An unmutated germ line configuration of the immunoglobulin variable heavy-chain gene (VH) has emerged to be a crucial adverse prognostic factor in chronic lymphocytic leukemia (CLL) under conventional treatment. The purpose of the present study was to investigate whether the VH mutational status retains its prognostic value in CLL also in the setting of autologous stem cell transplantation (SCT). Therefore, we investigated the mutational status in 58 patients with CLL who underwent myeloablative radiochemotherapy with SCT. Rearranged VH genes were analyzed by multiplex polymerase chain reaction (PCR) and direct sequencing using FR1 family–specific primers and JH consensus primers. Twenty patients (34%) showed less than 98% homology compared with germ line VH sequences and were considered as mutated, whereas 38 patients (66%) had an unmutated VH status (median mutational rate of 0%; range, 0%-1.7%). An unmutated VHconfiguration was strongly correlated with the presence of short lymphocyte doubling time (P = .003) and high lymphocyte count (P = .005). Time to clinical relapse and time to recurrence of monoclonal B cells as assessed by consensus IgH CDR3 PCR was significantly shorter in the group with unmutated VH genes (2-year probability 19% versus 0%,P = .0008, and 34% versus 9%, P = .0006, respectively). These results show that in CLL, an unmutated VH gene status of the tumor clone remains an adverse prognostic factor after SCT. Nevertheless, the hitherto only 3 deaths and the median treatment-free interval of 49 months in the unmutated cohort suggest a beneficial effect of SCT for this high-risk population in comparison to conventional treatment.
Introduction
The clinical course of B-cell chronic lymphocytic leukemia (CLL) is often indolent with a median survival of more than 10 years. However, patients with advanced stage or biologic risk factors such as short lymphocyte doubling time, diffuse bone marrow infiltration, or distinct genomic aberrations show a more aggressive form of CLL with considerably shorter survival under conventional chemotherapy.1 2
Recently, the mutational status of the variable region of the immunoglobulin heavy-chain gene (VH) has been established as a crucial prognostic factor in conventionally treated CLL. During B-cell maturation immunoglobulin heavy-chain genes are rearranged in a distinct matter. Germ line sequences of the variable (V), diversity (D), and joining (J) genes located on chromosome 14 are rearranged to form a unique DNA sequence coding for the individual heavy chain of a given B-cell clone. During physiologic B-cell maturation this VDJ sequence is further modified after antigen contact in a germinal center reaction leading to nucleotide changes in the rearranged VHgene. These mutated VH genes differ from known germ line sequences and the mutational rate can be calculated. Several investigators have demonstrated a significant prognostic impact of the VH mutational status in CLL; an unmutated germ line VH configuration is associated with progressive disease and a dismal prognosis, whereas patients with somatic hypermutations of the VH gene are characterized by stable disease and long survival.3,4 Furthermore, there appears to be a relationship between unmutated VH and other biologic high-risk features such as distinct genomic aberrations.5 6
To improve the dismal outcome of younger patients with poor-risk CLL, autologous stem cell transplantation (SCT) has been increasingly used during recent years.7-13 Because relapses after SCT continue to occur, this kind of aggressive therapy does not seem to be curative in the majority of patients with CLL. Nevertheless, overall survival data of single-center studies look promising, suggesting that the prognosis of patients in the poor-risk category might show substantial improvement with SCT.14 15 These clinical data, however, were published without investigation of VHmutational status. Considering the mutational status is one of the most important risk factors in CLL, re-evaluating these data is mandatory.
The purpose of the present study was to investigate whether the VH mutational status as one of the most important biologic risk factors in CLL has a prognostic influence also after SCT. We retrospectively investigated the mutational status in 58 patients with CLL who had undergone myeloablative radiochemotherapy with autotransplantation of immunomagnetically purged stem cells. The results show that an unmutated VH gene status of the tumor clone remains an adverse prognostic factor after SCT.
Patients, materials, and methods
We investigated the immunoglobulin (Ig) VHmutational status of 17 women and 41 men with confirmed diagnosis of CD5+/CD19+/CD23+/CD20dimCLL who had diagnostic material for molecular analysis available and had been treated according to a local protocol (n = 30) or a multicenter protocol (n = 28) on SCT for poor-risk CLL (CLL3 study of the German CLL Study Group) at the University of Kiel (n = 53) and University of Ulm/Heidelberg (n = 5), respectively.8,16All patients were diagnosed between 1987 and 2000 with a median time to SCT of 17 months (range, 2-125 months). Briefly, patients were eligible for high-dose therapy if they had a history of either symptomatic disease according to the revised National Cancer Institute (NCI) guidelines (stage Binet B with B symptoms and/or massive or progressive lymphadenopathy or splenomegaly, stage Binet C)17 or by asymptomatic disease with adverse prognostic factors, namely, lymphocyte count more than 50 × 109/L, lymphocyte doubling time (LDT) less than 12 months, diffuse bone marrow (BM) infiltration, or presence of a 11q22.3-q23.1 deletion (del 11q−). LDT was assessed according to Montserrat et al.18 Cytogenetic analyses were performed with diagnostic samples by interphase fluorescence in situ hybridization (FISH) as previously described.19
For molecular analysis, 10 mL blood or BM samples were taken before transplantation for clonality assessment and Ig VHsequencing. For molecular follow-up, blood or BM samples were taken at regular time points after SCT within the first year. After 2 years clonality screening was performed once a year.
Treatment protocol
Autologous hematopoietic stem cells were mobilized with the Dexa-BEAM regimen (dexamethasone 3 × 8 mg days 1-10; BCNU [carmustine] 60 mg/m2 day 2; etoposide 75 mg/m2 days 4-7; cytarabine 100 mg/m2 every 12 hours days 4-7; melphalan 20 mg/m2 day 3), followed by daily administration of granulocyte colony-stimulating factor (G-CSF; 5-10 μg/kg subcutaneously) through the nadir until the last day of peripheral blood stem cell (PBSC) collection as previously described.8 The collection products underwent positive, negative, or positive/negative immunomagnetic purging with the MaxSep, Isolex, or Clinimacs systems (Nexell Therapeutics, Irvine, CA, and Miltenyi Biotec, Bergisch Gladbach, Germany). Myeloablative therapy consisted of fractionated total body irradiation (TBI; 6 × 2 Gy on 3 consecutive days) and cyclophosphamide (total dose 2 × 60 mg/kg on 2 consecutive days) followed by reinfusion of at least 2 × 106 immunoselected CD34+ cells/kg. The protocol was approved by the responsible institutional review boards.
Ig VH identification and sequencing
gDNA was isolated from blood or BM samples using Qiagen Blood mini Kits (Qiagen, Hilden, Germany). Assessment of the clonally rearranged Ig heavy chain was performed by multiplex polymerase chain reaction (PCR) using 6 family-specific 5′-FR1 and one consensus 3′-JH primer20 21 and subsequent gene scanning using an ABI 310 (Applied Biosystems, Weiterstadt, Germany) sequencer. PCR was performed in a final volume of 50 μL with 0.8 mM deoxyribonucleoside triphosphate (dNTP), 1.5 mM MgCL2, 0.4 mM of each primer, 5 μL 10 × PCR buffer II (Applied Biosystems), and 0.2 μL AmpliTaq gold polymerase (Applied Biosystems). The PCR protocol was done as follows: 10 minutes at 94°C initial denaturing and Taq activating, 35 cycles of 1 minute at 94°C denaturing, 1 minute at 60°C annealing, 30 seconds at 72°C extension, and a final extension step of 10 minutes at 72°C. PCR products of monoclonal samples were used for direct sequencing with the same primer sets as in the initial PCR amplification. In samples showing biallelic rearranged IgH chains, 6 PCR reactions using the 3′-JH and for each reaction one of the family-specific 5′-FR1 primers were performed to identify the rearranged VH genes. Subsequently, family-specific primers of the identified family were used for sequencing. In cases with biallelic rearranged IgH chains of the same VH family or polyclonal background signals, PCR products of interest were isolated from polyacrylamide gel electrophoresis.
To avoid the misinterpretation of Taq polymerase reading errors as Ig VH mutations, the products of 5 independently performed PCR reactions for each sample were pooled before sequencing analysis. PCR products were purified using Microcon columns (Millipore, Bedford, MA). Sequencing was done with Big Dye terminator sequencing kits (Applied Biosystems) and DyeEx spin columns (Qiagen) for purification on an ABI 310 sequencer. Each pooled sample was sequenced forward and backward using the FR1 primer mix or the appropriate family-specific FR1 and JH primers. In all cases forward/backward sequencing analysis was repeated once. The resulting 4 sequence replicates were compared with each other to identify misreading errors.
Ig VH mutational analysis
The sequences were compared with published germ line VH, D, and JH genes using DNAPLOT software and IMGT database (IMGT, the international ImMunoGeneTics databasehttp://imgt.cines.fr:8104; Initiator and coordinator: Marie-Paule Lefranc, Montpellier, France). Mutational status was calculated as percent deviation from the closest matching germ line VHsegment. Sequences showing 98% or more homology to the nearest germ line gene were assigned as unmutated.
Consensus IgH-CDR3 PCR
All patients were monitored by consensus IgH-CDR3 PCR and gene scanning for molecular clonality as previously described by our group.22 Seminested PCR was performed with one upstream FR3 consensus primer and one outer and one inner 5′-FAM–labeled consensus JH primer as described elsewhere.22 The sensitivity of this method varies between 1% and 0.1%.
Statistical analysis
The 2-tailed nonparametric Mann-Whitney tests and Fisher exact test were used to compare quantitative and qualitative parameters, respectively, between subgroups of patients. Survival time data were estimated using the Kaplan-Meier method. Events relevant for the end point “clinical relapse” were clinical progression or disease recurrence according to NCI criteria. Events relevant for “molecular clonality” after transplantation were persistence or recurrence of molecular clonality using FR3 consensus primer PCR. The “treatment-free interval” was defined as the time from SCT to the next salvage therapy. Kaplan-Meier curves were compared with the log-rank test to identify explanatory variables prognostic for molecular outcome. Proportional hazards models (Cox regression) were set up to investigate the confounding effects of prognostic variables. Due to the low number of events and missing data for LDT and cytogenetics, only a limited number of explanatory variables could be included into multivariate analysis. These were time from diagnosis to transplantation, Binet stage, lymphocyte count, presence of del 11q−, and mutational status. Significance levels were set at .05. Calculations were done using GraphPad Prism software (release 3.02; San Diego, CA) and NCSS software (release 5.5; Kaysville, UT), respectively. Data were analyzed as of April 5, 2002.
Results
The median age was 50 years (range, 29-64 years) at time of diagnosis. An advanced Binet stage (B or C) was present in 72% of the patients (stage A = 16; B = 27; C = 15). Adverse biologic risk factors were found as follows: high lymphocyte count in 55% (32 of 57 patients), short LDT in 67% (29 of 42), and del 11q− in 23% (11 of 48). Autografting was performed as part of first-line therapy in 46 patients, whereas 12 patients received stem cell transplants as salvage treatment. Accordingly, the median time from diagnosis to mobilization was relatively short at 14 months (range, 2-117 months). Patients had been pretreated with one line (range, 0-4 lines) of conventional chemotherapy, which consisted of alkylating agents only in 38 patients, whereas 13 patients had been exposed to fludarabine.
Mutational status and VH segment usage
Among the 58 investigated patients, 7 patients had biallelic VH rearrangements. In all biallelic cases the mutational status of both alleles was identical. Thus, overall 65 IgH alleles were sequenced. Thirty-eight patients (43 alleles) showed rearranged VH genes with 98% or more homology to the closest matched germ line sequence and were considered as unmutated, whereas in the remaining 20 patients (22 alleles) less than 98% germ line homology was found. The mutational frequency ranged from 2.2% to 13.9% (median, 6.5%) in the mutated group and from 0% to 1.7% (median, 0%) in the unmutated group. The VH -1 (in the majority VH 1-69) gene was preferably used by unmutated alleles (33% versus 13% in mutated), whereas VH-3 genes were overrepresented in the mutated group (54% versus 34% in unmutated) although statistical significance was not reached with the number of cases analyzed here. There was no difference in terms of D segment and J segment usage between mutated and unmutated alleles (data not shown).
Mutational status and other prognostic factors
The patient distribution according to age, sex, time from diagnosis, and upfront versus salvage transplantation was similar between the mutated and unmutated cohorts. In contrast, clinical and biologic risk factors such as short LDT, high lymphocyte count, and del 11q− were strongly correlated with an unmutated VHstatus (Table 1).
Distribution of clinical and biologic risk factors in 58 patients with CLL according to VH mutation status
| . | Mutated, n = 20 . | Unmutated, n = 38 . | P* . |
|---|---|---|---|
| Age, y (range) | 49 (34-60) | 51 (29-64) | NS |
| Time to ASCT, mo (range) | 17 (5-109) | 16 (6-102) | NS |
| Upfront transplantation (%) | 17 (85) | 29 (76) | NS |
| Binet stage (%) | |||
| A | 8 (40) | 8 (21) | NS |
| B | 6 (30) | 21 (55) | NS |
| C | 6 (30) | 9 (23) | NS |
| LDT less than 12 mo (%) | 5/14 (20) | 24/28 (85) | .003 |
| High lymphocyte count (%) | 6/20 (30) | 26/37 (70) | .005 |
| del 11q−(%) | 1/17 (6) | 10/31 (32) | .07 |
| . | Mutated, n = 20 . | Unmutated, n = 38 . | P* . |
|---|---|---|---|
| Age, y (range) | 49 (34-60) | 51 (29-64) | NS |
| Time to ASCT, mo (range) | 17 (5-109) | 16 (6-102) | NS |
| Upfront transplantation (%) | 17 (85) | 29 (76) | NS |
| Binet stage (%) | |||
| A | 8 (40) | 8 (21) | NS |
| B | 6 (30) | 21 (55) | NS |
| C | 6 (30) | 9 (23) | NS |
| LDT less than 12 mo (%) | 5/14 (20) | 24/28 (85) | .003 |
| High lymphocyte count (%) | 6/20 (30) | 26/37 (70) | .005 |
| del 11q−(%) | 1/17 (6) | 10/31 (32) | .07 |
ASCT indicates autologous stem cell transplantation; and NS, not significant.
Fisher exact test.
Mutational status and posttransplantation outcome
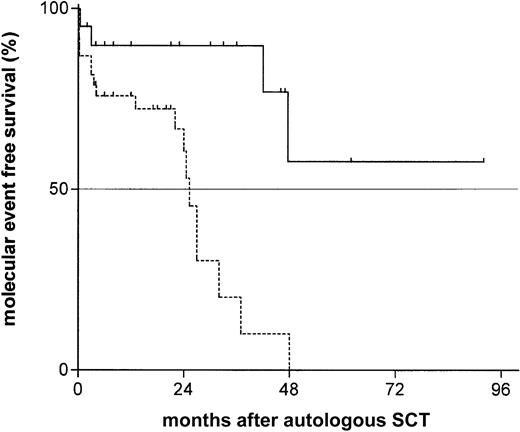
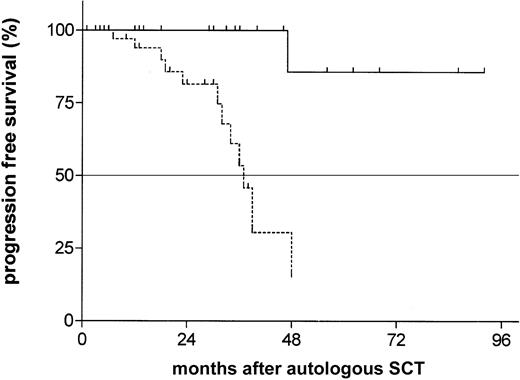
Considering molecular clonality assessed by consensus IgH-CDR3 PCR as an early surrogate marker for disease recurrence, we compared the molecular event-free survival after transplantation of both groups. We found a strong difference with a median time to recurrence of molecular clonality of 25 months in the unmutated group, whereas the median time to recurrence of molecular clonality was not reached in the group with mutated VHgenes after a median follow-up of 24 months (range, 2-80 months;P = 0.0006; hazard ratio [HR], 4.8; 95% CI, 1.9-11.1; Figure 1). This difference translated into a benefit for the mutated cohort also in clinical outcome parameters. Although the median time to clinical relapse or disease progression was 37 months in the unmutated group, a mutated VH status was associated with a much more favorable posttransplantation course with only one documented event at 4 years (P = .0008; HR, 13.2; 95% CI, 2.6-21.8; Figure2). Similarly, the treatment-free interval after SCT was significantly longer in the mutated cohort (not reached versus 49 months; P = .009; HR, 8.1; 95% CI 1.8-52.7). However, with only 5 deaths at all (2 in the mutated versus 3 in the unmutated group), overall survival is still excellent even in the unmutated cohort (2-year probability 89%; 95% CI, 82-96).
Probability of recurrence of molecular clonality assessed by IgH-CDR3 consensus PCR and gene scanning after autologous SCT according to mutational status.
The proportion of recurrence of molecular recurrence of the unmutated group (dotted line, median event-free survival 25 months) versus the unmutated group (solid line, median event-free survival not reached) by Kaplan Meier estimation (P = .0006; HR, 4.8; 95% CI, 1.9-11.1).
Probability of recurrence of molecular clonality assessed by IgH-CDR3 consensus PCR and gene scanning after autologous SCT according to mutational status.
The proportion of recurrence of molecular recurrence of the unmutated group (dotted line, median event-free survival 25 months) versus the unmutated group (solid line, median event-free survival not reached) by Kaplan Meier estimation (P = .0006; HR, 4.8; 95% CI, 1.9-11.1).
Probability of progression-free survival from time of autologous transplantation according to Ig VH mutational status.
The estimated time of progression-free survival of patients with unmutated VH status (dotted line) was 37 months versus not reached in the mutated group (median follow-up 24 months;P = .0008; HR, 13.2; 95% CI, 2.6-21.8).
Probability of progression-free survival from time of autologous transplantation according to Ig VH mutational status.
The estimated time of progression-free survival of patients with unmutated VH status (dotted line) was 37 months versus not reached in the mutated group (median follow-up 24 months;P = .0008; HR, 13.2; 95% CI, 2.6-21.8).
In addition to the VH mutational status, other risk factors, such as del 11q−, high lymphocyte count, and short LDT, had a significant predictive value for recurrence of molecular clonality but not for clinical relapse, whereas sex, age, time from diagnosis, timing of SCT, and Binet stage had no effect in univariate log-rank comparisons. Unmutated VH remained a highly significant adverse factor even if only patients without del 11q− were considered (P = .002), and del 11q− was still unfavorable if only the unmutated cohort was considered (P = .01), suggesting that both parameters have an independent prognostic influence. Accordingly, the prognosis was worst in those patients who have both and unmutated VH status and del 11q− (Table2). Multivariate analysis confirmed that mutational status and del 11q− are independent prognostic factors for molecular outcome (Table 3).
Variables predictive for molecular outcome (univariate analysis)
| Prognostic factor . | HR . | 95% CI . | P* . |
|---|---|---|---|
| VHunmutated | 4.8 | 1.9-11.1 | .0006 |
| del 11q− present | 4.9 | 4.3-71.1 | < .0001 |
| Both VHunmutated and del 11q− present (versus VHmutated) | 6.2 | 3.2-63.4 | .0005 |
| LDT less than 12 mo | 4.2 | 1.4-10.7 | .007 |
| Lymphocyte count 50 × 109/L | 2.5 | 1.1-6.2 | .02 |
| Prognostic factor . | HR . | 95% CI . | P* . |
|---|---|---|---|
| VHunmutated | 4.8 | 1.9-11.1 | .0006 |
| del 11q− present | 4.9 | 4.3-71.1 | < .0001 |
| Both VHunmutated and del 11q− present (versus VHmutated) | 6.2 | 3.2-63.4 | .0005 |
| LDT less than 12 mo | 4.2 | 1.4-10.7 | .007 |
| Lymphocyte count 50 × 109/L | 2.5 | 1.1-6.2 | .02 |
Variables not predictive included sex, age, time from diagnosis to transplantation, timing of transplantation (first-line or salvage), and Binet stage.
Log-rank test.
Variables predictive for molecular outcome (Cox regression)
| Prognostic factor . | Regression coefficient (HR) . | SE of coefficient . | P . |
|---|---|---|---|
| VHunmutated | 1.385 (4.0) | 0.672 | .0394 |
| del 11q− present | 1.858 (6.4) | 0.614 | .0025 |
| Lymphocyte count 50 × 109/L | 1.333 (3.8) | 0.558 | .0169 |
| Prognostic factor . | Regression coefficient (HR) . | SE of coefficient . | P . |
|---|---|---|---|
| VHunmutated | 1.385 (4.0) | 0.672 | .0394 |
| del 11q− present | 1.858 (6.4) | 0.614 | .0025 |
| Lymphocyte count 50 × 109/L | 1.333 (3.8) | 0.558 | .0169 |
Variables not remaining in the model were time from diagnosis to transplantation and Binet stage.
Discussion
To improve the dismal outlook of younger patients with poor-risk CLL, autologous SCT has been increasingly used during recent years. Preliminary results of single-center studies suggest that the prognosis of patients with advanced or resistant disease might be substantially improved by SCT.14,15 Recent evidence has indicated that disease activity and resistance to treatment are determined by genetic risk factors, in particular the mutational status of the VHgene.3-5 The purpose of the present study was to investigate whether the VH mutational status as one of the most important risk factors in conventionally treated CLL has a prognostic influence also after SCT.
Taking 98% or more homology to the closest matching germ line VH segment as cutoff level, we found mutated VHgenes in only 35% of the cases compared with 50% or more as published by others,3,4,23,24 reflecting the high-risk selection criteria used in the patient cohort selected for SCT in this study. Despite the relatively low number of patients with mutated VH genes, our data clearly show that an unmutated VH gene status of the CLL clone remains an adverse prognostic factor also after SCT. Within 4 years, virtually all patients with unmutated VH genes have evidence of disease recurrence at the molecular level. This implies that even with myeloablative radiochemotherapy and highly effective ex vivo purging of the graft, this approach is not curative in patients with an unmutated VH status and translates into an almost 10-fold risk of clinical relapse compared with the group with VH mutation. Thus, similar to the situation in follicular lymphoma and mantle cell lymphoma, autologous SCT alone does not allow complete disease eradication in genetically unfavorable CLL.25-28Nevertheless, the median treatment-free interval of 49 months in the unmutated group of patients (n = 38) points to a beneficial effect of SCT in this otherwise very unfavorable population.5 This assumption is underlined by the promising overall survival observed in the unmutated group with 91% of the patients surviving 4 years after diagnosis if SCT is performed as first-line treatment (median follow-up 3 years). On the other hand, the outcome of the 20 patients with mutated VH was very favorable with to date ongoing molecular remissions in the vast majority of cases and with need for retreatment in only 1 of 20 patients more than 6 years after diagnosis (4 years after SCT). However, one has to consider that also with conventional treatment the prognosis might be good in this subgroup of patients.
Similarly to the mutational status, the other risk factors investigated had a significant impact on the molecular outcome in univariate analysis, implying that the mutational status might be a surrogate for other genetic markers. However, subgroup comparisons suggested that unmutated VH had an adverse influence on the molecular outcome independent of del 11q− and vice versa. It is likely that short LDT and high lymphocyte count are secondary to these genetic risk factors in CLL. Although the results of the Cox analysis must be regarded as preliminary due to the low number of events as well as the limited number of explanatory variables included, multivariate testing confirmed that high lymphocyte count, del 11q−, and unmutated VH status and are independent adverse prognostic factors for molecular outcome.,
We conclude that the Ig VH mutational status in CLL remains an adverse risk factor even after autologous transplantation. Having in mind the selection bias associated with this kind of study, our survival data nevertheless suggest a possible prognostic improvement for high-risk patients with unmutated VH genes by high-dose chemotherapy, which can, however, only be proven by randomized studies. On the other hand, the benefit for patients with mutated VHgenes is less evident, implying that autografting should be considered only with great caution as upfront treatment in this subgroup. Thus, for randomized studies aiming at evaluating the role of SCT in CLL, molecular genetic and cytogenetic stratification appears to be mandatory.
SCT in its current form is not curative per se in patients with unmutated VH but might be a step toward cure. The marked reduction of the tumor cell load, which can be achieved by SCT, may provide a basis for the successful elimination of residual disease by additional interventions, such as the in vivo use of monoclonal antibodies.29,30 For selected very high-risk patients with advanced disease and presence of both unmutated VH and del 11q− as genetic predictor for a very low probability of long-term remission after autologous SCT, alternative strategies, such as primary allografting with dose-reduced conditioning should be considered.31 32
Prepublished online as Blood First Edition Paper, October 31, 2002; DOI 10.1182/blood-2002-06-1744.
Supported by the “Deutsche José Carreras Leukämie-Stiftung e.V.,” “Interdisziplinäres Zentrum Krebsforschung” (IZKF), University of Kiel and EC grant QLG2-CT-1999-00786, EC grant QLRT-1999-00786, and BMBF 01KW9938/0.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Matthias Ritgen, Second Department of Medicine, Chemnitzstr 33, D-24105 Kiel, Germany; e-mail:m.ritgen@med2.uni-kiel.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal