulin and antimouse FcR block (antimouse CD16/CD32 monoclonal antibody [mAb]; Caltag, Burlingame, CA) for 10 minutes on ice. These cells were then stained in multiple reactions that all included a phycoerythrin (PE)–conjugated antibody to human CD45 (Miltenyi). The other antibodies used in these reactions included fluorescein isothiocyanate (FITC)–labeled mAbs against human CD19 and CD66b (Immunotech, Marseille, France) and Tri-Color–labeled mAbs against human CD3 and CD14 (Caltag). An additional reaction with PE-labeled isotype antibody was performed to control for CD45 staining. Human cells were identified by specific CD45 staining and gated to plots for analysis of the other lineage markers. Appropriate isotype control antibodies for the lineage markers were used in a separate reaction containing the PE-labeled anti-CD45 mAb. Background signal in the isotype quadrants was subtracted from the staining of the lineage markers for purposes of quantitative analysis. Bone marrow samples from certain highly engrafted mice were also stained with PE-labeled antibody against human CD34 and FITC-labeled antibody against human glycophorin A (Immunotech). Tissues from a mouse that did not receive transplants were stained with all of the above antibodies as a negative control. All staining reactions proceeded for 30 minutes on ice and were washed twice in FACS buffer before reading on a FACScan (Becton Dickinson, San Jose, CA). Analysis was performed using CELLquest software (Becton Dickinson). Statistical analysis of lineage engraftment data was performed by using a 2-tailed t test with equal variance.
For flow cytometric measurement of GUSB+ cells, red cell–free preparations of bone marrow, spleen, and peripheral blood cells were suspended at a concentration of 1 × 106cells/mL in Iscoves modified Dulbecco media containing 2% BSA and 100 μM ImaGene Green C12-FDGlcU substrate (Molecular Probes, Eugene, OR). Cells from NOD/SCID/MPSVII mice that did not receive transplants were assayed as a negative control. Samples were incubated at 37°C in a 5% CO2 atmosphere for 2.5 hours and then washed once in cold FACS buffer. Cell pellets were resuspended in FACS buffer and then incubated with the PE-labeled antihuman CD45 antibody or PE-isotype antibody as described earlier.
GUSB-specific histochemical assay
Portions of sternum, spleen, liver, kidney, lungs, heart, thymus, mesenteric lymph node, brain, eye, and skeletal muscle from NOD/SCID/MPSVII mice that did and did not receive transplants were frozen in optimal cutting temperature (OCT) embedding medium (Sakura, Torrance, CA) for histochemical analysis. Sections were made at a thickness of 10 μm and stained for enzymatically active GUSB as previously described by using naphthol-AS-BI-β-D-glucuronide (Sigma) as a substrate.32 Slides were subsequently counterstained with methyl green.
GUSB-specific biochemical assay
Samples of peripheral blood leukocytes, plasma, bone marrow, spleen, liver, kidney, lungs, heart, thymus, mesenteric lymph node, brain, and skeletal muscle from NOD/SCID/MPSVII mice that did and did not receive transplants were homogenized with a motorized pestle in 10 mM Tris (tris(hydroxymethyl)aminomethane) (pH7.5), 150 mM NaCl, 0.2% Triton X-100, and 1 mM dithiothreitol. Homogenates were centrifuged in a microfuge at 14 000 rpm for 1 minute to remove debris. A portion of each homogenate was incubated at 37°C for 1 to 12 hours in 100 μL 5 mM 4-MU-β-D-glucuronide substrate (Sigma). Reactions were stopped with 0.1 M sodium carbonate and then assayed fluorometrically as previously described.32 The protein concentrations of the homogenates were measured by using the Coomassie dye binding assay (Bio-Rad, Hercules, CA).35 Specific activity (1 unit = 1 nmole substrate cleaved/hour/mg protein) was calculated for each tissue. Specific activities from the tissues of animals that did not receive transplants were subtracted from those of the engrafted tissues to correct for background fluorescence.
Immunohistochemistry
Frozen sections identical to those used for the histochemical assay were taken from the liver, spleen, lymph nodes, thymus, kidney, and lung of NOD/SCID and NOD/SCID/MPSVII mice that did and did not receive transplants. Sections were fixed for 15 minutes in acetone at 4°C and blocked at room temperature in PBS with 10% goat serum, 1% BSA, 0.2% powdered milk (blocking buffer) supplemented with papain-treated antimouse immunoglobins prepared as previously described.36 Unconjugated mouse antihuman CD45 antibody (Anti-HLe-1; Becton Dickinson) diluted 1/200 in blocking buffer was applied as a primary antibody. Secondary antibody consisting of an alkaline phosphatase-conjugated goat antimouse immunoglobulin antibody (Sigma) was followed with commercially available alkaline phosphatase development reagents (Vector Labs, Burlingame, CA). Certain NOD/SCID/MPSVII tissues were first immunostained for human CD45 and then subsequently stained for GUSB activity histochemically.13 In these cases, the alkaline phosphatase development reagent was rinsed off with water and sections were allowed to equilibrate in 50 mM sodium acetate (pH 4.5) for 20 minutes at 4°C prior to GUSB histochemical staining.
Histopathologic analysis
Portions of the ribs, spleen, liver, kidney, lung, heart, brain, and eye from NOD/SCID/MPSVII mice that received transplants, received mock transplants, and did not receive transplants were fixed in 4% paraformaldehyde and 2% glutaraldehyde in PBS prior to embedding in Spurr resin. Sections of 0.5 μm thickness were taken and stained with toluidine blue.19 Slides were then examined microscopically for the appearance of lysosomes.
Results
NOD/SCID/MPSVII mice
The NOD/SCID/MPSVII mouse was produced through 10 backcrosses of the MPSVII mutation from its original B6.C-H-2bm1 strain onto the NOD/SCID mouse. GUSB activity was undetectable in tissues from NOD/SCID/MPSVII mice using a specific histochemical stain (data not shown). NOD/SCID/MPSVII mice demonstrated similar phenotypic characteristics as B6.C-H-2bm1 MPSVII mice, including smaller size, a blunted face, shortened tail and limbs, and deafness. An average life span of 220 ± 53 days was calculated from a group of 17 NOD/SCID/MPSVII mice, a value comparable to that reported for NOD/SCID mice.29 A histopathologic survey of NOD/SCID/MPSVII mice revealed pronounced lysosomal distention in tissues throughout the body in a pattern similar to that seen in B6.C-H-2bm1MPSVII mice (data not shown). For example, most cells in the brain of NOD/SCID/MPSVII mice, both neuronal and non-neuronal, had visible lysosomal distention, much like in the parental MPSVII brain. Perivascular and glial cells had higher levels of storage than neurons, whereas hippocampal neurons showed more lysosomal distention than cortical neurons. However, some subtle differences in the histopathology between the 2 strains were observed. The level of lysosomal storage in the tubules and glomeruli of the NOD/SCID/MPSVII kidney, for example, was slightly less severe than that of the parental MPSVII strain.
After the 10th backcross, NOD/SCID/MPSVII mice received transplants of human CD34+ cells from both cord blood and mobilized peripheral blood sources. Flow cytometric analysis of the levels of human cells in the bone marrow 6 or 12 weeks after transplantation revealed that offspring from 6 of a total of 18 mating pairs supported engraftment. Mice were considered engrafted if at least 0.5% of the bone marrow cells were positive for human CD45 by flow cytometric measurement. Because the scid mutation was determined by PCR to be homozygous at the fifth backcross, the relatively small number of permissive lines suggests that a NOD allele necessary for the engraftment of human hematopoietic progenitor cells may be closely linked to the MPSVII locus. When a mating pair produced NOD/SCID/MPSVII offspring that engrafted with human cells, all animals tested from that cross were included in the experiments that follow. Those permissive crosses have since been propagated and have continued to produce mice capable of engrafting human cells. Lines that did not support human engraftment were terminated and any transplantation results from them were not included in the present study.
Engraftment of human CD34+ cells in NOD/SCID/MPSVII mice
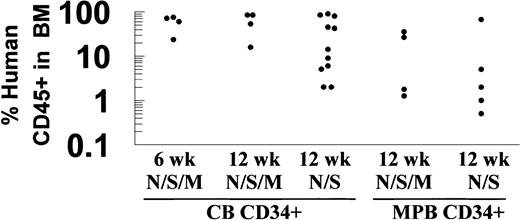
Flow cytometric analysis for human CD45 was used to compare NOD/SCID/MPSVII mice with standard NOD/SCID mice in their ability to engraft human CD34+ cells from cord blood and mobilized peripheral blood (Figure 1). A similar level of human cell engraftment was evident in the bone marrow of NOD/SCID and NOD/SCID/MPSVII mice 12 weeks following transplantation of cord blood CD34+ cells (34.2% ± 33.6% in NOD/SCID, 60.3% ± 28.9% in NOD/SCID/MPSVII) or mobilized peripheral blood CD34+ cells (14.9% ± 25.6% in NOD/SCID, 16.3% ± 17.5% in NOD/SCID/MPSVII).
Human CD34+ cells engrafted NOD/SCID/MPSVII mice at levels comparable with those of NOD/SCID mice.
NOD/SCID (N/S) and NOD/SCID/MPSVII (N/S/M) mice received transplants of human CD34+ cells from cord blood (CB) or mobilized peripheral blood (MPB) sources. The percentage of cells expressing human CD45 in the bone marrow of these mice was determined by flow cytometry 6 or 12 weeks following transplantation. Each dot represents data from a single mouse.
Human CD34+ cells engrafted NOD/SCID/MPSVII mice at levels comparable with those of NOD/SCID mice.
NOD/SCID (N/S) and NOD/SCID/MPSVII (N/S/M) mice received transplants of human CD34+ cells from cord blood (CB) or mobilized peripheral blood (MPB) sources. The percentage of cells expressing human CD45 in the bone marrow of these mice was determined by flow cytometry 6 or 12 weeks following transplantation. Each dot represents data from a single mouse.
More detailed flow cytometric analysis of the levels of human lymphoid and myeloid populations in engrafted mice was also conducted (Figure2). A gate encompassing human CD45+ cells was drawn to exclude most of the autofluorescence from unstained cells (G1 of Figure 2A-C). Although this gate captured most CD45+ events, a minor population of cells low in side scatter and dim for CD45 expression was excluded in the bone marrow of highly engrafted animals. A portion of this excluded population stained positively for human glycophorin A and thus may represent human erythroid progenitors (data not shown). Cells positive for human CD45 were gated to plots that surveyed CD19, CD3, CD14, and CD66b expression (Figure 2D-F). Substantial CD19+ B-cell populations were present in all engrafted animals. Staining for the monocytic marker CD14 and granulocytic marker CD66b typically yielded several myeloid subpopulations. For purposes of analysis, quadrants were drawn to divide these populations into regions that were CD66b+/CD14−, CD66b−/CD14+, and CD66b+/CD14+. Dual CD66b+/CD14+ myeloid populations were seen only in the bone marrow of mice with very high levels of human cells (> 40% CD45+). CD66b+/CD14−granulocyte populations made up a substantial proportion of the bone marrow in these highly engrafted animals. Animals with less engraftment (< 40% CD45+) had lower proportions of human granulocytes with a compensatory increase in the proportion of CD19+ cells. No detectable CD3+ cells were found in any animal tested. Staining of highly engrafted bone marrow samples for human CD34 also revealed measurable populations of positive cells in both NOD/SCID and NOD/SCID/MPSVII mice (data not shown).
Transplantation of human CD34+ cells into NOD/SCID/MPSVII mice led to engraftment of both lymphoid and myeloid lineages.
Bone marrow from a NOD/SCID/MPSVII mouse 12 weeks after the transplantation of 5 × 105 CD34+ cells from human cord blood was analyzed by flow cytometry. (A) A gate (G1) was drawn to exclude most of the positive cells in a sample stained with an isotype control for an antihuman CD45 antibody. (B) Staining for human CD45 showed 86.7% of bone marrow cells to fall within G1. Such positive cells were gated to the lower set of plots for analysis with additional lineage markers and isotype controls. (C) The specificity of the antibody used against human CD45 was demonstrated by a lack of staining of bone marrow from a NOD/SCID/MPSVII mouse that did not receive a transplant. (D) Human CD45+ cells gated from G1 exhibited minimal staining with isotype control antibodies for lineage markers. (E) A human CD19+ population but no human CD3+ cells were seen in G1-gated human CD45+cells. (F) Several populations expressing either human CD66b alone, human CD14 alone, or both markers together were evident in G1-gated human CD45+ cells.
Transplantation of human CD34+ cells into NOD/SCID/MPSVII mice led to engraftment of both lymphoid and myeloid lineages.
Bone marrow from a NOD/SCID/MPSVII mouse 12 weeks after the transplantation of 5 × 105 CD34+ cells from human cord blood was analyzed by flow cytometry. (A) A gate (G1) was drawn to exclude most of the positive cells in a sample stained with an isotype control for an antihuman CD45 antibody. (B) Staining for human CD45 showed 86.7% of bone marrow cells to fall within G1. Such positive cells were gated to the lower set of plots for analysis with additional lineage markers and isotype controls. (C) The specificity of the antibody used against human CD45 was demonstrated by a lack of staining of bone marrow from a NOD/SCID/MPSVII mouse that did not receive a transplant. (D) Human CD45+ cells gated from G1 exhibited minimal staining with isotype control antibodies for lineage markers. (E) A human CD19+ population but no human CD3+ cells were seen in G1-gated human CD45+cells. (F) Several populations expressing either human CD66b alone, human CD14 alone, or both markers together were evident in G1-gated human CD45+ cells.
The measurements of the flow cytometric analysis on the bone marrow, spleen, and peripheral blood of animals receiving transplants are compiled in Table 1. No significant differences in the levels of the human CD45+ cells were found between NOD/SCID/MPSVII and NOD/SCID mice for any of the tissues tested. The proportion of other human lineage markers is expressed as the percentage of the human CD45+ cells in Table 1. All mice displayed both human lymphoid and myeloid cell types in all tissues. Small but statistically significant differences in the proportions of human cells that were CD19+, CD66b+/CD14−, and CD66b+/CD14+ were evident between the bone marrow of NOD/SCID and NOD/SCID/MPSVII mice 12 weeks after transplantation with cord blood CD34+ cells. In these circumstances, NOD/SCID/MPSVII mice had a slightly increased proportion of CD66b+ populations and fewer CD19+ cells than NOD/SCID mice. No significant differences between NOD/SCID/MPSVII and NOD/SCID mice were detected in transplants with mobilized peripheral blood CD34+ cells.
Expression of human lineage markers in the bone marrow, spleen, and peripheral blood of NOD/SCID and NOD/SCID/MPSVII mice at 6 and 12 weeks after transplantation of cord blood CD34+cells or mobilized peripheral blood CD34+ cells
| . | CD45+ . | % of Human CD45+ cells . | ||||
|---|---|---|---|---|---|---|
| CD19+ . | CD14+/CD66b− . | CD66b+/CD14− . | CD66b+/CD14+ . | Undetermined . | ||
| Cord blood CD34+cells | ||||||
| NOD/SCID/MPSVII | ||||||
| 6 wk | ||||||
| BM | 57.9 ± 20.4 | 54.8 ± 6.9 | 4.8 ± 1.1 | 12.9 ± 8.5 | 0.4 ± 0.2 | 27.3 ± 5.9 |
| SPL | 18.8 ± 14.5 | 82.5 ± 5.0 | 1.9 ± 1.0 | 0.6 ± 0.4 | 0 | 15.0 ± 4.9 |
| PB | 10.4 ± 7.5 | 65.5 ± 6.8 | 7.0 ± 4.2 | 1.4 ± 0.4 | 0 | 26.1 ± 3.3 |
| 12 wk | ||||||
| BM | 60.3 ± 28.9 | 42.3 ± 15.5* | 6.4 ± 1.6 | 29.8 ± 19.5† | 1.2 ± 0.7‡ | 20.5 ± 4.4 |
| SPL | 29.5 ± 18.7 | 79.6 ± 4.0 | 1.5 ± 0.9 | 0.6 ± 0.4 | 0 | 18.1 ± 3.4 |
| PB | 10.1 ± 8.0 | 58.5 ± 4.0 | 8.4 ± 3.1 | 1.5 ± 1.1 | 0 | 31.6 ± 4.0 |
| NOD/SCID | ||||||
| 12 wk | ||||||
| BM | 34.2 ± 33.6 | 59.7 ± 11.7* | 7.0 ± 5.0 | 9.1 ± 9.0† | 0.3 ± 0.4‡ | 24.0 ± 6.8 |
| SPL | 26.0 ± 28.2 | 83.9 ± 7.2 | 1.3 ± 0.7 | 0.4 ± 0.5 | 0 | 14.5 ± 7.0 |
| PB | 9.0 ± 13.3 | 61.9 ± 11.3 | 6.1 ± 2.7 | 0.9 ± 0.8 | 0 | 31.0 ± 11.0 |
| Mobilized peripheral blood CD34+cells | ||||||
| NOD/SCID/MPSVII | ||||||
| 12 wk | ||||||
| BM | 16.3 ± 17.5 | 52.3 ± 13.6 | 9.6 ± 5.9 | 4.8 ± 6.9 | 0.1 ± 0.3 | 33.3 ± 6.7 |
| SPL | ND | ND | ND | ND | ND | ND |
| PB | 1.4 ± 1.1 | 56.5 ± 8.5 | 10.5 ± 3.7 | 0.8 ± 1.0 | 0 | 32.3 ± 5.1 |
| NOD/SCID | ||||||
| 12 wk | ||||||
| BM | 14.9 ± 25.6 | 55.6 ± 11.8 | 6.4 ± 3.6 | 3.1 ± 4.3 | 0.2 ± 0.2 | 30.8 ± 11.2 |
| SPL | ND | ND | ND | ND | ND | ND |
| PB | 2.6 ± 4.9 | 57.3 ± 6.3 | 9.7 ± 6.5 | 0.3 ± 0.5 | 0 | 32.7 ± 5.7 |
| . | CD45+ . | % of Human CD45+ cells . | ||||
|---|---|---|---|---|---|---|
| CD19+ . | CD14+/CD66b− . | CD66b+/CD14− . | CD66b+/CD14+ . | Undetermined . | ||
| Cord blood CD34+cells | ||||||
| NOD/SCID/MPSVII | ||||||
| 6 wk | ||||||
| BM | 57.9 ± 20.4 | 54.8 ± 6.9 | 4.8 ± 1.1 | 12.9 ± 8.5 | 0.4 ± 0.2 | 27.3 ± 5.9 |
| SPL | 18.8 ± 14.5 | 82.5 ± 5.0 | 1.9 ± 1.0 | 0.6 ± 0.4 | 0 | 15.0 ± 4.9 |
| PB | 10.4 ± 7.5 | 65.5 ± 6.8 | 7.0 ± 4.2 | 1.4 ± 0.4 | 0 | 26.1 ± 3.3 |
| 12 wk | ||||||
| BM | 60.3 ± 28.9 | 42.3 ± 15.5* | 6.4 ± 1.6 | 29.8 ± 19.5† | 1.2 ± 0.7‡ | 20.5 ± 4.4 |
| SPL | 29.5 ± 18.7 | 79.6 ± 4.0 | 1.5 ± 0.9 | 0.6 ± 0.4 | 0 | 18.1 ± 3.4 |
| PB | 10.1 ± 8.0 | 58.5 ± 4.0 | 8.4 ± 3.1 | 1.5 ± 1.1 | 0 | 31.6 ± 4.0 |
| NOD/SCID | ||||||
| 12 wk | ||||||
| BM | 34.2 ± 33.6 | 59.7 ± 11.7* | 7.0 ± 5.0 | 9.1 ± 9.0† | 0.3 ± 0.4‡ | 24.0 ± 6.8 |
| SPL | 26.0 ± 28.2 | 83.9 ± 7.2 | 1.3 ± 0.7 | 0.4 ± 0.5 | 0 | 14.5 ± 7.0 |
| PB | 9.0 ± 13.3 | 61.9 ± 11.3 | 6.1 ± 2.7 | 0.9 ± 0.8 | 0 | 31.0 ± 11.0 |
| Mobilized peripheral blood CD34+cells | ||||||
| NOD/SCID/MPSVII | ||||||
| 12 wk | ||||||
| BM | 16.3 ± 17.5 | 52.3 ± 13.6 | 9.6 ± 5.9 | 4.8 ± 6.9 | 0.1 ± 0.3 | 33.3 ± 6.7 |
| SPL | ND | ND | ND | ND | ND | ND |
| PB | 1.4 ± 1.1 | 56.5 ± 8.5 | 10.5 ± 3.7 | 0.8 ± 1.0 | 0 | 32.3 ± 5.1 |
| NOD/SCID | ||||||
| 12 wk | ||||||
| BM | 14.9 ± 25.6 | 55.6 ± 11.8 | 6.4 ± 3.6 | 3.1 ± 4.3 | 0.2 ± 0.2 | 30.8 ± 11.2 |
| SPL | ND | ND | ND | ND | ND | ND |
| PB | 2.6 ± 4.9 | 57.3 ± 6.3 | 9.7 ± 6.5 | 0.3 ± 0.5 | 0 | 32.7 ± 5.7 |
ND indicates not determined.
Statistically significant difference between the CD19+ population in the bone marrow of NOD/SCID and NOD/SCID/MPSVII mice at 12 weeks after transplantation (P = .049).
Statistically significant difference between the CD66b+/CD14− population in the bone marrow of NOD/SCID and NOD/SCID/MPSVII mice at 12 weeks after transplantation (P = .022).
Statistically significant difference between the CD66b+/CD14+ population in the bone marrow of NOD/SCID and NOD/SCID/MPSVII at 12 weeks after transplantation (P = .015).
Distribution of GUSB+ cells in recipient NOD/SCID/MPSVII mice
Organs from the 12 NOD/SCID/MPSVII mice that received transplants were sectioned and examined histochemically for the presence of human cells. Because essentially all human cell types are GUSB+, expression of this enzyme provides an effective means of tracking human cells in the GUSB− background of the NOD/SCID/MPSVII mouse. Tissues from NOD/SCID/MPSVII mice that did not receive transplants were completely devoid of GUSB+ cells by this method (data not shown). Histochemically stained sections of NOD/SCID/MPSVII mice that received transplants revealed numerous GUSB+ cells in many tissues (Figure3). The bone marrow and spleen revealed numbers of red-staining GUSB+ cells at a level concordant with flow cytometric measurements of human CD45. Other hematopoietic tissues such as the lymph nodes and thymus also contained substantial numbers of GUSB+ cells. Interestingly, a variety of nonhematopoietic organs not normally analyzed in such xenotransplantation experiments were heavily engrafted. The liver in particular was homogeneously infiltrated with GUSB+ cells. The kidney and lung contained a regular but less numerous distribution of positive cells. Scattered GUSB+ cells were also present in areas of the heart, at regular intervals in the meninges, and in close association with the retinal pigment epithelial layer. Additionally, GUSB staining was detected in rare cells at other sites such as the brain parenchyma, skeletal muscle, and pancreas (data not shown).
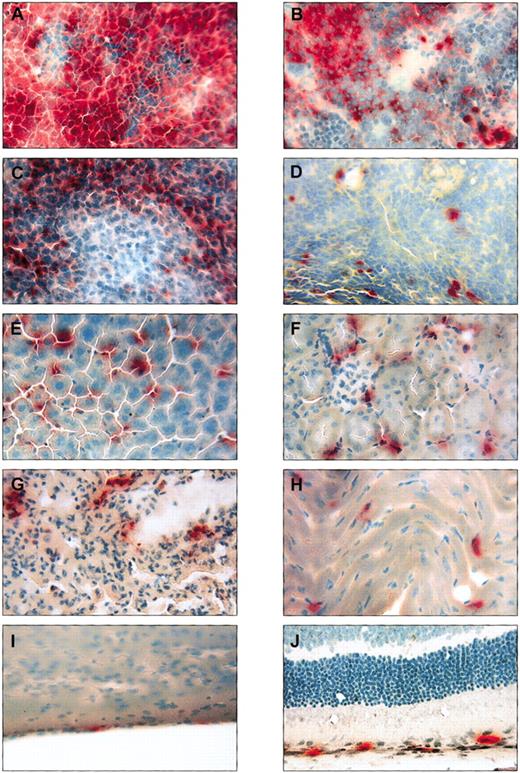
Histochemical staining for GUSB revealed positive cells in many tissues throughout NOD/SCID/MPSVII mice that received transplants of human CD34+ cells.
Tissues from a single NOD/SCID/MPSVII mouse 12 weeks following the transplantation of human CD34+ cells from cord blood are shown stained for GUSB activity (red color) and counterstained with methyl green (blue-green color). The bone marrow (A) and spleen (B) contained high levels of positive cells that correlated with a flow cytometric measurement of human CD45 expression (87% human CD45+ in the bone marrow and 61% human CD45+in the spleen). Groups of GUSB+ cells were visible throughout the lymph node (C), whereas scattered GUSB+cells were evident in the thymus (D). The liver (E) was uniformly infiltrated with GUSB+ cells, and the kidney (F) and lung (G) contained a less dense pattern of positive cells. GUSB staining was also seen in scattered cells in the heart (H), at regular intervals in the meninges (I), and in association with the pigment epithelial layer of the retina (J). Original magnification, × 250.
Histochemical staining for GUSB revealed positive cells in many tissues throughout NOD/SCID/MPSVII mice that received transplants of human CD34+ cells.
Tissues from a single NOD/SCID/MPSVII mouse 12 weeks following the transplantation of human CD34+ cells from cord blood are shown stained for GUSB activity (red color) and counterstained with methyl green (blue-green color). The bone marrow (A) and spleen (B) contained high levels of positive cells that correlated with a flow cytometric measurement of human CD45 expression (87% human CD45+ in the bone marrow and 61% human CD45+in the spleen). Groups of GUSB+ cells were visible throughout the lymph node (C), whereas scattered GUSB+cells were evident in the thymus (D). The liver (E) was uniformly infiltrated with GUSB+ cells, and the kidney (F) and lung (G) contained a less dense pattern of positive cells. GUSB staining was also seen in scattered cells in the heart (H), at regular intervals in the meninges (I), and in association with the pigment epithelial layer of the retina (J). Original magnification, × 250.
Although sections from a highly engrafted mouse are presented in Figure3, mice with a lower level of human reconstitution exhibited a similar distribution pattern, albeit with proportionately fewer cells (data not shown). A similar pattern of GUSB+ cells was also seen in mice that were engrafted with CD34+ cells purified from either mobilized peripheral blood or cord blood 12 weeks following transplantation. In addition, the tissue distribution of GUSB+ cells was comparable between animals analyzed at 6 or 12 weeks after the transplantation of cord blood CD34+cells. Furthermore, tissues from wild-type NOD/SCID mice stained immunohistochemically for human CD45 showed a widespread distribution of positive cells similar to the pattern of GUSB+ cells in NOD/SCID/MPSVII mice (data not shown).
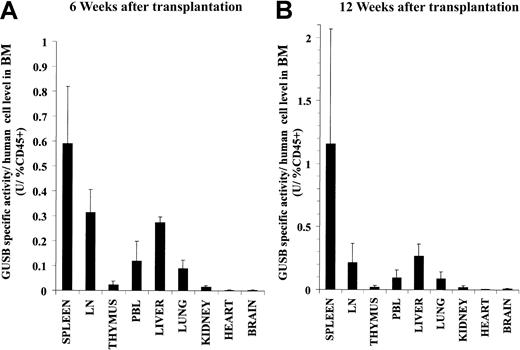
To quantitatively determine the distribution of GUSB+ cells in NOD/SCID/MPSVII mice that received transplants, a fluorometric assay was used to measure the GUSB-specific activity in tissue homogenates. Because of the widely varying levels of engraftment between individual animals, the specific activity values were normalized to facilitate comparisons. For this adjustment, the GUSB-specific activities in the tissues of each mouse were divided by the percentage of human CD45+ cells measured in the respective mouse's bone marrow by flow cytometry. Such normalized data from groups of mice could then be averaged to get a relative distribution pattern of GUSB activity in recipient tissues. As expected, at 6 and 12 weeks after cord blood CD34+ cell transplantation, a similar level of enzyme-specific activity per percentage of human CD45+engraftment was found in the bone marrow (6 weeks = 6.5 ± 2.3 U/percentage of CD45+, 12 weeks = 7.3 ± 2.9 U/percentage of CD45+). Adjusting the specific activities of other tissues to the percentage of bone marrow CD45+ engraftment revealed relative proportions of GUSB activity that were several fold lower than that of the bone marrow itself (Figure4). Similar distribution patterns of adjusted enzyme activity were found at both 6 and 12 weeks following transplantation. The lymph nodes, spleen, and liver contained the highest proportions of enzyme following that of the bone marrow. The peripheral blood, lungs, kidney, and thymus had relatively lower but substantial levels of GUSB activity. The brain, heart, and skeletal muscle (data not shown) had very low but detectable proportions of enzyme. No mouse had detectable levels of GUSB activity in the plasma at any time point. NOD/SCID/MPSVII mice analyzed 12 weeks after transplantation with mobilized peripheral blood CD34+ cells showed a distribution of GUSB activity similar to that found for cord blood CD34+ cells (data not shown). The biochemical measurement of engrafted cells correlated with the histochemical data, in that tissues heavily infiltrated with GUSB+ cells as examined in situ had relatively high proportions of GUSB-specific activity.
A biochemical assay for GUSB activity revealed a similar widespread distribution of enzyme in the tissues of NOD/SCID/MPSVII mice at both 6 and 12 weeks following transplantation of human CD34+ cells.
The tissues of NOD/SCID/MPSVII mice that received transplants of human C34+ cells derived from cord blood were analyzed 6 (A) or 12 (B) weeks later with the GUSB-specific biochemical assay. The GUSB-specific activities (U = nmol substrate cleaved/hour/mg protein) for the tissues of each mouse were divided by the percentage of human CD45+ cells in the bone marrow of the respective mouse. The adjusted values from 4 mice at each time point were averaged and are shown with the standard error. Data are presented for the spleen, mesenteric lymph nodes (LN), thymus, peripheral blood leukocytes (PBL), liver, lung, kidney, heart, and brain. The bone marrow of these mice had an average of 6.5 ± 2.3 U/percentage of CD45+ and 7.3 ± 2.9 U/percentage of CD45+ at 6 and 12 weeks following transplantation, respectively.
A biochemical assay for GUSB activity revealed a similar widespread distribution of enzyme in the tissues of NOD/SCID/MPSVII mice at both 6 and 12 weeks following transplantation of human CD34+ cells.
The tissues of NOD/SCID/MPSVII mice that received transplants of human C34+ cells derived from cord blood were analyzed 6 (A) or 12 (B) weeks later with the GUSB-specific biochemical assay. The GUSB-specific activities (U = nmol substrate cleaved/hour/mg protein) for the tissues of each mouse were divided by the percentage of human CD45+ cells in the bone marrow of the respective mouse. The adjusted values from 4 mice at each time point were averaged and are shown with the standard error. Data are presented for the spleen, mesenteric lymph nodes (LN), thymus, peripheral blood leukocytes (PBL), liver, lung, kidney, heart, and brain. The bone marrow of these mice had an average of 6.5 ± 2.3 U/percentage of CD45+ and 7.3 ± 2.9 U/percentage of CD45+ at 6 and 12 weeks following transplantation, respectively.
Validation of GUSB-specific assays
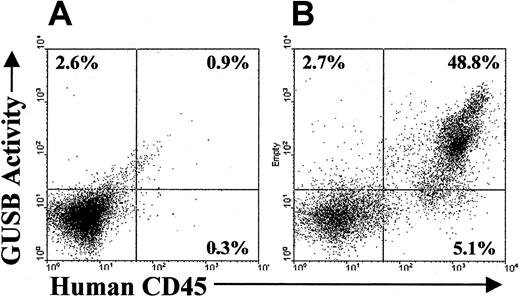
As a lysosomal enzyme, a small fraction of donor-derived GUSB is secreted and then taken up by neighboring cells in a mannose receptor- or mannose 6-phosphate receptor-dependent manner.26-28 This process will result in the host cells acquiring small amounts of GUSB that could potentially be detectable by the enzyme-specific assays used earlier. Therefore, experiments were conducted to confirm that the GUSB+ cells identified in the distribution study were actually human donor cells and not cross-corrected murine host cells. A flow cytometric assay was used to investigate whether murine host cells in highly engrafted tissues accumulated detectable levels of GUSB. Bone marrow cells from engrafted NOD/SCID/MPSVII animals were stained for human CD45 expression and incubated with a GUSB-specific fluorogenic substrate that is compatible with flow cytometric analysis (Figure5). Distinct populations of GUSB+/human CD45+ cells were revealed in the bone marrow of engrafted mice, but no substantial GUSB+/human CD45− murine cells were evident. A similar absence of human GUSB+/human CD45−cells was seen in the peripheral blood and spleen of engrafted NOD/SCID/MPSVII animals (data not shown).
GUSB activity correlated with human CD45 expression in a flow cytometric assay on the bone marrow of NOD/SCID/MPSVII mice that received transplants of human CD34+ cells.
(A) Bone marrow from a NOD/SCID/MPSVII mouse that did not receive a transplant when incubated with a fluorescent substrate for GUSB followed by staining with an antihuman CD45 antibody showed slight background on the GUSB channel (2.6%). Minimal staining on the human CD45 channel was found (0.3%). (B) In similarly assayed bone marrow from a NOD/SCID/MPSVII mouse 12 weeks after the transplantation of cord blood CD34+ cells, only the population positive for human CD45 had significant GUSB activity. Cells negative for human CD45 displayed a level of GUSB activity (2.7%) comparable to that of the control NOD/SCID/MPSVII bone marrow (A).
GUSB activity correlated with human CD45 expression in a flow cytometric assay on the bone marrow of NOD/SCID/MPSVII mice that received transplants of human CD34+ cells.
(A) Bone marrow from a NOD/SCID/MPSVII mouse that did not receive a transplant when incubated with a fluorescent substrate for GUSB followed by staining with an antihuman CD45 antibody showed slight background on the GUSB channel (2.6%). Minimal staining on the human CD45 channel was found (0.3%). (B) In similarly assayed bone marrow from a NOD/SCID/MPSVII mouse 12 weeks after the transplantation of cord blood CD34+ cells, only the population positive for human CD45 had significant GUSB activity. Cells negative for human CD45 displayed a level of GUSB activity (2.7%) comparable to that of the control NOD/SCID/MPSVII bone marrow (A).
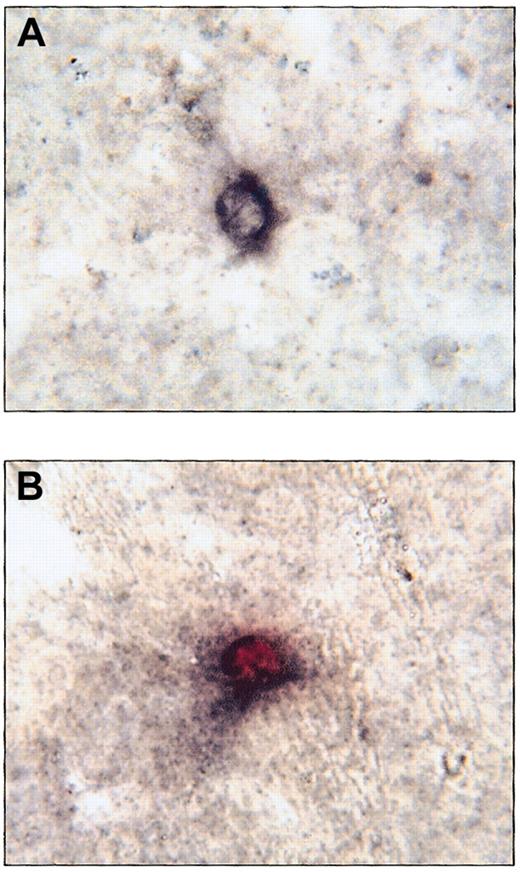
To further validate the accuracy of the GUSB-specific assays used in this study, sections from tissues of engrafted mice were costained for GUSB and human CD45 expression. Throughout the livers of engrafted NOD/SCID/MPSVII mice, immunohistochemical analysis for human CD45 expression revealed numerous cells with distinct black membrane staining (Figure 6A). When these immunohistochemically assayed slides were subsequently stained for GUSB activity, human CD45 expression correlated exactly with GUSB activity (Figure 6B). No GUSB+, human CD45− cells were seen in the livers of any animals examined. Similar results were seen in other tissues, including the spleen, lymph nodes, and thymus following costaining for GUSB activity and human CD45 expression (data not shown).
Immunohistochemical staining for human CD45 colocalized with GUSB histochemical staining in the liver of a NOD/SCID/MPSVII mouse following transplantation of human CD34+ cells.
(A) A cell in the liver of a NOD/SCID/MPSVII mouse 12 weeks after the transplantation of human cord blood CD34+ cells shows black membrane staining following immunohistochemistry for human CD45. (B) After staining for both human CD45 and GUSB activity, a cell in the same NOD/SCID/MPSVII liver as in (A) shows both black membrane signal for human CD45 and red cytoplasmic signal for GUSB activity. GUSB enzyme activity was seen only in the presence of black membrane staining for human CD45. In addition, all human CD45+ cells exhibited GUSB staining. Original magnification, × 833.
Immunohistochemical staining for human CD45 colocalized with GUSB histochemical staining in the liver of a NOD/SCID/MPSVII mouse following transplantation of human CD34+ cells.
(A) A cell in the liver of a NOD/SCID/MPSVII mouse 12 weeks after the transplantation of human cord blood CD34+ cells shows black membrane staining following immunohistochemistry for human CD45. (B) After staining for both human CD45 and GUSB activity, a cell in the same NOD/SCID/MPSVII liver as in (A) shows both black membrane signal for human CD45 and red cytoplasmic signal for GUSB activity. GUSB enzyme activity was seen only in the presence of black membrane staining for human CD45. In addition, all human CD45+ cells exhibited GUSB staining. Original magnification, × 833.
Correction of tissue pathology
Although the amount of GUSB transferred to host cells was too low to be detected by histochemical and flow cytometric analysis, the possibility of there being sufficient cross-correction to reduce accumulated storage material in diseased cells was investigated. The degree of microscopically visible lysosomal distention in tissues from engrafted NOD/SCID/MPSVII mice was compared with that of NOD/SCID/MPSVII mice that were untreated or that received mock transplants (Figure 7). Hepatocytes, Kupffer cells, and bone marrow sinus lining cells consistently had a moderate to marked reduction in the amount of lysosomal storage. Osteoblasts were also corrected in highly engrafted animals. A substantial decrease in lysosomal distention was seen in the sinus lining cells of the spleen but was typically not as dramatic as that seen in the fixed tissue macrophage system of the liver and bone marrow. The kidney showed minimal to no reduction in the amount of storage in the tubular epithelial cells and visceral epithelial cells of the glomeruli. Meninges in one animal had a slight reduction in the amount of storage over the cerebellar folia. Heart valve stromal cells, corneal stromal cells, retinal pigment epithelium, and neurons and glial cells in the neocortex had no evidence of response to the therapy. A similar pattern of disease correction was evident following the transplantation of cord blood–derived or mobilized peripheral blood-derived CD34+ cells (data not shown). Even in NOD/SCID/MPSVII mice that were engrafted at very low levels (1%-2% human CD45+ in the bone marrow), a reduction of storage material was found in the bone marrow, liver, and spleen. Mock-treated NOD/SCID/MPSVII mice that were irradiated, injected with vehicle, and given regular cytokine doses showed no reduction in storage material compared with age-matched untreated NOD/SCID/MPSVII mice (data not shown).
NOD/SCID/MPSVII mice that received transplants of human CD34+ cells showed correction of tissue pathology in some engrafted tissues.
The tissues of NOD/SCID/MPSVII mice that were either untreated or received transplants of human CD34+ cells were stained with toluidine blue. Lysosomes distended with storage material appear as clear, foamy areas by this method. In the bone marrow of NOD/SCID/MPSVII mice that did not receive transplants, lysosomal storage material (arrows) filled the sinus lining cells (A) and osteoblasts (C). The spleen (E) and liver (G) of untreated NOD/SCID/MPSVII mice also contained large amounts of storage material (arrows). In NOD/SCID/MPSVII mice that received human CD34+cells, the bone marrow sinus lining cells (B) and osteoblasts (D) were essentially free of lysosomal distention (arrows). The spleen (F) of such treated mice typically showed marked correction with only residual levels of storage material present (arrow). The liver (H) of transplanted NOD/SCID/MPSVII mice showed essentially complete removal of lysosomal storage material. Original magnifications: × 833 (A-D); × 416 (E-H).
NOD/SCID/MPSVII mice that received transplants of human CD34+ cells showed correction of tissue pathology in some engrafted tissues.
The tissues of NOD/SCID/MPSVII mice that were either untreated or received transplants of human CD34+ cells were stained with toluidine blue. Lysosomes distended with storage material appear as clear, foamy areas by this method. In the bone marrow of NOD/SCID/MPSVII mice that did not receive transplants, lysosomal storage material (arrows) filled the sinus lining cells (A) and osteoblasts (C). The spleen (E) and liver (G) of untreated NOD/SCID/MPSVII mice also contained large amounts of storage material (arrows). In NOD/SCID/MPSVII mice that received human CD34+cells, the bone marrow sinus lining cells (B) and osteoblasts (D) were essentially free of lysosomal distention (arrows). The spleen (F) of such treated mice typically showed marked correction with only residual levels of storage material present (arrow). The liver (H) of transplanted NOD/SCID/MPSVII mice showed essentially complete removal of lysosomal storage material. Original magnifications: × 833 (A-D); × 416 (E-H).
Discussion
In this study, the NOD/SCID/MPSVII mouse displayed the ability to engraft human CD34+ hematopoietic progenitor cells in a fashion similar to that of standard NOD/SCID mice with prolonged production of human lymphoid and myeloid cells. The only statistically significant differences in human engraftment were found in the bone marrow of mice 12 weeks following the transplantation of cord blood CD34+cells. NOD/SCID/MPSVII bone marrow contained a higher proportion of CD66b+ granulocyte populations and a decrease in CD19+ B cells compared with that of NOD/SCID controls. However, for both NOD/SCID and NOD/SCID/MPSVII mice, highly engrafted animals (> 40% CD45+ in the bone marrow) had comparably increased numbers of CD66b+ cells and decreased levels of CD19+ cells (data not shown). Because 3 of the 4 NOD/SCID/MPSVII mice in this comparison were highly engrafted, whereas only 5 of 11 NOD/SCID mice had similar levels of human cells, statistical differences between the groups may simply be due to sample size.
The GUSB deficiency of the NOD/SCID/MPSVII mouse allowed the use of sensitive enzyme-specific assays to detect engrafted normal, GUSB+ cells.11,32 To determine whether these GUSB+ cells were exclusively of donor origin, the potential error from cross-corrected murine host cells was evaluated. A tight correlation of detectable GUSB activity with the nondiffusible, human pan-leukocyte surface marker, CD45, indicated that only donor human cells were identified by the GUSB-specific assays. These assays should be similarly accurate in xenotransplantation experiments with a wide variety of human cell types, allowing the in vivo analysis of trafficking and plasticity properties. However, the GUSB-specific assays will need to be further evaluated for their fidelity in certain gene therapy experiments in which cells greatly overexpress GUSB.37 38
In the human CD34+ hematopoietic progenitor cell transplants of this study, substantial human engraftment was observed not only in hematopoietic tissues like the bone marrow, spleen, lymph nodes, and thymus but also in other organs, including the liver, lung, kidney, heart, brain, and eye. Human CD34+ cells from cord blood engrafted NOD/SCID/MPSVII mice with a pattern similar to that of mobilized peripheral blood CD34+ cells. Furthermore, a comparable distribution of human cells at 6 and 12 weeks after transplantation of cord blood–derived CD34+ cells suggests that a relatively stable spatial pattern of engraftment was established by as early as 6 weeks in NOD/SCID/MPSVII mice.
Such a diverse localization of human cells has not been thoroughly described in similar xenotransplantation studies. The present experiments also found a comparable distribution of human engraftment in transplanted NOD/SCID tissues stained immunohistochemically for human CD45, suggesting that the MPSVII disease background does not significantly influence the trafficking of human cells (data not shown). Engraftment analysis in most similar xenotransplantation studies to date has focused on the host hematopoietic tissues or examined other organs only at very early time points.4-6However, data from one study that collected flow cytometric measurements in the liver, kidney, and lung of NOD/SCID tissues 6 to 7 weeks after human CD34+ cell transplantation do correspond with the relative levels of human engraftment determined in the current experiments.7
The widespread pattern of donor cells could be explained in part by the plasticity of hematopoietic progenitors.39 However, in dual-staining experiments, every GUSB+ cell examined costained with human CD45, indicating that the engrafted cells were limited to the hematopoietic lineage. It should be noted that the growing body of evidence for stem cell plasticity has largely been obtained in models with severe organ damage.40 41 The lack of detectable plasticity in NOD/SCID/MPSVII mice suggests that the pathology is not extensive enough to evoke a substantial plasticity response. In addition, the fact that engrafted cells correct the disease in many tissues may in part explain the apparent absence of a strong injury stimulus in this model.
The distribution of human hematopoietic cells in NOD/SCID/MPSVII mice resembles that of syngeneic bone marrow transplantation in MPSVII mice.16,24 25 In the latter setting, donor cells in organs like the liver, kidneys, and lungs result from reconstitution of the host's fixed tissue macrophage system. It is likewise possible that myeloid progeny of engrafted human hematopoietic progenitor cells integrated into the fixed-tissue macrophage system of NOD/SCID/MPSVII hosts in the current study. In the histochemical stains of the liver, for example, the elongated morphology and intense level of enzyme activity of GUSB+ cells suggests that they were Kupffer cells. More detailed staining experiments with lineage-specific cell markers would be necessary to confirm this hypothesis.
Although cross-correction of GUSB was undetectable by the enzyme-specific assays, levels of transferred GUSB were sufficient to reduce the pathologic storage material in several organs of NOD/SCID/MPSVII hosts that received transplants. Although heavily engrafted animals tended to demonstrate more disease reversal, mice that were relatively poorly engrafted also showed evidence of reduced storage material. This finding is consistent with previous studies showing that low levels of GUSB activity can correct the pathology of certain tissues.42-44 Transplantation of CD34+cells from both cord blood and mobilized peripheral blood sources had a similar therapeutic effect. Tissues with high numbers of human cells like the liver, spleen, and bone marrow demonstrated a marked degree of correction, whereas the low levels of human engraftment in tissues like the brain and heart showed little therapeutic effect. The correlation between the level of human engraftment in a tissue and the resultant amount of disease correction suggests that enzyme was supplied locally to diseased cells. The finding that GUSB activity was not detectable in the plasma of any NOD/SCID/MPSVII mouse that received transplants further supports this idea.
The successful demonstration of disease correction by transplanted human hematopoietic progenitor cells in the NOD/SCID/MPSVII mouse establishes a therapeutic baseline for comparison to other treatment approaches. For example, the efficacy of transplanting different human cell populations such as neural progenitors or mesenchymal stem cells could be assessed. Furthermore, valuable preclinical data could be gathered through gene therapy experiments in which diseased cells from human MPSVII patients are transduced and transplanted into NOD/SCID/MPSVII hosts. Although the NOD/SCID/MPSVII mouse represents the first reported model of human disease in which therapeutic strategies with human cells can be directly evaluated, NOD/SCID models harboring other disease mutations would also be highly valuable for analogous studies.
In summary, these experiments demonstrate that the NOD/SCID/MPSVII mouse can support the growth of primitive CD34+ human hematopoietic progenitors. Sensitive and precise GUSB-specific assays revealed human cells in tissues throughout the host, including a wide range of nonhematopoietic organs. In several of these tissues, a therapeutic effect of donor cells was observed through the reduction of lysosomal storage material. Therefore, the NOD/SCID/MPSVII mouse provides the unique opportunity to examine both the in vivo biology of human cell types and the therapeutic benefit of different treatment strategies through such xenotransplantation experiments.
We thank the Pharmacia Corporation for providing the generous gift of daniplestim, Cory Johnson for his help in obtaining cord blood products, Jeff Haug for assisting with flow cytometric assays, and Anthony Donsante for his help in maintaining the mouse colonies. We also thank Jan Nolta, Jane Barker, and Pampee Young for their critical reading of the manuscript.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-08-2597.
Supported by grant DK57586 (M.S.S.) and training grant 5T32 HL07088 (A.A.H.) from the National Institutes of Health, and a grant from the Mucopolysaccharidosis Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mark S. Sands, Washington University School of Medicine, Department of Internal Medicine, Box 8007, 660 S Euclid Ave, Saint Louis, MO 63110; e-mail:msands@imgate.wustl.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal