Fanconi anemia (FA) is an autosomal recessive disorder characterized by cellular hypersensitivity to DNA cross-linking agents and cancer predisposition. Recent evidence for the interactions of ataxia-telangiectasia mutated protein ATM and breast cancer susceptibility proteins BRCA1 and BRCA2 (identified as FANCD1) with other known FA proteins suggests that FA proteins have a significant role in DNA repair/recombination and cell cycle control. The International Fanconi Anemia Registry (IFAR), a prospectively collected database of FA patients, allows us the unique opportunity to analyze the natural history of this rare, clinically heterogeneous disorder in a large number of patients. Of the 754 subjects in this study, 601 (80%) experienced the onset of bone marrow failure (BMF), and 173 (23%) had a total of 199 neoplasms. Of these neoplasms, 120 (60%) were hematologic and 79 (40%) were nonhematologic. The risk of developing BMF and hematologic and nonhematologic neoplasms increased with advancing age with a 90%, 33%, and 28% cumulative incidence, respectively, by 40 years of age. Univariate analysis revealed a significantly earlier onset of BMF and poorer survival for complementation group C compared with groups A and G; however, there was no significant difference in the time to hematologic or nonhematologic neoplasm development between these groups. Multivariate analysis of overall survival time shows that FANCCmutations (P = .007) and hematopoietic stem cell transplantation (P = < .0001) define a poor-risk subgroup. The results of this study of patients registered in the IFAR over a 20-year period provide information that will enable better prediction of outcome and aid clinicians with decisions regarding major therapeutic modalities.
Introduction
Fanconi anemia (FA) is a genetically and phenotypically heterogeneous autosomal recessive disorder defined by cellular hypersensitivity to DNA cross-linking agents.1,2Clinically, FA is characterized by congenital malformations, progressive marrow failure, and predisposition to acute myelogenous leukemia (AML) and other malignancies.3 The hypersensitivity to DNA cross-linking agents such as diepoxybutane (DEB) is used as a diagnostic test for this disease and allows the clinician to make a diagnosis of FA in persons with subtle clinical features, including those without clinically detectable congenital anomalies.4,5 Complementation studies have shown that there are at least 8 separate FA complementation groups (A through G, including D1 and D2).6-8 The genes responsible for the defect in each of these FA complementation groups have been identified.8-15 Complementation groups A (65%), C (15%), and G (10%) account for most patients with this disorder reported to the International Fanconi Anemia Registry (IFAR).
Although the most common and well-characterized malignancies in FA are hematologic, FA patients have been found to develop a wide array of different neoplasms. Several recent case reports and literature reviews have suggested that FA patients are highly predisposed to nonhematologic (solid) tumors, particularly squamous cell carcinoma (SCC) of the upper aerodigestive and anogenital tract.16,17 This increased cancer susceptibility is most likely due to the high degree of genomic instability characteristic of FA patients. However, the relationship of genomic instability to cancer is not well characterized. Other syndromes with a high degree of genomic instability and strong cancer predisposition include ataxia-telangiectasia, Nijmegen breakage syndrome, Bloom syndrome, hereditary nonpolyposis colorectal cancer (HPNCC), and hereditary breast/ovarian cancer syndromes.2,18 Recent evidence for the interactions of ataxia-telangiectasia mutated protein ATM19 and breast cancer susceptibility proteins BRCA120 and BRCA2 (identified as FANCD1)15with other known FA proteins suggests that FA proteins have a significant role in DNA repair/recombination and cell cycle control. The study of the natural history of the disease in FA patients is important to furthering our limited understanding of the role of FA proteins in healthy individuals.
The rare occurrence of FA in the general population makes the analysis of the clinical aspects of the disease difficult. Most studies evaluating FA outcomes involve a small number of subjects with brief follow-up. Many of these patients do not have a DEB-confirmed diagnosis of FA, and therefore, a number of patients may receive a misdiagnosis of FA. In addition, most literature reviews are biased toward more severe clinical phenotypes, because the earlier published cases were often diagnosed only when aplastic anemia or leukemia developed in individuals with characteristic physical abnormalities. The goal of this study is to understand the relationship between genotype and the clinical phenotype in FA patients in terms of bone marrow failure (BMF), neoplasm development, and overall survival. To accomplish this goal, we report the cumulative incidence of BMF and tumor development in 754 subjects enrolled in the IFAR and analyze prognostic variables that influence overall survival. An increased understanding of the factors that influence the development of BMF, tumor development, and survival will help to better predict clinical outcome and aid decision making regarding major therapeutic modalities for this unique population of patients.
Materials and methods
Registry characteristics
The IFAR was established at The Rockefeller University in 1982 to collect clinical and genetic information from a large number of FA patients. At the time of this analysis, 755 FA patients from North America were enrolled in this registry. Registration in the IFAR usually occurs at the time of the diagnosis and includes information on congenital and hematologic abnormalities, as well as any history of neoplasm development. Approval for these studies was obtained from The Rockefeller University institutional review board. Informed consent was provided according to the Declaration of Helsinki. The diagnosis of FA is confirmed by the study of chromosomal breakage induced by DEB testing in the peripheral blood (PB) lymphocytes. The protocol used for DEB testing is described in detail in Current Protocols in Human Genetics.21 Comprehensive attempts to obtain follow-up data and report results have periodically been made.4,5 22-26
Hematologic abnormalities
The onset of hematologic abnormalities is defined as the time at which one of the following laboratory parameters is observed: platelet count lower than 100 × 109/L, hemoglobin level (Hb) lower than 6.206 mM (10 g/dL), or absolute neutrophil count (ANC) lower than 1 × 109/L.3 Less severe decreases in blood cells and alterations in red blood cell mean corpuscular volume (MCV) and fetal Hb (HbF) are often noted earlier in FA patients, but are not defined as hematologic abnormalities in determining the age of hematologic onset. Pancytopenia is defined as an abnormality in 2 or 3 blood cell lineages. In this analysis, unless otherwise specified, we use the term BMF to indicate hematologic abnormalities other than myelodysplastic syndrome (MDS) or AML. The time to BMF is defined as the time elapsed between the patient's date of birth (which is also considered as the date of FA onset) and the date of BMF.
Definitions of neoplasms (hematologic and solid tumors)
Hematologic and nonhematologic (solid) neoplasms were identified by reviewing the clinical records in the IFAR and by contacting patients who were alive at last follow-up. In patients receiving a diagnosis of neoplasm, a complete history of the tumor, including stage, management, recurrence, and outcome, was ascertained. The pathology was confirmed by obtaining pathology reports from the original biopsies or bone marrow aspirations or by review of the surgical specimen. If the type of neoplasm could not be confirmed, the tumor was not considered in the analysis.
MDS is a known complication of Fanconi anemia.24 27 The diagnosis of MDS was based on the clinical and pathology reports submitted to the IFAR. For purposes of this analysis, AML was defined as more than 30% myeloid blasts in the BM or more than 20% myeloid blasts in the blood. The time to hematologic or solid tumors was defined as the time elapsed between the date of birth and the date of neoplasm onset. The overall survival time was defined as the time elapsed between the date of birth and the date of death or the date of last follow-up.
Statistical analysis
The following end points were calculated for all patients: overall survival time, time to BMF, time to hematologic malignancy, time to solid tumor, and time to squamous cell carcinoma. All these end points were calculated by treating the date of birth as the date of FA onset. The prognostic factors evaluated were as follows: complementation group, sex, and hematopoietic stem cell transplantation (HSCT). Complementation group was treated as a categoric variable (A, C, D2, F, G, and nontyped). Sex was treated as a binary variable (male and female). HSCT was treated as a time-dependent covariate.
The Kaplan-Meier28 estimates of overall survival time in various prognostic factor categories, except HSCT, were calculated and compared by means of the log-rank test statistic. Variables significant in the univariate analysis were carried over to multivariate analysis. The multivariate analysis was performed by means of a Cox proportional hazards model. HSCT was performed only in those patients who experienced the onset of BMF. This induces a colinear relationship between HSCT and BMF. Therefore, the effect of HSCT on overall survival was analyzed only for the subset of patients who experienced the onset of BMF. For this purpose, we recalculated the overall survival time within this subgroup of patients as the time elapsed between the date of BMF onset and the date of death or last follow-up.
A competing-risk approach was used to analyze all the other end points. A competing risk is an event whose occurrence precludes the event of interest from occurring. In our data, death is a competing cause of risk for the other end points. For example, death prior to hematologic malignancy is a competing risk for this end point. The Kaplan-Meier approach assumes that the event of interest and the censoring event are statistically independent. This assumption is violated in the presence of competing risks. Therefore, we have used the competing-risk approach to estimate the cumulative incidence29 of the other end points. The hazard ratios were computed by means of competing-risk regression, and compared by means of a chi-square test. All analyses were carried out by means of S-plus 2000 (Mathsoft, Seattle, WA).
Results
Subject characteristics
A total of 755 subjects from North America with the DEB-confirmed diagnosis of FA were registered into the IFAR between the years of 1982 and 2001. One patient died on the day of birth and was excluded from all the analyses, resulting in an effective sample size of 754 patients. Patient characteristics are provided in Table1. There were 367 (49%) female and 387 (51%) male FA patients. Complementation groups were determined in 341 patients by mutation screening as previously described for IFAR patients 18,25,30-32 or by correction of cross-linker hypersensitivity of the patient cells by transduction with retroviral vectors containing the normalFANCA, FANCC, or FANCG cDNAs. The method of cross-linker hypersensitivity correction is similar to that established for peripheral blood–derived patient T cells.33 In the remaining 413 patients, complementation groups were not determined; these patients were grouped together and referred to as nontyped. This group of subjects is likely to be genetically heterogeneous, composed of all complementation groups represented in the IFAR. The study closing date was December 31, 2001. By this date, 283 (38%) of the 754 patients had died. A total of 601 patients (80%) experienced the onset of BMF. Of these, 219 patients (36%) received an HSC transplant during the course of this study.
Characteristics of patients enrolled in the International Fanconi Anemia Registry (1982–2001)
| Variable . | No. patients (%) . |
|---|---|
| Sex | |
| Female | 367 (49) |
| Male | 387 (51) |
| Complementation group | |
| A | 207 (27) |
| C | 78 (10) |
| D2 | 2 (0.3) |
| F | 8 (1) |
| G | 46 (6) |
| Nontyped* | 413 (55) |
| BMF | |
| Yes | 601 (80) |
| No | 153 (20) |
| HSCT | |
| Yes | 219 (29) |
| No | 535 (71) |
| Hematologic malignancy | |
| Yes | 120 (16) |
| No | 634 (84) |
| Solid tumor | |
| Yes | 73 (10) |
| No | 681 (90) |
| Alive | |
| Yes | 471 (62) |
| No | 283 (38) |
| Variable . | No. patients (%) . |
|---|---|
| Sex | |
| Female | 367 (49) |
| Male | 387 (51) |
| Complementation group | |
| A | 207 (27) |
| C | 78 (10) |
| D2 | 2 (0.3) |
| F | 8 (1) |
| G | 46 (6) |
| Nontyped* | 413 (55) |
| BMF | |
| Yes | 601 (80) |
| No | 153 (20) |
| HSCT | |
| Yes | 219 (29) |
| No | 535 (71) |
| Hematologic malignancy | |
| Yes | 120 (16) |
| No | 634 (84) |
| Solid tumor | |
| Yes | 73 (10) |
| No | 681 (90) |
| Alive | |
| Yes | 471 (62) |
| No | 283 (38) |
HSCT indicates hematopoietic stem cell transplantation.
Complementation group unknown.
Tumor development
Of the total population of 754 subjects in the IFAR, 173 patients (23%) developed 199 neoplasms. Of these, 120 (60%) were hematologic and 79 (40%) were nonhematologic. Squamous cell carcinoma of the head and neck, anogenital region, and skin (39 cases) were the most common nonhematologic neoplasms identified, followed in descending order by benign and malignant liver neoplasms (18 cases), brain neoplasms (5 cases), and renal neoplasms (6 cases). Table2 describes the specific types of neoplasms (benign or malignant) and the number of cases found in the IFAR database. Twenty-one patients developed more than 1 neoplasm during their lifetime (range, 1-4 neoplasms). Seventeen patients developed 2 tumors; 3 patients developed 3 tumors; and 1 patient developed 4 tumors. Seventeen (80%) of the 21 patients who developed multiple neoplasms had SCC as one of their neoplasms. In contrast to the general population, predisposing risk factors for head and neck cancer were rare in this population. Only 3 (16%) of 19 patients who developed head and neck SCC had a history of alcohol or tobacco abuse. Review of the IFAR clinical charts revealed that risk factors for anogenital cancer were high among the FA population. Of the FA patients who developed cervical, vulvar, and anal squamous cell carcinoma, 54% had a preceding history of vaginal and/or anal human papillomavirus (HPV)–associated condylomas.
Neoplasms identified in FA patients
| Tumor type . | No. cases (%) . |
|---|---|
| Hematologic tumors | 120 (60) |
| AML | 60 |
| MDS | 53 |
| ALL | 5 |
| CMMOL | 1 |
| Burkitt lymphoma | 1 |
| Nonhematologic tumors | 79 (40) |
| Liver tumors | 18 (9) |
| Liver adenoma | 11 |
| Hepatocellular carcinoma | 6 |
| Liver adenocarcinoma | 1 |
| Brain tumors | 5 (2) |
| Medulloblastoma | 4 |
| Astrocytoma | 1 |
| Renal tumors | 6 (3) |
| Wilm tumor | 4 |
| Renal cell carcinoma | 1 |
| Nephroblastoma | 1 |
| Squamous cell carcinoma | 39 (20) |
| Head and neck SCC | 19 |
| Vulvar | 8 |
| Cervix | 6 |
| Cutaneous | 3 |
| Anus | 2 |
| Esophagus | 1 |
| Miscellaneous tumors | 11 (6) |
| Breast carcinoma | 3 |
| Basal cell carcinoma | 2 |
| Neuroblastoma | 1 |
| Desmoid tumor | 1 |
| Gonadoblastoma | 1 |
| Melanoma | 1 |
| Neurilemmona | 1 |
| Osteogenic sarcoma | 1 |
| Tumor type . | No. cases (%) . |
|---|---|
| Hematologic tumors | 120 (60) |
| AML | 60 |
| MDS | 53 |
| ALL | 5 |
| CMMOL | 1 |
| Burkitt lymphoma | 1 |
| Nonhematologic tumors | 79 (40) |
| Liver tumors | 18 (9) |
| Liver adenoma | 11 |
| Hepatocellular carcinoma | 6 |
| Liver adenocarcinoma | 1 |
| Brain tumors | 5 (2) |
| Medulloblastoma | 4 |
| Astrocytoma | 1 |
| Renal tumors | 6 (3) |
| Wilm tumor | 4 |
| Renal cell carcinoma | 1 |
| Nephroblastoma | 1 |
| Squamous cell carcinoma | 39 (20) |
| Head and neck SCC | 19 |
| Vulvar | 8 |
| Cervix | 6 |
| Cutaneous | 3 |
| Anus | 2 |
| Esophagus | 1 |
| Miscellaneous tumors | 11 (6) |
| Breast carcinoma | 3 |
| Basal cell carcinoma | 2 |
| Neuroblastoma | 1 |
| Desmoid tumor | 1 |
| Gonadoblastoma | 1 |
| Melanoma | 1 |
| Neurilemmona | 1 |
| Osteogenic sarcoma | 1 |
ALL indicates acute lymphoblastic leukemia; CMMOL, chronic myelomonocytic leukemia; SCC, squamous cell carcinoma.
Overall survival time
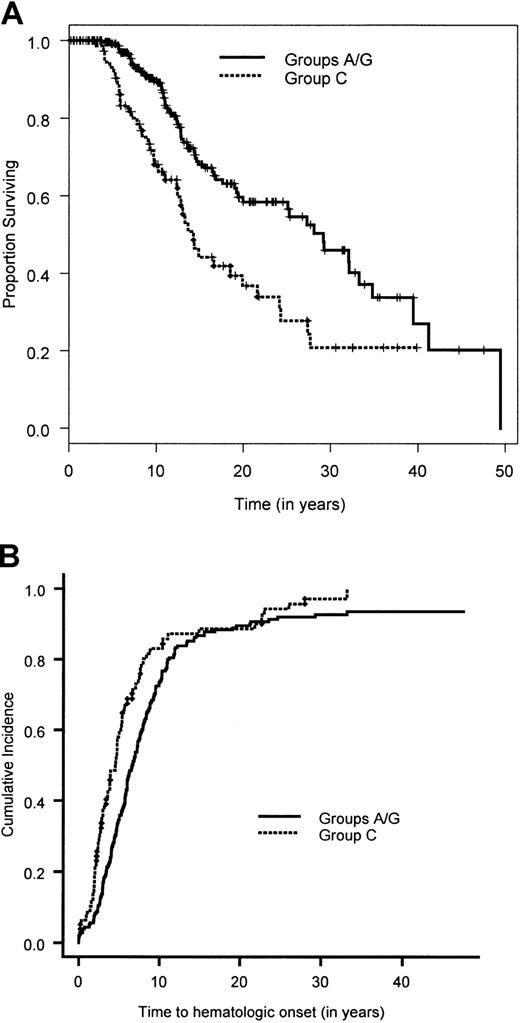
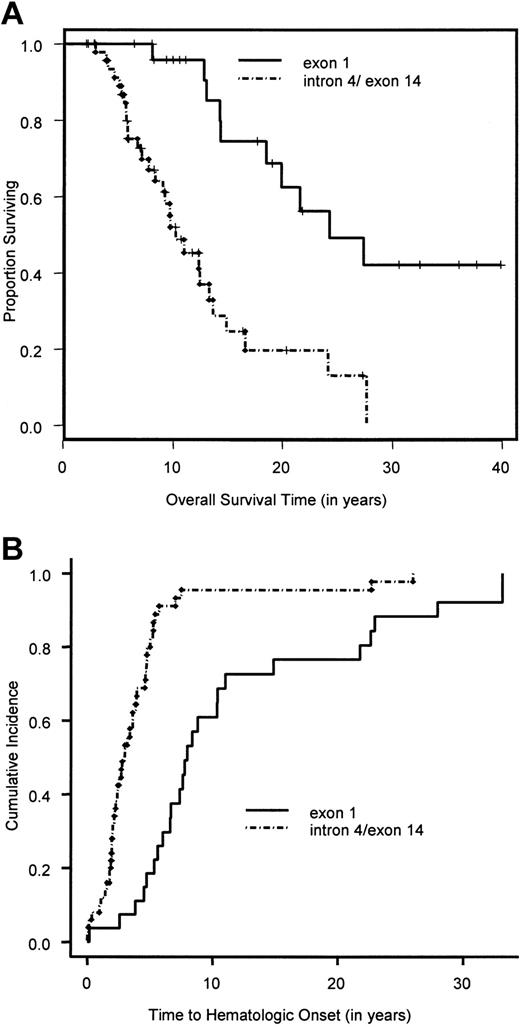
Figure 1 shows the overall survival time for the 754 patients. The median survival time was 24 years. The median follow-up time, based on the 471 patients alive at the end of the study, was 10.6 years. The results of the univariate analyses are given in Tables 3 and4. There was no significant difference in the survival time between the 341 patients in whom complementation group was typed (groups A, C, and G combined) and the 413 nontyped (genetically heterogeneous) patients (P = .36). Owing to the very small sample size in complementation groups D2 (2 patients) and F (8 patients), we focused the univariate and multivariate analyses on the remaining 744 patients. Pair-wise analysis of the groups showed no significant difference in overall survival time between groups A and G (P = .94). Hence, patients in the complementation groups A and G were combined. Comparing the overall survival time in the 3 groups, namely A/G versus C versus nontyped, showed a significant difference in overall survival (Table 3; Figure 2A). Patients in complementation group C had a significantly poorer survival than the other 2 groups. Furthermore, there was a significantly poorer overall survival for patients with at least one intron 4 (IVS4+4A>T) or one exon 14 (R548X and L554P) mutation compared with patients with at least one exon 1 mutation (322delG and Q13X) and no mutations in exon 14 or IVS4 (Table 3; Figure3A). Males had a significantly poorer survival than females. An initial multivariate Cox proportional hazards model was fit for complementation group (A/G versus C versus nontyped) and sex. Patients in complementation group C had a significantly increased hazard for overall survival time compared with patients in groups A and G (hazard ratio = 2.1; 95% CI, 1.4-3.1), as did patients in the nontyped group (hazard ratio = 1.4; 95% CI, 1.04-1.81). Furthermore, males had a significantly worse hazard than females (hazard ratio = 1.4; 95% CI, 1.1-1.7). The results of this multivariate analysis are summarized in Table5.
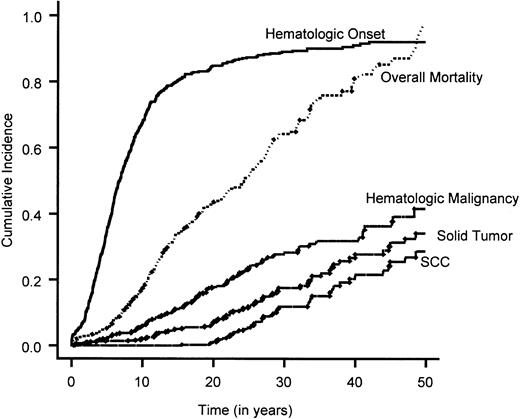
Cumulative incidence of the end points of interest.
The overall mortality is estimated by means of the Kaplan-Meier method. Overall survival rate is 1 − overall mortality rate. The cumulative incidence of all the other end points are calculated by treating death as a competing cause of risk.
Cumulative incidence of the end points of interest.
The overall mortality is estimated by means of the Kaplan-Meier method. Overall survival rate is 1 − overall mortality rate. The cumulative incidence of all the other end points are calculated by treating death as a competing cause of risk.
Overall survival time in FA patients, univariate results (log-rank test)
| Variable . | No.patients . | No.events . | P . | Survival rate, % . | ||
|---|---|---|---|---|---|---|
| 5-year . | 10-year . | 15-year . | ||||
| Group | ||||||
| Typed3-150 | 341 | 115 | .36 | 97.8 | 84.7 | 62.5 |
| Nontyped3-151 | 413 | 168 | 92.0 | 81.1 | 68.2 | |
| A | 207 | 57 | .94 | 99.5 | 91.5 | 68.0 |
| G | 46 | 16 | 97.8 | 82.1 | 67.9 | |
| A/G | 253 | 73 | < .001 | 99.1 | 89.7 | 68.1 |
| C | 78 | 40 | 93.1 | 68.1 | 44.3 | |
| Intron 4/exon 14 | 50 | 29 | < .0001 | 91.2 | 52.1 | 24.7 |
| Exon 1 | 27 | 10 | 100 | 95.8 | 74.5 | |
| Sex | ||||||
| Male | 387 | 151 | .02 | 94.1 | 80.4 | 60.0 |
| Female | 367 | 132 | 95.1 | 85.0 | 71.6 | |
| Variable . | No.patients . | No.events . | P . | Survival rate, % . | ||
|---|---|---|---|---|---|---|
| 5-year . | 10-year . | 15-year . | ||||
| Group | ||||||
| Typed3-150 | 341 | 115 | .36 | 97.8 | 84.7 | 62.5 |
| Nontyped3-151 | 413 | 168 | 92.0 | 81.1 | 68.2 | |
| A | 207 | 57 | .94 | 99.5 | 91.5 | 68.0 |
| G | 46 | 16 | 97.8 | 82.1 | 67.9 | |
| A/G | 253 | 73 | < .001 | 99.1 | 89.7 | 68.1 |
| C | 78 | 40 | 93.1 | 68.1 | 44.3 | |
| Intron 4/exon 14 | 50 | 29 | < .0001 | 91.2 | 52.1 | 24.7 |
| Exon 1 | 27 | 10 | 100 | 95.8 | 74.5 | |
| Sex | ||||||
| Male | 387 | 151 | .02 | 94.1 | 80.4 | 60.0 |
| Female | 367 | 132 | 95.1 | 85.0 | 71.6 | |
Complementation groups A, C, D2, F, and G.
Complementation group unknown.
Overall survival time for FA patients with HSCT, univariate results (Cox proportional hazards model)
| HSCT . | Result . |
|---|---|
| No. patients | 589 |
| Hazard ratio (95% CI) | 4.6 (3.5-5.9) |
| P | < .0001 |
| HSCT . | Result . |
|---|---|
| No. patients | 589 |
| Hazard ratio (95% CI) | 4.6 (3.5-5.9) |
| P | < .0001 |
CI indicates confidence interval.
Overall survival time and cumulative incidence of bone marrow failure for complementation groups FA-A/FA-G and FA-C.
(A) Overall survival time estimated by the Kaplan-Meier method for patients in complementation groups A/G and C. (B) Cumulative incidence of bone marrow failure calculated by a competing-risk approach, in which death is treated as a competing cause of risk, for patients in complementation groups A/G and C.
Overall survival time and cumulative incidence of bone marrow failure for complementation groups FA-A/FA-G and FA-C.
(A) Overall survival time estimated by the Kaplan-Meier method for patients in complementation groups A/G and C. (B) Cumulative incidence of bone marrow failure calculated by a competing-risk approach, in which death is treated as a competing cause of risk, for patients in complementation groups A/G and C.
Overall survival time and cumulative incidence of bone marrow failure for exon 1 and intron 4/exon 14 FANCCpatients.
(A) Overall survival time of exon 1 and intron 4/exon 14 patients calculated by the Kaplan-Meier method. (B) Cumulative incidence of bone marrow failure in exon 1 and intron 4/exon 14 patients, calculated by a competing-risk approach, in which death is treated as a competing cause of risk.
Overall survival time and cumulative incidence of bone marrow failure for exon 1 and intron 4/exon 14 FANCCpatients.
(A) Overall survival time of exon 1 and intron 4/exon 14 patients calculated by the Kaplan-Meier method. (B) Cumulative incidence of bone marrow failure in exon 1 and intron 4/exon 14 patients, calculated by a competing-risk approach, in which death is treated as a competing cause of risk.
Overall survival time for FA patients, multivariate results (Cox proportional hazards model)
| Variable . | No.patients . | Hazard ratio (95% CI) . | P . |
|---|---|---|---|
| Group | |||
| A/G | 253 | 1.05-151 | NA |
| C | 78 | 2.1 (1.4-3.1) | .0002 |
| Nontyped5-150 | 413 | 1.4 (1.04-1.81) | .03 |
| Sex | |||
| Female | 363 | 1.05-151 | NA |
| Male | 381 | 1.4 (1.1-1.7) | .01 |
| Variable . | No.patients . | Hazard ratio (95% CI) . | P . |
|---|---|---|---|
| Group | |||
| A/G | 253 | 1.05-151 | NA |
| C | 78 | 2.1 (1.4-3.1) | .0002 |
| Nontyped5-150 | 413 | 1.4 (1.04-1.81) | .03 |
| Sex | |||
| Female | 363 | 1.05-151 | NA |
| Male | 381 | 1.4 (1.1-1.7) | .01 |
NA indicates not applicable.
Complementation group unknown.
Baseline.
The effect of the time-dependent variable HSCT on overall survival time was examined within the subgroup of patients who had an onset of BMF. A total of 593 of the 744 patients experienced an onset of BMF. Of these, 4 patients were not followed beyond the date of BMF. Hence, the effect of HSCT on overall survival was analyzed in the subgroup of 589 patients. The time-dependent variable HSCT had a significant effect on survival, with a hazard ratio of 4.6 (95% CI, 3.5-5.9) in a univariate model. The results of the univariate analyses are shown in Table 4. The following variables were entered into the multivariate model for this subgroup of patients: complementation group (A/G versus C versus nontyped); sex (male versus female); and HSCT (time-dependent variable). The results of the multivariate model are summarized in Table 6. HSCT continued to be a significant risk factor for overall survival within this subgroup of patients in the multivariate model (hazard ratio = 5.0; 95% CI, 3.8-6.6).
Overall survival time in the subgroup of FA patients with BMF, multivariate results (Cox proportional hazards model)
| Variable . | No. patients . | Hazard ratio (95% CI) . | P . |
|---|---|---|---|
| Group | |||
| A/G | 212 | 1.06-151 | NA |
| C | 72 | 1.7 (1.2-2.6) | .007 |
| Nontyped6-150 | 305 | 1.5 (1.2-2.1) | .004 |
| Sex | |||
| Female | 290 | 1.06-151 | NA |
| Male | 299 | 1.3 (1.0-1.7) | .06 |
| HSCT | 589 | 5.0 (3.8-6.6) | < .0001 |
| Variable . | No. patients . | Hazard ratio (95% CI) . | P . |
|---|---|---|---|
| Group | |||
| A/G | 212 | 1.06-151 | NA |
| C | 72 | 1.7 (1.2-2.6) | .007 |
| Nontyped6-150 | 305 | 1.5 (1.2-2.1) | .004 |
| Sex | |||
| Female | 290 | 1.06-151 | NA |
| Male | 299 | 1.3 (1.0-1.7) | .06 |
| HSCT | 589 | 5.0 (3.8-6.6) | < .0001 |
NA indicates not applicable.
Complementation group unknown.
Baseline.
It can be seen that sex is not a significant variable in this multivariate model (P = .06). However, we have retained this variable in the model since sex is known to be a prognostic factor among FA patients.
Time to BMF
A total of 601 patients (80%) had BMF. The cumulative incidence of BMF by age 40 was 90% (Figure 1). Comparison of the cumulative incidence of BMF in various complementation groups showed no significant difference between groups A and G (P = .58). Hence, patients in these 2 complementation groups were combined. Comparison of the combined group A/G with group C and the nontyped (genetically heterogeneous) group showed that patients in group C had a significantly higher incidence of BMF compared with the combined group A/G (hazard ratio = 1.5; 95% CI, 1.1-2.0) (Figure 2B), while patients in the nontyped group had a significantly lower incidence of BMF compared with group A/G (hazard ratio = 0.75; 95% CI, 0.63-0.89). These results are summarized in Table7. Furthermore, within complementation group C, there was a significantly earlier onset of BMF in patients with at least one intron 4 (IVS4+4A>T) or at least one exon 14 (R548X or L554P) mutation compared with patients with at least an exon 1 mutation (322delG and Q13X) and no mutations in exon 14 or intron 4 (Figure 3B). There was no difference between males and females in the cumulative incidence of BMF.
Hazard ratios of various end points calculated by means of competing-risk regression
| End point and variable . | No.patients . | No.events . | Hazard ratio (95% CI) . | P . |
|---|---|---|---|---|
| BMF | ||||
| Group | ||||
| A/G | 253 | 213 | 1.07-151 | NA |
| C | 78 | 73 | 1.5 (1.1-2.0) | .005 |
| Nontyped7-150 | 413 | 307 | 0.75 (0.63-0.89) | .001 |
| FANCC mutations | ||||
| Exon 1 | 27 | 25 | 1.07-151 | NA |
| Intron 4/exon 14 | 50 | 47 | 3.8 (2.1-6.9) | < .0001 |
| Hematologic malignancy | ||||
| Sex | ||||
| Female | 367 | 49 | 1.07-151 | NA |
| Male | 387 | 71 | 1.5 (1.1-2.2) | .02 |
| Solid tumor | ||||
| Sex | ||||
| Female | 367 | 48 | 1.07-151 | NA |
| Male | 387 | 25 | 0.52 (0.33-0.83) | .007 |
| SCC | ||||
| Sex | ||||
| Female | 349 | 30 | 1.07-151 | NA |
| Male | 371 | 9 | 0.30 (0.15-0.61) | .001 |
| End point and variable . | No.patients . | No.events . | Hazard ratio (95% CI) . | P . |
|---|---|---|---|---|
| BMF | ||||
| Group | ||||
| A/G | 253 | 213 | 1.07-151 | NA |
| C | 78 | 73 | 1.5 (1.1-2.0) | .005 |
| Nontyped7-150 | 413 | 307 | 0.75 (0.63-0.89) | .001 |
| FANCC mutations | ||||
| Exon 1 | 27 | 25 | 1.07-151 | NA |
| Intron 4/exon 14 | 50 | 47 | 3.8 (2.1-6.9) | < .0001 |
| Hematologic malignancy | ||||
| Sex | ||||
| Female | 367 | 49 | 1.07-151 | NA |
| Male | 387 | 71 | 1.5 (1.1-2.2) | .02 |
| Solid tumor | ||||
| Sex | ||||
| Female | 367 | 48 | 1.07-151 | NA |
| Male | 387 | 25 | 0.52 (0.33-0.83) | .007 |
| SCC | ||||
| Sex | ||||
| Female | 349 | 30 | 1.07-151 | NA |
| Male | 371 | 9 | 0.30 (0.15-0.61) | .001 |
NA indicates not applicable.
Complementation group unknown.
Baseline.
Time to hematologic malignancy
A total of 120 (16%) patients experienced a hematologic malignancy. The cumulative incidence of hematologic malignancy by age 40 was 33% (Figure 1). There was no significant difference between the various complementation groups in the cumulative incidence of hematologic malignancy. However, males had a significantly increased cumulative incidence compared with females (hazard ratio = 1.5; 95% CI, 1.1-2.2) (Table 7).
Time to solid tumor
A total of 73 (10%) of the 754 patients had a diagnosis of solid tumor. The cumulative incidence of solid tumors by age 40 was 28% (Figure 1). There was no significant difference between the various complementation groups in the cumulative incidence of solid tumors. However, males had a significantly lower incidence of solid tumors compared with females (hazard ratio = 0.52; 95% CI, 0.33-0.82) (Table 7).
Analysis of SCC was restricted to 720 patients who developed SCC, died prior to SCC, or did not develop any other solid tumors. SCC was diagnosed in 39 patients, with 30 cases occurring in females and 9 in males. The cumulative incidence of SCC by age 40 was 20%. There was no significant difference in SCC among the complementation groups. However, males had a significantly lower incidence of SCC than females (hazard ratio = 0.30; 95% CI, 0.15-0.61). To determine whether this significant difference among males and females was related to the large number of gynecologic malignancies (vulva, cervix, vagina) found in this subset of patients, we reanalyzed this comparison after excluding 19 patients with gynecologic malignancies. There was no significant difference between males and females.
HSCT and its role in malignancy
Of the 754 FA patients, 219 underwent HSCT. A total of 6 (2.7%) of the 219 patients who underwent HSCT developed a solid neoplasm. In contrast, a total of 67 (13%) of the 535 who did not undergo HSCT developed a solid neoplasm.
The analysis of the relationship between HSCT and SCC was based on 720 patients, after the exclusion of patients who have developed non-SCC solid neoplasms. There were 29 SCCs (5.6%) diagnosed among the 510 patients who did not undergo HSCT. The remaining 210 patients underwent HSCT. Of these 210 patients, 12 had their last follow-up on their date of HSCT. Five of these 12 patients developed SCC prior to HSCT. Of the remaining 198 patients who underwent HSCT and had a postprocedure follow-up, 5 patients (2.5%) received a diagnosis of SCC. Since the number of neoplasms among patients undergoing HSCT is small, the hazard ratio could not be estimated.
Discussion
The ability of a cell to maintain genomic integrity under normal circumstances and the failure of this mechanism in FA are currently not well understood. On a molecular level, recent investigations have shown that FANCA, FANCC, FANCE, FANCF, and FANCG proteins assemble in a multisubunit nuclear complex.34-37This FA protein complex is required for the monoubiquitination of FANCD2 and this monubiquitination is essential for the function of the FA pathway and for DEB resistance.20 In response to DNA damage, monoubiquitinated FANCD2 is then translocated to nuclear foci containing BRCA1, BRCA2 (FANCD1), and other proteins important in DNA repair pathways.37 However, in FA cells, the formation of the FA protein complex is impaired; FANCD2 is not monubiquitinated and, therefore, does not form nuclear foci with BRCA1 and BRCA2.
Genotype-phenotype correlations in FA
The relationship between a defective FA pathway and the clinical FA phenotype (congential abnormalities, BMF, cancer predisposition, and early death) is currently not well elucidated, and further clinical studies are needed to correlate this pathway with the clinical presentation and outcome of FA patients. Since there is variability in both the age of onset of hematologic abnormalities and survival among various complementation groups, understanding the natural history of the different complementation groups is important in this regard. The incidence of solid and hematologic malignancies is also currently not well described, and a better understanding of the risk of neoplasm development not only will aid in counseling and management decisions for patients, but may further our understanding of the significance of the reported link between the 7 cloned FA genes with BRCA1.20
Although a large number of subjects and long follow-up make this database unique in its ability to describe the natural history of FA patients, there are potential limitations. One is potential selective subject reporting in a registry database. Another is that the frequency and extent of the clinical testing are determined by participating physicians and are therefore not standardized. A third consideration is completeness and accuracy of data reporting; no audits of reporting centers were performed. Even with these limitations, the IFAR allows us to follow a large number of DEB-confirmed FA patients and analyze their clinical outcomes. As the IFAR forms a cohort, analyses are internally valid. This information is important in helping physicians understand and manage this clinically complex disease.
The heterogenous nature of FA makes an understanding of the correlation between genotype and phenotype important in the clinical management of FA patients. This study confirms that specific complementation groups play a significant role in both the timing of BMF and the prognosis in these patients. FA-C patients had a significantly earlier onset of BMF and poorer survival compared with FA-A, FA-G, and nontyped patients. Subdividing the FA-C group on the basis of region of the gene that is mutated revealed that patients with intron 4 or exon 14 mutations had an earlier onset of BMF and poorer survival compared with patients with exon 1 mutations. Therefore, FA-C patients, and more specifically FA-C patients with mutations in intron 4 or exon 14, may be expected to have a more rapid course of hematologic abnormalities as well as a decreased overall survival. This information can aid families and clinicians in the timing of various treatment options. For example, patients with this more severe complementation group may need more frequent clinical assessment and marrow evaluation, while patients with the milder phenotypes and no related human leukocyte antigen (HLA) matches may wish to defer HSCT as long as possible.
Neoplasm development in FA
An understanding of the relationship between genomic instability in FA and solid tumor development has not been well elucidated, probably as a result of the rare occurrence of FA in the general population and, thus, the small number of FA-associated neoplasms. This study reveals that there is a progression to the development of solid malignancy with age, with a continuous change in the risk for patients who have not succumbed to earlier complications. Solid malignancies appear to play an important role in the natural history of FA. In fact, the cumulative incidence of hematologic neoplasms (33%) was not that different from the cumulative incidence of solid tumors (28%). Evaluation of the types of neoplasms that FA patients developed revealed a wide array of different solid neoplasms (Table 2), but FA patients appear to be strongly predisposed to SCC, especially of the upper aerodigestive and anogenital regions. In fact, there is a 500-fold increase in the cumulative incidence of head and neck cancer in FA patients compared with the Surveillance Epidemiology End Result (SEER) population.38 Therefore, FA patients who live into their third and fourth decades should be followed closely to screen for solid malignancies, particularly of the head and neck and the anogenital regions.
The recent report of Howlett et al15 that cell lines derived from FA-B and FA-D1 patients have biallelic mutations in BRCA2 and express truncated BRCA2 proteins leads these investigators to suggest that germ line mutations in any of the 7 cloned FA genes(FANCA, FANCC, FANCD1, FANCD2, FANCE, FANCF, FANCG) may result in cancer risks similar to those observed in families with BRCA1 or BRCA2 mutations. Owing to the lack of any clinical information regarding cancer in the families from which the BRCA2 mutant cell lines were derived, this report is still speculative. In our present analysis of malignancies in 754 FA homozygote subjects in the IFAR, we found 3 patients who developed breast cancer and 1 with malignant melanoma, while there were no observed cases of other cancers commonly associated withBRCA1 and BRCA2 mutations, such as ovarian, pancreatic, prostate, gallbladder and bile duct, or stomach.39 SCCs of the head and neck, anogenital region, and skin were the most common nonhematologic neoplasms identified, followed in descending order by benign and malignant liver neoplasms (11 cases), brain neoplasms (5 cases), and renal neoplasms (6 cases). Of the 79 subjects with solid tumors in our study, 39 developed SCCs at a median age of 37.8 years. The risk of breast cancer in carriers of germ line mutations in any of the FA genes is also unknown at this time. A screen for mutations in FANCA in 19 breast tumors with specific loss of heterozygosity (LOH) at 16q24.3,40 the region where FANCA is located, did not reveal any germ line mutations. Five polymorphisms were identified, but frequencies of occurrence did not deviate from those in a healthy control population. Also, there is little evidence in previously published studies for an excess of cancers in FA carriers.41 We are currently performing a retrospective cohort study of IFAR families to evaluate whether FA carriers with known mutations of the various FA genes are at increased risk for cancer.
Not only do FA patients have an increased predisposition to both hematologic and nonhematologic neoplasms, but also many patients develop multiple tumors during their lifetime. In this study, 21 patients were found to develop more than 1 neoplasm before the age of 50, and 4 patients eventually developed more than 2 neoplasms. Seventeen (80%) of these 21 patients developed at least one SCC. Of these 17 patients, 9 patients developed a second primary squamous cell carcinoma of the aerodigestive or anogenital tract. Other studies have also shown that FA patients have a marked affinity for the development of malignancies of the anogenital and oral areas. In their review, Kennedy and Hart42 found that of the 14 patients with head and neck SCC, 5 (35%) developed carcinoma in more than one mucosal site. The reason for this strong propensity for FA patients to develop multiple solid tumors, especially of the mucous membranes of the head and neck and anogenital region, is unclear. Tobacco and alcohol use, which are known risk factors for head and neck SCC, were found in only 3 of 19 patients who developed head and neck malignancy. Without the influence of known risk factors, therefore, a controversial question is whether the tendency to develop malignancy in FA results only from the genetic disorder of chromosomal breakage or is due to increased susceptibility to local predisposing factors such as viral infections, radiation exposure, or environmental toxins. The virus hypothesis is an interesting one because the mucous membranes are a common route of viral infections, especially for HPV and herpes simplex virus (HSV). In addition, the immunosuppression associated with persistent bone marrow failure may predispose these patients to the incorporation of viral DNA into the genome. Interestingly, 54% of the FA patients in this study who developed SCC of the cervix, vulva, and anus showed evidence of HPV-associated condylomas prior to developing SCC of the anogenital region. However, whether HPV infection or other local predisposing factors are related to the development of malignancy in these patients is not yet known and needs further elucidation.
HSCT and its role in malignancy
An understanding of the relationship between HSCT and malignancy development is important, since it may play a role in the type of HSCT-conditioning protocol used and in the management of FA patients. In previous studies, high-dose cytotoxic therapy used in HSCT is associated with secondary malignancies in the healthy population.43,44 FA patients may be more predisposed to the development of secondary malignancies following HSCT owing to their ongoing chromosomal instability and abnormal response to DNA damage in other tissues. Deeg et al45 evaluated the risk of secondary malignancy in 700 patients with aplastic anemia (AA), including 79 FA patients who received transplants between 1970 and 1993. Among patients with FA, a single hazard peak for solid tumors occurred between 8 and 9 years after HSCT. Their analysis revealed a 42% cumulative probability of developing a solid or lymphohematopoietic malignancy by 20 years after HSCT. This previous study was limited by the fact that it analyzed only patients who underwent HSCT and did not compare the risk of malignancy in FA patients who underwent HSCT with FA patients who did not. A controversy still exists as to whether the development of solid tumors, including SCC, is caused by the basic FA genetic disorder or whether it is a potential side effect of the HSCT therapy. Even though the statistical analysis of the relationship between HSCT and solid neoplasm development could not be done owing to the small number of HSC transplantation patients who developed solid tumors, it appears from this study that the percentage of patients who have undergone HSCT and developed solid neoplasms (2.7%) is actually less than the percentage of patients without HSCT who developed solid neoplasms (13%). Therefore, the underlying chromosomal instability characteristic of FA may play a more important role in the development of solid tumors than HSCT. In addition, FA patients have normal immune function following transplantation and have the potential for improved immune surveillance. However, more studies evaluating the role of HSCT in cancer development in FA are required.
Conclusions
By using the IFAR and its collection of clinical information on 754 DEB-confirmed FA patients, this report represents the largest analysis of the natural history of FA patients in terms of BMF, malignancy development, and outcomes. This study highlights the susceptibility of FA patients to both benign and malignant tumor development, and emphasizes the importance of regular and thorough tumor evaluations of all patients receiving a diagnosis of this disease. Early therapeutic intervention may be translated into improved survival, or at least reduce the necessity for more aggressive surgical approaches. This study also identifies several factors, including HSCT and complementation group C (especially mutations in intron 4 and exon 14), that adversely affect overall survival. These factors need to be considered when counseling patients and families about prognosis and in determining the need for early therapeutic interventions. It also begins to provide an insight into FA as a model for defective DNA repair mechanisms, which may later allow broadening of our knowledge in this area as a whole.
The authors would like to thank all the patients and their families who provided tissue samples for the studies reported here. The authors gratefully acknowledge the contributions of the many physicians who referred the patients to the IFAR and helped in sending the tissue samples. We thank John Wagner for valuable critical reading of the manuscript.
Prepublished online as Blood First Edition Paper, September 26, 2002; DOI 10.1182/blood-2002-07-2170.
Supported in part by grants R37 HL32987 (A.D.A.), RO1 CA82678 (M.B., A.D.A.), and 5T32 CA09685 (D.I.K.) from the National Institutes of Health; by Research Center grants M0l-RR00102 and M0l-RR06020 from the National Institutes of Health to The Rockefeller University Hospital General Clinical Research Center and The New York Presbyterian Hospital Children's Clinical Research Center; and by the Forschungsverbund Somatische Gentherapie des Bundesministeriums für Bildung und Forschung (Beo 0311661) and the Elterninitiative Kinderkrebsklinik eV (H.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Arleen D. Auerbach, The Rockefeller University, Laboratory of Human Genetics and Hematology, 1230 York Ave, New York, NY 10021; e-mail:auerbac@mail.rockefeller.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal