Few results on cardiac catheterization have been published for patients with sickle cell disease (SCD) with pulmonary hypertension (PHTN). Their survival once this complication develops is unknown. We analyzed hemodynamic data in 34 adult patients with SCD at right-sided cardiac catheterization and determined the relationship of PHTN to patient survival. In 20 patients with PHTN the average systolic, diastolic, and mean pulmonary artery pressures were 54.3, 25.2, and 36.0 mm Hg, respectively. For 14 patients with SCD without PHTN these values were 30.3, 11.7, and 17.8 mm Hg, respectively. The mean pulmonary capillary wedge pressure in patients with PHTN was higher than that in patients without PHTN (16.0 versus 10.6 mm Hg;P = .0091) even though echocardiography showed normal left ventricular systolic function. Cardiac output was high (8.6 L/min) for both groups of patients. The median postcatheterization follow-up was 23 months for patients with PHTN and 45 months for those without PHTN. Eleven patients (55%) with PHTN died compared to 3 (21%) patients without PHTN (χ2 = 3.83;P = .0503). The mean pulmonary artery pressure had a significant inverse relationship with survival (Cox proportional hazards modeling). Each increase of 10 mm Hg in mean pulmonary artery pressure was associated with a 1.7-fold increase in the rate (hazards ratio) of death (95% CI = 1.1-2.7; P = .028). The median survival for patients with PHTN was 25.6 months, whereas for patients without PHTN the survival was still over 70% at the end of the 119-month observation period (P = .044, Breslow-Gehan log-rank test). Our findings suggest that PHTN in patients with SCD shortened their survival.
Introduction
Pulmonary hypertension (PHTN) is a relatively common complication in adult patients with sickle cell disease (SCD).1,2 Its precise prevalence is unknown but estimates range from 5% to 30% of adult patients.1-3There are few reports on the pulmonary pressures in patients with SCD with PHTN.4-8 Furthermore, their prognosis, once the diagnosis of PHTN has been established by cardiac catheterization, has not been determined. The median survival of untreated primary PHTN, that is, without SCD, is 2.8 years.9 In patients with SCD, PHTN could add to their sickling-related pulmonary pathology, which includes recurrent vascular occlusion (chest syndrome) and pulmonary fibrosis.10 Perhaps for this reason anecdotal reports suggest shorter survival times in these patients.1 2
In this report, we review the results of right-sided cardiac catheterization in 34 adults with SCD, 20 patients with and 14 patients without PHTN. The catheterization procedures were not carried out in the context of a study but were performed for clinical indications. We also compare the survival of patients with SCD with PHTN to that from patients with normal pulmonary pressures.
Patients and methods
Patient population
All 34 patients were adults, aged 18 years or older, with SCD followed at the Howard University Center for Sickle Cell Disease. Their hemoglobinopathy type was determined by hemoglobin electrophoresis and their demographic and some of their clinical characteristics, including age at cardiac catheterization, are shown in Table1. All catheterizations were carried out between 1991 and 2001. At the time of the procedure all patients were hospitalized, mostly in the resolution phase of a painful sickle cell episode (crisis). Patients whose anemia was worse than that characteristic of their steady state generally received a transfusion before the procedure. Fourteen patients had undergone transfusions within a 3-month period before the cardiac catheterization, 7 (35%) in the PHTN group and 7 (50%) in the group without this abnormality. The indication for cardiac catheterization in most cases was the suspicion of PHTN by criteria such as increased intensity of the second heart sound at the pulmonary area, right-sided cardiac chamber enlargement by chest radiography or electrocardiogram, or an abnormal echocardiogram suggesting this diagnosis. In 6 patients (nos. 10, 15, 16, 19, 25, and 30), the indication for catheterization was evaluation of left ventricular function or suspicion of coronary artery disease (or both), in which case they had both right and left cardiac catheterization. All patients had at least one right-sided cardiac catheterization and all gave their written informed consent in accordance with our hospital's policy for clinically indicated procedures. The diagnosis of PHTN was defined as a mean pulmonary artery pressure (MPAP) of more than 25 mm Hg at cardiac catheterization.11 Five patients had one or more additional procedures as follow-up and in these cases we report the results from only the first cardiac catheterization. One of the patients who had normal pressures initially had PHTN at a repeat cardiac catheterization. Only the data from the second catheterization are reported for this patient (no. 18), and she was assigned to the PHTN group. Another patient (no. 9), who initially had increased pulmonary pressures, had normal values at a repeat cardiac catheterization 4.5 years later. Only the initial catheterization data are reported and she was assigned to the PHTN group. We used only the abnormal results in these 2 instances so that our mortality analyses would be based on as large a number of patients with PHTN as this retrospective study would allow. In patient no. 9, use of the abnormal results also allowed reporting of the hemodynamic response to prostacyclin.
Clinical data on patients with SCD at cardiac catheterization
| . | Age at catheterization, y . | Sex . | Hemoglobin type . | Other diagnoses . | Status . |
|---|---|---|---|---|---|
| Patients with PHTN | |||||
| 1 | 23 | F | SS | Deceased | |
| 2 | 44 | F | SS | Alive | |
| 3 | 37 | M | SS | Deceased | |
| 4 | 32 | M | SS | Deceased | |
| 5 | 40 | F | SS | Alive | |
| 6 | 28 | F | SS | Alive | |
| 7 | 33 | F | SS | Deceased | |
| 8 | 55 | F | SS | ESRD | Deceased |
| 9 | 21 | F | SS | Alive | |
| 10 | 47 | M | SC | Stroke-hypertension | Alive |
| 11 | 27 | F | SS | Pulmonary fibrosis | Alive |
| 12 | 30 | F | SS | Deceased | |
| 13 | 30 | F | SS | Deceased | |
| 14 | 42 | F | SS | Liver cirrhosis | Alive |
| 15 | 57 | F | SS | ESRD | Deceased |
| 16 | 37 | M | SC | Deceased | |
| 17 | 40 | F | SS | ESRD | Deceased |
| 18 | 26 | F | SS | Deceased | |
| 19 | 59 | M | SD | Azotemia | Alive |
| 20 | 37 | M | Sβ+-thalassemia | Pulmonary fibrosis | Alive |
| Patients without PHTN | |||||
| 21 | 21 | F | SS | Hypertension | Alive |
| 22 | 28 | M | SS | Alive | |
| 23 | 35 | F | SS | Alive | |
| 24 | 33 | F | SS | Alive | |
| 25 | 45 | M | SC | Alive | |
| 26 | 31 | M | SS | Alive | |
| 27 | 48 | M | SC | Pulmonary fibrosis | Alive |
| 28 | 25 | M | SS | Alive | |
| 29 | 25 | M | SS | Liver cirrhosis | Deceased |
| 30 | 42 | F | SS | Pulmonary vascular sclerosis | Alive |
| 31 | 34 | M | SS | HIV+, arrhythmias | Deceased |
| 32 | 20 | M | SS | Strokes, liver cirrhosis | Deceased |
| 33 | 21 | M | SS | Alive | |
| 34 | 21 | M | SS | Alive |
| . | Age at catheterization, y . | Sex . | Hemoglobin type . | Other diagnoses . | Status . |
|---|---|---|---|---|---|
| Patients with PHTN | |||||
| 1 | 23 | F | SS | Deceased | |
| 2 | 44 | F | SS | Alive | |
| 3 | 37 | M | SS | Deceased | |
| 4 | 32 | M | SS | Deceased | |
| 5 | 40 | F | SS | Alive | |
| 6 | 28 | F | SS | Alive | |
| 7 | 33 | F | SS | Deceased | |
| 8 | 55 | F | SS | ESRD | Deceased |
| 9 | 21 | F | SS | Alive | |
| 10 | 47 | M | SC | Stroke-hypertension | Alive |
| 11 | 27 | F | SS | Pulmonary fibrosis | Alive |
| 12 | 30 | F | SS | Deceased | |
| 13 | 30 | F | SS | Deceased | |
| 14 | 42 | F | SS | Liver cirrhosis | Alive |
| 15 | 57 | F | SS | ESRD | Deceased |
| 16 | 37 | M | SC | Deceased | |
| 17 | 40 | F | SS | ESRD | Deceased |
| 18 | 26 | F | SS | Deceased | |
| 19 | 59 | M | SD | Azotemia | Alive |
| 20 | 37 | M | Sβ+-thalassemia | Pulmonary fibrosis | Alive |
| Patients without PHTN | |||||
| 21 | 21 | F | SS | Hypertension | Alive |
| 22 | 28 | M | SS | Alive | |
| 23 | 35 | F | SS | Alive | |
| 24 | 33 | F | SS | Alive | |
| 25 | 45 | M | SC | Alive | |
| 26 | 31 | M | SS | Alive | |
| 27 | 48 | M | SC | Pulmonary fibrosis | Alive |
| 28 | 25 | M | SS | Alive | |
| 29 | 25 | M | SS | Liver cirrhosis | Deceased |
| 30 | 42 | F | SS | Pulmonary vascular sclerosis | Alive |
| 31 | 34 | M | SS | HIV+, arrhythmias | Deceased |
| 32 | 20 | M | SS | Strokes, liver cirrhosis | Deceased |
| 33 | 21 | M | SS | Alive | |
| 34 | 21 | M | SS | Alive |
ESRD indicates end-stage renal disease.
Cardiac catheterization
All but one of the patients (no. 19) had their cardiac catheterizations performed at the Catheterization Laboratory in the Cardiology Division of Howard University Hospital.
The patient's right groin was prepped and draped in a sterile fashion with the right groin exposed. Under local anesthesia and using the Seldinger technique, a no. 8 French sheath was inserted into the right femoral vein. Hemodynamic and electrocardiographic monitoring was continuous. A no. 7 French Swan-Ganz catheter was advanced to the right atrium, right ventricle, pulmonary artery, and pulmonary capillary wedge position, with pressures obtained at each position. Cardiac output was determined by the thermodilution technique. In some patients with PHTN, a vasodilator, prostacyclin, was administered via the Swan-Ganz catheter to assess the reversibility of the PHTN. Incremental dosing was used until a significant reduction in pulmonary artery pressure and pulmonary vascular resistance (20%) occurred, symptoms developed, or systemic blood pressure decreased more than 30%, or heart rate increased more than 50%. Each dose was infused for 15 minutes and pulmonary artery pressure, MPAP, pulmonary capillary wedge pressure (PCWP), and cardiac output were obtained at each dose. At the end of the procedure the sheath was removed and hemostasis was achieved with direct pressure.
Statistical analysis
Categorical variables were compared with the χ2test. Continuous variables were compared with the Student ttest if they followed a normal distribution or with the Wilcoxon rank test if they followed a skewed distribution. The relationship of survival with MPAP, age, sex, hemoglobin type, and PCWP was examined with Cox proportional hazards modeling. Kaplan-Meier analysis was used to compare survival between patients with SCD with and without PHTN. For each patient the length of follow-up was calculated from the date of initial cardiac catheterization to either date of death, the date last seen (for patients lost to follow-up), or June 30, 2001. The Systat (SPSS, Chicago, IL) and the GB-STAT (Dynamic Microsystems, Silver Spring, MD) statistical software programs were used for the analyses.
Results
Characteristics of patients with PHTN
Patients with PHTN were older (mean age, 37.2 ± 11.0 years; range, 21-59 years) than those without this complication (mean age, 30.7 ± 9.45 years; range, 20-48 years; Table 1). The difference, however, was not statistically significant (P = .1).
Seventy percent of the patients with PHTN were women and this proportion was significantly greater than that (29%) in patients with normal pulmonary pressures (χ2 = 5.67;P = .017). The distribution of SS (homozygosity for SCD) and non-SS patients was similar for both groups. Eight of the 20 patients with PHTN and 6 of the 14 patients without PHTN had additional complications such as renal disease, liver disease, or stroke. However, all 3 patients with end-stage renal disease (ESRD) were in the PHTN group.
Results of cardiac catheterization
Table 2 shows pulmonary hemodynamic data for 20 SCD patients with PHTN and for 14 patients without this complication. PHTN was defined by a MPAP of more than 25 mm Hg.11 The PA pressures for patients with PHTN were 54.3 mm Hg, 25.2 mm Hg, and 36.0 mm Hg for systolic, diastolic, and mean pressures, respectively.
Cardiac catheterization results in patients with SCD with and without PHTN
| . | PAs, mm Hg . | PAd, mm Hg . | PAm, mm Hg . | CO, L/min . | PCWP, mm Hg . |
|---|---|---|---|---|---|
| Patients with PHTN | |||||
| 1 | 70 | 40 | 50 | 11.1 | 20 |
| 2 | 52 | 22 | 32 | 8.4 | 28 |
| 3 | 45 | 20 | 28 | 10.4 | 7 |
| 4 | 67 | 35 | 46 | 8.5 | 25 |
| 5 | 70 | 40 | 50 | 11.1 | 12 |
| 6 | 67 | 30 | 42 | 6.6 | 17 |
| 7 | 55 | 25 | 38 | 8.0 | 12 |
| 8 | 63 | 13 | 30 | 5.6 | 12 |
| 9 | 40 | 28 | 32 | 9.3 | 21 |
| 10 | 42 | 22 | 29 | 9.9 | 15 |
| 11 | 43 | 22 | 29 | 10.0 | 17 |
| 12 | 58 | 25 | 36 | 8.2 | 9 |
| 13 | 36 | 18 | 28 | 9.0 | 16 |
| 14 | 58 | 25 | 40 | 8.1 | 17 |
| 15 | 50 | 20 | 38 | 7.3 | 22 |
| 16 | 38 | 18 | 28 | 10.9 | 14 |
| 17 | 80 | 38 | 48 | NA | 24 |
| 18 | 51 | 22 | 32 | 8.41 | 10 |
| 19 | 55 | 23 | 37 | 7.6 | 14 |
| 20 | 46 | 18 | 27 | 4.9 | 8 |
| Patients without PHTN | |||||
| 21 | 22 | 2 | 9 | 9.5 | 3 |
| 22 | 25 | 10 | 15 | 13.5 | 12 |
| 23 | 32 | 17 | 22 | 5.6 | 12 |
| 24 | 20 | 5 | 10 | 7.1 | 5 |
| 25 | 30 | 10 | 17 | NA | 13 |
| 26 | 28 | 10 | 16 | 6.5 | 12 |
| 27 | 25 | 8 | 16 | 4.8 | 8 |
| 28 | 30 | 18 | 15 | 8.6 | 12 |
| 29 | 35 | 12 | 20 | NA | 12 |
| 30 | 32 | 12 | 22 | 7.2 | 8 |
| 31 | 33 | 10 | 18 | 14.3 | 7 |
| 32 | 40 | 17 | 25 | 8.1 | 15 |
| 33 | 35 | 17 | 23 | NA | 15 |
| 34 | 37 | 16 | 23 | 9.8 | 15 |
| . | PAs, mm Hg . | PAd, mm Hg . | PAm, mm Hg . | CO, L/min . | PCWP, mm Hg . |
|---|---|---|---|---|---|
| Patients with PHTN | |||||
| 1 | 70 | 40 | 50 | 11.1 | 20 |
| 2 | 52 | 22 | 32 | 8.4 | 28 |
| 3 | 45 | 20 | 28 | 10.4 | 7 |
| 4 | 67 | 35 | 46 | 8.5 | 25 |
| 5 | 70 | 40 | 50 | 11.1 | 12 |
| 6 | 67 | 30 | 42 | 6.6 | 17 |
| 7 | 55 | 25 | 38 | 8.0 | 12 |
| 8 | 63 | 13 | 30 | 5.6 | 12 |
| 9 | 40 | 28 | 32 | 9.3 | 21 |
| 10 | 42 | 22 | 29 | 9.9 | 15 |
| 11 | 43 | 22 | 29 | 10.0 | 17 |
| 12 | 58 | 25 | 36 | 8.2 | 9 |
| 13 | 36 | 18 | 28 | 9.0 | 16 |
| 14 | 58 | 25 | 40 | 8.1 | 17 |
| 15 | 50 | 20 | 38 | 7.3 | 22 |
| 16 | 38 | 18 | 28 | 10.9 | 14 |
| 17 | 80 | 38 | 48 | NA | 24 |
| 18 | 51 | 22 | 32 | 8.41 | 10 |
| 19 | 55 | 23 | 37 | 7.6 | 14 |
| 20 | 46 | 18 | 27 | 4.9 | 8 |
| Patients without PHTN | |||||
| 21 | 22 | 2 | 9 | 9.5 | 3 |
| 22 | 25 | 10 | 15 | 13.5 | 12 |
| 23 | 32 | 17 | 22 | 5.6 | 12 |
| 24 | 20 | 5 | 10 | 7.1 | 5 |
| 25 | 30 | 10 | 17 | NA | 13 |
| 26 | 28 | 10 | 16 | 6.5 | 12 |
| 27 | 25 | 8 | 16 | 4.8 | 8 |
| 28 | 30 | 18 | 15 | 8.6 | 12 |
| 29 | 35 | 12 | 20 | NA | 12 |
| 30 | 32 | 12 | 22 | 7.2 | 8 |
| 31 | 33 | 10 | 18 | 14.3 | 7 |
| 32 | 40 | 17 | 25 | 8.1 | 15 |
| 33 | 35 | 17 | 23 | NA | 15 |
| 34 | 37 | 16 | 23 | 9.8 | 15 |
Some data from 11 of the patients in this table were reported previously by Castro.2
In patients with PHTN, the mean ± SD for PAs, PAd, PAm, CO, and PCWP were 54.3 ± 12.26 mm Hg, 25.2 ± 7.72 mm Hg, 36.0 ± 7.78 mm Hg, 8.6 ± 1.76 L/min, and 16.0 ± 5.87 mm Hg. Respective values for patients without PHTN were 30.3 ± 5.77 mm Hg, 11.7 ± 4.86 mm Hg, 17.8 ± 4.86 mm Hg, 8.6 ± 3.03 L/min, and 10.6 ± 3.82 mm Hg.
PA indicates pulmonary artery pressure; s, systolic; d, diastolic; m, mean; CO, cardiac output; PCWP, pulmonary artery wedge pressure; NA, not available.
Probably as a result of their anemia, all patients with SCD had a high cardiac output. For patients with PHTN the mean cardiac output was 8.60 L/min, essentially the same as in SCD patients without PHTN (8.62 L/min). SCD patients with PHTN also had higher than normal PCWPs (mean, 16.01 mm Hg; range, 7-28 mm Hg). This was unexpected because all patients had normal left ventricular systolic function on echocardiography. In 8 of 13 (62%) patients with high PCWP (> 13 mm Hg) the PHTN was not merely passive because the difference between their pulmonary artery diastolic pressure and wedge pressure exceeded 5 mm Hg.
Reversibility of PHTN
Eight of the patients were given prostacyclin infusions at cardiac catheterization to test the reversibility of their PHTN (Table3). All 8 had their cardiac catheterizations performed in 1998 or thereafter. Since 1998, prostacyclin administration is a routine procedure for all patients with PHTN diagnosed at our catheterization laboratory. In 2 patients (nos. 11 and 12) prostacyclin did not substantially lower the pulmonary vascular resistance. In the remaining 6 patients pulmonary vascular resistance decreased by at least 20% so that for the 8 patients as a group prostacyclin infusions lowered pulmonary pressures by a mean of 34%. In an additional patient (no. 18, data not shown), prostacyclin lowered the pulmonary pressures, but it also decreased the systemic blood pressure so she was unable to tolerate the vasodilator infusion.
Effect of prostacyclin infusion on PHTN in 8 patients with SCD at cardiac or Swan-Ganz catheterization
| Patient no. . | Before prostacyclin . | After prostacyclin . | Change in PVR, % . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PAm, mm Hg . | CO, L/min . | PCWP, mm Hg . | PVR, dynes/s/cm−5 . | PAm, mm Hg . | CO, L/min . | PCWP, mm Hg . | PVR, dynes/s/cm−5 . | ||
| 2 | 43 | 9.1 | 12 | 253 | 42 | 9.9 | 16 | 199 | −21 |
| 6 | 28 | 4.8 | 22 | 242 | 27 | 8.6 | 114 | −53 | |
| 10 | 56 | 6.3 | 24 | 404 | 37 | 6.4 | 22 | 188 | −54 |
| 11 | 30 | 10 | 17 | 104 | 29 | 12.6 | 15 | 89 | −15 |
| 12 | 36 | 8.2 | 9 | 293 | 34 | 8.5 | 7 | 248 | −15 |
| 14 | 40 | 8.1 | 17 | 214 | 24 | 11.3 | 10 | 152 | −29 |
| 17 | 43 | 6.7 | 15 | 332 | 31 | 8.0 | 13 | 179 | −46 |
| 20 | 27 | 4.9 | 8 | 326 | 20 | 7.2 | 6 | 194 | −40 |
| Mean | 37.9 | 7.3 | 15.5 | 271 | 30.5 | 9.1 | 12.7 | 170 | −34 |
| SD | 9.7 | 1.9 | 5.7 | 90 | 7.1 | 2.1 | 5.6 | 51 | 16 |
| Patient no. . | Before prostacyclin . | After prostacyclin . | Change in PVR, % . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PAm, mm Hg . | CO, L/min . | PCWP, mm Hg . | PVR, dynes/s/cm−5 . | PAm, mm Hg . | CO, L/min . | PCWP, mm Hg . | PVR, dynes/s/cm−5 . | ||
| 2 | 43 | 9.1 | 12 | 253 | 42 | 9.9 | 16 | 199 | −21 |
| 6 | 28 | 4.8 | 22 | 242 | 27 | 8.6 | 114 | −53 | |
| 10 | 56 | 6.3 | 24 | 404 | 37 | 6.4 | 22 | 188 | −54 |
| 11 | 30 | 10 | 17 | 104 | 29 | 12.6 | 15 | 89 | −15 |
| 12 | 36 | 8.2 | 9 | 293 | 34 | 8.5 | 7 | 248 | −15 |
| 14 | 40 | 8.1 | 17 | 214 | 24 | 11.3 | 10 | 152 | −29 |
| 17 | 43 | 6.7 | 15 | 332 | 31 | 8.0 | 13 | 179 | −46 |
| 20 | 27 | 4.9 | 8 | 326 | 20 | 7.2 | 6 | 194 | −40 |
| Mean | 37.9 | 7.3 | 15.5 | 271 | 30.5 | 9.1 | 12.7 | 170 | −34 |
| SD | 9.7 | 1.9 | 5.7 | 90 | 7.1 | 2.1 | 5.6 | 51 | 16 |
PVR indicates pulmonary vascular resistance. Other abbreviations are explained in Table 2.
PHTN and survival of patients with SCD
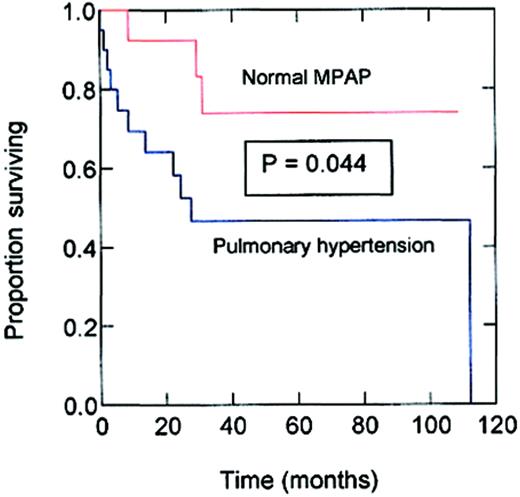
The median postcatheterization follow-up was 23 months for patients with PHTN and 45 months for those without PHTN. During follow-up 11 of 20 patients (55%) with PHTN died, compared to 3 of the 14 (21%) patients without this abnormality (χ2 = 3.83;P = .0503). In Cox proportional hazards modeling involving the results from all subjects, MPAP had a significant inverse relationship with survival. Each increase of 10 mm Hg in MPAP was associated with a 1.7-fold increase in the rate (hazards ratio) of death (95% CI = 1.1-2.7; P = .028). In this modeling, age, sex, hemoglobin type, and PCWP did not have a significant influence on survival. Kaplan-Meier survival curves of SCD patients with and without PHTN are shown in Figure1. The estimated median survival for patients with PHTN was 25.6 months from the date of cardiac catheterization. By comparison, the estimated median survival of SCD patients with normal pulmonary pressures had not been reached at the end of the follow-up period and the estimated survival was still more than 70% at the end of the 119 months of observation (P = .044, Breslow-Gehan log-rank test). Whether the reversibility of PHTN by test doses of prostacyclin affected survival could not be determined because only few of our patients were so tested.
Kaplan-Meier survival of patients with SCD with and without PHTN.
The upper (red) line is the survival estimate for SCD patients without PHTN, that is, with normal MPAP. The lower (blue) line is the survival estimate for SCD patients with PHTN, that is, with MPAP more than 25 mm Hg. The x-axis measures months of follow-up after cardiac catheterization.
Kaplan-Meier survival of patients with SCD with and without PHTN.
The upper (red) line is the survival estimate for SCD patients without PHTN, that is, with normal MPAP. The lower (blue) line is the survival estimate for SCD patients with PHTN, that is, with MPAP more than 25 mm Hg. The x-axis measures months of follow-up after cardiac catheterization.
The causes of death of patients with and without PHTN are shown in Table 4. Eight of the 11 patients with PHTN who died during follow-up probably died of complications usually associated with PHTN, such as cor pulmonale, sudden death, and respiratory failure. However, 1 of the 3 patients without PHTN also died suddenly; he was known to have recurrent cardiac arrhythmias.
Causes of death in patients with SCD with and without PHTN
| Patient no. . | PHTN . | Cause of death . |
|---|---|---|
| 1 | Yes | Cor pulmonale |
| 3 | Yes | Unknown (died at another hospital) |
| 4 | Yes | Cor pulmonale, sepsis, cerebral hemorrhage |
| 7 | Yes | Respiratory failure |
| 8 | Yes | Chronic renal failure |
| 12 | Yes | Cor pulmonale |
| 13 | Yes | Sudden death at home, liver cirrhosis, intestinal infarction |
| 15 | Yes | Sudden death, heart failure |
| 16 | Yes | Sudden death at home |
| 17 | Yes | Chronic renal failure, postoperative bleeding |
| 18 | Yes | Respiratory failure |
| 29 | No | Liver cirrhosis |
| 31 | No | Sudden death, cardiac arrhythmia |
| 32 | No | Liver cirrhosis, iron overload |
| Patient no. . | PHTN . | Cause of death . |
|---|---|---|
| 1 | Yes | Cor pulmonale |
| 3 | Yes | Unknown (died at another hospital) |
| 4 | Yes | Cor pulmonale, sepsis, cerebral hemorrhage |
| 7 | Yes | Respiratory failure |
| 8 | Yes | Chronic renal failure |
| 12 | Yes | Cor pulmonale |
| 13 | Yes | Sudden death at home, liver cirrhosis, intestinal infarction |
| 15 | Yes | Sudden death, heart failure |
| 16 | Yes | Sudden death at home |
| 17 | Yes | Chronic renal failure, postoperative bleeding |
| 18 | Yes | Respiratory failure |
| 29 | No | Liver cirrhosis |
| 31 | No | Sudden death, cardiac arrhythmia |
| 32 | No | Liver cirrhosis, iron overload |
Discussion
The normal blood pressure in the pulmonary artery is 25/9 mm Hg (systolic range, 17-32 mm Hg; diastolic range, 2-13 mm Hg) and the MPAP is 15 mm Hg.12 Hemodynamically, PHTN is defined as a MPAP above 25 mm Hg at rest and can be secondary to a large variety of abnormalities.11 Common causes of secondary PHTN include left ventricular failure, a left-to-right intracardiac shunt, pulmonary vascular abnormalities in disorders such as collagen vascular disease, thromboembolism and other lung disorders, chronic hypoxia, and portal hypertension.13 Patients with increased pulmonary arterial pressure in whom all of these disorders are excluded are diagnosed as having primary PHTN.11 These patients have a progressively increasing pulmonary pressure leading to cor pulmonale and death.
We reviewed the clinical characteristics, cardiac catheterization data, and the outcomes of 20 adult patients with SCD who also had PHTN. Their mean age was 37.2 years, not significantly different from that of 14 SCD patients with normal pulmonary pressures (30.7 years). The age range included 2 patients younger than 25 years, suggesting that in some SCD patients, PHTN may start developing even during the teenage years. In our series, 70% of subjects with PHTN were women, and this value was significantly higher than the proportion of women (29%) in the group of patients with normal pressures. A reasonable explanation for this difference could be that our small sample of patients without PHTN was not representative of the total adult population in our clinic, which in the years 1986-1995, for example, included 54.2% women.14 In fact, when we compared the proportion of women in the PHTN group with that of the total SCD patient population at our institution, the higher proportion of women with increased pulmonary pressure was no longer statistically significant (χ2 = 1.96; P = .16). Most of the patients had homozygous sickle cell disease (Hb SS) and the frequency of non-SS patients was similar for patients with and without PHTN.
In contrast to patients with primary PHTN, who by definition have normal left ventricular function, our group of patients included also 13 in whom PCWP was elevated (> 13 mm Hg). Only 3 of the patients with normal pulmonary artery pressures had high PCWPs. None of the patients had evidence of left ventricular systolic dysfunction (low ejection fraction) on echocardiography. It is thus possible that despite normal systolic function, the high wedge pressures were indicative of impaired left ventricular diastolic function. This has been previously reported in patients with SCD from our institution15 and from other centers.16,17Additionally, a reverse Bernheim effect in which paradoxical ventricular septal motion inhibits left ventricular filling,18 may explain the high wedge pressures.
The pulmonary artery pressures in SCD patients with PHTN (systolic, 54.3 ± 12.26 mm Hg; diastolic, 25.2 ± 7.72 mm Hg; and mean, 36 ± 7.78 mm Hg) though elevated, were not as high as those reported in the literature for patients with primary PHTN whose average values are 90, 45, and 61 mm Hg, respectively in men, and 92, 43, and 60 mm Hg, respectively in women.11 Also, the cardiac output was higher in SCD at 8.6 ± 1.76 L/min (regardless of PHTN) than that reported for primary PHTN (3.0 L/min).19 Despite these more favorable hemodynamic findings, patients with SCD did not appear to tolerate even milder elevations of pulmonary pressures because their median survival was 25.6 months compared with 33.6 months in patients with (untreated) primary PHTN.9 In 8 of our patients PHTN improved with short-term prostacyclin infusion. Two of our patients were tried on long-term intravenous prostacyclin (epoprostenol) infusion. One of them (no. 13) could not tolerate the drug because of jaw pain. The other patient (no. 2) has been on home treatment with intravenous prostacyclin for 1.5 years. Her pulmonary vascular resistance decreased from a baseline of 366 dynes/s/cm−5 to 185 dynes/s/cm−5after home prostacyclin infusion. Before this treatment she had syncopal episodes that have now ceased. She also developed jaw pain during the early phase of intravenous prostacyclin treatment. In both of these patients, the jaw pain was exacerbated while eating and they both had increased serum amylase and lipase levels, which suggested parotitis as a mechanism of their jaw pain.
In our patients, increased pulmonary pressures were associated with short survival. These early deaths may have been due to progressive PHTN. However, our data do not allow demonstration of a cause-and-effect relationship. PHTN may have been associated with short survival because it was a manifestation of severe SCD in these patients, who may have had sickling-related vasculopathy involving other vital organs. In any case, it seems reasonable to conclude that the SCD patients in our series tolerated poorly their PHTN. For this reason carefully conducted prospective treatment trials with prostacyclin via the intravenous,20,21subcutaneous,22 or inhalation23 route in patients such as ours are indicated. Other interventions, including treatment with the endothelin antagonist bosentan,24long-term transfusion programs,25 nitric oxide (NO) inhalation,26 or administration of the NO precursor, arginine,27 28 could also be explored.
We thank Dr Victor R. Gordeuk (Howard University College of Medicine, Washington, DC) for assistance in statistical analysis, Dr Anantha K. Rao (Washington Hospital Center, Washington, DC) for the catheterization results from patient no. 19, and Dr Mark Gladwin (Critical Care Section, Clinical Center, National Institutes of Health, Bethesda, MD) for helpful comments on the manuscript.
Prepublished online as Blood First Edition Paper, October 3, 2002; DOI 10.1182/blood-2002-03-0948.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Oswaldo Castro, Center for Sickle Cell Disease, Howard University College of Medicine, 2121 Georgia Ave NW, Washington, DC 20059; e-mail: olcastro@aol.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal