Abstract
The expression of the plasminogen activator inhibitor-1(PAI-1) gene is enhanced by insulin both in vivo and in various cell types. Because insulin exerts a number of its biologic activities via the phosphatidylinositol 3-kinase and protein kinase B (PI3K/PKB) signaling pathway, it was the aim of the present study to investigate the role of the PI3K/PKB pathway in the expression of the PAI-1 gene and to identify the insulin responsive promoter sequences. It was shown that the induction of PAI-1 mRNA and protein expression by insulin and mild hypoxia could be repressed by the PI3K inhibitor wortmannin. Overexpression of a constitutively active PKB led to induction of PAI-1 mRNA expression and of luciferase (Luc) activity from a gene construct containing 766 bp of the rat PAI-1 promoter. Mutation of the hypoxia response elements (HRE-1 and HRE-2) in rat PAI-1 promoter, which could bind hypoxia inducible factor-1 (HIF-1), abolished the induction of PAI-1 by insulin and PKB. Insulin and the constitutive active PKB also induced Luc expression in cells transfected with the pGl3EPO-HRE Luc construct, containing 3 copies of the HRE from the erythropoietin gene in front of the SV40 promoter. Furthermore, insulin and the active PKB enhanced all 3 HIF α-subunit protein levels and HIF-1 DNA-binding activity, as shown by electrophoretic mobility shift assays (EMSAs). Thus, the insulin-dependent activation of the PAI-1 gene expression can be mediated via the PI3K/PKB pathway and the transcription factor HIF-1 binding to the HREs in the PAI-1 gene promoter.
Introduction
The broad-spectrum serine protease plasmin is activated by the proteases, tissue-type (tPA) and urokinase-type (uPA) plasminogen activators.1 The tPA and uPA activity is regulated, in part, by plasminogen activator inhibitors (PAIs) that are glycoproteins of the serine protease inhibitor (serpin) superfamily.2 Among 2 identified inhibitors, PAI-1 and PAI-2, PAI-1 is the primary physiologic inhibitor of both tPA and uPA. It can be produced by platelets, vascular endothelial cells,3 vascular smooth muscle cells,4 and several nonvascular cell types, among them hepatocytes.5 6
PAI-1 regulates fibrinolysis in many normal and pathologic conditions such as atherosclerosis, coronary heart disease, wound healing, and cancer metastasis.7-9 It was shown that hyperinsulinemia especially associated with obesity, hypertension, and diabetes type 2 could increase PAI-1 levels in blood.10-13 Furthermore, insulin induced PAI-1 expression in vitro in various cell types, including primary human hepatocytes,14,15 HepG2 cells,16,17 and arterial endothelial cells.18 19
Insulin signaling involves second messengers, including members of the phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) cascades.20 The PI3K, which generates phosphatidylinositol-3,4,5-phosphate (PI3,4,5P3), has a key role in the metabolic actions of insulin.21PI3,4,5P3 regulates the activity or subcellular localization of a variety of signaling molecules such as phosphatidylinositol-dependent kinase (PDK) and protein kinase B (PKB) known as Akt, which are also involved in the transmission of the insulin signal.22 23
The expression of the PAI-1 gene and many other genes, expression of which can be induced by insulin, such as genes for the glucose transporters,24 several glycolytic enzymes,25 nitric oxide (NO) synthase,26 erythropoietin (EPO),27 and vascular endothelial growth factor (VEGF),28 can also be induced by hypoxia.29-33 The rat PAI-1gene was induced by hypoxia via an O2 responsive promoter sequence (−175/−158) containing 2 hypoxia response elements (HRE-1, −175/−168; HRE-2, −165/−158)34 binding the transcription factor hypoxia inducible factor-1 (HIF-1). HIF-1 is a dimer of HIF-1α and HIF-1β (arylhdrocarbon receptor nuclear translocator [ARNT]) both belonging to the basic helix-loop-helix (bHLH) PAS (Per-ARNT-Sim) transcription factor family. Two other HIF α-subunits (HIF-2α/EPAS/HRF/HLF and HIF-3α) as well as 2 other ARNT isoforms (ARNT2 and ARNT3/BMAL-1/MOP-3) have been identified, giving rise to the existence of several HIF-dimers composed of different HIF α-subunits and ARNT isoforms.35,36 HIF-1 was shown to be activated by insulin.37-39 Thereby, HIF-1α protein levels were found to be enhanced on treatment of HepG2 cells with insulin39 or on activation of the PI3K/PKB/FRAP (FKBP-rapamycin-associated protein) pathway in human prostate cancer cells.40 Because insulin may target HIF-1 via the PI3K/PKB pathway, it was the aim of the present study to find out whether PAI-1 expression was induced by insulin via the PI3K/PKB pathway and HIF-1 binding to the HREs using primary cultured rat hepatocytes as a model system.
It was shown that PAI-1 gene expression was induced by both insulin and mild hypoxia. Expression of a constitutively activated PKB enhanced PAI-1 expression and PAI-1 promoter-dependent luciferase (Luc) activity. Concomitantly, insulin and PKB enhanced HIF-1α protein levels and HIF-1 DNA-binding activity. Mutation of the HREs within thePAI-1 reporter gene constructs abolished the induction by insulin and PKB. Thus, insulin appears to induce rat PAI-1 expression via the PI3K/PKB/HIF-1 pathway.
Materials and methods
All biochemicals and enzymes were of analytical grade and were purchased from commercial suppliers.
Animals
Male Wistar rats (200-260 g) were kept on a 12-hour day/night rhythm with free access to water and food. Rats were anesthetized with pentobarbital (60 mg/kg body weight) prior to preparation of hepatocytes.
Cell culture experiments
Hepatocytes were isolated by collagenase perfusion. Cells (1 × 106 per dish) were cultured in a normoxic atmosphere of 16% O2, 79% N2, and 5% CO2 (by vol) in medium M 199 containing 0.5 nM insulin, 100 nM dexamethasone as permissive hormones, and 4% fetal calf serum for the initial 5 hours of culture. Cells were then cultured in serum-free medium from 5 to 24 hours at normal arterial 16% O2. At 24 hours medium was changed, and culture was continued at normoxia (16% O2) or at mild hypoxia (8% O2) for another 24 hours (87% N2, 5% CO2 [by vol]). Three hours before harvesting the cells were treated with either 10 nM insulin (dissolved in 0.9% NaCl containing 0.1% bovine serum albumin [BSA]), 20 nM wortmannin (dissolved in dimethyl sulfoxide [DMSO]), or in the controls with solvent.
Plasmid constructs
The pGl3PAI-766 plasmid, containing the rat PAI-1 promoter 5′-flanking region41 from −766 to +31, as well as pGl3PAI-766M1 and pGl3PAI-766M2, was described before.34The luciferase gene construct pGl3EPO-HRE containing 3 HREs from theEPO gene in front of the SV40 promoter was described.42 The vectors expressing the constitutively active form of PKB (myrPKB) and a dominant-negative PKB (PKB-K179A) were a kind gift from Dr D. Stokoe and have been already described.43
RNA preparation and Northern analysis
Isolation of total RNA and Northern analysis were performed as described.34 Digoxigenin (DIG)–labeled antisense RNAs served as hybridization probes; they were generated by in vitro transcription from pBS-PAI-1, pBS-rHIF2α-650, and pBS-GK using T3 RNA polymerase or from pCRII-rHIF1α-800 and pCRIITOPO-rHIF3α-200042 and pBS-β actin using T7 RNA polymerase and RNA labeling mixture containing 3.5 mM 11-DIG–uridine triphosphate (UTP), 6.5 mM UTP, 10 mM guanosine triphosphate (GTP), 10 mM cytosine triphosphate (CTP), and 10 mM adenosine triphosphate (ATP). The use of a full-length HIF-3α probe was appropriate because liver and HepG2 cells appeared not to express the HIF-3α splice variant IPAS.44 Hybridizations and detections were carried out essentially as described before.34 Blots were quantified with a videodensitometer (Biotech Fischer, Reiskirchen, Germany).
Western blot analysis
PAI-1, HIF-1α, HIF-2α, HIF-3α, PKB, and phosphoPKB (PKB-S473) Western blot analysis was carried out as described.45-47 In brief, media or lysates from primary cultured hepatocytes were collected, and the protein content was determined by using the Bradford method. Protein (50 μg) was loaded onto a 10% sodium dodecyl sulfate (SDS)–polyacrylamide gel and after electrophoresis blotted onto nitrocellulose membranes. The primary rabbit antibody against rat PAI-1 (American Diagnostics, Heidelberg, Germany) was used in a 1:200 dilution. The secondary antibody was a goat antirabbit immunoglobulin G (IgG; Santa Cruz Biotechnology, Santa Cruz, CA) and used in a 1:2000 dilution. The primary antibodies against HIF-1α, HIF-2α, and HIF-3α were described earlier42 and used in a 1:800 dilution. The primary rabbit antibody against Golgi membrane (GM; Bioscience, Göttingen, Germany) was used in a 1:8000 dilution. The secondary antibody was a goat antirabbit IgG horseradish peroxidase (HRP; Santa Cruz Biotechnology), used in a 1:2000 dilution. The PKB and PKB-S473 antibody (Cell Signaling, Frankfurt, Germany) was used in a 1:1000 dilution; the secondary antibody was an antirabbit IgG and used as above. The monoclonal mouse anti-EE antibody (Hiss Diagnostics, Freiburg, Germany) was used in a 1:1000 dilution; the secondary antibody was an antimouse IgG HRP and used as above. The enhanced chemiluminescence (ECL) Western blotting system (Amersham) was used for detection. Under these conditions PAI-1 was seen as a double band, the major 49-kDa band and the minor 46-kDa band,45HIF-1α was visible as a 120-kDa band, HIF-2α as a 130-kDa band, and HIF-3α as a 74-kDa band.42 PKB was visible as a 60-kDa band.48
Cell transfection and luciferase assay
Freshly isolated rat hepatocytes (about 1 × 106cells per dish) were transfected as described,46 thereby controlling transfection efficiency by cotransfection of 0.25 μgRenilla Luciferase expression vector (pRLSV40) (Promega). Additionally, 2 μg of the appropriate PAI-1 or EPO-HRE promoterFirefly luciferase construct was transfected together with 500 ng PKB expression vectors or in the controls with 500 ng empty control vector. For Northern blot and Western blot experiments cells were transfected with 2 μg PKB-K179A expression vector, myrPKB expression vector, or control vector. After 5 hours the medium was changed, and the cells were cultured under normoxia for 19 hours. Then, medium was changed again, and the cells were further cultured for 24 hours under normoxia or mild hypoxia. Three hours before harvesting the cells were treated with insulin (10 nM).
Preparation of nuclear extracts and electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared by modification of a standard protocol essentially as described.34 49 The sequence of the PAI-1 oligonucleotide used for the EMSA is 5′-TCTCACACACGTACACACACGTGTC-3′ (−182/−157). Equal amounts of complementary oligonucleotides were annealed and labeled by 5′-end labeling with γ-32P]ATP (Amersham) and T4 polynucleotide kinase (MBI). They were purified with the Nucleotide Removal Kit (Qiagen). Binding reactions were carried out in a total volume of 20 μL containing 50 mM KCl, 1 mM MgCl2, 1 mM EDTA (ethylenediaminetetraacetic acid), 5% glycerol, 10 μg nuclear extract, 250 ng poly d(I-C), and 5 mM dithioerythrol (DTE). After preincubation for 5 minutes at room temperature, 1 μL labeled probe (104 cpm) was added, and the incubation was continued for an additional 10 minutes. For supershift analysis 1 μL HIF-1α antibody was added to the EMSA reaction that was then incubated at 4°C for 2 hours. The electrophoresis was then performed with a 5% nondenaturing polyacrylamide gel in TBE buffer (89 mM Tris (tris(hydroxymethyl)aminomethane), 89 mM boric acid, 5 mM EDTA) at 200 V. After electrophoresis the gels were dried and exposed to a phosphorimager screen.
Results
Induction of PAI-1 expression by insulin via the PI3K pathway
Treatment of cells with different concentrations of insulin led to a concentration-dependent enhancement of PAI-1 mRNA levels. In the range of physiologic insulin concentrations up to 10 nM, PAI-1 mRNA was induced by about 3-fold under normoxia. The insulin concentration of 100 nM did not give rise to a more dramatic PAI-1 mRNA induction (Figure 1A). Under hypoxia the insulin-dependent PAI-1 mRNA induction started at 1 nM insulin, and then the induction was more pronounced than under normoxia. Maximal activation by about 7-fold under hypoxia was achieved with an insulin concentration of 100 nM. For further experiments an insulin concentration of 10 nM was chosen (Figure 1A). Treatment of hepatocytes with insulin enhanced PAI-1 mRNA levels to a transient maximum after 3 hours under both normoxia and hypoxia. PAI-1 mRNA levels then decreased again. Thus, for the experiments the cells were treated with insulin for 3 hours (data not shown).
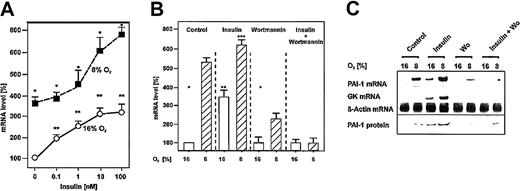
Induction of PAI-1 mRNA and protein expression by insulin under both normoxia and mild hypoxia.
Cells were cultured for 24 hours under arterial pO2. At 24 hours the medium was changed, and cells were further cultured for the next 24 hours under normoxic (16% O2) and hypoxic (8% O2) conditions. Three hours before harvesting, the cells were treated with insulin, wortmannin, or both. The PAI-1 mRNA levels were measured by Northern blotting. (A) The PAI-1 mRNA level under normoxia (16% O2) without addition of insulin was set equal to 100%. Concentrations of insulin are indicated. (B) The PAI-1 mRNA level under normoxia (16% O2) without addition of insulin was set equal to 100%. Insulin was used at a concentration of 10 nM, wortmannin was used at a concentration of 20 nM. Values are expressed as means ± SEMs of 3 independent culture experiments. Student t test for paired values: *significant difference 16% O2 versus 8% O2, **significant difference 16% O2 + insulin versus 16% O2(control), ***significant difference 8% O2 + insulin versus 8% O2 (control), P ≤ .05. (C) Representative Northern and Western blot. For Northern analysis 15 μg total RNA was hybridized to digoxigenin-labeled PAI-1, GK, and β-actin antisense RNA probes (see “Materials and methods” for more information). Protein (50 μg) from the medium was subjected to Western analysis with an antibody against rat PAI-1 (see “Materials and methods” for more information). Autoradiographic signals were obtained by chemiluminescence and scanned by videodensitometry. Wo indicates wortmannin.
Induction of PAI-1 mRNA and protein expression by insulin under both normoxia and mild hypoxia.
Cells were cultured for 24 hours under arterial pO2. At 24 hours the medium was changed, and cells were further cultured for the next 24 hours under normoxic (16% O2) and hypoxic (8% O2) conditions. Three hours before harvesting, the cells were treated with insulin, wortmannin, or both. The PAI-1 mRNA levels were measured by Northern blotting. (A) The PAI-1 mRNA level under normoxia (16% O2) without addition of insulin was set equal to 100%. Concentrations of insulin are indicated. (B) The PAI-1 mRNA level under normoxia (16% O2) without addition of insulin was set equal to 100%. Insulin was used at a concentration of 10 nM, wortmannin was used at a concentration of 20 nM. Values are expressed as means ± SEMs of 3 independent culture experiments. Student t test for paired values: *significant difference 16% O2 versus 8% O2, **significant difference 16% O2 + insulin versus 16% O2(control), ***significant difference 8% O2 + insulin versus 8% O2 (control), P ≤ .05. (C) Representative Northern and Western blot. For Northern analysis 15 μg total RNA was hybridized to digoxigenin-labeled PAI-1, GK, and β-actin antisense RNA probes (see “Materials and methods” for more information). Protein (50 μg) from the medium was subjected to Western analysis with an antibody against rat PAI-1 (see “Materials and methods” for more information). Autoradiographic signals were obtained by chemiluminescence and scanned by videodensitometry. Wo indicates wortmannin.
In cultured hepatocytes hypoxia induced PAI-1 mRNA and protein by about 5-fold in line with a previous study.34 Treatment of cells with insulin enhanced PAI-1 mRNA and protein by about 3-fold under normoxia and by about 6-fold under hypoxia. The PI3K inhibitor wortmannin did not affect PAI-1 mRNA and protein expression under normoxia but reduced the PAI-1 mRNA and protein induction under hypoxia to about 2.2-fold compared with the controls under normoxia. The presence of both insulin and wortmannin completely abolished PAI-1 mRNA and protein induction under both normoxia and hypoxia (Figure 1B).
To control the action of insulin the insulin-dependent glucokinase (GK) mRNA induction was measured. GK mRNA was hardly detectable in the controls, ie, without insulin. Treatment of the cells with insulin enhanced GK mRNA under normoxia and was more pronounced under hypoxia that was in line with previous findings.34Again, wortmannin abolished the induction of GK mRNA by insulin (Figure1C). Thus, these findings indicate that the activation of PAI-1 expression by insulin is mediated via the PI3K pathway.
Induction of PAI-1 mRNA expression by PKB
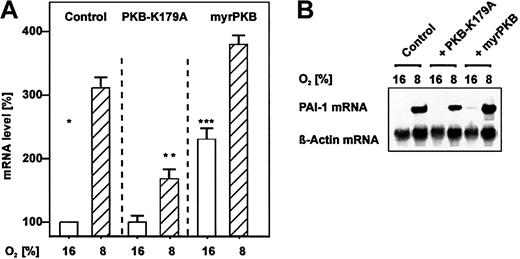
A further downstream-acting component in the PI3K pathway is PKB, also known as Akt. To investigate the involvement of PKB in the pathway contributing to the enhanced levels of PAI-1, cells were transfected with expression vectors encoding either a dominant-negative protein kinase B (PKB-K179A) or a constitutively active myristoylated variant of PKB (myrPKB). Similar to wortmannin transfection of the PKB-K179A expression vector resulted in decreased PAI-1 mRNA levels under hypoxia when compared with the control. The transfection of the vector for the constitutively active myrPKB enhanced PAI-1 mRNA levels by about 2-fold under normoxia and by about 4-fold under hypoxia. Thus, these results indicate that also the PKB pathway can contribute to enhanced PAI-1 levels (Figure 2).
Induction of PAI-1 mRNA expression by overexpression of PKB.
Hepatocytes that were transfected either with PKB-K179A or myrPKB expression vectors or with the empty control vector were cultured for 24 hours under arterial pO2. At 24 hours the medium was changed, and cells were further cultured for the next 24 hours under normoxic (16% O2) and hypoxic (8% O2) conditions. (A) The PAI-1 mRNA levels were measured by Northern blotting. The mRNA level under normoxia (16% O2) was set equal to 100%. Values are expressed as means ± SEMs of 3 independent culture experiments. Student t test for paired values: *significant difference 16% O2 versus 8% O2, **significant difference 8% O2 + PKBK179A versus 8% O2 (control), ***significant difference 16% O2 + myrPKB versus 16% O2 (control),P ≤ .05. (B) Representative Northern blot. For Northern analysis 15 μg total RNA was hybridized to digoxigenin-labeled PAI-1 and β-actin antisense RNA probes (see “Materials and methods” for more information). Autoradiographic signals were obtained by chemiluminescence and scanned by videodensitometry.
Induction of PAI-1 mRNA expression by overexpression of PKB.
Hepatocytes that were transfected either with PKB-K179A or myrPKB expression vectors or with the empty control vector were cultured for 24 hours under arterial pO2. At 24 hours the medium was changed, and cells were further cultured for the next 24 hours under normoxic (16% O2) and hypoxic (8% O2) conditions. (A) The PAI-1 mRNA levels were measured by Northern blotting. The mRNA level under normoxia (16% O2) was set equal to 100%. Values are expressed as means ± SEMs of 3 independent culture experiments. Student t test for paired values: *significant difference 16% O2 versus 8% O2, **significant difference 8% O2 + PKBK179A versus 8% O2 (control), ***significant difference 16% O2 + myrPKB versus 16% O2 (control),P ≤ .05. (B) Representative Northern blot. For Northern analysis 15 μg total RNA was hybridized to digoxigenin-labeled PAI-1 and β-actin antisense RNA probes (see “Materials and methods” for more information). Autoradiographic signals were obtained by chemiluminescence and scanned by videodensitometry.
Insulin-dependent activation of PAI-1 promoter Luc gene constructs
To investigate whether the insulin-dependent induction of thePAI-1 gene is mediated by a distinct promoter sequence, primary hepatocytes were transfected with Luc gene constructs driven by the hypoxia-inducible rat PAI-1 gene promoter. In pGl3PAI-766 Luc-transfected cells, Luc activity was enhanced by about 3-fold under hypoxia in line with previous studies.34 49 Treatment of the transfected cells with insulin enhanced Luc activity by about 2-fold under normoxia and by about 5-fold under hypoxia (Figure3).
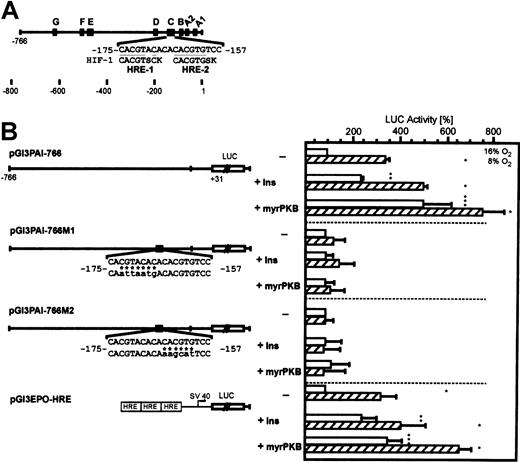
Induction of PAI-1 promoter-dependent luciferase activity by insulin and activated PKB is conferred by the HREs.
(A) The 5′-flanking region of the rat PAI-1 gene with its footprinted sites (A-G). In the “C-site” the 2 HIF-1 binding elements (HRE) matching the HIF-1 consensus sequence BACGTSSK50 where B = G/C/T, S = G/C, and K = G/T are underlined. B, S, or K is shown only if the actual PAI-1 sequence does not match any of the bases allowed by the consensus. (B) Hepatocytes were transiently cotransfected with either the myrPKB expression plasmid or the empty control vector and Luc gene constructs driven by a wild-type −766-bp rat PAI-1 promoter (pGl3PAI-766), or the 766-bp promoter mutated at either the HRE-1 (pGl3PAI-766M1) or HRE-2 (pGl3PAI-766M2) site, or Luc gene construct containing 3 copies of the EPO HRE element in front of the SV40 promoter (pGl3EPO-HRE). The cells were treated with 10 nM insulin, as indicated. In each experiment the percentage of Luc activity was determined relative to the pGl3PAI-766, pGl3PAI-766M1, pGl3PAI-766M2, or pGl3EPO-HRE controls that were set equal to 100%. In pGl3PAI-766M1 and pGl3PAI-766M2 the wild-type PAI-1 sequence is shown on the upper strand, mutated bases are indicated by * and are shown in lowercase letters. The values are represented as means ± SEMs of 3 independent experiments. Student t test for paired values: *significant difference 16% O2 versus 8% O2, **significant difference 16% O2 + insulin versus 16% O2 (control), ***significant difference 16% O2 + myrPKB versus 16% O2 (control),P ≤ .05.
Induction of PAI-1 promoter-dependent luciferase activity by insulin and activated PKB is conferred by the HREs.
(A) The 5′-flanking region of the rat PAI-1 gene with its footprinted sites (A-G). In the “C-site” the 2 HIF-1 binding elements (HRE) matching the HIF-1 consensus sequence BACGTSSK50 where B = G/C/T, S = G/C, and K = G/T are underlined. B, S, or K is shown only if the actual PAI-1 sequence does not match any of the bases allowed by the consensus. (B) Hepatocytes were transiently cotransfected with either the myrPKB expression plasmid or the empty control vector and Luc gene constructs driven by a wild-type −766-bp rat PAI-1 promoter (pGl3PAI-766), or the 766-bp promoter mutated at either the HRE-1 (pGl3PAI-766M1) or HRE-2 (pGl3PAI-766M2) site, or Luc gene construct containing 3 copies of the EPO HRE element in front of the SV40 promoter (pGl3EPO-HRE). The cells were treated with 10 nM insulin, as indicated. In each experiment the percentage of Luc activity was determined relative to the pGl3PAI-766, pGl3PAI-766M1, pGl3PAI-766M2, or pGl3EPO-HRE controls that were set equal to 100%. In pGl3PAI-766M1 and pGl3PAI-766M2 the wild-type PAI-1 sequence is shown on the upper strand, mutated bases are indicated by * and are shown in lowercase letters. The values are represented as means ± SEMs of 3 independent experiments. Student t test for paired values: *significant difference 16% O2 versus 8% O2, **significant difference 16% O2 + insulin versus 16% O2 (control), ***significant difference 16% O2 + myrPKB versus 16% O2 (control),P ≤ .05.
Because it was shown that the transcription factor HIF-1 binding to HREs can be a target in the PKB signaling pathway, it was further tested whether the mutation of the HREs in the PAI-1 promoter abolished the insulin-dependent Luc induction. Transfection of the PAI-1 promoter Luc construct in which the HRE-1 was mutated (pGl3PAI-766M1) abolished the induction of Luc activity by hypoxia as well as the insulin-dependent induction under both normoxia and hypoxia. Similarly, mutation of the HRE-2 site in pGl3PAI-766M2 also attenuated the induction of Luc activity by hypoxia in line with a previous study.34 Treatment of the transfected cells with insulin did not increase Luc activity as well (Figure 3). This finding indicated that indeed HIF-1 was involved in the insulin-dependent activation of the PAI-1 gene.
To further substantiate this finding, the cells were transfected with aLuc gene construct containing 3 isolated HREs from theEPO gene in front of the SV40 promoter. Transfection of the pGl3EPO-HRE Luc construct resulted in higher Luc activity by about 3-fold when the cells were exposed to hypoxia. Treatment of the pGl3EPO-HRE Luc-transfected cells with insulin enhanced Luc activity by about 2-fold under normoxia and by about 4-fold under hypoxia (Figure3).
The involvement of PKB was tested by cotransfection of the PAI-1 promoter Luc constructs with the expression vector encoding the constitutively activated PKB (myrPKB). Cotransfection of the wild-type PAI-1 promoter with the myrPKB expression vector elicited enhanced Luc activity by about 5-fold under normoxia and by about 8-fold under hypoxia. The mutation of either HRE site, HRE-1 or HRE-2, completely abolished the myrPKB-mediated induction of Luc activity. The cotransfection of the pGl3EPO-HRE Luc construct with the myrPKB expression vector enhanced Luc activity by about 3-fold under normoxia and by about 6-fold under hypoxia (Figure 3). Thus, it appears that thePAI-1 gene activation by insulin is mediated via the PI3K/PKB/HIF-1 pathway.
Enhancement of HIF α-subunit protein levels by insulin via PI3K and protein kinase B
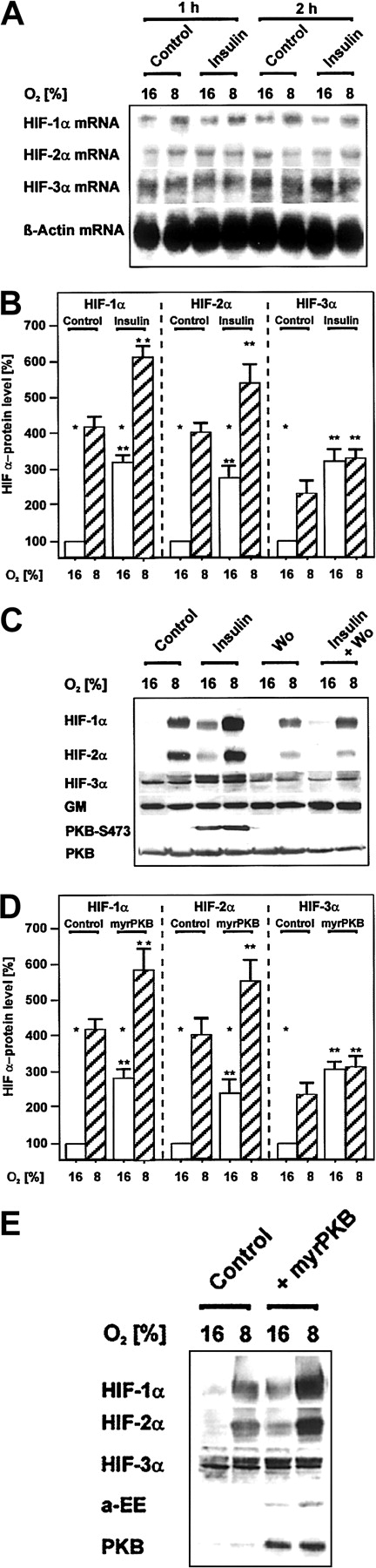
To test whether the activation of PAI-1 expression by insulin via the HREs can be mediated by enhanced levels of HIF-1α, HIF-2α, or HIF-3α, Northern and Western blot analyses were performed. Treatment of hepatocytes with insulin under normoxia and hypoxia did not enhance the mRNA levels of the 3 HIF α-subunits (Figure 4A). In contrast, the HIF-1α and HIF-2α protein levels were increased by about 4-fold and that of HIF-3α by about 2-fold under hypoxia. Insulin treatment for 3 hours mediated about a 3-fold enhancement of all HIF-α protein levels under normoxia. Under hypoxia insulin treatment increased the HIF-1α levels by about 6-fold, the HIF-2α levels by about 5-fold, and the HIF-3α levels by about 3-fold compared with the normoxic control (Figure4B,C). The insulin-dependent increase of the HIF α-subunit protein levels was mediated via PI3K because the PI3K inhibitor wortmannin abolished the insulin-dependent enhancement of the HIF α-subunit proteins (Figure 4C).
Induction of HIF α-subunit protein levels by insulin via PI3K and by overexpression of activated PKB.
Cells treated with either insulin, wortmannin, or both, as well as cells transfected with the myrPKB expression vector, were cultured for 24 hours under arterial pO2. At 24 hours the medium was changed, and cells were further cultured for the next 24 hours under normoxic (16% O2) and hypoxic (8% O2) conditions. Three hours before harvesting the cells were treated with insulin (10 nM), wortmannin (20 nM), or in the controls with solvent (see “Materials and methods” for more information). (A) The HIF α-subunit mRNA levels were analyzed by Northern blotting. Representative Northern blot of 15 μg total RNA hybridized to digoxigenin-labeled HIF-1α, HIF-2α, HIF-3α, and β-actin antisense RNA probes (see “Materials and methods” for more information). (B,D) The HIF-α protein levels were measured by Western blotting. The protein level under normoxia (16% O2) was set equal to 100%. Values are expressed as means ± SEMs of 3 independent culture experiments. Studentt test for paired values: *significant difference 16% O2 versus 8% O2, **significant difference 16% O2 + insulin versus 16% O2 (control), 8% O2 + insulin versus 8% O2 (control),P ≤ .05; (D) **significant difference 16% O2 + myrPKB versus 16% O2 (control), 8% O2 + myrPKB versus 8% O2 (control),P ≤ .05. (C,E) Representative Western blots. Protein (50 μg) was subjected to Western analysis with an antibody against HIF-1α, HIF-2α, HIF-3α, GM, PKB-S473, PKB, or anti–EE-tag (see “Materials and methods” for more information). Autoradiographic signals were obtained by chemiluminescence and scanned by videodensitometry.
Induction of HIF α-subunit protein levels by insulin via PI3K and by overexpression of activated PKB.
Cells treated with either insulin, wortmannin, or both, as well as cells transfected with the myrPKB expression vector, were cultured for 24 hours under arterial pO2. At 24 hours the medium was changed, and cells were further cultured for the next 24 hours under normoxic (16% O2) and hypoxic (8% O2) conditions. Three hours before harvesting the cells were treated with insulin (10 nM), wortmannin (20 nM), or in the controls with solvent (see “Materials and methods” for more information). (A) The HIF α-subunit mRNA levels were analyzed by Northern blotting. Representative Northern blot of 15 μg total RNA hybridized to digoxigenin-labeled HIF-1α, HIF-2α, HIF-3α, and β-actin antisense RNA probes (see “Materials and methods” for more information). (B,D) The HIF-α protein levels were measured by Western blotting. The protein level under normoxia (16% O2) was set equal to 100%. Values are expressed as means ± SEMs of 3 independent culture experiments. Studentt test for paired values: *significant difference 16% O2 versus 8% O2, **significant difference 16% O2 + insulin versus 16% O2 (control), 8% O2 + insulin versus 8% O2 (control),P ≤ .05; (D) **significant difference 16% O2 + myrPKB versus 16% O2 (control), 8% O2 + myrPKB versus 8% O2 (control),P ≤ .05. (C,E) Representative Western blots. Protein (50 μg) was subjected to Western analysis with an antibody against HIF-1α, HIF-2α, HIF-3α, GM, PKB-S473, PKB, or anti–EE-tag (see “Materials and methods” for more information). Autoradiographic signals were obtained by chemiluminescence and scanned by videodensitometry.
Similar to insulin, transfection of the cells with the vector for the constitutively active PKB enhanced the HIF-1α and HIF-2α protein levels by about 3-fold under normoxia and 5.5-fold under hypoxia, respectively, compared with the untransfected control under normoxia. The HIF-3α protein levels were enhanced by the constitutively active PKB by about 3-fold under both normoxia and hypoxia (Figure 4D-E). To control the presence of active PKB in the hepatocytes used for HIF α-subunit Western blots, the same lysates were used for Western blot analysis with an antibody against wild-type PKB and against the active form of PKB, which is phosphorylated at Ser473.51Induction of PKB-Ser473 in the cells treated with insulin was indeed observed (Figure 4E). To control the expression of the transfected myrPKB, Western blots with an antibody against wild-type PKB and against the EE-tag of the myrPKB construct were performed (Figure 4B). Thus, insulin and active PKB were able to contribute to enhanced HIF α-subunit protein levels.
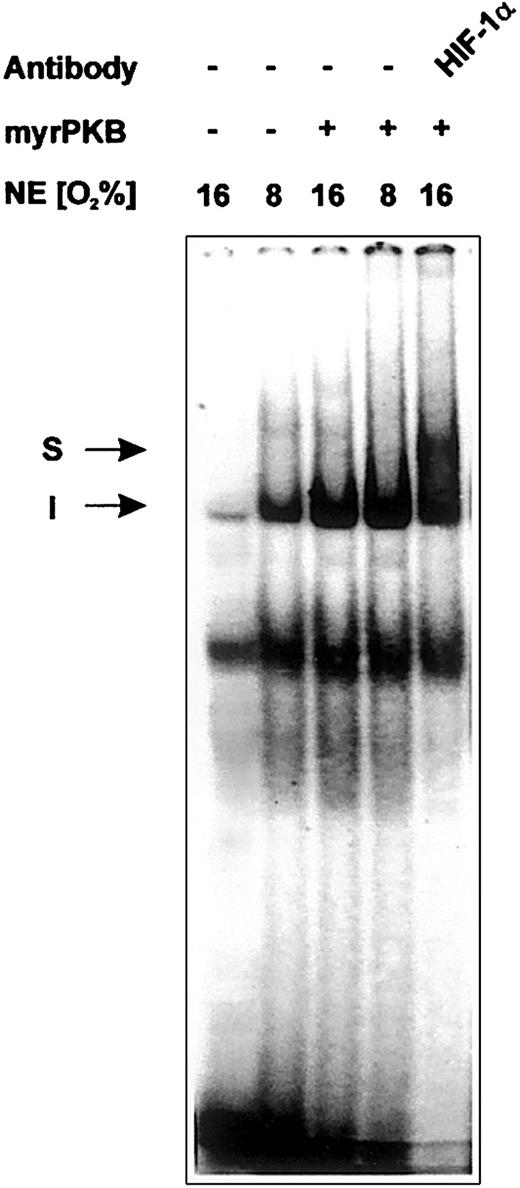
Insulin-mediated enhanced binding of HIF-1 to the hypoxia-responsive sequence of the PAI-1 promoter
To verify that the insulin-dependent and PKB-mediated induction of PAI-1 promoter controlled Luc activity and the accumulation of the HIF-1α protein is reflected by enhanced binding of HIF-1 to the PAI-1 HREs, EMSAs with nuclear extracts from cells cultured under either normoxia or hypoxia and transfected with the vector for the constitutively active PKB were performed. After incubation of the PAI-1 HRE1/2 oligonucleotide with nuclear extracts from cells cultured under normoxia, a DNA-protein complex could be detected, the formation of which was enhanced when nuclear extracts prepared from cells cultured under hypoxia were used (Figure 5). Usage of the nuclear extracts prepared from the myrPKB-transfected cells cultured under both normoxia and hypoxia also displayed the enhanced formation of the DNA-protein complex as observed with nuclear extracts from cells cultured under hypoxia. To confirm the presence of the HIF-1α protein in the observed complex, an anti–HIF-1α antibody was included in the binding reaction. When nuclear extracts from the cells transfected with myrPKB were treated with the anti–HIF-1α antibody, a supershifted complex was observed (Figure 5). These findings further substantiate the conclusion that PKB acts via HIF-1 and the HRE sites of the PAI-1 promoter.
Enhanced binding of HIF-1 to the HREs of the rat PAI-1 promoter because of overexpression of activated PKB.
EMSA: the 32P-labeled PAI-1 HRE (−182/−157) oligonucleotide was incubated with either 10 μg protein of nuclear extracts from normoxic (16% O2) or hypoxic (8% O2) cells transfected with the myrPKB expression vector or the empty control vector, as indicated (see “Materials and methods” for more information). In the EMSA with antibody the nuclear extracts were preincubated with 1 μL HIF-1α antibody for 2 hours at 4°C before adding the labeled probe. The DNA-protein binding was analyzed by electrophoresis on 5% native polyacrylamide gels. I represents induced HIF-1 complex; S, supershifted HIF-1 complex.
Enhanced binding of HIF-1 to the HREs of the rat PAI-1 promoter because of overexpression of activated PKB.
EMSA: the 32P-labeled PAI-1 HRE (−182/−157) oligonucleotide was incubated with either 10 μg protein of nuclear extracts from normoxic (16% O2) or hypoxic (8% O2) cells transfected with the myrPKB expression vector or the empty control vector, as indicated (see “Materials and methods” for more information). In the EMSA with antibody the nuclear extracts were preincubated with 1 μL HIF-1α antibody for 2 hours at 4°C before adding the labeled probe. The DNA-protein binding was analyzed by electrophoresis on 5% native polyacrylamide gels. I represents induced HIF-1 complex; S, supershifted HIF-1 complex.
Discussion
The present study has shown that the insulin-dependent activation of the rat PAI-1 gene expression is mediated via the PI3K/PKB pathway and the transcription factor HIF-1 binding to the HRE sites in the PAI-1 promoter.
Oxygen-independent activation of HIF-1–regulated genes
Hypoxic HIF-1 activation stimulates its binding to HREs, subsequently leading to the induction of genes whose products either increase availability of oxygen, such as EPO and VEGF, or promote metabolic adaptation to oxygen deprivation, such as glucose transporters or glycolytic enzymes.52 It is known that genes responsible for glucose and energy metabolism, as well as EPO and VEGF, are induced also by insulin which indicates that hypoxia and insulin signaling pathways could be interrelated.53,54 The results of our study confirm previous observations that exposure of human embryonic kidney 293 cells to insulin38 and HepG2 hepatoma cells to insulin39 resulted in the induction of HIF-1α protein levels. EMSA experiments with nuclear extracts from HepG2 cells and L8 rat skeletal muscle myoblasts, cultured under normoxia and treated with insulin, and an oligonucleotide corresponding to the HRE sequence of the EPO gene showed the formation of the active HIF-1 complex.37 Furthermore, insulin induced the expression of a Luc reporter construct containing 5 copies of the EPO-HRE as enhancer37 that is also in line with the present findings. Moreover, our study for the first time showed that primary hepatocytes treated with insulin and cultured under mild hypoxia contained higher levels of the HIF-1α protein and higher Luc activities from the PAI-1 promoter and the EPO-HRE Luc reporter construct when compared with the insulin-untreated hepatocytes cultured under hypoxia. This finding also indicates that insulin treatment and exposure of the cells to hypoxia have an additive effect on the HIF-1 activity.
Beside insulin, other hormones, growth factors, and clotting factors such as insulinlike growth factor (IGF),37,38angiotensin II,55 platelet-derived growth factor (PDGF),55 thrombin,56 and tumor necrosis factor α (TNFα)57 have been shown to enhance the HIF-1α levels and HIF-1 activity independent from the oxygen tension. The detailed mechanisms and the signaling pathways are not fully understood yet and may involve several MAPKs as well as the PI3K/PKB pathway.
Signaling pathways involved in the activation of HIF-1
The mechanisms by which hypoxia stimulates activation of HIF-1 are not understood to the last detail. Proposed models involve signal transduction pathways via oxygen-binding hemoproteins and generation of reactive oxygen species,52 as well as via oxygen-dependent prolyl hydroxylation58,59 and asparaginyl hydroxylation.60 The latter mechanisms have been shown to be of major importance for HIF α-subunit protein stability and coactivator recruitment. Under normoxia HIF-1α and HIF-2α destabilization is conferred by the O2-dependent hydroxylation of at least 2 proline residues within the O2-dependent degradation domains, enabling the binding of the von Hippel–Lindau tumor suppressor protein (pVHL), a component of an E3 ubiquitin ligase complex that targets the HIF α-subunits for degradation by the ubiquitin-proteasome pathway.61 In a similar manner the hydroxylation of an asparaginyl residue in the C-terminal transactivation domain of HIF-1α and HIF-2α prevents the recruitment of the coactivator CBP/p300, thus reducing the HIF-1α and HIF-2α transactivation potential.60 Furthermore, it was suggested that MAP kinases modulate the transcriptional activity of HIF-1 via phosphorylation of HIF-1α at its regulatory domain.62,63 In addition, the PI3K/PKB pathway was proposed to regulate the activity of HIF-1 mainly via stabilization of the HIF-1α protein.63,64 In line with this the present study and a study with HepG2 cells demonstrated that insulin induced HIF-1α accumulation, HIF-1 DNA-binding, and PAI-1, VEGF, and EPO synthesis independently from MAP kinases via the PI3K pathway.39 Furthermore, under hypoxia the PI3K/PKB pathway was responsible for the induction of VEGF expression in Ras-transformed NIH3T3 cells65 and inactivation of the PKB target glycogen synthetase kinase 3 (GSK-3) in HT1080 cells.66 The PI3K/PKB pathway was also shown to be involved in the NO-dependent,67thrombin-dependent,56 heregulin-dependent68and, as in the present and another recent study,69 the insulin-dependent activation of HIF-1 under normoxia. Thereby, insulin appeared to act via a translational-dependent pathway because it was currently shown that cycloheximide inhibited the insulin-dependent enhancement of the HIF-1α protein levels69 and, as in this study, did not affect the HIF-1α mRNA expression69(Figure 4A).
In our study the insulin-dependent accumulation of all 3 HIF α-subunits as well as the HIF-mediated PAI-1 expression could be abrogated by the PI3K inhibitor wortmannin (Figures 1 and 4). This is in line with experiments in human prostate cancer cells in which inhibition of PI3K by wortmannin and by LY294002 prevented induction of HIF-1α by insulin and EGF.70 Furthermore, we showed that overexpression of a dominant-negative PKB (PKB K179A) or of a constitutively active PKB (myrPKB) reciprocally regulated HIF-1–dependent Luc activity and PAI-1 expression (Figure 2). These results are also in line with data from experiments with human prostate cancer cells in which the HIF-1–dependent expression of a HRE-Luc reporter construct was blocked by the PI3,4,5P3 phosphatase and tumor suppressor phosphatase and tensin homologue (PTEN), by a dominant-negative form of the PI3K regulatory subunit p85, and by dominant-negative PKB, whereas constitutively active myrPKB and dominant-negative PTEN stimulated Luc expression.40However, more recent studies argued that the PI3K/PKB pathway was universally involved in the HIF-1 induction by hypoxia, although myrPKB was able to increase HIF-1–dependent transcription of a phosphoglycerate kinase (PGK) HRE-Luc reporter construct in a cell type–specific manner and also, despite PI3K/PKB inhibitors, wortmannin and LY294002 could block serum-induced accumulation of HIF-1α.71 72
In summary, the findings of this study with the PAI-1 gene are another example for the role of the PI3K/PKB pathway as a key regulatory cascade responsible for insulin signaling that may undergo a cross talk with the hypoxia-dependent signaling pathway in a cell type–specific manner.
PI3K/PKB pathway in the activation of the PAI-1gene expression
The involvement of HIF-1 and the PI3K/PKB pathway in the regulation of PAI-1 gene expression by insulin has not been shown previously. However, the proposal that the PI3K may have an effect on the stimulation of PAI-1 expression came from the observations that nerve growth factor–induced PAI-1 mRNA expression was inhibited by wortmannin in rat pheochromocytoma PC12 cells73 and that the insulin-induced human PAI-1 protein secretion was inhibited by LY294002 in HepG2 cells.74These results concur with our study. But in contrast to our experiments, the only other study, in which the signal transduction pathway leading to the PAI-1 induction by insulin was investigated, showed that downstream from PI3K protein kinase C (PKC) and MAP kinases were activated.74 Thus, it seems likely that at least in human cells downstream from PI3K more than one pathway is involved in the regulation of PAI-1 gene expression by insulin.
In contrast to our study, the transcription factor responsible for activation of human PAI-1 expression via the PKC/MAPK pathway in HepG2 cells as well as the exact insulin responsive element in the human PAI-1 promoter has not been identified. However, it was demonstrated by cotransfection and EMSA experiments that 3 regions of the human PAI-1 promoter, −777/−741, −157/−128, and −93/−62, were responsible for the PAI-1 gene activation by insulin.74 Hypoxia responsive elements in the human promoter, similar to the HREs of the rat PAI-1 promoter, are localized at positions −158/−151 and −194/−187.75 Thus, the partial overlap of the HRE sequence −158/−151 with one of the putative insulin response sequences −157/−128 suggests again also a possible involvement of HIF-1 in the insulin-dependent activation of the human PAI-1 promoter.
Insulin- and HIF-dependent PAI-1 gene expression under physiologic and pathophysiologic conditions
Induction of PAI-1 expression by insulin via HIF-1 might have importance for the development of cardiovascular disease. HIF-1 has been implicated to be involved in neovascularization of ischemic myocardium via activation of VEGF expression.33,76 The levels of plasma PAI-1 are elevated in hyperinsulinemia, hypertriglyceridemia, hypertension, obesity, and diabetes type 2 that are all characteristic for the insulin resistance syndrome associated with a highly increased risk of cardiovascular disease.77,78 Impaired fibrinolysis because of overexpression of PAI-1 has been implicated as one of the mechanisms responsible for coronary artery disease under hyperinsulinemia.79 However, data concerning the up-regulation of human PAI-1 by insulin in vivo are somewhat contradictory. Some in vivo studies investigating the effects of insulin infusion found no effect on the levels of PAI-1 in blood or even a decrease of PAI-1 levels and activity.80-82 However, induction of PAI-1 by insulin was found when the perfused forearm model was used to study the local effects of insulin infusion.13 Furthermore, in combination with hypertriglyceridemia and hyperglycemia, hyperinsulinemia was shown to increase PAI-1 plasma levels.83 Thus, further investigations are still necessary to elucidate the complete physiologic role of PAI-1 activation by insulin and its molecular mechanisms.
We thank Dr D. Stokoe (Cancer Research Institute, University of California, San Francisco) for the kind gift of the myrPKB and PKB-K179A plasmids and Dr T. D. Gelehrter (Department of Human Genetics, University of Michigan Medical School, Ann Arbor) for the kind gift of PAI-1 cDNA.
Prepublished online as Blood First Edition Paper, September 12, 2002; DOI 10.1182/blood-2002-06-1693.
Supported by the Deutsche Forschungsgemeinschaft SFB 402, Teilprojekt A1, and GRK 335.
Kurt Jungermann died on May 10, 2002.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thomas Kietzmann, Institut für Biochemie und Molekulare Zellbiologie, Humboldtallee 23, D-37073 Göttingen, Germany; e-mail: tkietzm@gwdg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal