Abstract
To explore the mechanisms that underlie the bleeding tendency in type 2A and 2B von Willebrand disease (VWD), we analyzed the mural thrombus generation process on a collagen surface under physiologic blood flow in a perfusion chamber using whole blood from these VWD patients. At a low shear rate (50 s−1), thrombus generation in all type 2A and 2B VWD patients was comparable to that of healthy controls. At a high shear rate (1500 s−1), thrombus generation was impaired in all type 2A patients, whereas that in type 2B VWD patients varied from normal to significantly defective, as judged by epifluorescence microscopy of thrombus surface coverage. However, in type 2B patients who showed normal thrombus generation at 1500 s−1, the height and volume of thrombi was significantly reduced, albeit with the normal surface coverage, compared with control thrombi, and von Willebrand factor (VWF) was poorly distributed within the type 2B thrombus mass when analyzed in detail by confocal laser scanning microscopy. Addition of purified VWF to patient blood completely reversed the defective spatial thrombus growth in type 2B VWD. Thus, our results confirm the impaired thrombus generation in type 2B VWD, which has never been demonstrable in previous in vitro soluble-phase platelet aggregation assays, and point to the critical function of larger VWF multimers in the proper spatial growth of mural thrombi under high shear rate conditions.
Introduction
The contributions of von Willebrand factor (VWF) to platelet plug formation at sites of vessel disruption are fundamental to primary hemostasis.1-4 Indeed, patients with von Willebrand disease (VWD) who congenitally lack VWF exhibit a bleeding tendency.5,6 This prevalent bleeding disorder is currently categorized as 3 major types: type 1 (partial) and type 3 (complete) are both quantitative defects of VWF, whereas type 2 is characterized by a qualitative or functional defect in VWF, despite significant plasma levels of this protein.7 8
Among type 2 VWD, a subtype (2A) is recognized in which larger VWF multimers are completely lacking in the plasma.7,8 The thrombogenic activities of VWF, which have so far been evaluated based mainly on the platelet aggregability in the presence of ristocetin, are strictly dependent on its multimeric structure, so that bleeding symptoms in type 2A VWD are clearly explained by defective ristocetin-induced platelet aggregation (RIPA) in a classical platelet aggregometer.2,8 By contrast, enhanced RIPA is observed in blood of subtype 2B patients due to the increased or spontaneous binding of their VWF to the platelet membrane receptor glycoprotein (GP) Ib α.2,8 Interestingly, a similar phenomenon occurs in platelet aggregation induced by experimental high shear stress, where the interaction of soluble VWF with both GP Ib α and integrin αIIbβ3 plays an essential role.9,10 In these studies, VWF-dependent platelet aggregation that absolutely required high shear stress (at least 90 dyne/cm2) in healthy controls appeared at relatively low shear stress (20 dyne/cm2 or less) in type 2B VWD, whereas no appreciable aggregation occurred under high shear stress conditions in type 2A VWD.9 10 Although the lack of in vitro platelet aggregation in type 2A is well correlated with its in vivo bleeding tendency, the paradoxical enhanced platelet aggregation in vitro in type 2B VWD cannot explain the pathogenic bleeding mechanisms in this patient subset.
To clarify the mechanisms that underlie the bleeding tendency in type 2A and 2B VWD, we analyzed the real-time process of mural thrombus generation, from initial platelet adhesion to spatial thrombus development, on a type I collagen–coated glass surface under physiologic flow conditions with various shear rates in blood of these VWD patients. Consistent with the clinical bleeding symptoms and defective RIPA, the observation by epifluorescence microscopy confirmed that thrombus generation in type 2A VWD blood was highly impaired under flow conditions of high shear rates. This impairment became more prominent with increasing shear rates, indicating the critical shear-dependent functions of larger VWF multimers under flowing blood. In type 2B VWD, thrombus generation under high shear was less defective compared with type 2A. However, when the height and volume of type 2B thrombi finally generated were analyzed in detail by confocal laser scanning microscopy (CLSM), the spatial development of mural thrombi was significantly reduced, even if initial platelet adhesion occurred normally, providing a mechanism to explain the bleeding tendency in these patients.
Patients, materials, and methods
Materials
The specific thrombin inhibitor argatroban (MD-805) was supplied by Mitsubishi Chemical (Tokyo, Japan); type I acid-insoluble collagen fibrils from bovine Achilles tendon and the fluorescent dye mepacrine (quinacrine dihydrochloride) were purchased from Sigma-Aldrich (Tokyo, Japan); FluoroLink MAb Cy2 and Cy3 labeling kits were from Amersham Pharmacia Biotech (Tokyo, Japan). Human native VWF containing the highest molecular weight multimers, as judged by sodium dodecyl sulfate (SDS)–1.5% agarose gel electrophoresis,11 was purified from cryoprecipitates as described.12-14 An anti-VWF monoclonal antibody LJ-2.2.9 was a kind gift from Dr Z. M. Ruggeri (The Scripps Research Institute, La Jolla, CA). This antibody recognizes the carboxy-terminal region of the VWF subunit and does not affect any adhesive function of VWF.12,14,15 Antifibrinogen monoclonal antibody IF-1 recognizes the fibrinogen conformation in the presence of calcium ions.16 Both monoclonal antibodies were used as F(ab′)2 fragments prepared by pepsin digestion of purified immunoglobulin G (IgG) at low pH and the collection of flow-through fractions in a protein A–Sepharose (Pharmacia-LKB Japan, Tokyo, Japan) column.12 15
Patient profiles
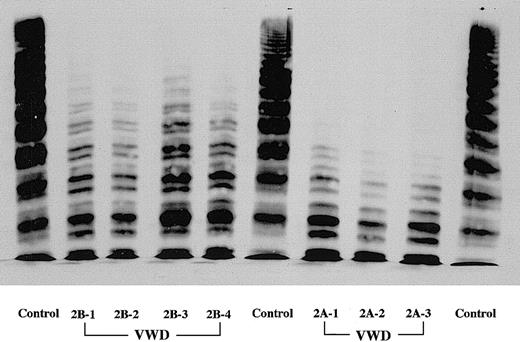
Three type 2A VWD patients from independent families completely lacked the larger and intermediate VWF multimers in plasma, as judged by SDS–1.5% agarose gel electrophoresis (Figure1). Plasma VWF antigen levels of these patients were 35% to 86% of normal (Table1). All type 2A patients exhibited complete lack of RIPA (1.2 mg/mL). Four type 2B VWD patients from 2 independent families showed a selective lack of larger VWF multimers with 34% to 42% of VWF antigen in plasma. Compared with type 2A patients, the lack of larger VWF multimers in all type 2B patients was less severe, with intermediate forms retained (Figure 1). All type 2B patients were confirmed to exhibit the enhanced RIPA—that is, platelet aggregation could be observed in the presence of 0.5 mg/mL ristocetin. DNA analyses confirmed a common type 2B mutation in 1 of 2 gene alleles in all patients, corresponding to an Arg545Cys (patients 1 and 2) and a Val553Met (patients 3 and 4) substitution in the A1 domain of the VWF subunit. Thus, these type 2B patients are heterozygous for the disease (Table 1). Although type 2B patients occasionally exhibit intermittent thrombocytopenia, platelet counts and plasma fibrinogen in all type 2A and 2B patients in this study were within the normal range. Five nonsmoking healthy volunteers, who were not taking any medications for the previous 2 weeks and whose platelet counts were all between 150 × 109/L and 300 × 109/L (150 × 103/μL to 300 × 103/μL), were included in this study as healthy controls. No blood donors, including patients, showed significant anemia, with hematocrit values always more than 35%.
Multimer patterns of plasma VWF in VWD patients.
SDS–1.5% agarose gel electrophoresis was performed in all VWD patients studied in this work. Three type 2A VWD patients completely lack the larger and intermediate VWF multimers in plasma. Compared with type 2A patients, the lack of larger VWF multimers in all type 2B patients was less severe, with intermediate forms retained.
Multimer patterns of plasma VWF in VWD patients.
SDS–1.5% agarose gel electrophoresis was performed in all VWD patients studied in this work. Three type 2A VWD patients completely lack the larger and intermediate VWF multimers in plasma. Compared with type 2A patients, the lack of larger VWF multimers in all type 2B patients was less severe, with intermediate forms retained.
VWF parameters in type 2A and 2B VWD patients
| Patient . | VWF:antigen, % . | R:cof,*% . | Gene analysis . |
|---|---|---|---|
| 2A-1 | 86 | 9.6 | ND |
| -2 | 42 | 4.8 | ND |
| -3 | 35 | < 4.8 | ND |
| 2B-1 | 42 | 4.8 | Arg545Cys |
| -2* | 34 | 4.8 | Arg545Cys |
| -3† | 36 | 12 | Val553Met |
| -4† | 42 | 12 | Val553Met |
| Patient . | VWF:antigen, % . | R:cof,*% . | Gene analysis . |
|---|---|---|---|
| 2A-1 | 86 | 9.6 | ND |
| -2 | 42 | 4.8 | ND |
| -3 | 35 | < 4.8 | ND |
| 2B-1 | 42 | 4.8 | Arg545Cys |
| -2* | 34 | 4.8 | Arg545Cys |
| -3† | 36 | 12 | Val553Met |
| -4† | 42 | 12 | Val553Met |
R:cof indicates ristocetin cofactor activity of VWF; ND, not determined.
Patient 2B-2 is the son of 2B-1.
Patients 2B-3 and 2B-4 are brothers.
Blood collection and platelet labeling
This work was approved by the institutional review board of Nara Medical University Hospital. After informed consent, blood was collected from patients and healthy volunteers using argatroban (final concentration 240 μmol/L) as an anticoagulant.17-19 Anticoagulated whole blood was kept at 37°C and used in perfusion studies within 30 minutes after blood collection. Mepacrine (final concentration 10 μmol/L) was added to the blood prior to perfusion to label platelets, allowing visualization of platelet-surface interaction with epifluorescence videomicroscopy.
Flow chamber and epifluorescence videomicroscopy
Type I collagen–coated glass coverslips were prepared as described17-19 and placed in a parallel plate flow chamber that varies shear rate in a linear manner.17-20 In brief, this chamber was designed to reproduce shear rates starting from a predetermined maximum value at the entrance and falling to zero at the exit.21 The chamber was assembled and mounted on a microscope (BX60, Olympus, Tokyo, Japan) equipped with epifluorescent illumination (BX-FLA, Olympus) and a CCD camera system (U-VPT-N, Olympus) as described.17-19 Whole blood containing mepacrine-labeled platelets was aspirated through the chamber by a syringe pump (model CFV-3200, Nihon Kohden, Tokyo, Japan) at a constant flow in a 37°C thermostatic air bath (model UI-50, Iuchi, Osaka, Japan).19-22 Unless otherwise indicated, the entire thrombus generation process, from initial platelet-surface interaction to platelet aggregate accumulation on the surface, was observed in real time at positions of the flow chamber corresponding to 50 s−1 and 1500 s−1 and recorded with a videocassette recorder (Hi8 VIEWCAM, Sharp, Osaka, Japan).19-22 The wall shear rate of 50 s−1 or 1500 s−1 is considered to be a typical low or high shear flow, respectively.17,19 21 In some experiments, perfusion with higher wall shear rates was also performed by changing flow rate.
Time-course images of thrombus formation in videotape were digitized by a frame grabber (DIG98, DITECT, Tokyo, Japan) and subjected to computer-assisted analysis with an image processing application (Win ROOF, Mitani, Fukui, Japan).
Confocal laser scanning microscopy (CLSM)
Platelet thrombi generated on a collagen-coated surface were fixed at 7 minutes after the initial platelet-surface interaction by gradual exchange of whole blood with fixation buffer (0.1 M phosphate-buffered saline [PBS] containing 4% paraformaldehyde, pH 7.4) at 37°C under continuous flow for 10 minutes. In preliminary experiments, the entire fixation process in perfusion of blood containing mepacrine-labeled platelets was observed in real time by epifluorescence microscopy to confirm fixation of the generated platelet thrombi without collapse or detachment from the collagen surface. After fixation, the perfusion chamber was disassembled, and a coverslip was rinsed 3 times with PBS, mounted in Dako fluorescent mounting medium (DAKO, Carpinteria, CA) as an antifade medium, and viewed by CLSM (MRC-600, Nippon Bio-Rad Laboratories, Tokyo, Japan). Mepacrine fluorescence corresponding to platelets was examined at an excitation wavelength of 488 nm with a barrier filter at 500 nm. Specimens were viewed at 1-μm intervals from the collagen surface to a height of 60 μm from the surface. Each image was digitized after background subtraction to calculate thrombus height and volume in a frame with the assistance of Win ROOF software.
Fluorescence labeling of monoclonal antibodies
The F(ab′)2 fractions of anti-VWF or antifibrinogen IgG were concentrated to 1 to 3 mg/mL, dialyzed with 20 nmol/L phosphate-buffered saline (pH 7.35), and labeled using FluoroLink MAb Cy2 or Cy3 labeling kit according to the manufacturer's protocol. Cy2 and Cy3 produce a green and orange signal, respectively. These fluorescence-labeled F(ab′)2 fractions were stored at 4°C until used.
Evaluation of VWF and fibrinogen distribution within thrombi
In some experiments when platelets were not labeled with mepacrine, a coverslip fixed with paraformaldehyde was double-stained with 100 μL of a solution mixture of Cy2-labeled anti-VWF and Cy3-labeled antifibrinogen F(ab′)2 (each at 0.24 μg mL) for 2 hours at 37°C and viewed with CLSM. These conditions for immunohistochemical staining were determined in preliminary experiments that confirmed the sufficient infiltration of fluorescence-labeled antibodies into thrombi—that is, the portions furthest from the outside surface were stained. Cy2 fluorescence (green) was examined at an excitation wavelength of 488 nm with a barrier filter at 500 nm, whereas Cy3 fluorescence (orange) was at an excitation wavelength of 529 nm with a barrier filter at 550 nm to assess the distribution of fibrinogen and VWF within thrombi.
Results
Thrombus generation on a collagen surface in type 2A VWD under flow conditions
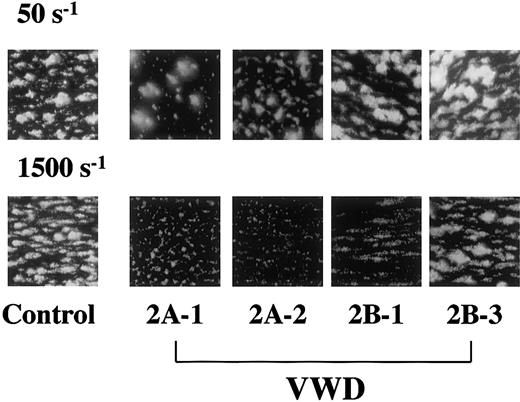
In perfusion of healthy control blood, platelets increasingly accumulate on a collagen surface as a function of time when observed with an epifluorescence microscopy in real time under both low and high shear rate conditions.19 The extent of thrombus generation in all type 2A patients examined here was comparable to that of a healthy control under a low shear rate (50 s−1), whereas thrombus generation was significantly reduced in all type 2A patients examined under a typical high shear rate condition (1500 s−1), as judged by visual recognition (Figure2) and time-course changes in surface coverage (Table 2). Application of various shear rates to a patient's blood confirmed the comparable surface coverage of thrombi finally generated in a type 2A patient and in a healthy control at lower shear rates (340 s−1 or less) as well as the apparent reduction of thrombus generation at shear rates above 630 s−1 (Figure3). These observations clearly indicate the critical involvement of larger VWF multimers in mural thrombus generation on a collagen surface under flow conditions with high shear rates.
Thrombus generation on a collagen surface in a healthy control and type 2A and 2B VWD patients at a typical low or high shear rate.
Whole blood containing mepacrine-labeled platelets was perfused through a collagen-coated glass surface at a low (50 s−1) or high (1500 s−1) shear rate condition. Images, displayed as accumulated mepacrine fluorescence, were obtained at 7 minutes after the beginning of platelet-surface interaction during perfusion. Two patients were selected as representative of each VWD subtype (Tables 1and 2), and control images (control-2 in Table 2) are representative of 5 independent perfusions using blood from 5 individual donors (original magnifications × 400). At a low shear rate, platelet thrombi in all VWD patients were generated to an extent comparable to that in healthy controls. In contrast, thrombus generation in type 2A patients was apparently reduced compared with a healthy control at a high shear rate. The extent of thrombus generation in type 2B patients under high shear was heterogeneous, ranging from normal to significantly reduced (Table 2).
Thrombus generation on a collagen surface in a healthy control and type 2A and 2B VWD patients at a typical low or high shear rate.
Whole blood containing mepacrine-labeled platelets was perfused through a collagen-coated glass surface at a low (50 s−1) or high (1500 s−1) shear rate condition. Images, displayed as accumulated mepacrine fluorescence, were obtained at 7 minutes after the beginning of platelet-surface interaction during perfusion. Two patients were selected as representative of each VWD subtype (Tables 1and 2), and control images (control-2 in Table 2) are representative of 5 independent perfusions using blood from 5 individual donors (original magnifications × 400). At a low shear rate, platelet thrombi in all VWD patients were generated to an extent comparable to that in healthy controls. In contrast, thrombus generation in type 2A patients was apparently reduced compared with a healthy control at a high shear rate. The extent of thrombus generation in type 2B patients under high shear was heterogeneous, ranging from normal to significantly reduced (Table 2).
Time-course changes in surface coverage in perfusion of blood from type 2A and 2B VWD patients and from controls under a high shear rate (1500 s−1)
| Patient . | 1-minute perfusion, % . | 7-minute perfusion, % . |
|---|---|---|
| 2A-1 | 0.9 ± 0.3 | 10.2 ± 2.4* |
| -2 | 0.2 ± 0.05 | 7.6 ± 3.0* |
| -3 | 0.1 ± 0.05 | 2.6 ± 0.3* |
| 2B-1 | 0.2 ± 0.15 | 8.5 ± 3.2* |
| -2 | 0.1 ± 0.05 | 12.7 ± 1.5* |
| -3 | 0.5 ± 0.1 | 38.2 ± 5.6 |
| -4 | 0.7 ± 0.2 | 43.6 ± 5.5 |
| Control-1 | 0.6 ± 0.25 | 37.3 ± 6.6 |
| -2 | 1.1 ± 0.3 | 42.4 ± 8.5 |
| -3 | 0.8 ± 0.2 | 48.3 ± 8.2 |
| -4 | 0.5 ± 0.15 | 40.1 ± 2.2 |
| -5 | 1.5 ± 0.4 | 42.7 ± 3.4 |
| Patient . | 1-minute perfusion, % . | 7-minute perfusion, % . |
|---|---|---|
| 2A-1 | 0.9 ± 0.3 | 10.2 ± 2.4* |
| -2 | 0.2 ± 0.05 | 7.6 ± 3.0* |
| -3 | 0.1 ± 0.05 | 2.6 ± 0.3* |
| 2B-1 | 0.2 ± 0.15 | 8.5 ± 3.2* |
| -2 | 0.1 ± 0.05 | 12.7 ± 1.5* |
| -3 | 0.5 ± 0.1 | 38.2 ± 5.6 |
| -4 | 0.7 ± 0.2 | 43.6 ± 5.5 |
| Control-1 | 0.6 ± 0.25 | 37.3 ± 6.6 |
| -2 | 1.1 ± 0.3 | 42.4 ± 8.5 |
| -3 | 0.8 ± 0.2 | 48.3 ± 8.2 |
| -4 | 0.5 ± 0.15 | 40.1 ± 2.2 |
| -5 | 1.5 ± 0.4 | 42.7 ± 3.4 |
Whole blood containing mepacrine-labeled platelets was perfused through a collagen-coated glass surface under a shear rate of 1500 s−1. Surface coverage by thrombi within a defined area (211 × 317 μm each) was evaluated based on epifluorescence images taken at 1 and 7 minutes after the beginning of platelet-surface interaction during perfusion. Data represent the mean ± SD of 5 areas randomly selected in each single perfusion.
Statistically significant differences from controls (P< .01) by 1-way factorial analysis of variance (ANOVA) and the Scheffé method as described in the legend to Figure 4.
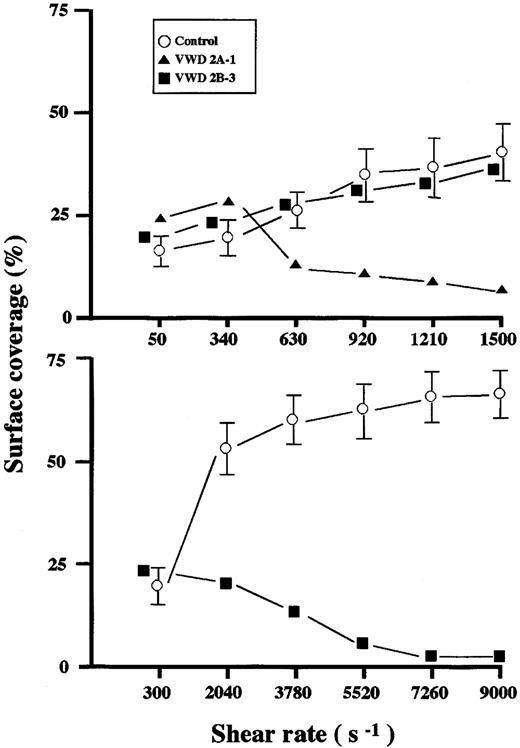
Shear dependency of thrombus generation on a collagen surface in healthy controls and a type 2A and 2B VWD patient.
Whole blood from a healthy control (control-2 in Table 2), a type 2A (2A-1 in Tables 1 and 2), and a type 2B (2B-3 in Tables 1 and 2) VWD patient was perfused for 7 minutes with various shear rates. Each data point represents the mean (± SD in the case of a healthy control) surface coverage value in 5 areas (211 × 317 μm each) randomly selected in each single perfusion. Thrombus generation in a type 2A patient was reduced at shear rates above 630 s−1 (top panel), whereas that in a type 2B patient was reduced at a shear rate above 2040 s−1 (bottom panel).
Shear dependency of thrombus generation on a collagen surface in healthy controls and a type 2A and 2B VWD patient.
Whole blood from a healthy control (control-2 in Table 2), a type 2A (2A-1 in Tables 1 and 2), and a type 2B (2B-3 in Tables 1 and 2) VWD patient was perfused for 7 minutes with various shear rates. Each data point represents the mean (± SD in the case of a healthy control) surface coverage value in 5 areas (211 × 317 μm each) randomly selected in each single perfusion. Thrombus generation in a type 2A patient was reduced at shear rates above 630 s−1 (top panel), whereas that in a type 2B patient was reduced at a shear rate above 2040 s−1 (bottom panel).
Thrombus generation on a collagen surface in type 2B VWD under flow conditions
Thrombus generation in type 2B VWD patients, like that in type 2A, was normal at a low shear rate (50 s−1) (Figure 2) but variable under a typical high shear rate (1500 s−1)—that is, significantly reduced in 2 patients (2B-1 and -2) and normal in the other 2 patients (2B-3 and -4) as judged by visual recognition (Figure2) and surface coverage (Table 2). However, when higher shear rates were applied, thrombus generation in a type 2B patient whose thrombus generation was comparable to normal at a shear rate of 1500 s−1 was apparently reduced at shear rates above 2040 s−1, although it was more resistant to increasing shear rates than that in a type 2A patient (Figure 3).
Confocal laser scanning microscopy (CLSM) of thrombi generated at a shear rate of 1500 s−1 in type 2B VWD patients
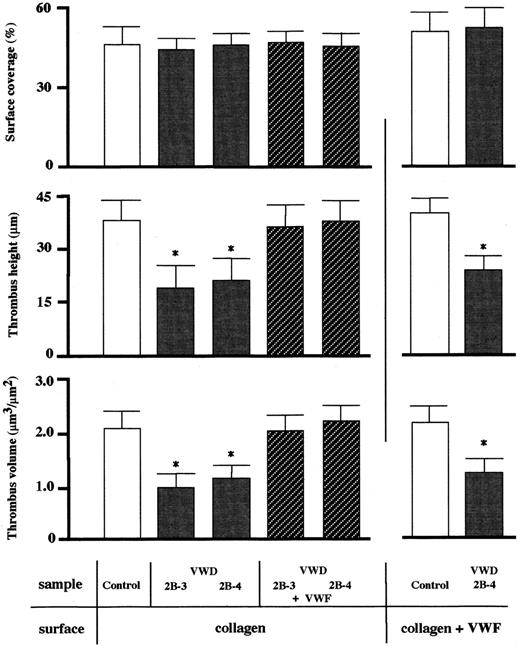
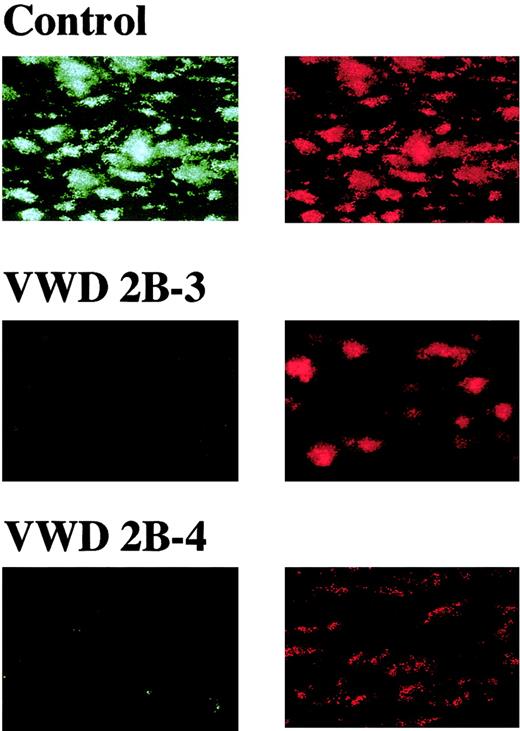
Surface coverage by thrombi as assessed by epifluorescence microscopy is a useful indicator of the extent of platelet adhesion and aggregation on a collagen surface but is assumed to be insufficient for detailed evaluation of spatial thrombus development. Indeed, reanalysis by CLSM of thrombi generated at a shear rate of 1500 s−1in type 2B VWD patients (2B-3, -4) whose thrombus generation at this shear rate was initially judged as normal by epifluorescence microscopy (Figure 2 and Table 2) showed that the height and volume of type 2B thrombi were about half those of control thrombi, although surface coverage was comparable to normal (Figure4). Thus, 3-dimensional thrombus development was impaired in these type 2B patients even at a shear rate of 1500 s−1, whereas 2-dimensional expansion occurred normally. Indeed, the significant reduction in spatial thrombus development in the type 2B patient was also observed on a collagen surface preincubated with purified normal VWF containing highest VWF multimers, where initial platelet adhesion must occur normally (Figure4). In contrast, addition of purified VWF to type 2B VWD blood completely reversed the defective spatial thrombus development in these patients (Figure 4). In addition, the immunohistochemical staining revealed that VWF, as compared with fibrinogen, was poorly integrated into type 2B VWD thrombi (Figure 5). These results suggest that an insufficient adhesive function of VWF, rather than a platelet insufficiency, circulating in blood of the type 2B VWD patient accounts for defective spatial thrombus development under high shear rate conditions.
CLSM analysis of thrombi generated on a collagen surface in a healthy control and type 2B patients at a 1500 s−1shear rate.
Experimental conditions were as described in the legend to Figure 2except that only a typical high shear rate (1500 s−1) was applied. Thrombi generated at 7 minutes of perfusion of blood from a healthy control and selected type 2B VWD patients (2B-3, -4) were fixed before viewing by CLSM. Surface coverage (top panel), maximum height (middle panel), and total volume (bottom panel) of thrombi within a defined area (211 × 317 μm each) were evaluated. Data represent the mean + SD of 5 areas randomly selected in each single perfusion. One-way factorial ANOVA and the Scheffé method were used for analysis of variance and for comparisons with controls, respectively, with assistance of Stat View computer software (Abacus Concepts, Berkeley, CA). Asterisks indicate statistically significant differences from respective controls (P < .01). Statistical analyses demonstrated that the height and volume of type 2B VWD thrombi were about half those of a healthy control, whereas surface coverage was comparable to normal (left panels). The reduced thrombus height and volume in type 2B VWD were completely normalized when purified VWF (20 μg/mL) was added to type 2B VWD blood prior to perfusion. The height and volume of type 2B VWD thrombi were significantly reduced compared with a healthy control, even when the collagen-coated surface was incubated with 200 μL purified normal VWF (20 μg/mL) for 1 hour prior to perfusion (right panels).
CLSM analysis of thrombi generated on a collagen surface in a healthy control and type 2B patients at a 1500 s−1shear rate.
Experimental conditions were as described in the legend to Figure 2except that only a typical high shear rate (1500 s−1) was applied. Thrombi generated at 7 minutes of perfusion of blood from a healthy control and selected type 2B VWD patients (2B-3, -4) were fixed before viewing by CLSM. Surface coverage (top panel), maximum height (middle panel), and total volume (bottom panel) of thrombi within a defined area (211 × 317 μm each) were evaluated. Data represent the mean + SD of 5 areas randomly selected in each single perfusion. One-way factorial ANOVA and the Scheffé method were used for analysis of variance and for comparisons with controls, respectively, with assistance of Stat View computer software (Abacus Concepts, Berkeley, CA). Asterisks indicate statistically significant differences from respective controls (P < .01). Statistical analyses demonstrated that the height and volume of type 2B VWD thrombi were about half those of a healthy control, whereas surface coverage was comparable to normal (left panels). The reduced thrombus height and volume in type 2B VWD were completely normalized when purified VWF (20 μg/mL) was added to type 2B VWD blood prior to perfusion. The height and volume of type 2B VWD thrombi were significantly reduced compared with a healthy control, even when the collagen-coated surface was incubated with 200 μL purified normal VWF (20 μg/mL) for 1 hour prior to perfusion (right panels).
Distribution of VWF and fibrinogen in thrombi generated under a high shear rate.
Whole blood, without mepacrine labeling, from a healthy control or type 2B VWD patients (2B-3, -4) was perfused over a collagen surface at a high shear rate (1500 s−1 thrombi generated at 7 minutes of perfusion were fixed, double-stained with Cy2-labeled anti-VWF and Cy3-labeled antifibrinogen antibodies, and viewed by CLSM. The images displayed are cross-sections at a height of 15 μm from the collagen surface (original magnifications × 400) and are representative of 2 independent perfusions of blood from a control or patient, respectively. Unlike control thrombi, VWF is poorly stained (green, left panels), compared with fibrinogen (orange, right panels), in type 2B VWD thrombi.
Distribution of VWF and fibrinogen in thrombi generated under a high shear rate.
Whole blood, without mepacrine labeling, from a healthy control or type 2B VWD patients (2B-3, -4) was perfused over a collagen surface at a high shear rate (1500 s−1 thrombi generated at 7 minutes of perfusion were fixed, double-stained with Cy2-labeled anti-VWF and Cy3-labeled antifibrinogen antibodies, and viewed by CLSM. The images displayed are cross-sections at a height of 15 μm from the collagen surface (original magnifications × 400) and are representative of 2 independent perfusions of blood from a control or patient, respectively. Unlike control thrombi, VWF is poorly stained (green, left panels), compared with fibrinogen (orange, right panels), in type 2B VWD thrombi.
Discussion
Our experimental approach involving perfusion of whole blood from VWD patients through a flow chamber visibly reproduces the in vivo hemostatic events occurring under physiologic blood flow conditions. Thus, our findings might be more physiologically relevant than those obtained in previous closed stirring experimental systems such as a classic platelet aggregometer. Our data demonstrating the selective reduction of mural thrombus generation in type 2A VWD under high shear rates are more likely to reflect the lack of larger VWF multimers than the mild decrease of VWF antigen in these patient plasmas because type 1 VWD patients, whose VWF antigen levels in plasma are more than 30%, showed thrombus generation comparable to normal control under such flow conditions (results not shown). However, thrombus generation in type 2A under high shear rate is apparently less impaired than in type 3 VWD in which almost no platelet-surface interaction was observed in the same experimental conditions.19 Indeed, significant platelet adhesion and subsequent aggregation were confirmed in all type 2A VWD patients in the present study (Figure 2 and Table 2). In contrast, RIPA of type 2A VWD, like that in type 3 VWD, was completely defective as determined by classic platelet aggregometer. In addition, no significant improvement of the reduced thrombus generation in type 2A VWD patients could be observed by the preadsorption of collagen surface with normal multimeric VWF (results not shown). Thus, the pathogenic bleeding in type 2A VWD is assumed to be due to impaired mural thrombus growth rather than the lack of initial platelet adhesion to a thrombogenic surface typically observed in type 3 VWD. The smaller VWF multimers circulating in a type 2A patient's plasma, albeit in a slow and insufficient way, can play a role especially at the stage of initial platelet adhesion, while only highly multimerized VWF can conduct subsequent thrombus growth by contributing to local VWF density.
Impaired platelet aggregation in type 2B VWD patients has never been demonstrated so far in closed stirring experimental systems in vitro such as a classic platelet aggregometer or cone-and-plate type viscometer using platelet-rich plasma from patients.9,10,23 Indeed, our epifluorescence microscopy analysis failed to clearly demonstrate reduced thrombus generation in some type 2B VWD patients at a typical high shear rate. However, application of higher shear rates or the use of CLSM in this experimental system successfully revealed the impairment of thrombus growth in type 2B patients. Note that 2-dimensional thrombus expansion, including initial platelet adhesion, does occur in type 2B VWD (Figure2 and Table 2), consistent with the results of the previous flow study that VWF synthesized by endothelial cells from a type 2B patient normally supported platelet adhesion24 and with recent flow study results indicating that a recombinant GP Ib α–binding domain of VWF with a type 2B mutation can support platelet adhesion under high shear when immobilized on a glass surface.25 The reduction of thrombus height and volume in type 2B VWD was also observed on the collagen surface preincubated with normal multimeric VWF (Figure 4), further confirming that the defective spatial thrombus development—not the defective platelet adhesion—is an essential pathogenesis in this subtype of disease. The reason for the heterogeneous extent of reduced thrombogenicity in type 2B patients is presently unclear but most likely reflects different type 2B mutations in aberrant VWF that have diverse binding affinities for platelet GP Ib α. Although the physical condition of the patient at the time of blood collection may have affected our results, no significant anemia, thrombocytopenia, or heterogeneity in multimeric distribution of VWF was observed in any of the blood samples.
The molecular mechanisms underlying impaired thrombus generation in type 2B VWD under high shear rates appear to be more complex, and it remains unclear why an enhanced reactivity of type 2B VWF with platelet GP Ib α leads to such impairment. Unlike in type 2A VWD that is clearly understood as the complete lack of larger VWF multimers in plasma, both normal and aberrant VWF circulate in the plasma of type 2B VWD patients. In this context, it has been speculated that spontaneous binding of aberrant VWF to platelets in the normal circulation, where normal VWF never interacts with platelets, may lead to a depletion or loss of larger VWF multimers in plasma that are essential for thrombus growth under high shear rate conditions.8,23 26 This scenario is consistent, at least in part, with the present observations that the reduction of thrombus generation in type 2A VWD was more severe than that in type 2B patients whose multimeric VWF composition in plasma is less affected.
It has also been hypothesized that platelets flowing in type 2B VWD blood may not function properly at a thrombogenic surface, because the VWF binding site within GP Ibα on the patient's platelets is already occupied and blocked in solution by the spontaneous binding of aberrant VWF to GP Ibα. Indeed, a recombinant type 2B VWF that showed spontaneous binding to GP Ibα in solution was recently shown to significantly inhibit thrombus generation under flow conditions in normal blood.26 Although this mechanism might be involved in the bleeding tendency in type 2B VWD, our findings that addition of normal multimeric VWF to type 2B blood completely corrects impaired thrombus formation (Figure 4) suggest that blockage of the GP Ibα receptor by aberrant VWF does not play a major role. Instead, our results are consistent with the clinical experience that infusion of VWF products is effective in managing hemostasis of type 2B VWD patients.8 At present, it appears that VWF in a patient's blood, which contains normal and aberrant molecules and lacks larger multimers, plays only a limited role at the stage of spatial thrombus development, as evidenced by the poor distribution of VWF within the type 2B VWD thrombus mass (Figure 5).
Together, our results confirm the shear rate–dependent reduction of thrombus generation in both type 2A and 2B VWD under physiologic flow conditions, a finding that has never been clearly demonstrable in previous in vitro soluble-phase platelet aggregation assays. This reduction may explain the bleeding tendency seen in these diseases and points to a critical function for larger VWF multimers at a stage of spatial growth of mural thrombi under high shear rate conditions, essential for proper hemostasis.
We thank M. Hoffman for editorial assistance.
Prepublished online as Blood First Edition Paper, September 12, 2002; DOI 10.1182/blood-2002-03-0944.
Supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan to M.S. (nos. 11670780 and 13671074).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mitsuhiko Sugimoto, Department of Pediatrics, Nara Medical University, 840 Shijo-cho, Kashihara, Nara 634-8522, Japan; e-mail: sugi-ped@naramed-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal