Plasma D-dimer concentration rises more than 100-fold during acute deep vein thrombosis, but there are no prospective data concerning D-dimer as a risk factor for incident venous thrombosis in a general population. Incident venous thrombosis was ascertained in 2 prospective observational studies, the Atherosclerosis Risk in Communities Study and the Cardiovascular Health Study. Of 21 690 participants enrolled between 1987 and 1993, after 8 years of follow-up, D-dimer was measured using baseline stored plasma of 307 participants who developed venous thrombosis and 616 who did not. Relative to the first quintile of the distribution of D-dimer, the age-adjusted odds ratios for future venous thrombosis for the second to fifth quintiles of D-dimer were 1.6, 2.3, 2.3, and 4.2, respectively (P for trend < .0001). Following added adjustment for sex, race, body mass index, factor V Leiden, prothrombin 20210A, and elevated factor VIII coagulant activity (factor VIII:c), these odds ratios were 1.5, 2.1, 1.9, and 3.0, respectively (P for trend < .0001). Among those with idiopathic thrombosis or secondary thrombosis unrelated to cancer, the adjusted fifth quintile odds ratios were 3.5 and 4.8, respectively. By contrast, D-dimer in the fifth versus first quintile was not related to occurrence of cancer-associated thrombosis (odds ratio, 1.1). Odds ratios for elevated D-dimer were consistently elevated in subgroups defined by age, sex, race, duration of follow-up, and thrombosis type (deep vein thrombosis or pulmonary embolus). D-dimer is strongly and positively related to the occurrence of future venous thrombosis.
Introduction
Our understanding of hemostatic disorders related to venous thrombosis has increased significantly since the early 1990s and the discovery of the factor V Leiden mutation.1However, even though up to 35% of unselected patients with a first thrombosis possess either factor V Leiden or the prothrombin 20210A gene variant,2 approximately 40% of patients with venous thrombosis in the presence of a positive family history do not have identifiable hemostatic disorders.3 Therefore, further study of hemostatic factors in relation to occurrence of venous thrombosis is warranted.
Plasma levels of fibrin fragment D-dimer are elevated during acute venous thrombosis because D-dimer is a marker of fibrin formation and reactive fibrinolysis. In clinical settings, a low D-dimer concentration may be useful for exclusion of acute thrombosis.4 Among healthy individuals, there is significant between-person variability of D-dimer concentration within the normal range, and concentrations of plasma D-dimer in the top third or fourth of the normal population distribution were associated with increased risk of future myocardial infarction in several prospective studies.5-9 In this context, D-dimer may represent the summation of procoagulant balance or genetic factors, the extent of subclinical atherosclerosis, or the presence of underlying coagulation disorders that predispose to coronary thrombosis.10 Two case-control studies reported that those with a history of deep vein thrombosis were more likely than controls to have elevated D-dimer concentration,11,12 and a third study reported a high negative predictive value for recurrent venous thrombosis among those with low D-dimer after initial treatment.13
The Longitudinal Investigation of Thromboembolism Etiology (LITE) is a population-based prospective study of the incidence and risk factors for venous thrombosis. We evaluated the association of baseline D-dimer with incidence of venous thrombosis in this study.
Patients and methods
Study population and baseline assessments
The LITE study is composed of 2 pooled, multicenter longitudinal population-based cohort studies, the Atherosclerosis Risk in Communities (ARIC) Study and the Cardiovascular Health Study (CHS). In ARIC, 15 792 participants, aged 45 to 64 years, were examined at baseline in 1987-1989, and in CHS, 5201 participants, aged 65 years or older, were examined in 1989-1990. To enhance their representation, an additional 687 black subjects were recruited to CHS in 1992-1993. Informed consent was obtained from all participants in accordance with institutional guidelines. Cardiovascular risk factors were assessed similarly in each study, as previously published.14,15 At baseline and 3 years later, blood was drawn from fasting participants in the morning in both studies, centrifuged for 30 000g-minutes, and stored in freezers at −70°C.16 17 Up to 3 follow-up examinations were performed every 3 years in ARIC and up to 9 follow-up examinations were performed annually in CHS.
Nested case-control design
The associations between incidence of venous thrombosis and D-dimer were assessed using a nested case-control study design and the stored blood samples. Potential cases of venous thrombosis were identified from baseline through September 1998. Hospital records were obtained and events validated by 2 physicians using standardized criteria. Radiologic documentation was required. Validated cases of deep vein thrombosis included those nearly always having a positive venogram, duplex, or Doppler ultrasound. Validated cases of pulmonary embolus nearly always had a positive pulmonary angiogram or a high-probability ventilation-perfusion scan (multiple segmental or subsegmental mismatched defects). Cases were also classified as incident or recurrent (based on information in the medical record), and idiopathic (no obvious cause) or secondary (associated with cancer, major trauma, surgery, marked immobility). Among 335 venous thrombosis events, 293 were incident and 42 recurrent; 153 were idiopathic and 182 secondary. There were 237 deep vein thromboses, with 220 of these involving veins of the legs, pelvis, or inferior vena cava. There were 52 cases of pulmonary embolus, and 46 additional cases of pulmonary embolus with concurrently documented deep vein thrombosis.
Controls were selected at random from the ARIC and CHS cohorts, with a ratio of 2.1 controls per case, and frequency matched to the cases by age (5-year groupings), sex, race (white, nonwhite), study (ARIC, CHS), and follow-up time (within 2 years).18Selection yielded 390 ARIC controls and 298 CHS controls.
Laboratory methods
Stored plasma and DNA were retrieved for the selected cases and controls. If baseline plasma samples were limited, previously thawed, or exhausted for a participant a sample was retrieved from the next visit, approximately 3 years after baseline (n = 79 cases and 131 controls; 12 cases whose thrombosis was before the second visit). If neither sample was available or the D-dimer assay could not be performed, results were considered missing (n = 28 cases and 72 controls). The percentage of missing samples for D-dimer measurement did not differ significantly comparing cases to controls (P = .29). DNA was missing or permission to use it was not given for 6.2% of ARIC and 8.5% of CHS participants.
D-dimer was measured at the University of Vermont with an enzyme-linked immunosorbent assay (ELISA) using 2 monoclonal antibodies against nonoverlapping determinants of D-dimer.19 The analytic coefficient of variation ranged from 7.5% to 10.2% from higher to lower D-dimer concentrations. D-dimer by this method has longitudinal within-individual variability comparable to serum cholesterol20 and is stable in long-term storage at −70°C.21
The factor V Leiden (1691G>A) mutation and prothrombin 20210A variant were detected using standard methods.2,22 Homozygosity for either trait was rare (3 with factor V Leiden, 0 with prothrombin variant) so heterozygotes and homozygotes were pooled for analysis. Factor VIII coagulant activity (factor VIII:c) was measured in all ARIC and CHS participants at baseline as previously reported.17 23
Statistical analyses
The association of baseline D-dimer with other factors was analyzed in the control group using analysis of variance. Unconditional logistic regression was used to calculate odds ratios and 95% confidence intervals (CIs) for venous thrombosis in relation to quintiles of the D-dimer distribution for combined case and control subjects. A test for trend in odds ratios was conducted using ordinal values for each quintile in logistic regression. Adjustment was made for factors previously associated venous thrombosis in the LITE study: age in all models, and race, sex, body mass index (BMI), factor V Leiden, prothrombin 20210A, and factor VIII:c in additional models. Analyses were repeated excluding the 12 cases whose D-dimer was measured after their thrombosis. Logistic regression analyses were repeated and stratified by study (ARIC and CHS) and by race (whites and nonwhites). Logistic regression analyses were also performed for prespecified subgroups (incident versus recurrent thrombosis, deep vein thrombosis versus pulmonary embolus, and idiopathic versus secondary thrombosis, with or without cancer) using all controls for comparison. Interactions of D-dimer with age, factor V Leiden, prothrombin 20210A variant, and elevated factor VIII:c were examined for idiopathic thrombosis by cross-classification.
Results
D-dimer was measured in 923 subjects, 307 who had venous thrombosis during 8 years of follow-up and 616 who did not. Among the 923 subjects, the average baseline age was 64 years (range, 45-94 years), 53% were women, and 78% were white (most nonwhites were black). The distributions of these characteristics were similar between cases and controls due to the matching. BMI and factor VIII:c were higher among cases than controls (P < .001 for both). The mean BMI in cases and controls was 29.0 and 27.6 kg/m2, respectively. These values for factor VIII:c were 145% and 133%, respectively. Approximately 14% of cases and 4% of controls carried factor V Leiden, and 4% of cases and 2% of controls carried the prothrombin 20210A polymorphism. Both gene variants were rare among nonwhites (211 nonwhites, 2 with factor V Leiden, and 1 with prothrombin 20210A); therefore interaction analyses of the gene variants with D-dimer included whites only.
Associations of D-dimer with several established risk factors for venous thrombosis are shown in Table 1. Higher D-dimer concentration was associated with older age, nonwhite race, and higher factor VIII:c. The mean factor VIII:c was 144% in the highest quintile of D-dimer compared to 123% in the lowest quintile. Factor V Leiden and prothrombin 20210A were more common among those with higher D-dimer. For 134 participants with factor V Leiden, prothrombin 20210A, or factor VIII:c above the 80th percentile, the geometric mean D-dimer concentration was 155.1 ng/mL compared to 111.6 ng/mL among 423 subjects without any of these factors (P = .0002).
Association of D-dimer with venous thrombosis risk factors in controls, LITE
| Characteristic (sample size) . | Quintile of D-dimer (range, ng/mL) . | P for trend . | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| (2.0-68.5) | (68.6-104.2) | (104.3-159.3) | (159.4-277.7) | (277.8-7428.9) | ||
| Age, y (616) | 59.3 | 62.6 | 65.0 | 67.5 | 68.8 | .0001 |
| Female sex (616) | 48% | 53% | 59% | 62% | 46% | .44 |
| White race (616) | 84% | 79% | 81% | 75% | 72% | .01 |
| BMI, kg/m2(613) | 26.6 | 27.8 | 27.8 | 28.3 | 27.4 | .10 |
| Factor VIII:c, % (586) | 123 | 133 | 133 | 134 | 144 | .0008 |
| Factor V Leiden (582) | 1.5% | 4.0% | 0.9% | 4.4% | 11.2% | .002 |
| Prothrombin 20210A (579) | 2.2% | 0.8% | 1.7% | 1.8% | 4.5% | .27 |
| Characteristic (sample size) . | Quintile of D-dimer (range, ng/mL) . | P for trend . | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| (2.0-68.5) | (68.6-104.2) | (104.3-159.3) | (159.4-277.7) | (277.8-7428.9) | ||
| Age, y (616) | 59.3 | 62.6 | 65.0 | 67.5 | 68.8 | .0001 |
| Female sex (616) | 48% | 53% | 59% | 62% | 46% | .44 |
| White race (616) | 84% | 79% | 81% | 75% | 72% | .01 |
| BMI, kg/m2(613) | 26.6 | 27.8 | 27.8 | 28.3 | 27.4 | .10 |
| Factor VIII:c, % (586) | 123 | 133 | 133 | 134 | 144 | .0008 |
| Factor V Leiden (582) | 1.5% | 4.0% | 0.9% | 4.4% | 11.2% | .002 |
| Prothrombin 20210A (579) | 2.2% | 0.8% | 1.7% | 1.8% | 4.5% | .27 |
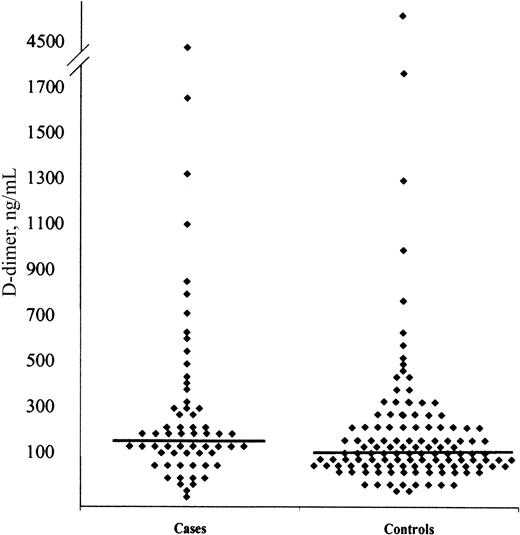
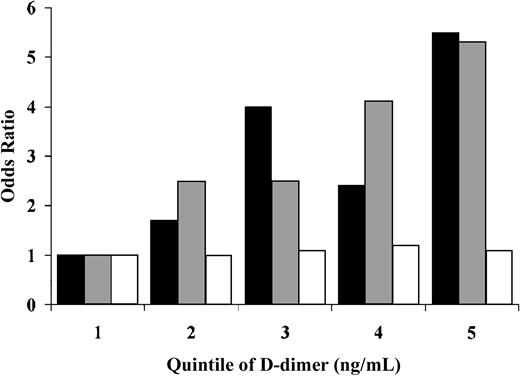
Figure 1 shows the distribution of D-dimer values among cases and controls, demonstrating higher values in cases. Overall, the risk of venous thrombosis increased substantially with increasing baseline D-dimer concentration (Table2). Nearly 30% of cases had D-dimer values in the highest quintile of the distribution, whereas only 12% had D-dimer in the lowest quintile. For D-dimer concentrations in the third through fifth, compared to the first quintile, the age-adjusted odds ratios for thrombosis were more than 2-fold increased, with a fifth quintile odds ratio of 4.2 (95% CI, 2.6-6.8). Assessment of the confounding effects of other factors on the association between D-dimer and venous thrombosis is shown in Figure2 and Table 2. Adjustment for age, sex, race, and BMI slightly reduced the quintile-specific odds ratios. Further adjustment for hemostatic risk factors (factor V Leiden, prothrombin 20210A, and factor VIII:c) yielded a fifth compared to first quintile odds ratio of 3.0 (95% CI, 1.7-5.2). With further adjustment for factor VIII:c alone this odds ratio was 3.3 (95% CI, 2.0-5.5). Results did not differ materially with exclusion of 12 participants (4% of cases) whose thrombosis occurred before D-dimer measurement, with exclusion of those with D-dimer above the 97.5 percentile, or with further adjustment for baseline C-reactive protein or fibrinogen concentrations or smoking status.
Distribution of D-dimer among cases and controls.
Each symbol represents the mean of 5 values. Horizontal lines represent the median D-dimer values.
Distribution of D-dimer among cases and controls.
Each symbol represents the mean of 5 values. Horizontal lines represent the median D-dimer values.
Odds ratio of venous thrombosis according to baseline D-dimer concentration, overall and in subgroups, LITE
| . | Quintile of D-dimer (range, ng/mL) . | P for trend . | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| (2.0-68.5) | (68.6-104.2) | (104.3-159.3) | (159.4-277.7) | (277.8-7428.9) | ||
| Overall venous thrombosis | ||||||
| No. of cases | 38 | 52 | 66 | 63 | 88 | <.0001 |
| No. of controls | 146 | 133 | 119 | 122 | 96 | |
| Age-adjusted | ||||||
| OR (95% CI) | 1.0 (Reference) | 1.6 (1.0-2.6) | 2.3 (1.5-3.8) | 2.3 (1.4-3.7) | 4.2 (2.6-6.8) | |
| Adjusted 1* | ||||||
| OR (95% CI) | 1.0 (Reference) | 1.5 (0.9-2.5) | 2.3 (1.4-3.7) | 2.2 (1.3-3.6) | 3.9 (2.4-6.4) | <.0001 |
| Adjusted 2† | ||||||
| OR (95% CI) | 1.0 (Reference) | 1.5 (0.9-2.5) | 2.1 (1.2-3.5) | 1.9 (1.1-3.3) | 3.0 (1.7-5.2) | <.0001 |
| Sex | ||||||
| Men | ||||||
| No. of cases | 28 | 20 | 29 | 25 | 43 | .001 |
| OR (95% CI) | 1.0 (Reference) | 0.9 (0.5-1.7) | 1.7 (0.9-3.3) | 1.7 (0.8-3.4) | 2.6 (1.4-5.1) | |
| Women | ||||||
| No. of cases | 10 | 32 | 37 | 38 | 45 | <.0001 |
| OR (95% CI) | 1.0 (Reference) | 3.4 (1.5-7.5) | 4.1 (1.9-9.0) | 4.0 (1.8-8.8) | 8.3 (3.7-18.6) | |
| Race | ||||||
| White | ||||||
| No. of cases | 32 | 41 | 53 | 47 | 60 | <.0001 |
| OR (95% CI) | 1.0 (Reference) | 1.6 (0.9-2.7) | 2.4 (1.4-4.1) | 2.4 (1.4-4.2) | 4.1 (2.4-7.2) | |
| Nonwhite | ||||||
| No. of cases | 6 | 11 | 13 | 16 | 28 | .005 |
| OR (95% CI) | 1.0 (Reference) | 1.5 (0.5-4.8) | 2.2 (0.7-7.0) | 2.1 (0.7-6.3) | 4.4 (1.5-13.0) | |
| Study | ||||||
| ARIC | ||||||
| No. of cases | 34 | 37 | 37 | 28 | 33 | <.0001 |
| OR (95% CI) | 1.0 (Reference) | 1.7 (1.0-2.9) | 2.2 (1.3-3.9) | 2.0 (1.1-3.6) | 3.4 (1.8-6.2) | |
| CHS | ||||||
| No. of cases | 4 | 15 | 29 | 35 | 55 | <.0001 |
| OR (95% CI) | 1.0 (Reference) | 1.9 (0.6-6.2) | 3.5 (1.1-11.1) | 3.7 (1.2-11.4) | 7.2 (2.3-22.1) | |
| . | Quintile of D-dimer (range, ng/mL) . | P for trend . | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| (2.0-68.5) | (68.6-104.2) | (104.3-159.3) | (159.4-277.7) | (277.8-7428.9) | ||
| Overall venous thrombosis | ||||||
| No. of cases | 38 | 52 | 66 | 63 | 88 | <.0001 |
| No. of controls | 146 | 133 | 119 | 122 | 96 | |
| Age-adjusted | ||||||
| OR (95% CI) | 1.0 (Reference) | 1.6 (1.0-2.6) | 2.3 (1.5-3.8) | 2.3 (1.4-3.7) | 4.2 (2.6-6.8) | |
| Adjusted 1* | ||||||
| OR (95% CI) | 1.0 (Reference) | 1.5 (0.9-2.5) | 2.3 (1.4-3.7) | 2.2 (1.3-3.6) | 3.9 (2.4-6.4) | <.0001 |
| Adjusted 2† | ||||||
| OR (95% CI) | 1.0 (Reference) | 1.5 (0.9-2.5) | 2.1 (1.2-3.5) | 1.9 (1.1-3.3) | 3.0 (1.7-5.2) | <.0001 |
| Sex | ||||||
| Men | ||||||
| No. of cases | 28 | 20 | 29 | 25 | 43 | .001 |
| OR (95% CI) | 1.0 (Reference) | 0.9 (0.5-1.7) | 1.7 (0.9-3.3) | 1.7 (0.8-3.4) | 2.6 (1.4-5.1) | |
| Women | ||||||
| No. of cases | 10 | 32 | 37 | 38 | 45 | <.0001 |
| OR (95% CI) | 1.0 (Reference) | 3.4 (1.5-7.5) | 4.1 (1.9-9.0) | 4.0 (1.8-8.8) | 8.3 (3.7-18.6) | |
| Race | ||||||
| White | ||||||
| No. of cases | 32 | 41 | 53 | 47 | 60 | <.0001 |
| OR (95% CI) | 1.0 (Reference) | 1.6 (0.9-2.7) | 2.4 (1.4-4.1) | 2.4 (1.4-4.2) | 4.1 (2.4-7.2) | |
| Nonwhite | ||||||
| No. of cases | 6 | 11 | 13 | 16 | 28 | .005 |
| OR (95% CI) | 1.0 (Reference) | 1.5 (0.5-4.8) | 2.2 (0.7-7.0) | 2.1 (0.7-6.3) | 4.4 (1.5-13.0) | |
| Study | ||||||
| ARIC | ||||||
| No. of cases | 34 | 37 | 37 | 28 | 33 | <.0001 |
| OR (95% CI) | 1.0 (Reference) | 1.7 (1.0-2.9) | 2.2 (1.3-3.9) | 2.0 (1.1-3.6) | 3.4 (1.8-6.2) | |
| CHS | ||||||
| No. of cases | 4 | 15 | 29 | 35 | 55 | <.0001 |
| OR (95% CI) | 1.0 (Reference) | 1.9 (0.6-6.2) | 3.5 (1.1-11.1) | 3.7 (1.2-11.4) | 7.2 (2.3-22.1) | |
Sex-, race-, and study-stratified models are adjusted for age.
OR indicates odds ratio.
Adjusted for age, race, sex, and BMI.
Adjusted for age, race, sex, BMI, factor VIII:c, factor V Leiden, and prothrombin 20210A.
Odds ratio of venous thrombosis in relation to baseline D-dimer concentration.
Baseline D-dimer values were divided into quintiles of the distribution and the risk of venous thrombosis for each quintile was compared to the first quintile using logistic regression. ▪ represent age-adjusted models; ░, models adjusted for age, sex, race, and BMI; ■, models adjusted for all previous factors and factor V Leiden, prothrombin 20210A, and factor VIII:c above the 80th percentile. D-dimer quintile definitions are given in Table 2.
Odds ratio of venous thrombosis in relation to baseline D-dimer concentration.
Baseline D-dimer values were divided into quintiles of the distribution and the risk of venous thrombosis for each quintile was compared to the first quintile using logistic regression. ▪ represent age-adjusted models; ░, models adjusted for age, sex, race, and BMI; ■, models adjusted for all previous factors and factor V Leiden, prothrombin 20210A, and factor VIII:c above the 80th percentile. D-dimer quintile definitions are given in Table 2.
The relationship of higher D-dimer to venous thrombosis was present among all subgroups analyzed in age-adjusted models in Table 2. The association of D-dimer with thrombosis was larger in women than in men, and in the older CHS participants than the younger ARIC participants, but the CIs overlapped widely. Similar findings were observed comparing incident to recurrent thrombosis: fifth quintile odds ratio 3.7 (95% CI, 2.2-6.2) for incident and 7.6 (95% CI, 2.4-24.1) for recurrent thrombosis. There were no differences in risk for elevated D-dimer comparing those with deep vein thrombosis to pulmonary embolus with or without a deep vein thrombosis. The association of D-dimer with thrombosis persisted throughout the duration of follow-up, but appeared larger for events occurring earlier during follow-up. For the 73 thrombosis events occurring within 2.5 years of phlebotomy, adjusting for age, race, sex, and BMI, the fifth quintile odds ratio was 7.5 (95% CI, 2.9-18.2), whereas for 222 later events the odds ratio was 3.1 (95% CI, 1.8-5.4).
Figure 3 shows the association of baseline D-dimer with incident venous thrombosis based on the presence or absence of acquired risk factors for thrombosis. The relative risks of idiopathic thrombosis and secondary thrombosis unrelated to cancer were similar, with fifth versus first quintile age, sex, race, and BMI-adjusted odds ratios of 5.5 (95% CI, 2.5-11.8) and 5.3 (95% CI, 2.1-13.3), respectively. Further adjustment for factor V Leiden, prothrombin 20210A, and factor VIII:c yielded odds ratios of 3.5 (95% CI, 1.5-8.3) and 4.8 (95% CI, 1.6-14.2), respectively. By contrast, there was no association of baseline D-dimer with the occurrence of cancer-associated venous thrombosis, with a fifth quintile adjusted odds ratio of 1.1 (95% CI, 0.5-2.7).
Odds ratio of venous thrombosis in relation to baseline D-dimer concentration, according to presence of precipitating factors for thrombosis.
Logistic regression models were fit for subgroups of thrombosis that were idiopathic (n = 113, ▪), associated with precipitants other than cancer (n = 89, ░), or associated with cancer (n = 68, ■). All models were adjusted for age, sex, race, and BMI. TheP values for trend with increasing quintiles of D-dimer were .003, .006, and .78, respectively.
Odds ratio of venous thrombosis in relation to baseline D-dimer concentration, according to presence of precipitating factors for thrombosis.
Logistic regression models were fit for subgroups of thrombosis that were idiopathic (n = 113, ▪), associated with precipitants other than cancer (n = 89, ░), or associated with cancer (n = 68, ■). All models were adjusted for age, sex, race, and BMI. TheP values for trend with increasing quintiles of D-dimer were .003, .006, and .78, respectively.
Considering only those 235 subjects with venous thrombosis that was unrelated to cancer, in comparison to all controls, the joint associations of D-dimer above the 60th percentile (159.3 ng/mL) with other hemostatic risk factors for venous thrombosis are shown in Table3. In the absence of elevated D-dimer, the presence of factor V Leiden, prothrombin 20210A, or elevated factor VIII:c was associated with increased risk of venous thrombosis. In the absence of a hemostatic defect, D-dimer above 159.3 ng/mL was associated with a 2-fold increased risk of thrombosis. The combination of factor V Leiden, prothrombin 20210A, or elevated factor VIII:c with elevated D-dimer conveyed lower odds ratios of venous thrombosis than expected by an additive model of individual risk factors. If higher values of D-dimer (80th or 90th percentile) were chosen to define elevated D-dimer, or if participants with isolated pulmonary embolus were excluded,24 inferences from this analysis were similar.
Joint associations of elevated D-dimer and hemostatic disorders with risk of venous thrombosis unrelated to cancer, LITE
| D-dimer above 60th percentile3-150 . | Hemostatic disorder . | OR (95% CI) (No. of events) . | ||
|---|---|---|---|---|
| Factor V Leiden . | Prothrombin 20210A . | Elevated factor VIII:c3-151 . | ||
| No | No | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| (65) | (73) | (82) | ||
| No | Yes | 7.3 (2.9-18.4) | 3.9 (1.2-13.0) | 1.8 (1.1-3.1) |
| (13) | (6) | (29) | ||
| Yes | No | 2.2 (1.4-3.4) | 2.5 (1.7-3.8) | 2.2 (1.5-3.2) |
| (62) | (79) | (84) | ||
| Yes | Yes | 5.6 (2.7-11.7) | 2.3 (0.5-10.2) | 2.3 (1.4-4.0) |
| (21) | (3) | (30) | ||
| D-dimer above 60th percentile3-150 . | Hemostatic disorder . | OR (95% CI) (No. of events) . | ||
|---|---|---|---|---|
| Factor V Leiden . | Prothrombin 20210A . | Elevated factor VIII:c3-151 . | ||
| No | No | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| (65) | (73) | (82) | ||
| No | Yes | 7.3 (2.9-18.4) | 3.9 (1.2-13.0) | 1.8 (1.1-3.1) |
| (13) | (6) | (29) | ||
| Yes | No | 2.2 (1.4-3.4) | 2.5 (1.7-3.8) | 2.2 (1.5-3.2) |
| (62) | (79) | (84) | ||
| Yes | Yes | 5.6 (2.7-11.7) | 2.3 (0.5-10.2) | 2.3 (1.4-4.0) |
| (21) | (3) | (30) | ||
D-dimer level above 159.3 ng/mL.
Factor VIII:c above the 80th percentile (170%).
Discussion
The main finding of this study was a graded increase in the future occurrence of venous thrombosis with increasing baseline concentration of D-dimer in a sample of the general US population. The association was independent of several common thrombosis risk factors, including factor V Leiden, the prothrombin 20210A variant, and factor VIII:c. Adjusting for demographic factors and BMI, the risk of venous thrombosis for the highest versus lowest 20% of D-dimer values was 3.9-fold increased, and adjusting further for hemostatic factors, the risk was 3.0-fold increased. These odds ratios were even higher when those with cancer-associated thrombosis were excluded. The association of D-dimer with venous thrombosis was observed consistently in subgroups defined by age, race, thrombosis type, time between phlebotomy and thrombosis, and by whether the thrombosis was a first or recurrent event or was idiopathic or associated with acquired risk factors other than cancer. There was no association of D-dimer with occurrence of cancer-associated venous thrombosis.
A case-control study including 66 women, aged 45 to 64, with deep vein thrombosis, reported an association between higher D-dimer concentration and a history of thrombosis.12 Similarly, in another report, 474 patients with a prior history of deep vein thrombosis were more likely than 474 controls to have elevated D-dimer concentration.11 In both studies, the odds ratios for thrombosis were similar to those observed here and were also present among those without known hemostatic defects. Our prospective data add important new information because D-dimer increases more than 100-fold during acute thrombosis, and it is unknown whether D-dimer returns to an individual's long-term normal value over time after a thrombotic event.
Our findings differ from the only other prospective study of D-dimer and the risk of venous thrombosis, which assessed the occurrence of deep vein thrombosis after elective hip replacement among 375 patients.25 In that study, mean preoperative D-dimer concentration was higher among 120 patients who subsequently developed postoperative deep vein thrombosis than those who did not, but this difference was not independent of other factors.25
There are a few possible pathophysiologic explanations for an association of D-dimer with venous thrombosis. It is unlikely that D-dimer itself is a causal factor in venous thrombosis. We hypothesize that it is a marker for other factors related to the pathophysiology of thrombosis. First, considering that venous thrombosis is an oligogenic disease,1 elevated D-dimer may reflect currently unknown hemostatic disorders that are associated with venous thrombosis. Indeed, D-dimer was higher in the presence of factor V Leiden, prothrombin 20210A, or elevated factor VIII:c, all recognized as common risk factors for thrombosis. However, it remained associated with thrombosis, independent of these factors. Other risk factors for thrombosis, including deficiencies of protein C, protein S, or antithrombin are probably too rare in the general population (< 1% each)1 to be considered responsible for the association of D-dimer with thrombosis. A recent finding that D-dimer has high heritability supports the notion that D-dimer reflects genetic factors.26 These might relate to regulatory proteins for coagulation factor production or clearance in general or to differences in coagulation factors themselves. Second, D-dimer might reflect other environmental risk factors for thrombosis. Higher D-dimer concentration is associated with smoking status27 and some coagulation and inflammation markers such as fibrinogen and C-reactive protein.7 However, none of these factors were associated with the risk of venous thrombosis in the LITE population, and none were confounders of the D-dimer association with venous thromboembolism.28 Third, because fibrinolytic factors, such as plasminogen activator inhibitor-1 and tissue plasminogen activator, are not generally considered risk factors for venous thrombosis,29,30 it is likely that higher D-dimer reflects increased fibrin formation rather than fibrinolytic reactivity. Our findings and those of others5-9 11-13 would support further study of phenotypic and genotypic determinants of fibrin formation.
Higher D-dimer is correlated with more advanced cancer stage.31 32 Even though D-dimer was not associated with cancer-associated thrombosis in this study, at the time of phlebotomy most participants did not have a history of cancer, and among CHS participants none were receiving active treatment for cancer. Therefore, further study of D-dimer as a risk factor for venous thrombosis among patients with active cancer may be useful.
A few points should be considered in interpreting these findings. First, D-dimer was measured using a research-based ELISA. To our knowledge, in nonacute clinical settings, this assay has not been compared to commercially available assays, and this would be necessary prior to considering use of D-dimer for thrombosis risk assessment. Second, inclusion as a case of venous thrombosis in this study required a clinical diagnosis of thrombosis, so some fatal pulmonary emboli and asymptomatic thromboses were under ascertained. Because venous thrombosis is a relatively rare disease, misclassification based on this or other factors, would not be expected to change the results significantly, and would lead to underestimation of the true risk associated with D-dimer.
In conclusion, higher D-dimer concentration was associated with increased risk of subsequent venous thrombosis in a general US population. Assessment of D-dimer may provide different clinical information than assessment of other thrombosis risk factors, such as factor V Leiden or obesity. Based on these data, calculation of the attributable risk fraction of venous thrombosis associated with D-dimer suggests that 13.3% of events may be accounted for by D-dimer in the highest quintile. The strength of the association observed here, and its consistency in various subgroups, suggests potential clinical roles for D-dimer assessment that could be tested in future clinical studies. These studies should include assessment of D-dimer with more readily available assay systems than the research-based ELISA used here. In addition, based on this and other recent findings,11,12 26further study of genetic determinants of elevated D-dimer in healthy populations is indicated.
We are grateful to ARIC and CHS investigators and the study participants for their many years of important contributions to these studies. We also thank Ms Cathy Tilley and Ms Elaine Cornell for technical support of this study.
Prepublished online as Blood First Edition Paper, September 26, 2002; DOI 10.1182/blood-2002-05-1416.
Supported by the Atherosclerosis Risk in Communities Study, funded by contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, N01-HC-55022, and the Cardiovascular Health Study, funded by contracts N01-HC-85079 to N01-HC-85086 from the National Heart, Lung, and Blood Institute. The Longitudinal Investigation of Thromboembolism Etiology was funded by R01 HL59367 from the National Heart, Lung, and Blood Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mary Cushman, Departments of Medicine and Pathology, 208 S Park Dr, Suite 2, Colchester, VT 05446; e-mail:mary.cushman@uvm.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal