Liver toxicity caused by high-dose myeloablative therapy leads to significant morbidity after hematopoietic cell transplantation. We examined the hypothesis that liver toxicity after cyclophosphamide and total body irradiation is related to cyclophosphamide through its metabolism to toxins. Cyclophosphamide was infused at 60 mg/kg over 1 to 2 hours on each of 2 consecutive days, followed by total body irradiation. Plasma was analyzed for cyclophosphamide and its major metabolites. Liver toxicity was scored by the development of sinusoidal obstruction syndrome (veno-occlusive disease) and by total serum bilirubin levels. The hazards of liver toxicity, nonrelapse mortality, tumor relapse, and survival were calculated using regression analysis that included exposure to cyclophosphamide metabolites (as the area under the curve). Of 147 patients, 23 (16%) developed moderate or severe sinusoidal obstruction syndrome. The median peak serum bilirubin level through day 20 was 2.6 mg/dL (range, 0.5-41.1 mg/dL). Metabolism of cyclophosphamide was highly variable, particularly for the metaboliteo-carboxyethyl-phosphoramide mustard, whose area under the curve varied 16-fold. Exposure to this metabolite was statistically significantly related to sinusoidal obstruction syndrome, bilirubin elevation, nonrelapse mortality, and survival, after adjusting for age and irradiation dose. Patients in the highest quartile ofo-carboxyethyl-phosphoramide mustard exposure had a 5.9-fold higher risk for nonrelapse mortality than did patients in the lowest quartile. Engraftment and tumor relapse were not statistically significantly related to cyclophosphamide metabolite exposure. Increased exposure to toxic metabolites of cyclophosphamide leads to increased liver toxicity and nonrelapse mortality and lower overall survival after hematopoietic cell transplantation.
Introduction
Hematopoietic stem cell transplantation following a myeloablative preparative regimen is the treatment of choice for some patients with refractory hematologic malignancy, aplastic anemia, and certain inborn errors of metabolism.1 A major limitation of myeloablative regimens is damage to the liver and development of multiorgan failure, a cause of death after transplantation.2-4
In the study reported here, we examined the hypothesis that liver toxicity that develops after a conditioning regimen containing cyclophosphamide and total body irradiation (TBI) is related to how cyclophosphamide is metabolized. The genesis of this hypothesis came from 4 observations: (1) cyclophosphamide is a component of the most hepatotoxic myeloablative regimens2,3; (2) in vitro, hepatic sinusoidal endothelial cells are injured by metabolites of cyclophosphamide that are generated within hepatocytes5; (3) damage to the microcirculation of the liver is central to the development of hepatic dysfunction after transplantation2,6-8; (4) there is patient-to-patient variability in cyclophosphamide metabolism and a relation of aberrant metabolism to toxicity in other organs.9-11
Patients, materials, and methods
Patient selection
This was a prospective study of the relationship between cyclophosphamide metabolism and clinical outcome. Between April 1997 and January 2000, all patients with hematologic malignancy undergoing allogeneic transplantation following a preparative regimen of cyclophosphamide (CY) plus total body irradiation were invited to participate. Patients signed informed consent approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center.
Technique of hematopoietic cell transplantation
Seven days before the infusion of stem cells, CY was infused through a central venous access catheter over 1 to 2 hours at a dose of 60 mg/kg body weight.12 On the following day, a second infusion of CY was given, at the same dose. After a day of rest, total body irradiation was given on each of the 3 or 4 subsequent days in hyperfractionated doses from opposing cobalt sources. Regimens of prophylaxis against graft-versus-host disease (GVHD) are given in Table1; BC3 is a murine antibody specific for human CD3.13 Infection prophylaxis included fluconazole or itraconazole, acyclovir, and trimethoprim-sulfamethoxazole.
Characteristics of 147 patients receiving cyclophosphamide and total body irradiation as myeloablative therapy followed by allogeneic transplantation
| Characteristic . | Results . |
|---|---|
| Age, y, median (range) | 35.2 ± 12.6 (2.8-58) |
| Sex, F/M | 58:89 |
| Diagnosis | |
| Chronic myeloid leukemia, chronic phase | 73 |
| Chronic myeloid leukemia, accelerated phase or blast crisis | 30 |
| Acute nonlymphocytic leukemia | 17 |
| Acute lymphocytic leukemia | 23 |
| Myelodysplastic syndrome | 3 |
| Lymphoma | 1 |
| Dose of hyperfractionated irradiation, Gy | |
| 9 | 1 |
| 12 | 61 |
| 13.2 | 68 |
| 14.4 | 17 |
| Source of donor hematopoietic stem cells | |
| HLA-matched sibling | 5 |
| HLA-mismatched family member | 3 |
| Unrelated donor | 139 |
| Prophylaxis against acute GVHD | |
| Cyclosporine or tacrolimus, methotrexate | 126 |
| Methotrexate | 1 |
| Cyclosporine or tacrolimus, methotrexate, RFT5 | 15 |
| Cyclosporine, prednisolone, BC3 | 3 |
| Prednisolone, BC3 | 2 |
| Characteristic . | Results . |
|---|---|
| Age, y, median (range) | 35.2 ± 12.6 (2.8-58) |
| Sex, F/M | 58:89 |
| Diagnosis | |
| Chronic myeloid leukemia, chronic phase | 73 |
| Chronic myeloid leukemia, accelerated phase or blast crisis | 30 |
| Acute nonlymphocytic leukemia | 17 |
| Acute lymphocytic leukemia | 23 |
| Myelodysplastic syndrome | 3 |
| Lymphoma | 1 |
| Dose of hyperfractionated irradiation, Gy | |
| 9 | 1 |
| 12 | 61 |
| 13.2 | 68 |
| 14.4 | 17 |
| Source of donor hematopoietic stem cells | |
| HLA-matched sibling | 5 |
| HLA-mismatched family member | 3 |
| Unrelated donor | 139 |
| Prophylaxis against acute GVHD | |
| Cyclosporine or tacrolimus, methotrexate | 126 |
| Methotrexate | 1 |
| Cyclosporine or tacrolimus, methotrexate, RFT5 | 15 |
| Cyclosporine, prednisolone, BC3 | 3 |
| Prednisolone, BC3 | 2 |
Measurement of plasma levels of cyclophosphamide and its metabolites
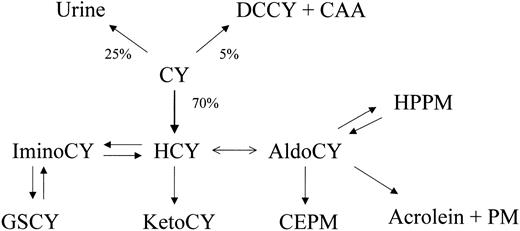
Blood samples were removed from a non-CY infusion port of a central venous access catheter before each CY infusion and at the following times: mid-infusion, immediately after the completion of infusion, and at 1, 3, 7, 20, and 24 hours afterward. Aliquots of each sample were placed into tubes containing eitherp-nitrophenyl hydrazine for analysis of 4-hydroxy cyclophosphamide or EDTA (ethylenediaminetetraacetic acid) for other analytes; then they were mixed and centrifuged at the bedside. Plasma was immediately removed, frozen, and stored at −80°C until analysis.14 15 Exposure to CY metabolites was expressed as the area under the curve (AUC; μM·h) derived from time zero (the time of the first CY dose) to 24 hours after the second dose of CY. The data reflect exposure to CY and its metabolites—4-hydroxy-cyclophosphamide (HCY),o-carboxyethyl-phosphoramide mustard (CEPM), deschloroethyl-cyclophosphamide (DCCY), 4-keto-cyclophosphamide (KetoCY), and hydroxypropyl-phosphoramide mustard (HPPM) (Figure1).
Quantitative disposition of cyclophosphamide (CY) indicating fraction of dose eliminated by pathway.
Chloroacetaldehyde (CAA), acrolein, and phosphoramide mustard (PM) are cytotoxins, but acrolein and PM are the major toxins because of the abundance of their formation. HCY (4-hydroxy-cyclophosphamide) is formed primarily in the liver but circulates in blood, entering cells as its tautomer aldocyclophosphamide (AldoCY). Acrolein and PM are formed from AldoCY when it decomposes through β-elimination. Other metabolites include o-carboxyethyl-phosphoramide mustard (CEPM), deschloroethyl-cyclophosphamide (DCCY), 4-keto-cyclophosphamide (KetoCY), hydroxypropyl-phosphoramide mustard (HPPM), imino-cyclophosphamide (IminoCy), and glutathionyl-cyclophosphamide (GSCY).
Quantitative disposition of cyclophosphamide (CY) indicating fraction of dose eliminated by pathway.
Chloroacetaldehyde (CAA), acrolein, and phosphoramide mustard (PM) are cytotoxins, but acrolein and PM are the major toxins because of the abundance of their formation. HCY (4-hydroxy-cyclophosphamide) is formed primarily in the liver but circulates in blood, entering cells as its tautomer aldocyclophosphamide (AldoCY). Acrolein and PM are formed from AldoCY when it decomposes through β-elimination. Other metabolites include o-carboxyethyl-phosphoramide mustard (CEPM), deschloroethyl-cyclophosphamide (DCCY), 4-keto-cyclophosphamide (KetoCY), hydroxypropyl-phosphoramide mustard (HPPM), imino-cyclophosphamide (IminoCy), and glutathionyl-cyclophosphamide (GSCY).
Definition of liver toxicity
Liver toxicity was scored by categoric and continuous variables—that is, the presence or absence of sinusoidal obstruction syndrome (SOS)2,7,8 and frequent measurement of total serum bilirubin levels through day 20 after transplantation. In patients who have undergone transplantation, SOS is an anatomically precise term for a clinical syndrome caused by toxin injury to hepatic sinusoids, conventionally described by the generic name veno-occlusive disease.6,8 SOS is our preferred term because hepatic venules are frequently patent in patients with this syndrome and because damage to sinusoidal endothelial cells is the initiating event.6,8 In the current study, a diagnosis of SOS was based on development of hepatomegaly, weight gain, and jaundice before day 20, as described previously.2 The severity of SOS was classified according to the subsequent course of the disease as mild (clinically obvious, requires no treatment, and resolves completely), moderate (causes signs and symptoms requiring treatment, such as diuretics or pain medications, but resolves completely), or severe (requires treatment but does not resolve before death or day 100).2,3,16 Patients without evidence of liver disease were categorized as not having SOS, and patients in whom liver disease developed before day 20 after transplantation but that did not meet the criteria for SOS (for example, acute graft-versus-host disease and cholangitis lenta17) were categorized as having liver disease of unknown etiology, as previously described.2Total serum bilirubin level was quantified in all patients in the study cohort as the maximum value between day 0 and day 20 and as the average daily bilirubin level, without reference to cause of hyperbilirubinemia. Assessment of liver toxicity was made without knowledge of cyclophosphamide pharmacokinetics.
Statistical methods
The primary end point of this prospective study was liver toxicity; secondary end points included nonrelapse mortality, relapse, engraftment, and overall survival. The study was designed to enroll 200 patients. If the true effect size, or the difference in means divided by standard deviation, for the AUC of a specified metabolite is 0.75, this number (200 patients) allows 94% power to detect a statistically significant difference in AUC at the 2-sided P = .05 significance level between patients with no SOS and patients with moderate to severe SOS. An interim analysis conducted after 140 patients were enrolled showed that significant differences had been demonstrated; thus, the study was stopped when this information became available, after 147 patients had been enrolled. The probability of survival was estimated using the Kaplan-Meier method, and the probabilities of nonrelapse mortality and relapse were summarized using cumulative incidence estimates. Relapse was regarded as a competing risk for nonrelapse mortality, and death without relapse was considered a competing risk for relapse. Liver toxicity as a categorical variable termed sinusoidal obstruction syndrome was assessed using logistic regression and as a continuous variable (total serum bilirubin) using linear regression. The hazards of overall mortality, nonrelapse mortality, and relapse were assessed using proportional hazards regression. For each of these end points, a base model was fit from among variables not including the pharmacokinetic parameters—that is, age, sex, diagnosis, dose of irradiation, and pretransplantation laboratory values for serum aspartate aminotransferase, albumin, creatinine, and blood urea nitrogen. Once the appropriate base model was fit, the model additionally containing one of the pharmacokinetic parameters was compared to the base model using the likelihood ratio test. Spearman rank correlation coefficient was used to assess the association between days to engraftment (by various measures) and pharmacokinetic parameters. All reported P values were 2-sided, and those estimated from regression models were derived from the Wald test. Several measures of liver toxicity were used, and these measures were obviously correlated. In addition, several metabolites of cyclophosphamide were measured, and some of these are correlated as well. It is, therefore, difficult to know how to best adjust for multiple comparisons in the estimation of reported P values, and for this reason no adjustments were made. P values between .01 and .05 should be considered suggestive of a true difference, particularly for secondary end points.
Results
Characteristics of the study cohort
The study cohort comprised 147 patients, 73 who underwent transplantation for chronic myeloid leukemia in chronic phase (CML-CP) and 74 who underwent it for other forms of hematologic malignancy (Table 1). For 139 patients, hematopoietic stem cells were from unrelated donors.
Frequency of liver toxicity through day 20
Of 147 patients studied, 56 (38%) had clinical criteria for SOS; 10 had severe, 13 had moderate, and 33 had mild liver disease. Seventy-six (51%) patients had no evidence of SOS. Fifteen patients were classified as having liver disease of uncertain etiology. The median value for maximum daily total serum bilirubin level through day 20 was 2.6 mg/dL (range, 0.5-41.1 mg/dL). The median value for average daily total serum bilirubin level through day 20 was 1.4 mg/dL (range, 0.4-13.0 mg/dL).
Cyclophosphamide metabolite exposure and liver toxicity
Variability of exposure (expressed as the AUC) to CY was 3.3 ×, but that of metabolites was higher, from 7.8 × for HCY, to 8.0 × for DCCY, to 16.1 × for CEPM. Data for exposure of patients to CY and its metabolites, according to the clinical diagnosis and severity of SOS, are given in Table 2.
AUCs for cyclophosphamide and its metabolites, expressed as μM·h
| AUCs . | All patients, SD (range) . | No SOS, n = 76 . | Mild SOS, n = 33 . | Moderate and severe SOS, n = 23 . | Moderate and severe SOS vs no SOS,P . | Mild SOS vs no SOS, P . |
|---|---|---|---|---|---|---|
| AUCDCCY | 598 ± 271 (210-1685) | 572 ± 278 | 672 ± 292 | 578 ± 202 | .4240 | .0373 |
| AUCHPPM | 104 ± 189 (14-1415) | 124 ± 245 | 73 ± 32 | 82 ± 33 | .1186 | .7871 |
| AUCCY | 6096 ± 1266 (2676-8864) | 5921 ± 1246 | 6032 ± 1175 | 6761 ± 1291 | .0041 | .6497 |
| AUCKetoCY | 238 ± 96 (85-648) | 233 ± 93 | 252 ± 113 | 235 ± 84 | .5480 | .4654 |
| AUCCEPM | 422 ± 193 (117-1881) | 369 ± 126 | 440 ± 124 | 569 ± 332 | .0002 | .0108 |
| AUCHCY | 158 ± 62 (38-298) | 165 ± 66 | 155 ± 57 | 139 ± 54 | .1164 | .4862 |
| AUCs . | All patients, SD (range) . | No SOS, n = 76 . | Mild SOS, n = 33 . | Moderate and severe SOS, n = 23 . | Moderate and severe SOS vs no SOS,P . | Mild SOS vs no SOS, P . |
|---|---|---|---|---|---|---|
| AUCDCCY | 598 ± 271 (210-1685) | 572 ± 278 | 672 ± 292 | 578 ± 202 | .4240 | .0373 |
| AUCHPPM | 104 ± 189 (14-1415) | 124 ± 245 | 73 ± 32 | 82 ± 33 | .1186 | .7871 |
| AUCCY | 6096 ± 1266 (2676-8864) | 5921 ± 1246 | 6032 ± 1175 | 6761 ± 1291 | .0041 | .6497 |
| AUCKetoCY | 238 ± 96 (85-648) | 233 ± 93 | 252 ± 113 | 235 ± 84 | .5480 | .4654 |
| AUCCEPM | 422 ± 193 (117-1881) | 369 ± 126 | 440 ± 124 | 569 ± 332 | .0002 | .0108 |
| AUCHCY | 158 ± 62 (38-298) | 165 ± 66 | 155 ± 57 | 139 ± 54 | .1164 | .4862 |
Based on the development of liver toxicity, that is, SOS. Data are expressed as the mean ± SD.
Among patients with no SOS or with moderate to severe SOS, a logistic regression model was fit to model the probability of moderate to severe SOS. After adjusting for age at transplantation and dose of irradiation (1200 or less vs more than 1200 cGy TBI), the model additionally containing AUCCEPM was statistically significantly improved compared with the model without AUCCEPM(P = .007); the model additionally containing AUCCY was suggestively improved (P = .04). Moreover, among all 147 patients, increasing AUCCEPM was statistically significantly associated with an increased maximum total serum bilirubin level before day 20 (P = .0001) and average daily total serum bilirubin level before day 20 (P = .0001) after adjusting for age and conditioning regimen. Increasing AUCCY was also associated with each of these bilirubin parameters, but the association was not as strong as seen with AUCCEPM (P = .01 andP = .04, respectively).
Cyclophosphamide pharmacokinetics and relation to nonrelapse mortality
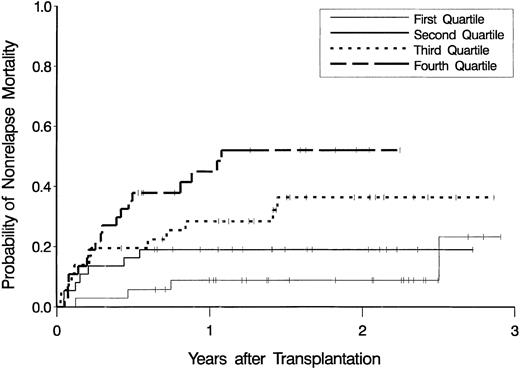
Figure 2 shows estimates of the probability of nonrelapse mortality according to quartiles of CEPM exposure. After adjusting for age at transplantation and type of disease (CML-CP vs other diagnoses), the hazard of nonrelapse mortality increased as CEPM exposure increased (P < .001). Summarized in Table 3 are the results from a multivariable proportional hazards regression model with AUCCEPM as a categorical variable. Addition of AUCCY (as a continuous variable) to the model containing age and disease did not lead to a statistically significant improvement in the model (P = .32).
Cumulative incidence estimates of the probability of nonrelapse mortality.
Estimates are displayed as a function of exposure to the cyclophosphamide metabolite, CEPM, expressed as the lowest (first) quartile through the highest (fourth) quartile of AUCCEPM.
Cumulative incidence estimates of the probability of nonrelapse mortality.
Estimates are displayed as a function of exposure to the cyclophosphamide metabolite, CEPM, expressed as the lowest (first) quartile through the highest (fourth) quartile of AUCCEPM.
Regression model of the relation of CEPM exposure to the hazard of nonrelapse mortality, adjusted for the variables age at transplantation and disease
| Quartile (AUCCEPM) . | Hazard ratio for nonrelapse mortality . | 95% CI . | P . |
|---|---|---|---|
| First | 1.0 | — | — |
| Second | 2.2 | 0.6-7.7 | .22 |
| Third | 3.2 | 1.0-10.3 | .06 |
| Fourth | 5.9 | 1.8-19.2 | .003 |
| Quartile (AUCCEPM) . | Hazard ratio for nonrelapse mortality . | 95% CI . | P . |
|---|---|---|---|
| First | 1.0 | — | — |
| Second | 2.2 | 0.6-7.7 | .22 |
| Third | 3.2 | 1.0-10.3 | .06 |
| Fourth | 5.9 | 1.8-19.2 | .003 |
Cyclophosphamide pharmacokinetics and engraftment of neutrophils and platelets
Although higher exposure to the metabolite CEPM was associated with increased liver toxicity and nonrelapse mortality, it is also possible that exposure to higher toxin levels could benefit patients by ensuring engraftment or preventing relapse. To test these possibilities, we next examined the Spearman rank correlation of AUCs for CEPM, HCY, and CY for the outcome variables time to sustained ANC greater than 1000/mm3 and time to sustained platelet count greater than 20 000/mm3. No statistically significant correlations were found for any of the CY metabolite exposures (data not shown). Moreover, most of the observed correlations were positive (ie, the higher the AUC, the longer the time to engraftment). Among 7 patients in whom engraftment was not successful, the median AUCCEPM was 442 μM·h, compared with a median AUCCEPM of 390 μM·h among 138 patients who did achieve a sustained ANC of more than 500/mm3. The median AUCHCY among the 7 patients who did not engraft was 108 μM·h compared with 142 μM·h among the 140 patients who did engraft. Although this difference is not statistically significant (P = .43, Wilcoxon rank-sum test), the possibility exists that the low number of patients who did not engraft precludes such a difference from being detected.
Cyclophosphamide metabolite exposure and tumor relapse
Twenty-nine relapses occurred. We examined the relation between CY metabolite exposure and relapse in the group of 147 as a whole, in a cohort of 73 patients with CML-CP, and in a cohort of 74 patients with malignancies other than CML-CP. If AUCCEPM (and its logarithm) are modeled as continuous variables in a multivariable Cox regression model for relapse (adjusting for disease [CML-CP vs other diagnoses] and age), AUCCEPM showed no suggestion of an association with the hazard of relapse (P = .88 andP = .75, respectively). If AUCCEPM is modeled in quartiles, the following is seen: hazard ratio (HR) = 1.9 for Q2 versus Q1; HR = 0.5 for Q3 versus Q1; HR = 1.3 for Q4 versus Q1. If AUCHCY (and its logarithm) are modeled as continuous variables, the adjusted hazards of relapse are actually increased as AUCHCY increases (P = .19 for each parameter). Finally, if AUCCY (and its logarithm) are modeled as continuous variables, AUCCY shows no suggestion of an association with the adjusted hazards of relapse (P = .64 and P = .46, respectively).
If one restricts the analysis to CML-CP patients only (n = 73), there were only 7 relapses among this group, so it is not likely that any statistically significant associations could be seen. As shown in Table 4, among the few CML-CP patients who did experience relapse, there does not appear to be any obvious differences in exposure to CY or its metabolites HCY and CEPM compared with the CML-CP patients who did not experience relapse. We also examined the relationship between exposure to CEPM, HCY, and CY and relapse in the cohort of 74 patients with malignancies other than CML-CP and found no significant associations for any metabolite.
Exposure to cyclophosphamide and its metabolites CEPM and HCY in relation to relapse of hematologic malignancy after transplantation
| AUCs . | Chronic myeloid leukemia in chronic phase . | Other hematologic malignancies . | ||
|---|---|---|---|---|
| Relapse, n = 7 . | No relapse, n = 66 . | Relapse, n = 22 . | No relapse, n = 52 . | |
| AUCCEPM | 381 (303-547) | 388 (117-664) | 366 (118-706) | 418 (123-1881) |
| AUCHCY | 153 (72-269) | 142 (62-284) | 161 (61-283) | 127 (38-298) |
| AUCCY | 5698 (4516-7099) | 6507 (3588-8864) | 6356 (4497-7968) | 5982 (2676-8761) |
| AUCs . | Chronic myeloid leukemia in chronic phase . | Other hematologic malignancies . | ||
|---|---|---|---|---|
| Relapse, n = 7 . | No relapse, n = 66 . | Relapse, n = 22 . | No relapse, n = 52 . | |
| AUCCEPM | 381 (303-547) | 388 (117-664) | 366 (118-706) | 418 (123-1881) |
| AUCHCY | 153 (72-269) | 142 (62-284) | 161 (61-283) | 127 (38-298) |
| AUCCY | 5698 (4516-7099) | 6507 (3588-8864) | 6356 (4497-7968) | 5982 (2676-8761) |
Numbers represent medians (ranges) of areas under the curve, expressed as μM·h.
Cyclophosphamide metabolite exposure and overall survival
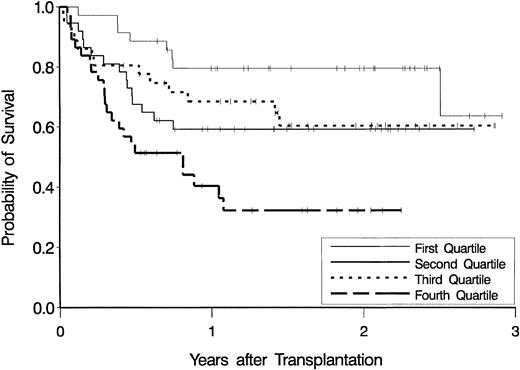
Figure 3 shows estimates of overall survival according to quartiles of AUCCEPM.
Kaplan-Meier estimates of the probability of overall survival.
Data are displayed as a function of exposure to the cyclophosphamide metabolite, CEPM, expressed as the lowest (first) quartile through the highest (fourth) quartile of AUCCEPM.
Kaplan-Meier estimates of the probability of overall survival.
Data are displayed as a function of exposure to the cyclophosphamide metabolite, CEPM, expressed as the lowest (first) quartile through the highest (fourth) quartile of AUCCEPM.
If AUCCEPM is modeled as a continuous variable and added to the regression model containing age and disease, the resultant model is statistically significantly improved (P = .002, likelihood ratio test). Inclusion of AUCCY as a continuous variable, however, does not lead to a statistically significant improved model over that containing age and disease (P = .20). Table5 summarizes results from the multivariable regression model with AUCCEPM modeled as quartiles.
Regression model of the relation of CEPM exposure to the hazard of overall mortality, adjusted for the variables age at transplantation and disease
| Quartile (AUCCEPM) . | Hazard ratio for overall mortality . | 95% CI . | P . |
|---|---|---|---|
| First | 1.0 | — | — |
| Second | 1.8 | 0.7-4.5 | .19 |
| Third | 1.6 | 0.6-4.2 | .30 |
| Fourth | 3.3 | 1.3-8.1 | .01 |
| Quartile (AUCCEPM) . | Hazard ratio for overall mortality . | 95% CI . | P . |
|---|---|---|---|
| First | 1.0 | — | — |
| Second | 1.8 | 0.7-4.5 | .19 |
| Third | 1.6 | 0.6-4.2 | .30 |
| Fourth | 3.3 | 1.3-8.1 | .01 |
Discussion
The primary objective of this study was to assess the impact of cyclophosphamide metabolism on liver toxicity caused by the preparative regimen. We found that increased exposure to toxic metabolites of cyclophosphamide led to increased liver toxicity and mortality and to decreased survival. Patients who had the lowest exposures to the metabolites CEPM and HCY were not at increased risk for failure to engraft or for relapse of malignancy. These results suggest that the dose of CY in the CY-TBI preparative regimen can be reduced without jeopardizing either engraftment or any antitumor effects of this preparative regimen.
The toxic metabolites formed after the administration of CY are acrolein and phosphoramide mustard.18-21 HCY is a protoxic metabolite converted to the actual toxins by the single step of β-elimination. Because CEPM is a nontoxic product of oxidation of HCY/aldophosphamide by ALHD1A1,22,23 our finding of a strong positive correlation between AUCCEPM and liver injury is the opposite of what might have been expected. Indeed, the formation of CEPM from HCY putatively explains the relative resistance of early hematopoietic precursors and L1210 leukemia cells from injury after the administration of CY.24-26 To verify that CEPM was not a hepatotoxin, we conducted incubations of CY, HCY, and CEPM with rat liver slices. CEPM was not toxic at millimolar concentrations, whereas CY and HCY were toxic at micromolar concentrations (S.R., J.T.S., unpublished observations, June 1999)—that is, CY and HCY were toxic because they were converted to the toxins acrolein and phosphoramide mustard. CEPM is the only chemically stable major CY metabolite formed between the activation of CY to HCY and the ultimate formation of glutathione conjugates of acrolein and phosphoramide mustard. The obvious hypothesis is that CEPM reports on the intrahepatocellular (or intrahepatic) exposure to chemically reactive species formed from HCY. However, for this to be so, there must be a means by which the intrahepatocellular AUCCEPM correlates positively with the intrahepatocellular AUCHCY, because the action of ALDH1A1 would produce a negative correlation. A positive correlation between the intrahepatocellular AUCCEPM and AUCHCY requires a route of elimination of HCY that competes with the formation of CEPM (Figure 1). Most likely this requirement is fulfilled by the transport of glutathionyl-cyclophosphamide (GSCY) out of the hepatocyte by the bile canalicular multiorganic anion transporter ABCC2, which has been shown to transport glutathione conjugates.27 Preliminary studies in Wistar rats and TR− rats (lacking ABCC2 expression in canalicular membranes) support the hypothesis that GSCY is a substrate of ABCC2: TR− rats have higher AUCCEPM and greater hepatic GSCY and HCY than do wild-type Wistar rats (R. Qiu, J.T.S., unpublished observations, June 2001).
The initial site of injury following high-dose myeloablative therapy and similar toxins is the hepatic sinusoid, specifically dissection of sinusoidal endothelial cells off the sinusoidal basement membrane and widespread hemorrhage in the centrilobular zone of the liver lobule.8,28,29 Cyclophosphamide alone is insufficient to produce the extensive sinusoidal injury that can be seen following cyclophosphamide and total body irradiation.30-32 Total body irradiation, when used alone in doses of 12 to 15 Gy, does not cause significant liver injury.33-35 However, cyclophosphamide followed by TBI is clearly synergistic. There are 2 potential mechanisms: one involves a 2-step injury—sublethal damage to sinusoidal endothelial cells caused by cyclophosphamide metabolites followed by irradiation damage. An alternative but not mutually exclusive mechanism involves depletion of reduced glutathione in hepatocytes and sinusoidal endothelial cells by cyclophosphamide, leaving sinusoidal endothelial cells more vulnerable to damage by irradiation. In animals exposed to hepatic irradiation or cyclophosphamide or monocrotaline, liver damage is enhanced by glutathione depletion and is lessened by repletion.5,29,34 36-38
There are several approaches to reducing the mortality caused by the CY-TBI regimen. One is to develop a method to adjust the second dose of CY based on the first day's exposure to the reporter molecule CEPM—to target the total exposure to CEPM to a value consistent with low toxicity and reliable engraftment. We estimate that elimination of liver toxicity by metabolism-based CY dosing in patients receiving CY-TBI would reduce nonrelapse deaths after transplantation by 20%. Patients with CML in chronic phase, for which we have the most data showing that CY metabolite exposures are unrelated to engraftment and relapse, are the best candidates for CY dosing that is targeted to a patient's metabolism. A similar targeting strategy has been used in the dosing of busulfan in the busulfan-cyclophosphamide conditioning regimen.39 40 The results of the current study cannot be extrapolated to other CY-containing regimens, such as busulfan-cyclophosphamide, without proof that there is a relation between CY metabolites and the end points toxicity, nonrelapse mortality, and survival following regimens other than CY-TBI. Another approach is to reduce the dose of CY for all patients who are receiving CY-TBI, but this may lead to failed engraftment and graft rejection and, possibly, to increased tumor recurrence. A third approach is to substitute for CY another agent of comparable immunosuppressive and antitumor activity that is not liver toxic.
We thank Linda Risler, Scott Cole, Rudy Linterman, Scott McDonald, Steven Ellis, and Raymond Salazar for their assistance and the medical and nursing staffs, patients, and patient families for their support. Dr Fred Appelbaum provided a critical review of the manuscript.
Prepublished online as Blood First Edition Paper, October 24, 2002; DOI 10.1182/blood-2002-06-1860.
Supported by grants CA18029 and CA15704 from the National Institutes of Health, National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
George B. McDonald, Gastroenterology/Hepatology Section (D2-190), Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109-1024.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal