Clinical studies have shown that the tyrosine kinase inhibitor STI571 effectively controls BCR-ABL–positive chronic myelogenous leukemia (CML). However, disease progression while on STI571 therapy has been reported, suggesting de novo or intrinsic resistance to BCR-ABL–targeted therapy. To investigate possible mediators of acquired STI571 resistance, K562 cells resistant to 5 μM STI571 (K562-R) were cloned and compared to the parental cell population. K562-R cells had reduced BCR-ABL expression and limited activation of BCR-ABL signaling cascades (Stat 5, CrkL, MAPK). STI571 failed to activate caspase cascades or to suppress expression of survival genes (bcl-xL) in resistant cells. Gene sequencing and tyrosine kinase activity measurements demonstrated that K562-R cells retained wild-type and active BCR-ABL tyrosine kinase that was inhibitable by in vitro incubation with STI571, suggesting that BCR-ABL was not coupled to proliferation or survival of K562-R cells. The src-related kinase LYN was highly overexpressed and activated in K562-R cells, and its inhibition reduced proliferation and survival of K562-R cells while having limited effects of K562 cells. Specimens taken from patients with advanced CML that progressed on STI571 therapy also were analyzed for LYN kinase expression, and they were found to be elevated to a level similar to that of K562-R cells. Comparison of samples from patients taken prior to and following STI571 failure suggested that expression and/or activation of LYN/HCK occurs during disease progression. Together, these results suggest that acquired STI571 resistance may be associated with BCR-ABL independence and mediated in part through overexpression of other tyrosine kinases.
Introduction
Cytogenetic abnormalities are common in adult-onset leukemias, and much attention has been focused on understanding both the cause and consequence of specific alterations.1 One of the first and most highly investigated cytogenetic changes is the 9:22 reciprocal chromosomal translocation in chronic myelogenous and, to a lesser extent, acute lymphocytic leukemias.1,2 Translocation places the c-abl gene under the transcriptional control of the bcr locus, allowing expression of a hybrid protein encoded by 1 to 3 exons of the bcr gene and all but the first exon of c-abl.3 This chimeric BCR-ABL protein (p190 or p210) expresses intrinsic tyrosine kinase activity with altered compartmentalization and distinctions in substrate accessible when compared to the predominantly nuclear c-abl protein.4 Tyrosine kinase activity is essential for the transforming function of BCR-ABL, and expression of BCR-ABL in stem cells of immune-deficient mice results in altered hematopoiesis resembling human leukemialike disorders.5 6 These observations support a role for BCR-ABL in early leukemogenesis and as a specific target for therapeutic intervention in chronic myelogenous leukemia (CML).
BCR-ABL expression alters many signaling pathways that increase cell survival and cell cycle progression.3,7 Many of these pathways are used by cytokines that regulate hematopoiesis, and constitutive enforcement of these cascades by BCR-ABL prolongs survival and provides a proliferative advantage early in leukemogenesis.8 Altered survival and cell cycle regulation may promote additional chromosomal alterations and mutations that parallel or amplify BCR-ABL transformation. These changes may lead to acceleration of the disease and play a role in the aggressive nature of late-stage CML. Although many changes have been described in late-stage disease, some evidence suggests that additional tyrosine kinases that function downstream of BCR-ABL or are activated in leukemic blasts (LYN, HCK) contribute to late-stage disease.9 10
STI571 (Gleevec, imatinib mesylate; Novartis AG, Basel, Switzerland) is a tyrosine kinase inhibitor, active against BCR-ABL and other specific kinase targets.11,12 The drug has effective clinical activity in CML and other BCR-ABL (+) leukemias and has recently been approved by the Food and Drug Administration for patients with BCR-ABL(+) leukemia. Patients recently diagnosed (< 1 year ago) or in early phases of the disease achieve early and stable hematologic remission with loss of the Philadelphia (Ph) chromosome in some patients.13 Patients with late-stage disease (accelerated phase or blast crisis) can achieve hematologic remission but frequently progress on therapy.11-13 These results suggest that although BCR-ABL expression is retained, STI571 responsiveness may be reduced. Several mechanisms have been proposed that account for loss of effective STI571 therapy in advanced disease, including pharmacologic barriers or BCR-ABL gene amplification/mutation, as suggested in recent studies.14-18 Cell model studies of minimally STI571-resistant leukemic cell clones have shown that BCR-ABL overexpression may account for loss of STI571 sensitivity, but other cell models suggest mechanisms unrelated to changes in BCR-ABL expression.14-18 More mechanistic studies of STI571 resistance are necessary to understand cellular and clinical responsiveness to STI571.
To define alternate mechanisms of STI571 resistance, K562 erythroid leukemic cells were selected for high-level resistance to STI571 (IC50 > 5 μM). Protein, signaling, and inhibitor studies suggest that these cells had become resistant to STI571 through loss of cellular dependence on BCR-ABL and not through mutations or loss of sensitivity to STI571-mediated kinase inhibition. Growth and survival in these cells was controlled by overexpression and/or activation of tyrosine kinases that are not inhibited by STI571, and analysis of clinical specimens support a role for the src-family of kinases in STI571 resistance and progressive disease. This cell model predicts that chronic BCR-ABL inhibition may promote outgrowth of BCR-ABL–independent CML cells, allowing cells to evade STI571-mediated apoptosis.
Materials and methods
Cell lines, kinase inhibitors, drugs, and antibodies
K562 and U-937 cells (provided by Dr Z. Estrov, Department of Bioimmunotherapy, M D Anderson Cancer Center) were maintained in RPMI 1640 with 10% fetal calf serum (FCS) at a density of < 107 cell/mL. K562 cells are highly sensitive to STI571-induced apoptosis (IC50 ∼0.2 μM), as previously described.19 HT-29 colon carcinoma cells were used as a control in some experiments and were maintained in DME/F12 Dulbecco modified Eagle media with 10% FCS. STI571 was kindly provided by Dr E. Buchdunger (Novartis, Basel, Switzerland) and was prepared as a 10 mM stock solution in dimethylsulfoxide (DMSO). Stock solution was diluted in cell culture media and added directly to cells with no more than 0.1% final DMSO concentration. This concentration of DMSO had no affect on signaling or apoptosis of K562 cells. Other kinase inhibitors used in these studies include PD180970, a src-family and c-abl tyrosine kinase inhibitor,20 provided by Dr Alan Kraker, Pfizer Central Research (Ann Arbor, MI). CGP-76030, a src-family kinase selective inhibitor,21,22 was kindly provided by Dr Susa Spring (Novartis AG, Basel, Switzerland) and was prepared as described for STI571 (above). PP2 kinase inhibitor23 was purchased from CalBiochem (San Diego, CA). Doxorubicin was kindly provided by Dr Waldemar Priebe (Department of Bioimmunotherapy, M D Anderson Cancer Center).
Antibodies used in these studies include poly (ADP-ribose) polymerase (PARP), phosphoMAPK, mitogen-activated protein kinase (MAPK) (Cell Signaling, Beverly, MA), phosphotyrosine, phosphoSTAT5, CrkL (Upstate Biotechnology Institute, Lake Placid, NY), c-abl8E9, c-src (Oncogene Sciences, San Diego, CA), bcl-xL, LYN, HCK, phosphoHCK (Santa Cruz Biotechnology, Santa Cruz, CA), and actin (Sigma, St Louis, MO). Polyclonal anti-STAT5 (a/b) was kindly provided by Dr Robert Kirken (University of Texas, Health Science Center, Houston, TX).
Isolation of STI571-resistant K562 cells
K562 cells were incubated with increasing concentrations of STI571 (starting at 0.2 μM), and surviving cells were collected by centrifugation and treated with 2-fold higher concentrations of STI571 (up to a concentration of 5 μM). Drug selection took place over a 6-month interval. The resistant cells were cloned by limiting dilution in the presence of 5 μM STI571. Clones surviving in the presence of STI571 were cultured in the absence of STI571 (> 3 days) and retested for STI571 sensitivity. Cells that retained STI571 resistance in the absence of drugs were characterized for distinctions to the parental population and used in this study. STI571-resistant cells are denoted as K562-R.
Apoptosis and cell survival measurement
Analysis of kinase expression and signal transduction
Protein levels of BCR-ABL, Stat 5, LYN, HCK, and phosphotyrosine were compared between parental and resistant cells by immunoblotting equal protein cell lysates (determined by bicinchoninic acid protein assay [BCA], Pierce Chemical, Rockford, IL) with specific antibodies. Antibodies against phosphorylated (activated) forms of signaling intermediates also were used to analyze changes in BCR-ABL signaling (Stat 5, MAPK) and other tyrosine kinases (phosphoHCK, HCK, LYN). CrkL was immunoprecipitated from cell lysates (see “Immune complex tyrosine kinase activity assay”), resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and immunoblotted with anti–p-Tyr to examine its tyrosine phosphorylation. The blot was stripped of primary antibody and reblotted with anti-CrkL to determine its relative expression and recovery by immunoprecipitation. All immunoblots were developed with horseradish-peroxidase–conjugated secondary antibodies (BioRad Laboratories, Hercules, CA) and enhanced chemiluminescence (ECL) reagent (Amersham Pharmacia, Arlington Heights, IL).
Immune complex tyrosine kinase activity assay
K562 and K562-R cell lysates (400 μg in lysis buffer; as described by Donato and Perez24) were incubated with 2 μg of antibody against c-abl, LYN or HCK (2 hours), and protein A/G–sepharose (40 μL; 50% slurry, 1 hour). Immune complexes were washed (with lysis buffer), and tyrosine kinase activity was measured in immune complexes by resuspension in kinase buffer consisting of 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.5, 10 mM MnCl2, 0.1 mM Na2VO4, and 10 μg enolase, as previously described.25 Kinase inhibitors (at the concentrations indicated) were preincubated with immune complexes for 30 minutes in some assays. Kinase reactions were initiated by the addition of 10 μCi (0.37 MBq) [32P]-adenosine triphosphate ([32P]-ATP) in a total volume of 50 μL and were incubated for 30 minutes at room temperature. Kinase reactions were quenched by the addition of SDS sample buffer, and after heating to 100°C for 5 minutes reactions were resolved by SDS-PAGE. Phosphoproteins were detected by autoradiography and quantitated by PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
RT-PCR amplification of BCR-ABL and sequencing of the ABL kinase domain
For bcr-abl reverse transcription–polymerase chain reaction (RT-PCR), mRNA was isolated as described below. RT-PCR reactions were performed in a 50 μL volume using SuperScript One-Step RT-PCR with Platinum Taq from Invitrogen (Carlsbad, CA). Reagents were at the following final concentrations: 1 × reaction mix, 1 μg total RNA, 0.2 μM sense primer, 0.2 μM antisense primer, 4 mM MgSO4, and 2 units RT/Platinum Taq mix. RT-PCR was performed on an MJ Research PTC-200 DNA Engine (Waltham, MA) as follows: for cDNA synthesis, 30 minutes at 55°C followed by 2 minutes at 94°C; for PCR, 40 cycles of 94°C for 15 seconds, 59°C for 30 seconds, and 72°C for 80 seconds. Reactions were run on a 1% agarose gel, and the 1.3-kbbcr-abl bands were excised, purified, and eluted in a 30 μL volume using a gel extraction kit from Qiagen (Valencia, CA). Platinum Taq DNA polymerase was used for nested PCR amplification of the abl kinase domain of the 1.3-kb bcr-abl PCR product. Reaction components were 1 × PCR buffer, 0.2 mM each dNTP, 1.5 mM MgCl2, 0.2 μM sense primer, 0.2 μM antisense), 5 μL of the eluted DNA from above, and 2.5 units of platinum Taq. PCR was performed on an MJ Research PTC-200 DNA Engine as follows: 1 cycle of 94°C for 2 minutes and 30 cycles of 94°C for 15 seconds, 56°C for 30 seconds, and 72°C for 30 seconds. Reaction products were purified as above and sequenced on a Biomeck 3700 automated DNA sequencer (Applied Biosystems, Foster City, CA). Primers were obtained from Sigma-Genosys (The Woodlands, TX), and the sequences used were as follows: forward: 5′-gaagcttctccctggcatcccgt-3′ and reverse 5′-gccaggctctcgggtgcagtcc-3′; for amplification of a 1.3-kbbcr-abl product representing the BCR-ABL junction and kinase domain. For nested PCR of the 323-bp kinase domain: forward: 5′-gcgcaacaagcccactgtctatgg-3′ and reverse 5′-gtagtccaggaggttcccgt-3′.
Lyn and BCR-ABL Northern blot
K562 cell RNA was extracted with Trizol reagent as previously described.26 For Northern blot, 15 μg total RNA was separated on a formaldehyde gel and transferred to a Schleicher and Schuell nylon membrane (Keene, NH) using standard protocols. The membrane was probed with 20 ng/μL of a biotinylated 1.3-kb bcr-abl PCR amplification product (as described in “RT-PCR amplification of BCR-ABL and sequencing of the ABL kinase domain”) or a biotinylated lyn probe using New England Biolabs' NEBlot Phototope Kit (Beverly, MA) according to the standard hybridization protocol. The template for the lyn probe was alyn insert separated from the pcDNA3-HA-lyn vector, kindly provided by Dr Seth Corey (University of Pittsburgh, Pittsburgh, PA). The probe was detected using the maximum sensitivity protocol from New England Biolabs' Phototope-Star detection kit.
LYN antisense treatment
Phosphorothioate antisense oligodeoxynucleotide LYN sequence (Lyn-AS), as previously described,27 28 and a sense oligomer representing the first 7 codons of the LYN gene (Lyn S1; complementary to Lyn-AS) were used in these studies (Sigma-Genosys). To examine cellular effects of oligomers, 20 000 cells growing in 96-well plates in RMPI 1640 media with 10% fetal bovine serum were treated with 20 μM oligomer for 24 hours. Cell media were supplemented with 5 μM oligomers for an additional 48 hours before cell growth and survival were estimated by MTT assays as described above. To monitor effects on LYN expression, cells were treated with 10 μM oligomer for 24 hours and supplemented with the same concentration for an additional 48 hours before lysates were prepared as described above. Equal protein (40 μg) cell lysates were resolved and immunoblotted with anti-LYN or antiactin as a protein loading control.
Analysis of LYN expression in specimens from STI571-treated CML patients
Blood samples were taken from accelerated-phase and blast-crisis CML patients prior to and during treatment with STI571 and, in some cases, during disease progression but before drug withdrawal. Single specimens from CML patients that had measurable hematologic responses to STI571 but subsequently progressed on therapy were also collected prior to withdrawal from STI571. All patients received 400 to 600 mg STI571 daily. All studies involving human subjects were approved by the Internal Review Board of M D Anderson Cancer Center, and informed consent was obtained from each patient prior to initiation of this procedure.
Briefly, fresh peripheral blood (∼ 18 mL) was overlaid onto Histopaque-1077 (Sigma) and centrifuged at 400g for 15 minutes. The cells at interphase were removed by aspiration and washed once with phosphate buffered saline. Cell preparations containing significant red blood cell contamination were subjected to treatment with ammonium chloride potassium (ACK) lysis buffer (0.154 M ammonium chloride, 0.01 M KHCO3, 0.13 μM EDTA [ethylenediaminetetraacetic acid]) for 30 minutes. Remaining cells were lysed in solubilization buffer (as described above) for 30 minutes on ice, and lysates were clarified by centrifugation at 12 000g (4°C) for 15 minutes. The supernatant fractions were retained, and protein content was measured by BCA protein dye reagent (Pierce). Next, 20 μg protein were resolved by SDS-PAGE (8% acrylamide), transferred to nitrocellulose membranes, and immunoblotted with anti-phosphoHCK, anti-HCK, or anti-LYN (Santa Cruz Biotechnology). The antigen was detected with secondary antibody and ECL reagent as described above. After primary antigen detection, the membrane was stripped and reprobed with antiactin to determine the relative protein load in each lane.
Results
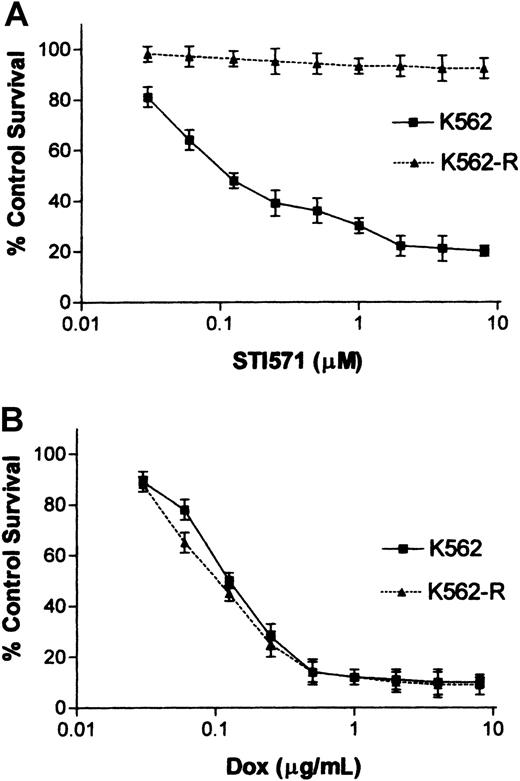
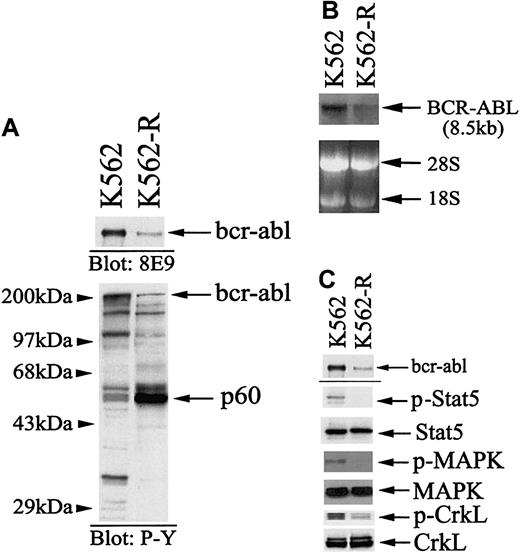
To examine potential mechanisms of acquired STI571 resistance, K562 cells were cloned in the presence of 5 μM STI571, and stable clones were compared to the parental population for STI571 responsiveness. As shown in Figure 1A, parental K562 cell responsiveness is detected at nM concentrations of STI571 (IC50 ∼ 0.1 μM), while 10 μM STI571 failed to reduce K562-R cell survival or proliferation. These cells expressed equal sensitivity to doxorubicin (Figure 1B), demonstrating defects in STI571 responsiveness that were not mediated by expression of multidrug resistance genes29 or global changes in responsiveness to an apoptotic stimulus. Because previous studies suggested that changes in BCR-ABL expression and mutations in the kinase domain correlate with STI571 responsiveness in resistant cells,14,15,17 18 BCR-ABL expression, signaling, and gene mutations were examined in K562 and K562-R cells. As shown in Figure2, BCR-ABL mRNA, protein expression, and signaling (Stat5, MAPK, CrkL) were reduced in resistant cells. Sequencing of the nested PCR product derived from a 1.3-kb BCR-ABL RT-PCR template (as described in “Materials and methods”) failed to detect mutations in the abl kinase domain (codons 225-328) of K562-R cells. To confirmed wild-type BCR-ABL tyrosine kinase activity in resistant cells, immune complex kinase assays of BCR-ABL immunoprecipitates were performed. As shown in Figure3, BCR-ABL tyrosine kinase activity was measurable in both parental and resistant cells, and incubation with STI571 reduced BCR-ABL tyrosine kinase activity and substrate (enolase) phosphorylation. This analysis suggested that STI571 resistance was not due to overexpression or mutations affecting STI571 binding in K562-R cells. Further, down-regulation and inhibition of BCR-ABL were not associated with an STI571 antiproliferative response in these cells. Although tyrosine phosphoprotein levels were reduced in resistant cells, a tyrosine phosphoprotein of ∼60 kDa was highly expressed in K562-R cells (Figure 2). Several techniques were used to identify this protein and to define its role in K562-R cells.
Effect of STI571 and doxorubicin on K562 and K562-R cell growth and survival.
Sensitivity of K562 and K562-R cells to STI571 (A) was quantitated by MTT assay as described in “Materials and methods.” Cells (in 96-well plates) were incubated with the indicated concentration of STI571 for 24 hours before analysis by MTT assay. Longer incubation intervals increased STI571 cytotoxicity in K562 cells but did not affect K562-R responsiveness (data not shown). The results represent the average ± SEM of 4 determinations and are compared to control untreated cells (set at 100% survival). (B) Doxorubicin sensitivity was analyzed after 24 hours' incubation with the indicated concentration of drug. Cell survival was estimated by MTT assays as described above.
Effect of STI571 and doxorubicin on K562 and K562-R cell growth and survival.
Sensitivity of K562 and K562-R cells to STI571 (A) was quantitated by MTT assay as described in “Materials and methods.” Cells (in 96-well plates) were incubated with the indicated concentration of STI571 for 24 hours before analysis by MTT assay. Longer incubation intervals increased STI571 cytotoxicity in K562 cells but did not affect K562-R responsiveness (data not shown). The results represent the average ± SEM of 4 determinations and are compared to control untreated cells (set at 100% survival). (B) Doxorubicin sensitivity was analyzed after 24 hours' incubation with the indicated concentration of drug. Cell survival was estimated by MTT assays as described above.
BCR-ABL expression, tyrosine phosphorylation, and signaling in K562 and K562-R cells.
(A) BCR-ABL and protein tyrosine phosphorylation levels were analyzed by immunoblotting equal protein (30 μg) cell lysates. For analysis of BCR-ABL protein expression (top), cell lysates (300 μg) were immunoprecipitated with anti-bcr (2 μg) prior to immunoblotting with anti-abl8E9. Relative migration of protein standards is shown at left. The most notable distinction between cell lines was expression of a 60-kDa tyrosine phosphoprotein (p60). (B) Northern blot analysis of BCR-ABL mRNA expression in K562 and K562-R cells. Total RNA (20 μg) from K562 and K562-R cells was blotted with a 1.3-kb BCR-ABL probe as described in “Materials and methods.” The extent of hybridization with probe is shown (top), and the migration of 28S and 18S RNA is shown at the bottom. (C) 30 μg protein from K562 and K562-R cells were immunoblotted with anti-abl (top), phosphoStat5 (p-Stat5), Stat5, phosphoMAPK (p-MAPK), or MAPK. CrkL phosphorylation (p-CrkL) was determined by immunoprecipitation from cell lysates (300 μg) and blotting with antiphosphotyrosine (4G10; Upstate Biotechnology Institute). CrkL levels were determined by stripping the membrane and reprobing with anti-CrkL.
BCR-ABL expression, tyrosine phosphorylation, and signaling in K562 and K562-R cells.
(A) BCR-ABL and protein tyrosine phosphorylation levels were analyzed by immunoblotting equal protein (30 μg) cell lysates. For analysis of BCR-ABL protein expression (top), cell lysates (300 μg) were immunoprecipitated with anti-bcr (2 μg) prior to immunoblotting with anti-abl8E9. Relative migration of protein standards is shown at left. The most notable distinction between cell lines was expression of a 60-kDa tyrosine phosphoprotein (p60). (B) Northern blot analysis of BCR-ABL mRNA expression in K562 and K562-R cells. Total RNA (20 μg) from K562 and K562-R cells was blotted with a 1.3-kb BCR-ABL probe as described in “Materials and methods.” The extent of hybridization with probe is shown (top), and the migration of 28S and 18S RNA is shown at the bottom. (C) 30 μg protein from K562 and K562-R cells were immunoblotted with anti-abl (top), phosphoStat5 (p-Stat5), Stat5, phosphoMAPK (p-MAPK), or MAPK. CrkL phosphorylation (p-CrkL) was determined by immunoprecipitation from cell lysates (300 μg) and blotting with antiphosphotyrosine (4G10; Upstate Biotechnology Institute). CrkL levels were determined by stripping the membrane and reprobing with anti-CrkL.
BCR-ABL kinase activity in K562 and K562-R cells.
BCR-ABL was immunoprecipitated from cell lysates (400 μg) with anti-abl, washed extensively, resuspended in kinase buffer, and incubated with 500 nM STI571 or buffer alone (control) for 30 minutes. Kinase reactions were initiated by the addition of 10 μCi (0.37 MBq) [32P]-ATP and enolase. After 30 minutes at 25°C, reactions were quenched with the addition of 2 × sample buffer, and phosphoproteins were resolved by SDS-PAGE. Tyrosine phosphoproteins were detected by autoradiography. Autophosphorylation of BCR-ABL and phosphorylation of enolase are shown. Similar results were obtained using anti-bcr for immunoprecipitation (data not shown).
BCR-ABL kinase activity in K562 and K562-R cells.
BCR-ABL was immunoprecipitated from cell lysates (400 μg) with anti-abl, washed extensively, resuspended in kinase buffer, and incubated with 500 nM STI571 or buffer alone (control) for 30 minutes. Kinase reactions were initiated by the addition of 10 μCi (0.37 MBq) [32P]-ATP and enolase. After 30 minutes at 25°C, reactions were quenched with the addition of 2 × sample buffer, and phosphoproteins were resolved by SDS-PAGE. Tyrosine phosphoproteins were detected by autoradiography. Autophosphorylation of BCR-ABL and phosphorylation of enolase are shown. Similar results were obtained using anti-bcr for immunoprecipitation (data not shown).
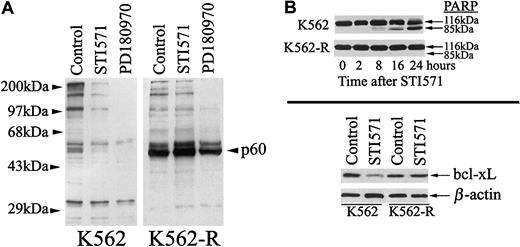
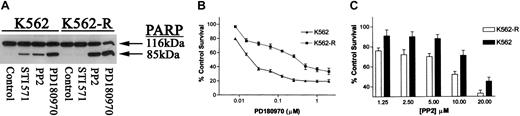
As previously described, STI571 induces apoptosis through inhibition of BCR-ABL–mediated tyrosine phosphorylation, changes in survival gene expression, and activation of caspase cascades.19 As shown in Figure 4, STI571 reduced tyrosine phosphorylation and bcl-xL expression, and it induced PARP cleavage in K562 cells but failed to affect these changes in K562-R cells.19 However, a tyrosine kinase inhibitor with reported activity against both src-family and abl kinases (PD180970) reduced tyrosine phosphorylation in both K562 and K562-R cells.20 30 Tyrosine phosphorylation of the p60 phosphoprotein was partially reduced by PD180970 in K562-R cells (Figure 4), suggesting a relationship to src-family kinases or kinase substrates. PD180970 induced PARP cleavage and growth inhibition in both STI571-sensitive and -resistant K562 cells (Figure 5A-B), and inhibitors with specificity for src kinases (PP2) induced greater antiproliferative and apoptotic effects on K562-R cells (Figure 5C). These results suggested that expression or activation of a src-family kinase in K562-R plays a role in STI571 resistance.
Effect of STI571 on tyrosine phosphorylation, PARP proteolysis, and bcl-xL expression in K562 and K562-R cells.
(A) K562 (left) or K562-R cells (right) were treated with STI571 or PD180970 (1 μM) for 30 minutes before equal protein cell lysates (30 μg) were analyzed for tyrosine phosphorylation by immunoblotting. (B, top) K562 and K562-R cells were treated with 5 μM STI571 for the interval noted prior to analysis of PARP cleavage by immunoblotting as a measure of activation of caspase cascades. Intact (116 kDa) and cleaved (85 kDa) PARP are shown. (B, bottom) Lysates from untreated (control) and STI571 treated (5 μM, 24 hours) K562 or K562-R cells were analyzed for bcl-xL levels by immunoblotting. Equal protein loads were monitored by probing β-actin on the same membrane.
Effect of STI571 on tyrosine phosphorylation, PARP proteolysis, and bcl-xL expression in K562 and K562-R cells.
(A) K562 (left) or K562-R cells (right) were treated with STI571 or PD180970 (1 μM) for 30 minutes before equal protein cell lysates (30 μg) were analyzed for tyrosine phosphorylation by immunoblotting. (B, top) K562 and K562-R cells were treated with 5 μM STI571 for the interval noted prior to analysis of PARP cleavage by immunoblotting as a measure of activation of caspase cascades. Intact (116 kDa) and cleaved (85 kDa) PARP are shown. (B, bottom) Lysates from untreated (control) and STI571 treated (5 μM, 24 hours) K562 or K562-R cells were analyzed for bcl-xL levels by immunoblotting. Equal protein loads were monitored by probing β-actin on the same membrane.
Effect of tyrosine kinase inhibitors on tyrosine phosphorylation, PARP cleavage, and survival of K562 and K562-R cells.
(A) K562 (left) and K562-R (right) cells were treated with 1 μM STI571, 10 μM PP2, or 1 μM PD180970 for 24 hours before PARP cleavage was monitored by immunoblotting. Intact and cleaved PARP are depicted. (B) K562 and K562-R cells were treated with PD180970 (at the concentration indicated) for 24 hours in a 96-well plate before survival was quantitated by MTT assay (described in “Materials and methods”) and compared to control (untreated) cells. Control cell survival was set at 100%. The results represent the average ± SEM of 4 determinations. (C) K562 and K562-R cells were incubated with the indicated concentration of PP2 for 48 hours in a 96-well plate before cell survival was estimated by MTT assay. Each data point represents the average ± SEM of 4 determinations. Untreated (control) cell survival was set at 100%.
Effect of tyrosine kinase inhibitors on tyrosine phosphorylation, PARP cleavage, and survival of K562 and K562-R cells.
(A) K562 (left) and K562-R (right) cells were treated with 1 μM STI571, 10 μM PP2, or 1 μM PD180970 for 24 hours before PARP cleavage was monitored by immunoblotting. Intact and cleaved PARP are depicted. (B) K562 and K562-R cells were treated with PD180970 (at the concentration indicated) for 24 hours in a 96-well plate before survival was quantitated by MTT assay (described in “Materials and methods”) and compared to control (untreated) cells. Control cell survival was set at 100%. The results represent the average ± SEM of 4 determinations. (C) K562 and K562-R cells were incubated with the indicated concentration of PP2 for 48 hours in a 96-well plate before cell survival was estimated by MTT assay. Each data point represents the average ± SEM of 4 determinations. Untreated (control) cell survival was set at 100%.
Previous studies demonstrated that specific members of the src kinase family, such as HCK and LYN, are expressed and activated in leukemic blasts.9 Additional studies demonstrated that these kinases (HCK, LYN) are activated by BCR-ABL kinase,10 and HCK appears to play an important role in BCR-ABL–mediated cytokine independence.31 Immunodepletion of K562-R cell lysates demonstrated that anti-LYN but not anti-HCK reduced p60 recovery in cell supernatants, confirmed by the loss of LYN from depleted lysates (Figure 6). K562-R cell LYN protein and mRNA expression were increased 4- to 8-fold, respectively, when compared to K562 cells (Figure 6B-C). As shown in Figure 6D, overexpression of LYN in K562-R cells correlated with a 7-fold increase in LYN tyrosine kinase activity when examined in immune complex kinase assays with exogenous substrate (enolase). HCK immune complexes from either K562 or K562-R cells had no detectable tyrosine kinase activity, demonstrating a specific increase in LYN expression and tyrosine kinase activity in STI571-resistant K562-R cells. From these results, we concluded that the p60 tyrosyl-phosphoprotein in STI571-resistant K562 cells is overexpressed LYN kinase.
LYN kinase expression and activity in K562 and K562-R cells.
(A) K562-R cell lysates (250 μg) were immunodepleted with anti-LYN (2 μg) or anti-HCK (2 μg) and protein A/G–sepharose, and resultant supernatants were analyzed for total tyrosine phosphoproteins (p-Tyr; left) or LYN (right). Migration of protein standards is shown on the left, and the 2 forms of LYN expressed in K562-R cells are shown on the right. (B) Twenty micrograms RNA from K562 and K562-R cells was resolved by electrophoresis, transferred to a membrane, and hybridized with a LYN probe as described in “Materials and methods.” The extent of hybridization with probe is shown (top), and the migration of 28S and 18S RNA is shown at the bottom. (C) Equal protein (30 μg) K562 and K562-R cell lysates were subjected to immunoblotting for HCK (left) and LYN (right). U-937 cell lysates were used as a positive control for HCK. Two forms of LYN (but not HCK) were detected in K562 cells, and the p53 form of LYN was overexpressed in K562-R cells. The same blots were probed with antiactin to monitor protein loading. (D) LYN or HCK immune complexes from K562 and K562-R cells were analyzed for tyrosine kinase activity in the presence of [32P]-ATP and enolase as described in “Materials and methods.” Src immune complexes from HT-29 cells were used as a positive control. Phosphorylation was detected by autoradiography on x-ray film. LYN autophosphorylation was detected in K562-R immune complexes, while exogenous substrate (enolase) phosphorylation was detected in both K562 and K562-R immune complexes. K562-R cell enolase phosphorylation was estimated (by PhosphorImager) to be approximately 7-fold higher than K562 cells.
LYN kinase expression and activity in K562 and K562-R cells.
(A) K562-R cell lysates (250 μg) were immunodepleted with anti-LYN (2 μg) or anti-HCK (2 μg) and protein A/G–sepharose, and resultant supernatants were analyzed for total tyrosine phosphoproteins (p-Tyr; left) or LYN (right). Migration of protein standards is shown on the left, and the 2 forms of LYN expressed in K562-R cells are shown on the right. (B) Twenty micrograms RNA from K562 and K562-R cells was resolved by electrophoresis, transferred to a membrane, and hybridized with a LYN probe as described in “Materials and methods.” The extent of hybridization with probe is shown (top), and the migration of 28S and 18S RNA is shown at the bottom. (C) Equal protein (30 μg) K562 and K562-R cell lysates were subjected to immunoblotting for HCK (left) and LYN (right). U-937 cell lysates were used as a positive control for HCK. Two forms of LYN (but not HCK) were detected in K562 cells, and the p53 form of LYN was overexpressed in K562-R cells. The same blots were probed with antiactin to monitor protein loading. (D) LYN or HCK immune complexes from K562 and K562-R cells were analyzed for tyrosine kinase activity in the presence of [32P]-ATP and enolase as described in “Materials and methods.” Src immune complexes from HT-29 cells were used as a positive control. Phosphorylation was detected by autoradiography on x-ray film. LYN autophosphorylation was detected in K562-R immune complexes, while exogenous substrate (enolase) phosphorylation was detected in both K562 and K562-R immune complexes. K562-R cell enolase phosphorylation was estimated (by PhosphorImager) to be approximately 7-fold higher than K562 cells.
To determine whether LYN kinase plays a role in K562-R cell growth and survival, LYN kinase activity or expression was suppressed by incubation with src-family kinase–specific inhibitor or LYN antisense, respectively. CGP-76030 (Novartis AG) is representative of a class of substituted 5,7-diphenyl-pyrrolo [2,3d]pyrimidines previously shown to inhibit src activity in vitro and to effect osteoclastic activity in animal models.21 22 The specificity of this compound was tested in BCR-ABL or LYN immune-complexes from K562 or K562-R cells. As shown in Figure 7, CGP-76030 inhibited LYN kinase activity from both K562 and K562-R cells with nM sensitivity. Dose-dependent LYN kinase inhibition was measurable in K562-R cell–derived immune complexes. However, incubation of BCR-ABL immune complexes with CGP-76030 at LYN inhibitory concentrations had limited BCR-ABL tyrosine kinase inhibitory affects. Similar results were obtained with immune complexes derived from K562 cells. These results demonstrate that CGP-76030 has greater LYN kinase inhibitory activity when compared to BCR-ABL in vitro. Distinctions in LYN kinase inhibition by CGP-76030 in immune complexes and intact cells may be due to the cellular ATP content that can reduce efficacy of kinase inhibition.
Effect of CGP-76030 on tyrosine kinase activity of BCR-ABL and LYN in vitro.
BCR-ABL or LYN was immunoprecipitated from equal protein cell lysates (300 μg) from K562 (left) or K562-R (right) cells. Immune complexes were washed extensively, resuspended in kinase buffer or CGP-76030 (at the final concentration noted), and incubated for 30 minutes before kinase reactions were initiated with the addition of enolase and 10 μCi (0.37MBq) [32P]-ATP. Phosphoproteins were detected as described in the legend to Figure 3. (Top) Migration of phosphorylated BCR-ABL and enolase is depicted. (Bottom) Migration of phospho-LYN and enolase is shown. Note: LYN autophosphorylation is not detected in immune complexes from K562 cells.
Effect of CGP-76030 on tyrosine kinase activity of BCR-ABL and LYN in vitro.
BCR-ABL or LYN was immunoprecipitated from equal protein cell lysates (300 μg) from K562 (left) or K562-R (right) cells. Immune complexes were washed extensively, resuspended in kinase buffer or CGP-76030 (at the final concentration noted), and incubated for 30 minutes before kinase reactions were initiated with the addition of enolase and 10 μCi (0.37MBq) [32P]-ATP. Phosphoproteins were detected as described in the legend to Figure 3. (Top) Migration of phosphorylated BCR-ABL and enolase is depicted. (Bottom) Migration of phospho-LYN and enolase is shown. Note: LYN autophosphorylation is not detected in immune complexes from K562 cells.
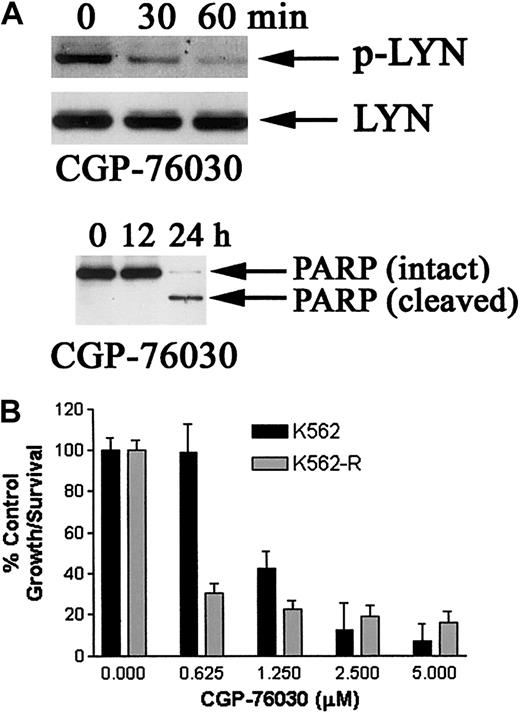
The effects of CGP-76030 on LYN kinase phosphorylation (activation), growth, and apoptosis were examined in K562-R cells. As shown in Figure8A, CGP-76030 reduced LYN tyrosine phosphorylation at a site previously shown to be involved in its autophosphorylation/activation (Y508; Porter et al32) and induced PARP proteolysis after extended inhibition of tyrosine kinase activity (> 12 hours). CGP-76030 treatment resulted in greater antiproliferative effects on K562-R cells than that measured in K562 cells. As shown in Figure 8B, CGP-76030 at 1.25 μM and lower concentrations had greater inhibitory effects on K562-R cells than parental STI571-sensitive K562 cells. Inhibitors of other protein kinases (50 μM AG-490, 50 μM LY298002) previously shown to play a role in leukemic cell growth and survival (Janus kinase 2 [Jak 2]; Wilson-Rawls et al33; phosphatidylinositol-3′-kinase, Neshat et al34) failed to induce apoptosis or reduce proliferation of K562 or K562-R cells by more than 50% (data not shown). Together with the in vitro effects of CGP-76030, these data provide evidence of a role for LYN in K562-R cell growth and survival.
Effect of src kinase inhibitor on K562 and K562-R cell survival, PARP cleavage, and LYN phosphorylation.
(A) K562-R cells were incubated with 1 μM CGP-76030 for 30 and 60 minutes (top) or longer intervals (12-24 hours, bottom), and equal protein (30 μg) cell lysates were analyzed for changes in LYN phosphorylation (top) or PARP cleavage (bottom). The blot was stripped (as previously described in Donato et al19) and reprobed with anti-LYN. (B) K562 and K562-R cells were incubated with the indicated concentration of CGP-76030 for 48 hours in a 96-well plate before cell survival was estimated by MTT assay. Each data point represents the average ± SEM of 4 determinations. Untreated (control) cell survival was set at 100%.
Effect of src kinase inhibitor on K562 and K562-R cell survival, PARP cleavage, and LYN phosphorylation.
(A) K562-R cells were incubated with 1 μM CGP-76030 for 30 and 60 minutes (top) or longer intervals (12-24 hours, bottom), and equal protein (30 μg) cell lysates were analyzed for changes in LYN phosphorylation (top) or PARP cleavage (bottom). The blot was stripped (as previously described in Donato et al19) and reprobed with anti-LYN. (B) K562 and K562-R cells were incubated with the indicated concentration of CGP-76030 for 48 hours in a 96-well plate before cell survival was estimated by MTT assay. Each data point represents the average ± SEM of 4 determinations. Untreated (control) cell survival was set at 100%.
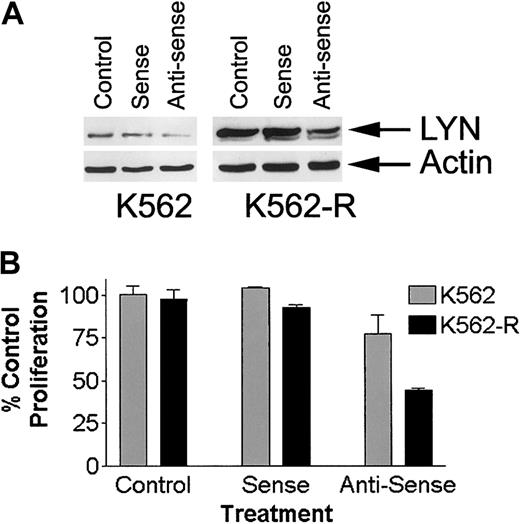
To confirm a role for LYN expression in K562-R cell growth and survival, the effects of LYN antisense (LYN-AS) oligonucleotide incubation were examined and compared to STI571-sensitive K562 cells. Incubation with LYN-AS reduced LYN expression by ∼ 50% in both K562 and K562-R cells (Figure 9), while LYN sense oligomers (LYN-S) had little effect of LYN expression. Importantly, LYN-AS significantly reduced K562-R cell growth (∼ 50% reduction) while having limited effects on K562 cells. In contrast, LYN-S had only minimal effects on growth of either population. Together, these results suggest that LYN expression plays a significant growth regulatory role in K562-R cells.
Effect of oligonucleotides on LYN expression and growth of K562 and K562-R cells.
(A) K562 and K562-R cells were incubated with sense of antisense oligonucleotides as described in “Materials and methods.” After 72 hours, cells were collected and washed, and LYN expression was determined by immunoblotting equal protein aliquots. Actin was probed on the same membrane to determine protein equivalence for each sample. (B) K562 or K562-R cells were incubated with media alone (control) or with sense or antisense oligonucleotides (72 hours total) before cell growth was estimated by MTT assay. Each data point represents the average ± SEM of 3 determinations. Untreated (control) cell survival was set at 100%.
Effect of oligonucleotides on LYN expression and growth of K562 and K562-R cells.
(A) K562 and K562-R cells were incubated with sense of antisense oligonucleotides as described in “Materials and methods.” After 72 hours, cells were collected and washed, and LYN expression was determined by immunoblotting equal protein aliquots. Actin was probed on the same membrane to determine protein equivalence for each sample. (B) K562 or K562-R cells were incubated with media alone (control) or with sense or antisense oligonucleotides (72 hours total) before cell growth was estimated by MTT assay. Each data point represents the average ± SEM of 3 determinations. Untreated (control) cell survival was set at 100%.
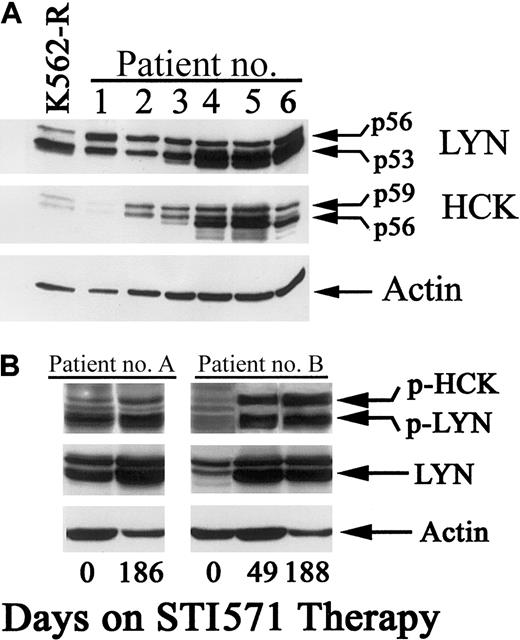
CML blast-crisis patients frequently progress on STI571 therapy, and clinical studies suggest that progression may be related to STI571 resistance.13,14,18 Resistance can be mediated by mutations within the ATP binding site in the abl kinase domain.14 However, other mechanisms may also play a role in progressive disease.16 18 Based on the K562 cell model, increased expression of src-family kinases may play a role in acquired STI571 resistance. To examine this possibility, CML samples from blast-crisis patients who progressed on STI571 (400-600 mg doses; progression within 3 months) were subjected to immunoblot and compared to K562-R cells for LYN and HCK expression. Samples were collected from relapsing patients prior to discontinuing STI571 therapy. As shown in Figure 10A, LYN kinase alone or LYN and HCK expression was detected in all samples tested. Expression levels in all samples were similar to those detected in K562-R cells (lane 1). To determine whether these changes correlated with disease progression, clinical samples were taken from blast-crisis patients prior to initiation of STI571 therapy. These samples were compared to specimens from the same patient after disease progression on therapy (49 to 186 days of STI571 therapy). Myeloblast contents varied by no more than 25% in these samples. As shown in Figure 10B, a moderate increase in LYN expression (and activation) was detected in patient A, while more significant changes in LYN (and HCK) were detected in patient B. Increased expression correlated with activation of these kinases when monitored by immunoblotting with LYN/HCK activation–specific antibody (p-HCK, p-LYN). These results suggest that src-family kinases are highly expressed and activated in CML blast-crisis patients and their increased expression correlates with progressive disease or STI571 resistance in some CML patients. Based on in vitro studies of K562 cells, the results also suggest that chronic STI571 exposure may induce expression or activation of other tyrosine kinases (that are unaffected by STI571), which contribute to BCR-ABL–independent growth and STI571 resistance.
Expression and activation of LYN and HCK kinases in STI571-resistant CML patients.
(A) Cell lysates were prepared from K562-R cells and specimens derived from several patients who failed to sustain hematologic remission with STI571 daily therapy (400-600 mg). Equal protein aliquots (20 μg) were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted for LYN or HCK with specific primary antibodies as described in “Materials and methods.” Actin levels on the same blot were used to determine protein equivalence in each sample. (B) Blood samples were collected from blast-crisis CML patients one day prior to beginning STI571 daily therapy (day 0) and at 1 or 2 points after disease progression (as noted). Specimens were prepared (as described in “Materials and methods”), protein content was estimated, and equal protein extracts were resolved by SDS-PAGE and immunoblotted with antibodies recognizing phosphorylated LYN (or HCK; p-HCK, p-LYN). The blot was stripped of primary antibody and reprobed with anti-LYN. Actin also was probed to determine protein equivalence in each sample.
Expression and activation of LYN and HCK kinases in STI571-resistant CML patients.
(A) Cell lysates were prepared from K562-R cells and specimens derived from several patients who failed to sustain hematologic remission with STI571 daily therapy (400-600 mg). Equal protein aliquots (20 μg) were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted for LYN or HCK with specific primary antibodies as described in “Materials and methods.” Actin levels on the same blot were used to determine protein equivalence in each sample. (B) Blood samples were collected from blast-crisis CML patients one day prior to beginning STI571 daily therapy (day 0) and at 1 or 2 points after disease progression (as noted). Specimens were prepared (as described in “Materials and methods”), protein content was estimated, and equal protein extracts were resolved by SDS-PAGE and immunoblotted with antibodies recognizing phosphorylated LYN (or HCK; p-HCK, p-LYN). The blot was stripped of primary antibody and reprobed with anti-LYN. Actin also was probed to determine protein equivalence in each sample.
Discussion
STI571 has demonstrated remarkable clinical activity in CML, but chronic use of this inhibitor may result in reduced efficacy, STI571 resistance, and disease progression. Clinical trials with STI571 have shown that blast-crisis patients frequently progress within 3 to 6 months of treatment, suggesting that BCR-ABL inhibition is not sufficient to prevent disease progression or to restrict clonal expansion of resistant cells.35 The underlying mechanisms of STI571 resistance and clonal expansion are not fully understood. Clinical samples from resistant patients suggest that CML cells retain expression of BCR-ABL and dependence on its downstream signaling for sustained growth and survival. BCR-ABL is overexpressed or is unaffected by STI571 due to BCR-ABL point mutations or increased drug efflux, allowing cells to escape STI571-mediated apoptosis and growth inhibition. However, cells expressing BCR-ABL mutations do not appear to predominate in CML patients because high-sensitivity procedures (nested PCR) are required to detect gene mutations.14 36-38 Even with high-sensitivity detection techniques, only a limited percentage of resistant patients express detectable BCR-ABL gene mutations, suggesting other resistance mechanism exist. The results presented in this report suggest that acquired resistance to STI571 is not associated with drug resistance mechanisms or mutations in the BCR-ABL gene but may be a consequence of expansion of BCR-ABL–independent cells.
K562-R cells express wild-type BCR-ABL based on sequence analysis (kinase domain) and in vitro kinase measurements. Immune complex kinase assays demonstrated comparable BCR-ABL tyrosine kinase activity in extracts from both K562 and K562-R cells, which was inhibited by in vitro incubation with STI571. Together with other characteristics of K562-R cells (reduced BCR-ABL expression and downstream signaling), these results suggest that BCR-ABL inhibition is not linked to STI571-resistant cell apoptosis. This is aligned with the observed loss of constitutive Stat5 or MAPK activation in K562-R, previously shown to be important contributors to BCR-ABL–mediated transformation.19 39-41 The results presented in Figures 2and 4 demonstrate that through a reduction in BCR-ABL expression and reduced signaling through chronic STI571 inhibition, K562-R cells are no longer BCR-ABL kinase or BCR-ABL signaling dependent. Expression of the src-related LYN kinase may play a role in acquisition of BCR-ABL independence. This phenotype is rare when compared to other cell models of STI571 resistance, which frequently report increased BCR-ABL expression and signaling activity. Our other cell models (Mo7e, BV-173) did not achieve complete STI571 resistance but rather a 2- to 3-fold shift in STI571 sensitivity and a corresponding increase in BCR-ABL expression (data not shown). Due to its unique characteristics, the K562-R variant was the focus of this report.
LYN kinase previously has been shown to be an important component in cytokine signal transduction in a variety of cell types and is reported to play a key role in the growth and apoptotic regulation of hematopoietic cells.42,43 In K562-R cells, overexpression and activation of LYN kinase appear to play a dominant role in their proliferation and survival. This conclusion is based on studies with kinase inhibitors, which were previously reported to target src kinases (PP2) or to inhibit both abl and src kinases (PD180970) in BCR-ABL–expressing cells.20 Growth inhibition and apoptotic studies demonstrate that targeting both kinase families overcomes apoptotic resistance in K562-R cells, while src-selective inhibitors show a consistently greater inhibitory effect on K562-R cells when compared to the parental population. A novel src inhibitory compound (CGP-76030; Missbach et al21 22) was shown to dose-dependently inhibit LYN kinase activity in vitro without significant affects on BCR-ABL (Figure 7). Selective inhibition of LYN in K562-R cells with CGP-76030 may explain their increased sensitivity to this compound when compared to K562 wild-type cells. We have been unable to detect cellular affects of CGP-76030 on LYN phosphorylation in parental K562 cells, perhaps due to its low level of expression and activation in this cell line. However, despite low expression levels, inhibition of LYN kinase activity by CGP-76030 in K562 cells only has limited affects on the growth of these cells when compared to the K562-R cell line (Figure 8). These observations suggest a shift induced through chronic STI571 exposure from BCR-ABL to LYN kinase dependence in STI571-resistant K562-R cells. In support of this conclusion, we also demonstrated that although LYN was expressed at considerably higher levels in K562-R cells, antisense oligonucleotides that suppress LYN expression had greater inhibitory effects on K562-R cells when compared to the K562 parental population (Figure 9). These results further support a role for LYN kinase in the growth and apoptotic protection of STI571-resistant K562-R cells.
Of significance to these studies, both LYN and the related HCK kinase have been shown to be activated by BCR-ABL,9,10 and some studies suggest that HCK activity is essential for cytokine independence of BCR-ABL–expressing cells.31 However, LYN and HCK kinase also may be activated through other mechanisms, as shown in studies of blasts from acute leukemias.9 LYN kinase activity appears to be essential for signal transduction of stress kinase pathways and tyrosine phosphorylation of proteins involved in DNA repair or damage recognition.44,45 Thus, LYN overexpression and activation in STI571-resistant cells are likely to promote growth and apoptotic protection through a signaling cascade that is distinct from that of BCR-ABL. These pathways are currently being examined, but initial studies have failed to detect a role for nuclear factor–κB, Jak kinases, Stat proteins (Stat 1, 3, or 5), phosphatidylinositol-3′-kinase, or Akt phosphorylation (data not shown) in LYN signaling in K562-R cells. LYN-mediated phosphorylation of BCR-ABL itself (as recently reported for the closely related HCK kinase; Schindler et al46) may also play a regulatory role in the growth and STI571 resistance of K562-R cells. HCK has been shown to phosphorylate tyrosine 393 (c-abl) in vitro, and phosphorylation of this autoregulatory loop inhibits STI571 binding and kinase inhibitory activity.46 Overexpression of LYN kinase may alter the stoichiometry of substrate phosphorylation and shift the cellular dependence for these kinases when challenged with a BCR-ABL inhibitor. As described above, LYN and HCK phosphorylation was previously shown to be mediated by BCR-ABL.9 10 In K562-R cells, these roles may be reversed.
LYN overexpression also was detected in lysates from STI571-resistant CML patients. However, other STI571-resistant mechanisms, including BCR-ABL mutations, also may exist. Prior to evaluating LYN expression in clinical specimens from these patients (progressed within 3 to 6 months of STI571 therapy), BCR-ABL T315 mutations (using the nested PCR restriction digest analysis as previously reported; Gorre et al14) were analyzed but none were detected. Further sequence analysis of the kinase domain in 4 specimens demonstrated only wild-type BCR-ABL expression (data not shown) and the possible existence of other resistance mechanisms in these patients. In 2 patients where clinical specimens were obtained prior to STI571 therapy, the effects on LYN expression and activation as patients progressed were evaluated. Changes in LYN and HCK expression and activation were detected in both patients with marked increased expression in patient B (Figure 10B). While it is unclear whether expression and/or activation of src kinases are sufficient to confer STI571 resistance in clinical samples, these results support evidence of a role for this kinase family in late-stage disease and BCR-ABL autonomous growth. Targeting both the abl and src kinases in late-stage patients may reduce disease progression and prevent acquired resistance to STI571.
As shown in Figure 10, altered signaling pathways, such as LYN, may be engaged in CML cells as a compensatory response to potent or chronic BCR-ABL inhibition. From the current study, it is hypothesized that CML cells may toggle between BCR-ABL and LYN dependence as a means of reducing susceptibility to STI571-induced growth arrest and apoptosis. Each of these kinases appear to signal through independent downstream cascades, providing an exploitable means of evaluating shifts in BCR-ABL dependence in clinical specimens. However, the appropriate signaling pathways engaged by LYN (or related kinases) first must be defined and are currently under investigation. Lineage-specific expression of other tyrosine kinases (such as BTK in acute lymphocytic leukemia patients) may play a parallel role in reducing BCR-ABL dependence and reducing STI571 efficacy in other Ph(+) leukemias.47
Overall, the results presented in this report suggest that STI571 resistance in K562 cells is mediated through BCR-ABL independent activation and overexpression of LYN. Tyrosine kinases with distinct ATP binding pockets that are not accessible to STI571 (such as those of the src family) may underlie development of BCR-ABL independence in some CML cells. Targeted inhibition of LYN kinase may circumvent STI571 resistance and disease progression in CML. Additional studies of BCR-ABL independence and secondary signaling events in STI571-resistant patient-derived cell lines will improve our understanding and therapy for advanced-stage CML.
Supported by a grant from the Leukemia Society of America (6153-02 [N.J.D.]).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nicholas J. Donato, Department of Bioimmunotherapy, University of Texas, M D Anderson Cancer Center, 1515 Holcombe Blvd, Box 422, Houston, TX 77030; e-mail:ndonato@mdanderson.org.

![Fig. 3. BCR-ABL kinase activity in K562 and K562-R cells. / BCR-ABL was immunoprecipitated from cell lysates (400 μg) with anti-abl, washed extensively, resuspended in kinase buffer, and incubated with 500 nM STI571 or buffer alone (control) for 30 minutes. Kinase reactions were initiated by the addition of 10 μCi (0.37 MBq) [32P]-ATP and enolase. After 30 minutes at 25°C, reactions were quenched with the addition of 2 × sample buffer, and phosphoproteins were resolved by SDS-PAGE. Tyrosine phosphoproteins were detected by autoradiography. Autophosphorylation of BCR-ABL and phosphorylation of enolase are shown. Similar results were obtained using anti-bcr for immunoprecipitation (data not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/2/10.1182_blood.v101.2.690/4/m_h80233696003.jpeg?Expires=1769085912&Signature=cNQCFD2gcuDCW4nuFQSkgo2XA9bhkt3eCe~BvzlVzuTbhAZW6~BpUzA84cFTbtMMuB9nZ2ugFd04kQmb9QT7L-Rov6amJUu2rsj0mqhbsnaqa5Jai2cAP7lSIzhR3TlBYi8vLYkVSH2ij~uKSlpIO6iqP5plwjHpLut9jfzMMJGbTWj9v9MU5JMDUfe0S2rHQgUIk2qt~kcdOKXagywfFruuJQW-f4pnB8BfhBt0c4YWScnYb3ky275ivZ7WVs256a0Nf0N47gATWEAj15pFlT2rHs1q8qHwDKGnfbGv7Yc6d6Yf3FCxNHSPx8U-EPY5ODVKG-pxXZi7nYKxzKcWew__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. LYN kinase expression and activity in K562 and K562-R cells. / (A) K562-R cell lysates (250 μg) were immunodepleted with anti-LYN (2 μg) or anti-HCK (2 μg) and protein A/G–sepharose, and resultant supernatants were analyzed for total tyrosine phosphoproteins (p-Tyr; left) or LYN (right). Migration of protein standards is shown on the left, and the 2 forms of LYN expressed in K562-R cells are shown on the right. (B) Twenty micrograms RNA from K562 and K562-R cells was resolved by electrophoresis, transferred to a membrane, and hybridized with a LYN probe as described in “Materials and methods.” The extent of hybridization with probe is shown (top), and the migration of 28S and 18S RNA is shown at the bottom. (C) Equal protein (30 μg) K562 and K562-R cell lysates were subjected to immunoblotting for HCK (left) and LYN (right). U-937 cell lysates were used as a positive control for HCK. Two forms of LYN (but not HCK) were detected in K562 cells, and the p53 form of LYN was overexpressed in K562-R cells. The same blots were probed with antiactin to monitor protein loading. (D) LYN or HCK immune complexes from K562 and K562-R cells were analyzed for tyrosine kinase activity in the presence of [32P]-ATP and enolase as described in “Materials and methods.” Src immune complexes from HT-29 cells were used as a positive control. Phosphorylation was detected by autoradiography on x-ray film. LYN autophosphorylation was detected in K562-R immune complexes, while exogenous substrate (enolase) phosphorylation was detected in both K562 and K562-R immune complexes. K562-R cell enolase phosphorylation was estimated (by PhosphorImager) to be approximately 7-fold higher than K562 cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/2/10.1182_blood.v101.2.690/4/m_h80233696006.jpeg?Expires=1769085912&Signature=GnkvKWT-zw1plKQPgonmFonIa3J3a-3MUJMt~Eumo7XH8W0BtToeVv59Zrijgqzw3Q2FqeU2gTegIn9Xo1ZueKejMZ~CdF6hvnPrCHqp1Gek1igbtTDXh0rmyhOYvb649lWEzxDlIPjqb2KnDJTc9SNM7CRQrbpIDUD7scHjI7wO7ddyIDKmjnagWqvglQB~pQz3sWhfLdernQxb1AjuIrtoaYepclHRKYwziHe9jjJi~li7GCPvi7WMkvazIw6lZ0dWbvzZLF~pBvQqmxxNXnryjpnSGSSfL5tCWwawDjWkQrPpUBNKC59GGkvseEoaHYQYoBiWsawL2IRaqh8Dqg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Effect of CGP-76030 on tyrosine kinase activity of BCR-ABL and LYN in vitro. / BCR-ABL or LYN was immunoprecipitated from equal protein cell lysates (300 μg) from K562 (left) or K562-R (right) cells. Immune complexes were washed extensively, resuspended in kinase buffer or CGP-76030 (at the final concentration noted), and incubated for 30 minutes before kinase reactions were initiated with the addition of enolase and 10 μCi (0.37MBq) [32P]-ATP. Phosphoproteins were detected as described in the legend to Figure 3. (Top) Migration of phosphorylated BCR-ABL and enolase is depicted. (Bottom) Migration of phospho-LYN and enolase is shown. Note: LYN autophosphorylation is not detected in immune complexes from K562 cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/2/10.1182_blood.v101.2.690/4/m_h80233696007.jpeg?Expires=1769085912&Signature=eEt72tj13uTFkrW6sWoQxlSTrdIxKJNXWS7nk-alfCI-Fs9EGD1fv08HCMRHNa4wq4sXy5CqG4zE2GGDGnHTtFKEtvEztWLKEa3CYURL~H8AGeFzGvSSFQTu0j~x4QBCR4nEbL0UniRjJzpMtmE05RSiMH4o~QjG3pwpA~3QhPgh0Qf0ZbtOXrD3aPX8SqMEBSjsqfwi9rCoKU5nvYEG5fJUovQjM-DGaLbZ9PP~vLWBR847IaHo93TaOh7lYRVTxOFQ2PmBLk5-9uHYwyXm-CGAxu2M~413O1q1FPBUN9DofrqlvgnWhv0PTIxziPTRIr2WFZbHjL~Ap87u4OHy8A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal