Immunosuppression with antithymocyte globulin, (methyl)prednisolone, and cyclosporin A is considered the treatment of choice for the patient with aplastic anemia without a donor for standard-risk stem cell transplantation. This consensus is supported by the results of several series, including a randomized German trial. Here we report 11-year results of the latter trial. With stringent response criteria and 4 months as the time to evaluate responses, this analysis confirms the superiority of the cyclosporine regimen regarding the response rate in all patients treated (70% vs 41%, with or without cyclosporine; P = .015) and in patients with severe aplastic anemia (65% vs 31%; P = .011). Patients responded more rapidly after treatment with cyclosporine (median, 60 vs 82 days; P = .019). Most patients treated with cyclosporine needed only one course of immunosuppression, whereas many patients treated without cyclosporine required repeated immunosuppressive treatment. Because of the efficacy of salvage treatment, overall survival was not different between the 2 treatment groups. However, failure-free survival favored the cyclosporine regimen (39% vs 24%; P = .04). The relapse rate, projected at 38% after 11.3 years, was similar between the 2 treatment groups. Remissions were cyclosporine dependent in 26% of the patients responding to a regimen that included cyclosporine. Clonal or malignant diseases developed in 25% of the patients. These data demonstrate that antithymocyte globulin, methylprednisolone, and cyclosporin A are an effective regimen for the treatment of aplastic anemia. However, remissions are unstable, and secondary diseases are common. In contrast to the results of stem cell transplantation, most patients are not cured.
Introduction
Aplastic anemia is thought to be an immune-mediated bone marrow disease, characterized by bone marrow aplasia and peripheral blood pancytopenia. Most patients can be successfully treated with either hematopoietic stem cell transplantation (HSCT) or immunosuppressive therapy (IST) and can survive long term.1
Because HSCT cures aplastic anemia, it is the treatment of choice for young patients with suitable stem cell donors. HLA-matched related donors are widely accepted as stem cell donors,2 whereas unrelated donor HSCT still carries a significant risk for morbidity and mortality.3 Most patients do not have donors for standard-risk HSCT and rely on IST as the first-line treatment.
For many years, antithymocyte globulin (ATG) has been the standard for IST of aplastic anemia. ATG significantly improves survival compared with supportive care4 or androgen therapy.5Response rates vary between 40% and 70%, and long term survival after ATG-based IST is similar to that in unselected patients treated with HSCT.6
Results of a German multicenter trial demonstrated that the response to IST can be significantly improved by the addition of cyclosporin A (CsA) to ATG.7 Although survival was not improved by the addition of CsA, the combination of ATG and CsA is regarded as a standard against which new treatment modalities can be evaluated.
For an informed decision between alternative treatment modalities, long-term observations of controlled trials are essential. Published information relies on retrospective or registry data6,8,9 or follow-up is short.7,10-13 Here we report 11-year results of the randomized German trial7that demonstrate the potential and the problems associated with the IST approach to aplastic anemia.
Patients, materials, and methods
Patients and treatment
From May 1986 to June 1989, 24 German centers recruited 84 patients with severe aplastic anemia (SAA) (n = 60) or nonsevere aplastic anemia (nSAA) (n = 24) in a prospective phase 3 trial.7 After stratification for severity of disease and its duration from diagnosis to treatment, patients were randomly assigned to treatment with horse ATG (Lymphoglobulin; IMTIX-SangStat [formerly Pasteur Mérieux], Ketsch, Germany) and methylprednisolone (Urbason; Hoechst Marion Roussel, Bad Soden, Germany) as the control group (n = 41) or with CsA (Sandimmune, Novartis [formerly Sandoz], Nuremberg, Germany), ATG, and methylprednisolone as the CsA group (n = 43). Details concerning the patient characteristics and the treatment protocol have been published.7
Collection of follow-up data
Patients, their hematologists, and their primary care physicians were sent forms asking for follow-up information accumulated since the last contact with the data center: complete blood counts, transfusion history, medication, illnesses or adverse events, and general health status. We specifically asked for data on relapse, CsA dependence of blood counts, and specific adverse events such as osteoporosis/necrosis, renal failure, hypertension, clinical or laboratory evidence for paroxysmal nocturnal hemoglobinuria (PNH) (Ham test, haptoglobin), myelodysplastic syndrome (MDS), leukemia, and solid tumors. All patients gave written consent to publish follow-up data anonymously.
Definitions
In contrast to the initial report of the trial7 and following current consensus guidelines of the European Blood and Marrow Transplant (EBMT) Severe Aplastic Anaemia Working Party,14response was defined as a significant improvement of blood counts (partial or complete remission) within 4 months. Complete remission (CR) was defined as blood counts normal for age and sex. For patients 15 years and older, we used hemoglobin (Hb) levels 12 g/dL or greater for women and Hb levels 13 g/dL or greater for men, granulocyte counts 1.5 × 109/L or higher, and platelet counts 150 × 109/L or higher. For 6 children, age-adjusted lower thresholds were derived from a textbook.15 Partial remission (PR) was defined by transfusion independence and by an unsupported increase of the counts of at least one cell line over baseline values (Hb by at least 3 g/dL, granulocytes by at least 0.5 × 109/L, if previously lower than 0.5 × 109/L, and platelets by at least 20 × 109/L, if previously lower than 20 × 109/L) or by doubling or normalization of counts of at least one cell line if previous counts of the respective cell line(s) did not meet the criteria for SAA. All remissions had to be confirmed by at least 2 blood counts at least 4 weeks apart.
Clinical relapse was defined as a decrease in any of the peripheral blood counts to less than 50% of the median sustained counts during remission, by return of the counts to levels meeting the definition of SAA, or by the need for transfusion support. Complete diagnostic and follow-up information were later used to divide patients with clinical relapse into those with true relapse of AA and those with decreased blood counts because of the development of PNH, MDS, or leukemia. If blood counts deteriorated on lowering the dose of CsA or after discontinuation of CsA and if they improved again with CsA therapy, the remission was called CsA dependent, even if the definition of relapse was not met. Time to relapse was calculated from the day the patient fulfilled the criteria for partial or complete remission to the day of relapse. For calculating the time to relapse, patients who died and patients who were alive at last follow-up without having experienced relapse were censored at the time of death or last follow-up.
Time to remission was defined as the time from the first day of treatment until the day a patient achieved PR. All PRs were counted as events, including those that occurred late and might have been induced by salvage treatment. For calculating response kinetics, only remissions (at least PR) reached within 1 year and after a single course of treatment were counted. Patients who achieved PR after salvage treatment and those who died before reaching PR were censored at the time of salvage treatment or death.
Standard criteria were used to calculate overall survival (OS), time to relapse, or time to clonal disease. Time to treatment failure (TTTF) was defined as the time from the first day of treatment until salvage treatment for nonresponse, relapse, development of a clonal hematologic disease (PNH, MDS, or leukemia), solid tumor, or disease- or treatment-related death (excluding deaths resulting from unrelated causes), whatever came first. In 5 nonresponders who received no salvage treatment and finally died, 4 months were used as the time of treatment failure. This definition of TTTF is a modification of a standard definition,16 taking into account specific features of aplastic anemia.
Data analysis and statistical methods
All evaluations were performed on an intent-to-treat basis, as previously reported.7
Results
Follow-up
Updated information was obtained from all patients. The median observation time of surviving patients is 11.3 (range, 9.4-13.4) years, 11.4 years in the CsA group and 11.1 years in the control group.
Response
Four months after the start of treatment, 47 (56%) patients achieved at least PR and were considered responders. Response was more frequent in patients treated with CsA (30 patients; 70%) than in patients treated without CsA (17 patients; 41%) (P = .015). Using the more stringent criteria of this report, 8 patients achieved CR; 3 had been treated with CsA and 5 without CsA. Thirty-nine patients achieved PR; 27 had been treated with CsA and 12 without CsA. Thirty-seven (44%) patients were alive without response.
Subgroup analysis demonstrated that the advantage of adding CsA was only apparent in patients with SAA. Response at 4 months was 65% (20 of 31 patients) after treatment with CsA compared with 31% (9 of 29 patients) after treatment with ATG and methylprednisolone (P = .011). There was no difference according to treatment modality in patients with nSAA (83% vs 67%; P = .6).
Eleven (13%) patients died within 4 months, 4 in the CsA group and 7 in the control group (9% vs 17%; P = .3). Causes of death were confirmed or suspected cerebral hemorrhage in 6 patients, respiratory failure from pneumonia in 2 patients, multiorgan failure from sepsis in 2 patients, and sepsis and gastrointestinal bleeding in 1 patient.
Kinetics of remission
Blood counts of 12 patients improved more than 4 months and up to 3.5 years after treatment. Six of these patients reached CR and continue to be in stable CR 9.4 to 12.7 years after treatment. Most (10 of 12) patients with late remissions had been part of the control group, initially treated without CsA. Six of 10 late remissions in the control group were observed after one or more additional courses of IST. Second or later courses of IST all included CsA. Late remissions in 2 patients of the CsA group were not preceded by salvage treatment.
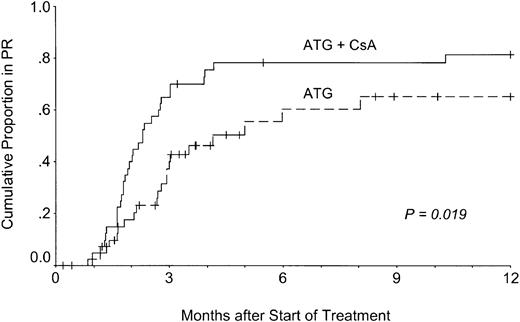
Late remissions illustrate that it is impossible to separate response to the initial treatment from response to salvage treatment and delayed or spontaneous remissions. Because knowledge of the kinetics of response to front-line treatment is important for patient management, the actuarial probability of remission was calculated using data from only those patients who responded after one course of IST and within 1 year of treatment (Figure 1).
Kinetics of response to treatment.
Patients treated with ATG, methylprednisolone, and CsA (ATG + CsA) responded more quickly to treatment than patients treated without CsA (ATG).
Kinetics of response to treatment.
Patients treated with ATG, methylprednisolone, and CsA (ATG + CsA) responded more quickly to treatment than patients treated without CsA (ATG).
The figure demonstrates that blood counts did not improve earlier than 1 month after treatment in either group. However, the slope of the response curve was steeper in patients in the CsA group than in patients in the control group (P = .019; log-rank test). Median actuarial time to response within 1 year was 60 days in the CsA group and 82 days in the control group. Ninety-six percent of all 1-year responses to first-line treatment occurred within 6 months. Actuarial response rates 1 year after treatment were 81% or 65% in patients treated with or without CsA, respectively. When salvage treatment was included in the analysis, the difference in the response kinetics was still significant (P = .031), and the median times to response within 1 year did not change (60 and 87 days).
Survival and failure-free survival
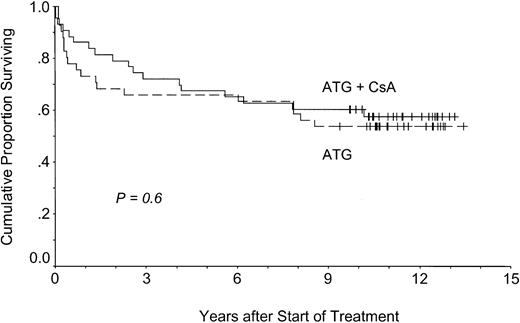
Survival was similar in the patients treated with or without CsA (Figure 2). At 11.3 years, actuarial survival was 58% in the CsA group and 54% in the control group (P = .6). In contrast to response data, there was no survival advantage in patients with SAA (P = .4). All deaths were considered disease- or treatment-related—infection in 17 patients (including Aspergillus pneumonia in 2 patients), confirmed or suspected cerebral hemorrhage in 12 patients, intestinal hemorrhage in 2 patients, MDS or AML in 4 patients, breast cancer in 1 patient, and gastric cancer in 1 patient.
Overall survival.
Patients treated with (ATG + CsA) or without CsA (ATG) had similar overall survival times.
Overall survival.
Patients treated with (ATG + CsA) or without CsA (ATG) had similar overall survival times.
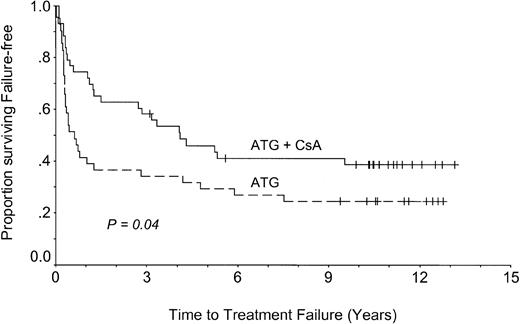
The addition of CsA increased the response rate and accelerated response (Figure 1) and may thus have decreased the risk for early treatment failure. To evaluate this hypothesis, failure-free survival was calculated using TTTF (Figure 3). It is apparent that treatment with ATG and CsA significantly decreased the probability of early treatment failure compared with ATG alone (39% vs 24% at 11 years; P = .04). Subset analysis indicated that the advantage of adding CsA was only significant in patients with SAA (P = .02), whereas there was no significant advantage of adding CsA in patients with nSAA (P = .9) (data not shown).
Failure-free survival.
Patients treated with CsA (ATG + CsA) had longer failure-free survival times than patients treated without CsA (ATG).
Failure-free survival.
Patients treated with CsA (ATG + CsA) had longer failure-free survival times than patients treated without CsA (ATG).
Relapse
Blood counts deteriorated to levels fulfilling the criteria of clinical relapse in 30 of 59 responding patients. However, laboratory and bone marrow findings finally confirmed relapse of AA in only 23 patients. In 7 patients, deterioration of blood counts turned out to be caused by the development of PNH, MDS, or leukemia (Table1).
Follow-up after clinical relapse, depending on the initial quality of response
| Final relapse classification . | n . | Secondary treatment, n . | Result (n) . | Patients taking CSA longer than 9 y . |
|---|---|---|---|---|
| Clinical relapse from CR | ||||
| AA | 11 | 2nd, 5; 3rd, 3; >3rd IST, 2 | CR (9) PR (2) | 2 of 10 |
| No treatment, 1 | ||||
| PNH | 2 | CsA, 1; No treatment, 1 | PR (2) | 1 of 1 |
| MDS | 1 | Supportive, 1 | Death (3) | NA |
| Leukemia | 2 | HSCT, 1; supportive, 1 | ||
| Clinical relapse from PR | ||||
| AA | 12 | 2nd, 7; 3rd, 1; >3rd IST, 1 | CR (2) PR (5) death (5) | 4 of 6 |
| HSCT, 1; No treatment, 2 | ||||
| PNH | 1 | CsA, 1 | PR (1) | 1 of 1 |
| MDS | 1 | Supportive, 1 | Death (1) | NA |
| Final relapse classification . | n . | Secondary treatment, n . | Result (n) . | Patients taking CSA longer than 9 y . |
|---|---|---|---|---|
| Clinical relapse from CR | ||||
| AA | 11 | 2nd, 5; 3rd, 3; >3rd IST, 2 | CR (9) PR (2) | 2 of 10 |
| No treatment, 1 | ||||
| PNH | 2 | CsA, 1; No treatment, 1 | PR (2) | 1 of 1 |
| MDS | 1 | Supportive, 1 | Death (3) | NA |
| Leukemia | 2 | HSCT, 1; supportive, 1 | ||
| Clinical relapse from PR | ||||
| AA | 12 | 2nd, 7; 3rd, 1; >3rd IST, 1 | CR (2) PR (5) death (5) | 4 of 6 |
| HSCT, 1; No treatment, 2 | ||||
| PNH | 1 | CsA, 1 | PR (1) | 1 of 1 |
| MDS | 1 | Supportive, 1 | Death (1) | NA |
Fourteen patients experienced clinical relapse from CR; 16 experienced it from PR. Data for all patients with clinical diagnoses of relapse are shown. They are separated according to the final classification of the disease, causing deterioration of blood counts. Repeated courses of IST included CsA. Six patients were also treated with growth factors. All patients in second or later remission are alive.
NA indicates not applicable.
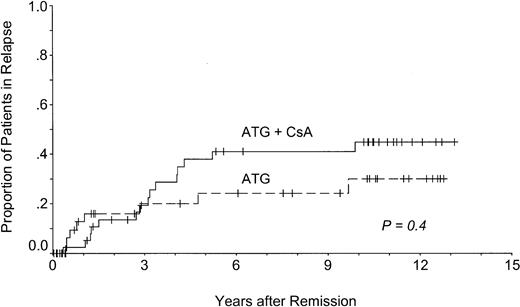
The actuarial risk for relapse of aplastic anemia was 38%. It was similar after treatment with or without CsA (45% vs 30%;P = .4) (Figure 4). A shift of the early relapse curve to the right in patients initially treated with CsA reflects maintenance of the initial remission by CsA. In these patients, median time from discontinuation of CsA to relapse was 27 months (range, 2-49 months). There was no relapse during the administration of CsA.
Relapse of AA after immunosuppressive treatment.
Patients treated with (ATG + CsA) or without CsA (ATG) had similar relapse rates.
Relapse of AA after immunosuppressive treatment.
Patients treated with (ATG + CsA) or without CsA (ATG) had similar relapse rates.
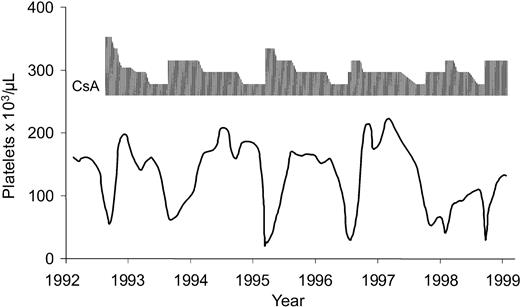
The course in patients who had relapses differed, depending on their initial quality of response (Table 1). All patients who experiencedrelapse from CR again reached CR or PR spontaneously or after repeated courses of ATG/CsA (with or without growth factors) or CsA alone. Except for 2 patients, CsA therapy could be discontinued after a median of 1.6 years (range, 8 months-7 years); these patients have now had stable blood counts for 5.0 to 9.4 years. In 2 patients, remissions are CsA dependent for the long term. They have required maintenance treatment for 9.7 and 10.1 years to keep their blood counts up. Both patients have only minor adverse effects to the drug. The course of the more informative patient is shown in Figure5.
CsA dependence.
Example of CsA-dependent remission in a 21-year-old woman with aplastic anemia. Platelet counts are shown because they were most sensitive to changes in CsA dose.
CsA dependence.
Example of CsA-dependent remission in a 21-year-old woman with aplastic anemia. Platelet counts are shown because they were most sensitive to changes in CsA dose.
Twelve patients who experienced relapse from PR had a less favorable course. Only 7 patients are in long-term CR or PR, one of them after uneventful HSCT from her HLA-identical sibling. Four of 6 patients need maintenance treatment with CsA for 9.7 to 12 years. Five patients died after unsuccessful IST or after they decided against salvage treatment (each was an elderly patient with a complicated course).
CsA dependence
Of all patients who had a response to a regimen containing CsA during first- or second-line treatment, 26% (11 of 43) required administration of the drug for more than 6 months because their counts decreased with the discontinuation of CsA or when the dose of the drug was lowered, and they returned to previous counts with the readministration of CsA. In 5 of these patients, CsA could be discontinued after 5.5 to 8.7 years. Six patients require maintenance treatment with CsA and are taking the drug for 9.7 to 12 years.
Adverse effects
Short-term toxicity has been described.7 Long-term adverse effects were as expected from prolonged administration of corticosteroids and CsA. In 4 patients corticosteroid-induced osteonecrosis of the femur (3 patients) or humerus (1 patient) developed and required orthopedic surgery. One additional patient has lower back pain from severe osteoporosis. Although patients were treated with CsA for a median of 1.1 years (range, 7 days-12 years), CsA-associated toxicity was modest: Eighteen of 47 patients reported hypertrichosis, which was considered disturbing by 4 female patients. Other adverse effects were gingival hyperplasia (7 patients), hypertension requiring treatment (4 patients), laboratory evidence of grade 1 hepatotoxicity (2 patients), edema (2 patients), and muscle cramps (1 patient). Impairment of renal function with creatinine values up to 2.3 mg/dL was observed in 2 patients. CsA had to be discontinued because of adverse effects in only 2 patients.
Clonal and malignant diseases
In 5 patients PNH developed, in 4 patients MDS or leukemia developed, and in 4 patients solid tumor developed (not counting breast cancer in one patient 3 months after the start of IST). The actuarial probability of clonal or malignant disease observed in this trial was 25% at 11.3 years, with no apparent plateau of events.
PNH was diagnosed 4.3 to 9.4 years after the diagnosis of aplastic anemia (actuarial probability, 10% at 11 years). All patients had previously been in remission, and all had been treated with CsA either up front (2 patients) or after relapse (3 patients). The diagnosis of PNH was later confirmed by flow cytometry in all these patients. Flow cytometry analysis of recent blood samples of 13 additional surviving patients of the trial revealed one more patient with glycosyl-phosphatidylinositol (GPI)–deficient granulocytes but without any clinical or laboratory evidence of PNH (data not shown). Only 2 of the patients have clinical PNH (mild hemolysis and cerebral vein thrombosis, respectively). Interestingly, blood counts are CsA sensitive in both patients, who are still being treated with CsA.
The actuarial probability of malignant diseases was 18% at 11.3 years. It was 8% for MDS or leukemia and 11% for solid tumors. There were no significant differences between the treatment groups. All 5 patients from the control group were exposed to CsA during salvage treatment or treatment of relapse.
The interval from treatment of AA to the diagnosis of MDS or leukemia was 6.6 to 9.5 years. All patients had been in CR (3 patients) or PR (1 patient). The patient with MDS was classified as having refractory anemia with severe and finally lethal thrombocytopenia. Leukemia in 3 patients was classified according to the former French-American-British (FAB) classification as secondary AML-M4 evolving from MDS (subtype, refractory anemia with excess of blasts), AML-M0, and AML-M7. Cytogenetics were only available from the patient with AML-M7 (monosomy 7 and t[9;22]). All patients died rapidly after supportive care or unsuccessful treatment of the malignancy, including HSCT in the patient with AML-M7.
The interval from treatment of AA to the diagnosis of a solid tumor varied widely: 1.2 years (gastric cancer), 1.3 years (squamous cell cancer), 10.9 years (squamous cell cancer), and 11.5 years (renal cell cancer). The patient with advanced gastric cancer died of the cancer. The other patients are alive and well.
Quality of remission
Blood counts did not reach normal values in all patients responding to IST. Among 47 surviving responders, 47 (100%) have normal granulocyte levels, 40 (85%) have normal hemoglobin levels for age and sex, 30 (64%) have normal platelet levels, and 27 (57%) have normal trilinear blood counts. Excluding 2 patients on maintenance treatment with CsA, and 1 patient who underwent transplantation, 24 (29%) of the 84 patients who entered the study are long-term survivors with normal trilinear blood counts, off any treatment, and without disease- or treatment-associated adverse effects.
Discussion
This report extends the previous analysis of the German multicenter trial of IST in aplastic anemia7 using a mature data set. Median observation time is now 11.3 years, the longest observation time of a phase 3 trial evaluating IST in aplastic anemia published to date. It provides the long-term follow-up information physicians and patients need when they choose among treatment modalities in aplastic anemia.
Approximately two thirds of the patients with aplastic anemia responded to a combination of ATG, CsA, and short-course methylprednisolone. This combination was significantly more effective than ATG and methylprednisolone alone (response rate, 70% vs 41%;P = .015). The advantage of adding CsA was only evident in patients with SAA, whose response rate to the triple-drug combination was approximately twice as high as that of the control group (65% vs 31%; P = .011). Patients not only responded more frequently, they responded earlier to the protocol, including CsA (Figure 1).
More rapid response to treatment may result in lower rates of early death, less need of supportive care, less use of resources, and thereby lower cost. Although the rate of early death (13% vs 24% at 4 months) and the slope of the survival curve seemed to favor the CsA group, the differences were not significant. Evaluation of pharmacoeconomics was not planned in this trial.
Favorable early results of the treatment with ATG, methylprednisolone, and CsA have been confirmed in several small series and in large American17 and Italian trials.18 CsA alone has activity in aplastic anemia,11 but European trials showed that the combination of ATG and CsA is more effective than CsA alone.12,19 Aplastic anemia may be particularly sensitive to CsA in children.13 20 Combined ATG, methylprednisolone, and CsA has thus become the standard immunosuppressive protocol in children and adults with SAA against which new treatment regimens are compared.
Despite improved short-term results, long-term results and, in particular, overall survival could not be improved by up-front treatment with CsA (Figure 2). Although this seems surprising at first glance, it is not considering the natural history and the current treatment strategies in aplastic anemia.
Because of improved supportive care and, above all, better control of infections and the use of leukocyte-depleted blood products, patients can be kept alive during prolonged periods of pancytopenia. This allows delayed recovery of bone marrow function and salvage treatment with a second course of IST.21 In 12 of 26 patients without response 4 months after treatment, blood counts improved with or without salvage treatment. Only 2 of these patients were in the CsA group, but 10 were in the control group, which may explain why the survival curve of the control group approached the curve of the CsA group during long-term follow-up.
Late improvements of blood counts up to 3.5 years after the initial treatment demonstrate that it is impossible to separate response to treatment from spontaneous recovery of the bone marrow late in the course of aplastic anemia. This is why we restricted our analysis of response kinetics to 12 months after up-front therapy (Figure 1). Analysis of the shape of the response curves demonstrates that few responses occur longer than 4 months after primary IST, including CsA. Four months is thus a reasonable checkpoint to consider salvage treatment or alternative treatment strategies.14
If overall survival cannot be improved by the addition of CsA, why should one use it up-front? CsA can cause adverse effects, and it is expensive. Considering improvements in supportive care, a strategy of starting treatment with ATG and methylprednisolone and of only treating nonresponders at 4 months with a salvage protocol including CsA may result in similar long-term outcome.
Although this sounds reasonable, we favor the up-front, triple-drug regimen for several reasons. As illustrated in Figure 1, a triple-drug regimen induces almost all remissions that can be induced by current ATG-based protocols. Recovery time is shorter than it is with ATG alone. By administering CsA up-front, many patients are spared prolonged time at risk for complications of pancytopenia, and they less often need salvage treatment, with its associated cost and morbidity. This is also illustrated by the analysis of failure-free survival, which shows superiority of the triple-drug regimen (Figure 3).
Achieving all potential IST-induced remissions as early as possible is essential for the patient who is a candidate for allogeneic HSCT but who is treated with IST because of age or lack of a sibling donor. Graft failure because of sensitization by blood products is still a concern, even with improved transfusion strategies and better conditioning regimens.22 Even more important is that patients acquire opportunistic viral and fungal infections during prolonged IST23 and are thus at increased risk for dying of infectious complications during transplantation.
To choose the best treatment strategy for a patient who is a candidate for HSCT, long-term data are critical. This report demonstrates that IST cures only a minority of patients. In most patients, it converts a life-threatening disease to a chronic, often drug-dependent disease prone to relapse and to the development of secondary diseases.
Nevertheless, 29% of all patients treated in this trial have attained normal peripheral blood counts, no longer require treatment, and do not have disease- or treatment-associated adverse effects. They are alive and well, and some may be cured. For patients treated with CsA up-front, freedom from treatment failure is projected at 39% at 11 years. Their actuarial risk for relapse with AA is 38% at 11 years, a figure similar to registry data of the EBMT.24
Twenty-six percent of the patients responding to a regimen containing CsA went through a period of CsA-dependent remission. In approximately half of these patients (5 of 11), CsA could be discontinued after treatment for up to 8.7 years; the other patients are currently on maintenance treatment with CsA for 9.7 to 12 years. Similar observations have been reported by other groups.17 18 They add circumstantial evidence for an (auto)immune mechanism in aplastic anemia.
Although clinically apparent adverse effects of CsA were surprisingly mild (it had to be discontinued for this reason in only 2 patients), long-term administration is worrisome. The drug causes subclinical organ toxicity, it may contribute to the development of cancer, and it is expensive. We therefore recommend tapering and finally discontinuing CsA in patients with stable remissions for 6 months.
The actuarial probability of clonal or malignant diseases in this trial was 25% at 11.3 years. This is similar to registry data,25 wherein most patients were not treated with CsA, making a dramatic effect of CsA on clonal diseases unlikely. However, there is no plateau of events, suggesting that the risk for clonal diseases may persist during the patient's entire life.
Follow-up revealed only 5 cases of PNH, defined by standard criteria. This low incidence may be the consequence of stringent exclusion of PNH patients with the help of an antibody-enhanced Ham test, the most sensitive tool available at the time this trial was performed.7
Among malignant diseases, we found an equal number of hematologic malignancies and solid tumors. The development of MDS or AML in patients who had been in remission for 6.6 to 9.5 years illustrates that long-term follow-up is required to assess the value of any treatment strategy in aplastic anemia. Long-term follow-up is also required to confirm that the impressive results of a quadruple regimen of ATG, CsA, methylprednisolone, and granulocyte–colony-stimulating factor (G-CSF)18 are not compromised by an increased incidence of MDS and leukemia. Current data do not support such a concern.26-28
Two solid tumors, squamous cell carcinoma and renal cell cancer, occurred after an interval of 11 years, suggesting secondary cancer. Two more cancers were found approximately 1 year after the start of treatment, suggesting coincidence rather than a causal relationship. Squamous cell carcinoma is a secondary disease, well known in patients with long-term immunosuppression after organ transplantation,29 30 that may be underestimated in IST trials.
If one considers the long-term results of IST, it is apparent that treatment decisions in aplastic anemia are similar to those in chronic myeloid leukemia (CML). Drug regimens control the disease in most patients. They are the treatment of choice for patients who are not candidates for standard risk allogeneic HSCT. Short-term toxicity and death is lower after drug treatment than after allogeneic HSCT. In the long run, however, HSCT from HLA-identical sibling donors is superior in AA, as it is in CML, because 72% to 88% of patients with AA survive at least 5 years,22,31 and almost all of these patients are cured. Improved HLA matching and safer conditioning regimens are expected to improve HSCT results even further. Five-year survival after IST is excellent (75%),31 but only a minority of the patients is cured.
Some parameters, such as age and granulocyte count, help in the decision between IST and HSCT.6 However, they only predict outcome up to approximately 6 years. Data from this report confirm the recommendation that patients should only be treated with IST if parameters for short-term outcome predict significantly inferior results after HSCT. If short-term outcomes are comparable, HSCT should be the treatment of choice because time clearly favors HSCT over IST.6 27
We thank the patients and their current physicians for their help collecting follow-up information. Members of the German Aplastic Anemia Study Group who entered patients are listed in the with their current addresses.
study group members
Members of the German Aplastic Anemia Study group who entered patients into this trial:
R. Bätge (Medizinische Klinik, Universitätsklinik Göttingen), D. Böttcher (Krankenhaus Bethesda, Wuppertal), M. Burk (Medizinische Klinik II, Stadtkrankenhaus Hanau), M. R. Clemens (Innere Medizin I, Krankenanstalt Mutterhaus der Borromäerinnen, Trier), M. Freund (Abt. Hämatologie und Onkologie, Universitätsklinik Rostock), N. Frickhofen, H.-G. Fuhr (Klinik Innere Medizin III, Dr.-Horst-Schmidt-Kliniken, Wiesbaden), A. Grüneisen (Hämatologie und Onkologie, Krankenhaus Neukölln, Berlin), T. Harrer (Medizinische Klinik III, Universitätsklinik Erlangen), H. Heimpel (Medizinische Klinik III, Universitätsklinik Ulm), W. Heit (Medizinische Klinik, Evangelisches Krankenhaus, Essen-Werden), K. Höffken (Medizinische Klinik II, Universitätsklinik Jena), G. Janka-Schaub (Kinderklinik, Universitäts-Krankenhaus Eppendorf, Hamburg), J. P. Kaltwasser (Abt. Hämatologie, Universitätsklinik Frankfurt), P. Koch (Medizinische Klinik A, Universitätsklinik Münster), E. D. Kreuser (Abt. Hämatologie und Onkologie, Krankenhaus der Barmherzigen Brüder, Regensburg), M. Maasberg (Abt. Hämatologie und Onkologie, Universitätsklinik Marburg), H. Malchus (Klinikum Charlottenburg, Berlin), P. Meusers (Klinik und Poliklinik für Strahlentherapie, Universitätsklinik Essen), H. W. Pees (Innere Medizin I, Universitätsklinik Homburg), A. Raghavachar (Medizinische Klinik 1, Klinikum Wuppertal), G. Schlimok (Abt. Hämatologie, Zentralklinikum Augsburg), H. Schrezenmeier (Abt. Transfusionsmedizin, Universitätsklinik Ulm), R. Sonnen (Hämatologische Abt., Allg. Krankenhaus St. Georg, Hamburg), B. Stollmann-Gibbels (Universitätskinderklinik Essen), H-G. Vogt (Strahlenklinik, Klinikum Offenbach), H. Wandt (5. Medizinische Klinik, Nürnberg), H. J. Weh (Medizinische Klinik II, Franziskus-Hospital, Bielefeld)
Prepublished online as Blood First Edition Paper, October 10, 2002; DOI 10.1182/blood-2002-04-1134.
Supported by IMTIX-SangStat GmbH (formerly Institut Mérieux), Ketsch, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
N. Frickhofen, Department of Medicine III (Hematology/Oncology), HSK, Dr.-Horst-Schmidt-Kliniken, D-65199 Wiesbaden, Germany; e-mail: n.frickhofen@t-online.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal