Murine hematopoietic stem cells (HSCs) originate from mesoderm in a process that requires the transcription factor SCL/Tal1. To define steps in the commitment to blood cell fate, we compared wild-type and SCL−/− embryonic stem cell differentiation in vitro and identified CD41 (GpIIb) as the earliest surface marker missing from SCL−/− embryoid bodies (EBs). Culture of fluorescence-activated cell sorter (FACS) purified cells from EBs showed that definitive hematopoietic progenitors were highly enriched in the CD41+ fraction, whereas endothelial cells developed from CD41− cells. In the mouse embryo, expression of CD41 was detected in yolk sac blood islands and in fetal liver. In yolk sac and EBs, the panhematopoietic marker CD45 appeared in a subpopulation of CD41+ cells. However, multilineage hematopoietic colonies developed not only from CD45+CD41+ cells but also from CD45−CD41+ cells, suggesting that CD41 rather than CD45 marks the definitive culture colony-forming unit (CFU-C) at the embryonic stage. In contrast, fetal liver CFU-C was CD45+, and only a subfraction expressed CD41, demonstrating down-regulation of CD41 by the fetal liver stage. In yolk sac and EBs, CD41 was coexpressed with embryonic HSC markers c-kit and CD34. Sorting for CD41 and c-kit expression resulted in enrichment of definitive hematopoietic progenitors. Furthermore, the CD41+c-kit+ population was missing from runx1/AML1−/− EBs that lack definitive hematopoiesis. These results suggest that the expression of CD41, a candidate target gene of SCL/Tal1, and c-kit define the divergence of definitive hematopoiesis from endothelial cells during development. Although CD41 is commonly referred to as megakaryocyte–platelet integrin in adult hematopoiesis, these results implicate a wider role for CD41 during murine ontogeny.
Introduction
Blood cell development during embryogenesis is initiated within the mesodermal germ layer in a process that requires the basic helix-loop-helix (bHLH) transcription factor SCL/tal1.1-3 However, the precise steps leading to commitment to blood cell fate are incompletely characterized. Intimate spatial and temporal development of the hematopoietic and endothelial cell lineages in the embryo, shared marker gene expression, and knockout mice with defects in both lineages have led to the hypothesis of a common origin for blood and endothelium, the hemangioblast.4-12 By definition, the hemangioblast is a common progenitor for primitive and definitive hematopoietic and endothelial cell lineages. The divergence of the lineages and the phenotype of the earliest precursors in each lineage, however, are not fully defined.
In the mouse embryo, the commitment to hematopoiesis occurs within days 7 to 8.25 (E7-E8.25) of embryonic development, during which time hematopoietic progenitors of primitive and definitive hematopoietic lineage appear in the extraembryonic yolk sac and the embryo proper.13,14 After a transient wave of primitive erythropoiesis in the yolk sac, definitive hematopoietic progenitors from the yolk sac and para-aortic splanchnopleural/aorta-gonad-mesonephros (PAS/AGM) region seed the fetal liver, which is the principal site of definitive hematopoiesis during fetal life.15-17 During the fetal liver stage, hematopoietic stem cells acquire the capacity to engraft and reconstitute bone marrow (BM) and thus support lifelong hematopoiesis. The origin of hematopoietic stem cells (HSCs) during development is a subject of controversy. It has been generally accepted that de novo hematopoiesis occurs in 2 independent sites, the extraembryonic yolk sac and the intraembryonic PAS/AGM and that cells originating from both locations can contribute to adult hematopoiesis when stimulated appropriately. The earliest site of detectable HSC activity in developing mouse embryo is the PAS/AGM region, where adult reconstituting HSCs can be found at E10.5, before the fetal liver stage.17-21
Although no adult BM reconstituting stem cells are found in the yolk sac before fetal liver hematopoiesis, yolk sac cells can reconstitute multilineage, long-term hematopoiesis in conditioned newborn recipients.22,23 This has led to the hypothesis that yolk sac stem cells are immature and require an additional maturation step within fetal liver before BM engraftment can be achieved. Yolk sac cells have been shown to acquire some adult BM reconstitution potential by coculture on an AGM-derived stromal cell line24 or by overexpression of HOXB4.25
In recent years, model systems have been developed that permit the study of hematopoietic and endothelial commitment in vitro.26-32 Murine embryonic stem (ES) cells grown in suspension culture without feeder cells and leukemia inhibitory factor (LIF) spontaneously form cell aggregates called embryoid bodies (EBs) and differentiate into cell types representing all germ layers, including hematopoietic and endothelial cells. The EBs form cystic structures resembling yolk sac blood islands where the first steps of hematopoietic development occur in a fashion similar to that in the mouse embryo. Furthermore, in vitro differentiation of ES cells affords study of gene-targeted cells without confounding embryonic lethality of the developing mouse. In spite of the close similarity of hematopoietic development in the EBs to hematopoiesis in vivo, the validity of this model system has been questioned because of a failure to demonstrate the presence of authentic hematopoietic stem cells. Recent work shows that EB-derived hematopoietic progenitors can achieve some adult BM reconstitution potential by transient overexpression of HOXB4 or BCR-ABL.25 33
Studies in gene-targeted mice and ES cells have demonstrated a critical role for the bHLH transcription factor SCL in the initiation of the blood program. Without SCL, no blood cells of any lineage develop in vivo in chimeric mice or in vitro in embryoid bodies.34,35In contrast, endothelial cells are present but fail to remodel properly in the yolk sac.36 In vitro study of SCL−/−ES cells suggests that the block in blood cell development occurs at the hemangioblast stage.3,37 Our aim here was to characterize the initial steps in the commitment to blood cell fate by studying the events downstream of SCL function. We have compared wild-type and SCL−/− mouse ES cells and identified cell populations absent in SCL−/− embryoid bodies by analyzing the surface expression of markers previously implicated in blood and endothelial cell development. We demonstrate that the expression of CD41, also known as GpIIb, correlates with SCL expression and is the earliest blood-specific marker absent in SCL−/− EBs. Expression of CD41 precedes the expression of the panhematopoietic marker CD45, which is expressed later in a subpopulation of CD41+ cells. Although CD41 is recognized as a megakaryocyte/platelet marker in adult hematopoiesis with established roles in platelet function,38 early expression of CD41 in embryos is not correlated with megakaryopoiesis. In vitro cultures of sorted cells demonstrate that the expression of CD41 and c-kit mark definitive hematopoietic progenitors in vitro in EBs and in vivo in yolk sac blood islands, independent of CD45 expression. In contrast, endothelial cells develop from the CD41− fraction. These results suggest that CD41 expression marks the divergence of definitive hematopoietic precursors from the hemangioblasts or hemogenic endothelium during mouse embryonic development.
Materials and methods
Culture and differentiation of ES cells
Wild-type (J1 and CJ7), SCL−/−(J1),34 and runx1/AML1−/−(CJ7)39 ES cells were cultured in gelatin-coated flasks in Dulbecco modified Eagle medium (DMEM, high glucose; Gibco-BRL, Grand Island, NY) supplemented with 15% fetal calf serum (FCS; Hyclone, Logan, UT), 2 mM L-glutamine (Gibco-BRL), penicillin/streptomycin (Gibco-BRL), 10 ng/mL LIF, 1.5 × 10−4 M monothioglycerol (MTG) (Sigma, St Louis, MO), 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, and 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (Gibco-BRL). Twenty-four to 48 hours before the initiation of differentiation, ES cells were adapted to Iscove modified Dulbecco medium (IMDM; Cellgro; Mediatech, Herndon, VA) containing FCS, penicillin/streptomycin, L-glutamine, MTG, and LIF as described. Before EB differentiation, the ES cells were trypsinized and seeded in suspension cultures in 60-mm Petri dishes (Fisherbrand, Bramalea, ON, Canada) at a concentration of 10 000-20 000 cells/mL. The EB differentiation media contained IMDM supplemented with 15% FCS (Summit Biotechnologies, Fort Collins, CO), 2 mM L-glutamine, 1% penicillin/streptomycin, 25 μg/mL mM ascorbic acid (Sigma), 300 μg/mL transferrin (Roche Diagnostic, Indianapolis, IN), and 4.5 × 10−4 M MTG. After differentiation for the desired time, EBs were collected and trypsinized into single-cell suspension and analyzed by reverse transcription–polymerase chain reaction (RT-PCR), FACS analysis, and hematopoietic and endothelial cell cultures.
FACS analysis and sorting
EBs differentiated for various times (2.75-7.75 days) were analyzed for surface expression of hematopoietic and endothelial markers. Trypsin-dissociated EBs were stained with fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC), Cy5, or biotin-conjugated monoclonal rat antimouse antibodies against c-kit (PE or APC), Flk1 (PE), α4-integrin (PE or biotin), CD34 (FITC or biotin), platelet endothelial cell adhesion molecule (PECAM) (FITC or biotin), MECA-32 (biotin), CD41 (FITC or Cy-5), CD45 (PE or APC), and Ter119 (PE or biotin) (PharMingen, Becton Dickinson, San Diego, CA). Biotin-conjugated antibodies were detected by secondary staining with PE, APC, or Pharred-conjugated streptavidin (PharMingen). For analysis of VE-cadherin or endoglin, purified antibody was used and detected with PE-conjugated polyclonal rabbit antirat antibody (PharMingen). Cells were analyzed using FACScalibur (Becton Dickinson). For separation of cell populations, the cells were analyzed and sorted on a MoFlow cytometer (Cytomation, Fort Collins, CO).
Methylcellulose colony assays
EBs were harvested at specific time points for plating into the blast cell colony assay (days in embryoid body cultures in vitro [d]; d3.25-3.5) or hematopoietic colony assays (d5-7). For blast cell colony assay, the cells were plated at 0.75 to 1.5 × 105 cells/mL in 1% methylcellulose containing 10% FCS (Summit Biotechnologies), 4.5 × 10−4M MTG, 1% penicillin/streptomycin, 1% L-glutamine supplemented with 5 ng/mL vascular endothelial growth factor (VEGF), 5 ng/mL interleukin-6 (IL-6), 100 ng/mL stem cell factor (SCF; R&D Systems, Minneapolis, MN), 25 μg/mL ascorbic acid, and 300 μg/mL transferrin (Roche) to support hemangioblast differentiation. To verify hematopoietic and endothelial cell potential in the blast cell colonies, individual blast colonies were picked at 4 days of methylcellulose culture and were plated into growth-factor–reduced Matrigel (Becton Dickinson)–coated dishes in IMDM, 10% FCS, 10% horse serum, 5 ng/mL VEGF, 10 ng/mL insulinlike growth factor-1 (IGF-1), 10 ng/mL basic fibroblast growth factor (bFGF), 2 U/mL erythropoietin (EPO), 50 ng/mL SCF, 10 ng/mL IL-3 (R&D Systems), and endothelial cell growth supplement (Collaborative Biomedical Products, Becton Dickinson).
For analysis of hematopoietic precursors, the methylcellulose mix was supplemented with hematopoietic growth factors 2 U/mL EPO, 100 ng/mL mSCF, 1 ng/mL mIL-3, 5 ng/mL murine IL-6 (mIL-6), 5 ng/mL murine thrombopoietin (mTPO), 30 ng/mL human granulocyte–colony-stimulating factor (hG-CSF), 3 ng/mL murine granulocyte macrophage–colony-stimulating factor (mGM-CSF), 5 ng/mL IL-11, and 5 ng/mL mM-CSF. Hematopoietic colonies were scored individually by microscopy and May-Grünwald-Giemsa staining at 5 to 8 days of differentiation.
Endothelial cell cultures
FACS-sorted cells from EBs were plated on Matrigel-coated dishes (Becton Dickinson) in DMEM, 10% FCS (Summit), penicillin/streptomycin, 25 ng/mL VEGF, 10 ng/mL IGF, 10 ng/mL bFGF (R&D Systems), and endothelial cell growth supplement (Collaborative Biomedical Products, Becton Dickinson). Endothelial cell potential was monitored by the growth of adherent cells and FACS staining for Flk1 and PECAM (CD31) (Becton Dickinson).
Analysis of mouse embryos
To analyze embryonic and fetal hematopoietic development in mice, inbred (C57Bl6) and outbred (Swiss Webster) mice were time-mated, and embryos were collected at E8.5, E9.5, and E10.5 for studies of yolk sac hematopoiesis and at E12.5 or E14.5 for fetal liver hematopoiesis. Yolk sacs were dissected from the embryo proper, trypsinized, separated into single-cell suspension by a 21-gauge syringe, filtered, and analyzed by FACS. Fetal livers were collected and separated into single-cell suspension by pipetting and filtration.
Wholemount immunohistochemistry for E8.5 and E9.5 embryos using anti-CD41 antibody (PharMingen) was performed essentially as described.36 Peroxidase staining was used for antibody detection. Fluorescein isothiocyanate (FITC)–conjugated anti-CD41 antibody was used to stain 10-μm cryosections of dissected E9.5 yolk sac that had been fixed with 4% paraformaldehyde.
Results
Kinetics of expression of hematopoietic and endothelial surface markers in wild-type and transcription factor mutant EBs
To analyze the commitment of mesoderm to hematopoietic and endothelial cell lineages, wild-type and transcription factor mutant ES cells were examined during differentiation in vitro. SCL−/− ES cells were used as a negative control for hematopoietic development because previous studies have shown complete absence of hematopoietic activity in SCL−/−EBs.3,34 35 EBs were harvested at specific times and subjected to FACS analysis. Table 1demonstrates similar kinetics of expression of the early mesoderm/hemangioblast markers c-kit, Flk1, and α4-integrin (CD49d) in wild-type and SCL−/− EBs. Similarly, endothelial cell markers endoglin (CD105), MECA32, and VE-cadherin and combined endothelial/hematopoietic markers CD34 and PECAM (CD31) were expressed in wild-type and SCL−/− EBs. However, all markers specific for hematopoietic cells (CD41, CD45, and Ter119) were absent in SCL−/− EBs. In these temporal studies, CD41 was identified as the earliest blood-specific marker in the wild-type EBs. Surface expression of CD41 was first detected at approximately d4.25 to d4.75 of EB differentiation and increased progressively to 5% to 30% by d6 to d7 (Table 1; Figure 1).
Expression of hematopoietic and endothelial surface markers in wild-type and SCL−/− EBs during differentiation in vitro
| Marker . | Positive cells, % . | ||||
|---|---|---|---|---|---|
| d2.75 . | d3.75 . | d4.75 . | d5.75 . | d6.75 . | |
| Wild-type EBs | |||||
| Flk1 | 3.2 ± 1.6 | 39.1 ± 2.4 | 24.7 ± 1.7 | 26.1 ± 1.8 | 25.8 ± 2.1 |
| c-kit | 41.3 ± 5.6 | 43.4 ± 3.7 | 53.1 ± 5.2 | 51.5 ± 2.6 | 40.3 ± 3.6 |
| α4-integrin | — | 2.5 ± 0.5 | 20.2 ± 0.6 | 42.9 ± 5.3 | 56.9 ± 6.2 |
| Endoglin | — | 25.1 ± 7 | 24.3 ± 9.9 | 15.0 ± 1 | 22.5 ± 1.2 |
| PECAM | — | 2 ± 0.8 | 14.3 ± 6.3 | 22.4 ± 7.8 | 24.4 ± 1.7 |
| MECA32 | — | 8.4 ± 3.2 | 8.1 ± 3.6 | 18.1 ± 2.7 | 28 ± 1.8 |
| VE-cadherin | — | — | — | 3.9 ± 1 | 4.9 ± 1 |
| CD34 | — | — | — | 1.7 ± 0.9 | 7.6 ± 1.4 |
| CD41 | — | — | 1.3 ± 0.7 | 8 ± 2.0 | 17.7 ± 3.4 |
| CD45 | — | — | — | 0.8 ± 0.5 | 1.9 ± 0.5 |
| Ter119 | — | — | — | — | 1.6 ± 0.2 |
| SCL−/−EBs | |||||
| Flk1 | 2.4 ± 1.3 | 53.7 ± 5.2 | 22.2 ± 2.9 | 29.6 ± 6.9 | 33.9 ± 1.4 |
| c-kit | 47.8 ± 3.1 | 35.2 ± 2 | 68.9 ± 5.5 | 66.3 ± 8.2 | 62.4 ± 2.8 |
| α4-integrin | — | 2 ± 0.5 | 42.6 ± 4.4 | 58.8 ± 6.2 | 65.5 ± 3.1 |
| Endoglin | — | 37.1 ± 5.6 | 35.6 ± 2.8 | 23.0 ± 1 | 36.7 ± 6.6 |
| PECAM | — | 2.5 ± 0.7 | 14.7 ± 6.1 | 22.3 ± 7.3 | 18.1 ± 2.2 |
| MECA32 | — | 7.2 ± 0.4 | 14.8 ± 6.1 | 21.2 ± 0.1 | 27.2 ± 1.9 |
| VE-cadherin | — | — | — | 2.8 ± 1.1 | 3.9 ± 0.1 |
| CD34 | — | — | — | — | 5.6 ± 1.5 |
| CD41 | — | — | — | — | — |
| CD45 | — | — | — | — | — |
| Ter119 | — | — | — | — | — |
| Marker . | Positive cells, % . | ||||
|---|---|---|---|---|---|
| d2.75 . | d3.75 . | d4.75 . | d5.75 . | d6.75 . | |
| Wild-type EBs | |||||
| Flk1 | 3.2 ± 1.6 | 39.1 ± 2.4 | 24.7 ± 1.7 | 26.1 ± 1.8 | 25.8 ± 2.1 |
| c-kit | 41.3 ± 5.6 | 43.4 ± 3.7 | 53.1 ± 5.2 | 51.5 ± 2.6 | 40.3 ± 3.6 |
| α4-integrin | — | 2.5 ± 0.5 | 20.2 ± 0.6 | 42.9 ± 5.3 | 56.9 ± 6.2 |
| Endoglin | — | 25.1 ± 7 | 24.3 ± 9.9 | 15.0 ± 1 | 22.5 ± 1.2 |
| PECAM | — | 2 ± 0.8 | 14.3 ± 6.3 | 22.4 ± 7.8 | 24.4 ± 1.7 |
| MECA32 | — | 8.4 ± 3.2 | 8.1 ± 3.6 | 18.1 ± 2.7 | 28 ± 1.8 |
| VE-cadherin | — | — | — | 3.9 ± 1 | 4.9 ± 1 |
| CD34 | — | — | — | 1.7 ± 0.9 | 7.6 ± 1.4 |
| CD41 | — | — | 1.3 ± 0.7 | 8 ± 2.0 | 17.7 ± 3.4 |
| CD45 | — | — | — | 0.8 ± 0.5 | 1.9 ± 0.5 |
| Ter119 | — | — | — | — | 1.6 ± 0.2 |
| SCL−/−EBs | |||||
| Flk1 | 2.4 ± 1.3 | 53.7 ± 5.2 | 22.2 ± 2.9 | 29.6 ± 6.9 | 33.9 ± 1.4 |
| c-kit | 47.8 ± 3.1 | 35.2 ± 2 | 68.9 ± 5.5 | 66.3 ± 8.2 | 62.4 ± 2.8 |
| α4-integrin | — | 2 ± 0.5 | 42.6 ± 4.4 | 58.8 ± 6.2 | 65.5 ± 3.1 |
| Endoglin | — | 37.1 ± 5.6 | 35.6 ± 2.8 | 23.0 ± 1 | 36.7 ± 6.6 |
| PECAM | — | 2.5 ± 0.7 | 14.7 ± 6.1 | 22.3 ± 7.3 | 18.1 ± 2.2 |
| MECA32 | — | 7.2 ± 0.4 | 14.8 ± 6.1 | 21.2 ± 0.1 | 27.2 ± 1.9 |
| VE-cadherin | — | — | — | 2.8 ± 1.1 | 3.9 ± 0.1 |
| CD34 | — | — | — | — | 5.6 ± 1.5 |
| CD41 | — | — | — | — | — |
| CD45 | — | — | — | — | — |
| Ter119 | — | — | — | — | — |
— indicates negative staining.
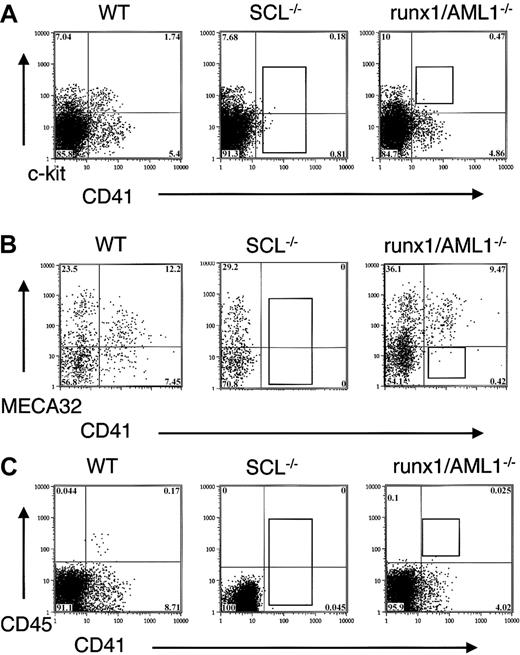
Expression of CD41, c-kit, MECA32, and CD45 in transcription factor mutant EBs.
(A) D6 EBs from wild-type, SCL−/−, and runx1/AML1−/− ES cells were stained for the expression of CD41 and c-kit. In SCL−/− ES cells, no expression of CD41 can be detected, whereas in runx1/AML−/− EBs, the CD41+ c-kit+ population is absent. (B) D6.75 EBs were stained for CD41 and MECA 32. Wild-type EBs demonstrate intermediate/low expression of MECA32 in CD41+ cells, whereas CD41+ cells in runx1/AML1−/− EBs fail to down-regulate MECA32. (C) D6 EBs were stained for CD41 and CD45. Wild-type EBs show that CD45+ cells form a subpopulation within CD41+ cells. Runx1/AML1−/− EBs fail to express CD45.
Expression of CD41, c-kit, MECA32, and CD45 in transcription factor mutant EBs.
(A) D6 EBs from wild-type, SCL−/−, and runx1/AML1−/− ES cells were stained for the expression of CD41 and c-kit. In SCL−/− ES cells, no expression of CD41 can be detected, whereas in runx1/AML−/− EBs, the CD41+ c-kit+ population is absent. (B) D6.75 EBs were stained for CD41 and MECA 32. Wild-type EBs demonstrate intermediate/low expression of MECA32 in CD41+ cells, whereas CD41+ cells in runx1/AML1−/− EBs fail to down-regulate MECA32. (C) D6 EBs were stained for CD41 and CD45. Wild-type EBs show that CD45+ cells form a subpopulation within CD41+ cells. Runx1/AML1−/− EBs fail to express CD45.
RT-PCR analysis for CD41 demonstrated a correlation of CD41 surface expression with the presence of CD41 mRNA in the EBs. In wild-type EBs, the expression of CD41 RNA was detected approximately 12 hours after the appearance of SCL RNA transcripts, whereas no CD41 RNA was found in SCL−/− EBs (not shown).
Combined analysis of CD41 and other hematopoietic and endothelial markers showed that CD41+ cells developed from c-kit+ cells in the EBs with partial overlap with Flk1 expression. All CD41+ cells coexpressed α4-integrin and low levels of PECAM (not shown). Initially, CD41+ cells also expressed low levels of MECA32, which was down-regulated in a subpopulation of CD41+ cells (Figure 1B). CD45 was expressed exclusively in a subpopulation of CD41+ cells with a 1- to 2-day delay from the appearance of CD41 expression (Figure1C).
We also examined CD41 expression during in vitro differentiation of knockout ES cells for runx1/AML1.39 Whereas no expression of CD41 was detected in SCL−/− EBs, CD41 expression was observed in runx1/AML 1−/− EBs (Figure 1). Runx1/AML1−/− EBs exhibited decreased expression of CD41+ and failed to coexpress CD41 and c-kit (Figure 1A). Instead, CD41+ cells in runx1/AML1−/− EBs sustained the expression of endothelial markers MECA32, PECAM, and Flk1 (Figure 1B and not shown). No CD45 cells developed in runx1/AML1−/− EBs (Figure 1C).
Characterization of hematopoietic and endothelial surface marker expression in blast cell colonies
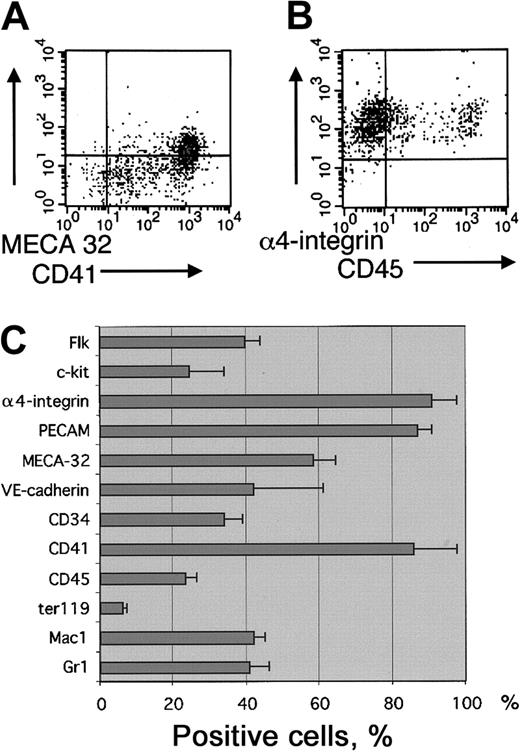
EB-derived blast cell colonies that represent early SCL-dependent hemangioblast/hematopoietic activity in cultures with VEGF were analyzed for surface expression of hematopoietic and endothelial cell markers. Cells from d3.25 to d3.5 EBs were first cultured in methylcellulose with VEGF, IL-6, and SCF. Four days later, cultures were scored for the presence of colonies containing loosely attached cells characteristic of blast cell colonies. Hematopoietic and endothelial potential in the blast cell colonies was verified by plating individual colonies into Matrigel with hematopoietic and endothelial growth factors as previously described.32 As shown earlier,3,37 SCL−/− EBs lack the potential to form blast cell colonies. The phenotype of the cells in blast colonies was analyzed by pooling the loosely attached cells from methylcellulose cultures and subjecting them to FACS analysis. Although the cells that give rise to blast colonies are Flk1 positive37 (data not shown), only a portion of the nonadherent cells in their progeny (40% ± 4%) express Flk1, demonstrating down-regulation of Flk1 during hematopoietic development. In comparison, α4-integrin, PECAM, and CD41 were up-regulated during blast colony development and were expressed on the surfaces of most (more than 85%) cells (Figure 2). Other hematopoietic markers were present in lower frequencies (Mac1 42% ± 3%, Gr1 41% ± 5%, Ter119 6% ± 1.4%, CD45 23% ± 3%, c-kit 25% ± 9%). A subfraction of cells in the blast colonies also expressed the endothelial markers MECA32 (58% ± 6%) and VE-cadherin (42% ± 19%) and the endothelial/hematopoietic marker CD34 (34% ± 5%) (Figure2C).
Surface marker expression in blast cell colonies.
Blast-cell colonies were grown from d3.5 EBs in the presence of VEGF, IL-6, and SCF. The loosely attached cells from blast-cell colony cultures were pooled and analyzed for surface marker expression by FACS. (A) Staining with CD41 and MECA32. (B) Staining with α4-integrin and CD45. (C) Percentage of cells expressing hematopoietic and endothelial cell surface markers. Results are shown as means ± SEMs from 3 independent experiments.
Surface marker expression in blast cell colonies.
Blast-cell colonies were grown from d3.5 EBs in the presence of VEGF, IL-6, and SCF. The loosely attached cells from blast-cell colony cultures were pooled and analyzed for surface marker expression by FACS. (A) Staining with CD41 and MECA32. (B) Staining with α4-integrin and CD45. (C) Percentage of cells expressing hematopoietic and endothelial cell surface markers. Results are shown as means ± SEMs from 3 independent experiments.
Developmental potential of CD41+ and CD41−EB fractions
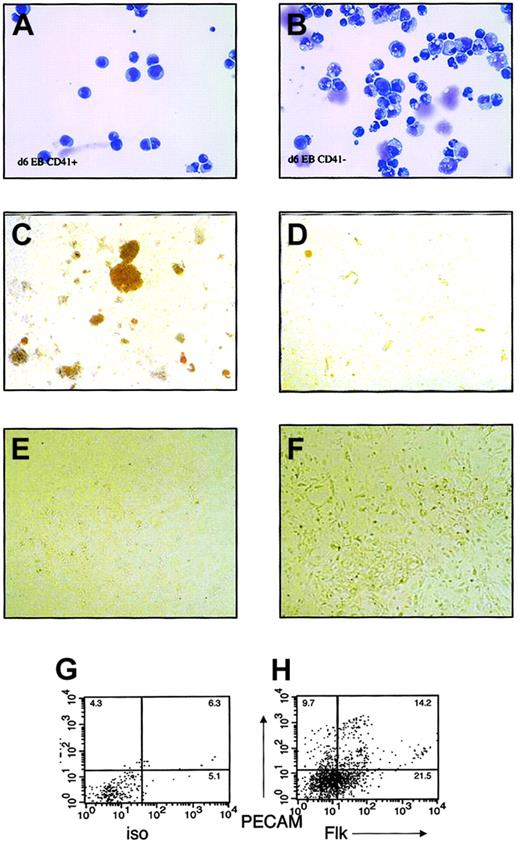
Because CD41 was the first marker absent in SCL−/−EBs and the most frequently expressed blood-specific marker in SCL-dependent blast colonies, wild-type EBs were fractionated based on CD41 expression, and the developmental potentials of CD41+and CD41− populations were individually assessed. EBs were differentiated for 5 to 7 days, trypsinized into single-cell suspensions, and sorted for surface expression of CD41. Expression of CD41 was present on 2% to 4% of cells at day 5 and increased to 5% to 30% at days 6 and 7. Morphologically, EB-derived CD41+cells comprised a homogenous, blastlike population (Figure3A). In contrast, CD41−cells were heterogeneous in appearance (Figure 3B).
Hematopoietic and endothelial potential of CD41+ and CD41− fractions in embryoid bodies.
Embryoid bodies were differentiated for 6 to 7 days, separated into single-cell suspension, and sorted based on surface expression of CD41. FACS-sorted cells were subjected to May-Grünwald-Giemsa staining and culture in hematopoietic or endothelial cell growth conditions. (A) May-Grünwald-Giemsa staining of CD41+ cells. (B) May-Grünwald-Giemsa staining of CD41− cells. (C) Methylcellulose colony assay of CD41+ cells. (D) Methylcellulose colony assay of CD41− cells. (E) Endothelial cell culture of CD41+ cells. (F) Endothelial cell culture of CD41− cells. (G) Isotype control and (H) Flk/PECAM staining of endothelial cell cultures from CD41− cells. Original magnifications A-B, × 400; C-D, × 40; E-F, × 100.
Hematopoietic and endothelial potential of CD41+ and CD41− fractions in embryoid bodies.
Embryoid bodies were differentiated for 6 to 7 days, separated into single-cell suspension, and sorted based on surface expression of CD41. FACS-sorted cells were subjected to May-Grünwald-Giemsa staining and culture in hematopoietic or endothelial cell growth conditions. (A) May-Grünwald-Giemsa staining of CD41+ cells. (B) May-Grünwald-Giemsa staining of CD41− cells. (C) Methylcellulose colony assay of CD41+ cells. (D) Methylcellulose colony assay of CD41− cells. (E) Endothelial cell culture of CD41+ cells. (F) Endothelial cell culture of CD41− cells. (G) Isotype control and (H) Flk/PECAM staining of endothelial cell cultures from CD41− cells. Original magnifications A-B, × 400; C-D, × 40; E-F, × 100.
To assay hematopoietic potential, sorted cells were plated into methylcellulose cultures supplemented with a combination of hematopoietic growth factors. At all times, definitive hematopoietic activity was highly enriched in the CD41+ fraction (Figure3C), whereas the CD41− fraction yielded no growth or yielded secondary EBs consisting of tightly packed cells (Figure 3 D). At day 5, primitive erythroid elements were observed from CD41+ and CD41− fractions (not shown). Rarely, definitive erythroid colonies or single lineage macrophage colonies were also derived from the CD41− fraction. By day 7, hematopoietic progenitor activity correlated with surface expression of CD41.
To study the potential of CD41+ cells to differentiate into endothelial cells, CD41+ and CD41− FACS-sorted cells were plated on Matrigel in endothelial growth conditions. Endothelial cell potential was assessed by the ability of the cells to proliferate and differentiate into adhesive cells on Matrigel and to express endothelial cell markers Flk1 or PECAM on their surfaces. At all times studied, d5 to d7, only CD41− cells proliferated in endothelial cell culture conditions; CD41+ cells died in such culture conditions (Figure 3E-F). On FACS analysis of the adherent cells from CD41− cultures, 25% to 30% of the cells expressed PECAM or Flk on their surfaces (Figure 3G-H), suggesting the presence of endothelial cells among the progeny of CD41−cells.
Expression of CD41 in the mouse embryo
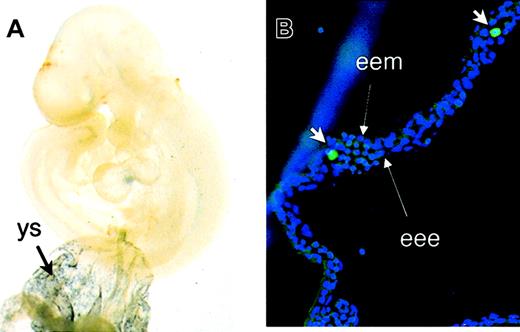
To correlate in vitro ES cell differentiation with embryonic hematopoiesis in vivo, CD41 expression was studied in time-mated mouse embryos. Wholemount staining demonstrated the expression of CD41 in yolk sac blood islands at E8.5 and E9.5 (not shown and Figure4) in locations similar to that of GATA-1 (not shown). At E9.5, CD41 expression was detected in the vitelline and umbilical vessels; however, the endothelial cell network that stains with PECAM (not shown) in the embryo proper did not express detectable CD41. Staining with FITC-conjugated anti-CD41 antibody demonstrates the presence of individual CD41+ cells inside yolk sac blood islands at E9.5 (Figure 4B) and occasionally inside the vessels in the embryo proper (not shown). Endothelial cells in blood islands at E9.5 did not express detectable CD41.
Expression of CD41 during mouse embryonic development.
(A) E9.5 yolk sac (ys) and embryos were subjected to wholemount staining with anti-CD41 antibody. Abundant expression of CD41 was detected in yolk sac blood islands. (B) Staining with FITC-conjugated anti-CD41 antibody (short arrows) demonstrates the presence of individual CD41+ hematopoietic cells inside the blood islands. Long arrows point to extraembryonic endoderm (eee) and extraembryonic mesoderm (eem). Original magnifications A, × 40; B, × 200.
Expression of CD41 during mouse embryonic development.
(A) E9.5 yolk sac (ys) and embryos were subjected to wholemount staining with anti-CD41 antibody. Abundant expression of CD41 was detected in yolk sac blood islands. (B) Staining with FITC-conjugated anti-CD41 antibody (short arrows) demonstrates the presence of individual CD41+ hematopoietic cells inside the blood islands. Long arrows point to extraembryonic endoderm (eee) and extraembryonic mesoderm (eem). Original magnifications A, × 40; B, × 200.
FACS analysis of hematopoietic organs revealed abundant (5%-25%) surface expression of CD41 in the yolk sac at E8.5, E9.5, and E10.5. Similarly, some CD41 expression was detectable in the AGM region at E10.5, and in E12.5, and E14.5 fetal liver, indicating localization of CD41 expression into sites of hematopoietic activity in the mouse embryo and the fetus (Figure 5A and not shown).
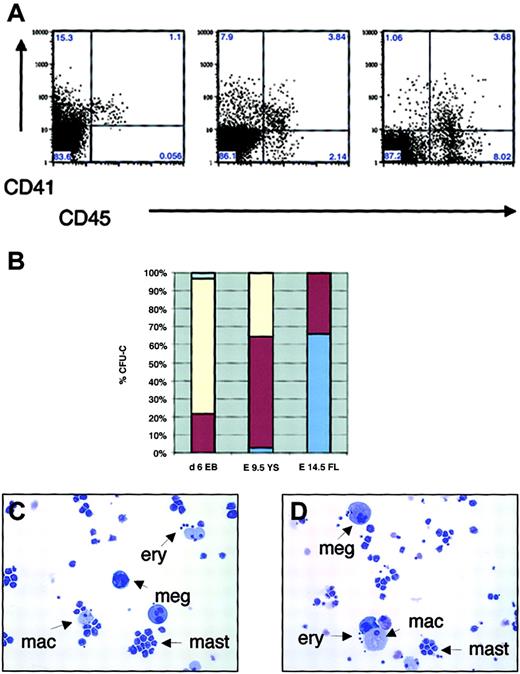
Expression of CD41 and CD45 in hematopoietic progenitors during embryonic and fetal hematopoiesis in vitro and in vivo.
(A) FACS sorting of d6 EBs (left), E9.5 yolk sac (middle), and E14.5 fetal liver (right). (B) Percentage of definitive CFU-C in EB subpopulations expressing CD41 and/or CD45, yolk sac, and fetal liver (average of 3 independent experiments). Pale blue indicates CD45−CD41−; yellow, CD45−CD41+; dark red, CD45+CD41+; and medium blue, CD45+CD41−. (C-D) May-Grünwald-Giemsa staining of individual CFU-mix colonies from CD45−CD41+ (C) and CD45+CD41+ (D) subpopulations of E9.5 yolk sac. Arrows demonstrate the presence of definitive erythroid cells (ery), megakaryocytes (meg), macrophages (mac), and mast cells (mast) in individual colonies from both populations. Original magnifications C-D, × 200.
Expression of CD41 and CD45 in hematopoietic progenitors during embryonic and fetal hematopoiesis in vitro and in vivo.
(A) FACS sorting of d6 EBs (left), E9.5 yolk sac (middle), and E14.5 fetal liver (right). (B) Percentage of definitive CFU-C in EB subpopulations expressing CD41 and/or CD45, yolk sac, and fetal liver (average of 3 independent experiments). Pale blue indicates CD45−CD41−; yellow, CD45−CD41+; dark red, CD45+CD41+; and medium blue, CD45+CD41−. (C-D) May-Grünwald-Giemsa staining of individual CFU-mix colonies from CD45−CD41+ (C) and CD45+CD41+ (D) subpopulations of E9.5 yolk sac. Arrows demonstrate the presence of definitive erythroid cells (ery), megakaryocytes (meg), macrophages (mac), and mast cells (mast) in individual colonies from both populations. Original magnifications C-D, × 200.
Definition of the surface phenotype of hematopoietic progenitors during murine ontogeny
Analysis of the kinetics of surface marker expression in the EBs and yolk sac demonstrated that CD45 expression is first detectable in a subpopulation of CD41+ cells (Figures 1C, 5A). To define whether hematopoietic progenitor activity resided only in the CD45+ fraction of CD41+ cells, cells were sorted by CD45 and CD41 expression. Surprisingly, in E6 EBs and E9.5 yolk sac, clonogenic progenitor activity was detected also in CD45− cells (76% vs 22% in CD45−CD41+ and CD45+CD41+ subfractions in EBs, and 33% vs 62% in the yolk sac, respectively; Figure 5B). These results indicate that the first hematopoietic progenitors during ontogeny did not express CD45, a panhematopoietic marker in the adult. In contrast, in E14.5 fetal liver, all colony-forming activity resided in the CD45+ fraction, with only a subpopulation coexpressing CD41 (66% in CD45+CD41− vs 32% in CD45+CD41+ fractions). In EBs and yolk sac (YS), CD45+CD41+ and CD45−CD41+ fractions gave rise to multilineage colonies with erythroid, megakaryocytic, and myeloid elements (not shown and Figure 5C-D). In contrast, in fetal liver, the CD41+ fraction rarely gave rise to multilineage colonies but mainly consisted of myeloid progenitors that differentiated into mast cells and macrophages in culture, whereas multilineage CFU-C were enriched in the CD45+CD41− fraction (not shown).
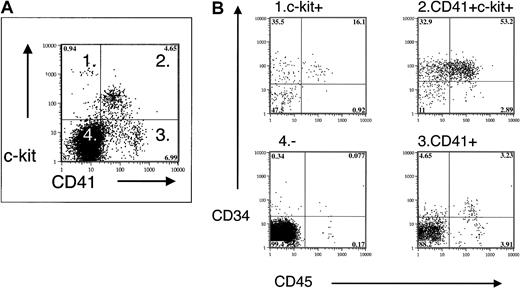
Multicolor FACS of EBs and yolk sac cells demonstrated the coexpression of stem cell and progenitor cell markers c-kit and CD34 with CD41 (not shown and Figure 6). Fractionation of the CD41+ population by positive c-kit expression increased the CFU-C frequency and showed that most definitive hematopoietic progenitors in the EBs and yolk sac are included within the CD41+ c-kit+ population (Table2).
Coexpression of CD41 and hematopoietic progenitor/stem cell markers in E9.5 yolk sac.
Fractionation of E9.5 yolk sac by CD41 and c-kit expression demonstrates enrichment of CD34 and CD45 expression in CD41+c-kit+ cells.
Coexpression of CD41 and hematopoietic progenitor/stem cell markers in E9.5 yolk sac.
Fractionation of E9.5 yolk sac by CD41 and c-kit expression demonstrates enrichment of CD34 and CD45 expression in CD41+c-kit+ cells.
CFU-C in yolk sac and embryoid bodies
| Surface phenotype . | E9.5 YS/103 cells . | D6 EBs/103 cells . |
|---|---|---|
| CD45+CD41+c-kit+ | 250 ± 20 | 50 ± 11 |
| CD45−CD41+c-kit+ | 200 ± 15 | 100 ± 15 |
| CD45−CD41+c-kit− | <1 | 20 ± 7* |
| CD45−CD41−c-kit+ | <1 | 10 ± 4* |
| CD45−CD41−c-kit− | <0.1 | <1 |
| Unfractionated | 11 ± 1 | 4 ± 1 |
| Surface phenotype . | E9.5 YS/103 cells . | D6 EBs/103 cells . |
|---|---|---|
| CD45+CD41+c-kit+ | 250 ± 20 | 50 ± 11 |
| CD45−CD41+c-kit+ | 200 ± 15 | 100 ± 15 |
| CD45−CD41+c-kit− | <1 | 20 ± 7* |
| CD45−CD41−c-kit+ | <1 | 10 ± 4* |
| CD45−CD41−c-kit− | <0.1 | <1 |
| Unfractionated | 11 ± 1 | 4 ± 1 |
Data consist of mean ± SEM from 3 independent experiments.
YS indicates yolk sac.
Most colonies were primitive erythroid colonies.
Discussion
SCL/tal 1 is strictly required for expression of CD41, whereas runx1/AML1 is essential for development of CD41+c-kit+ definitive hematopoietic progenitors
Studies of gene-targeted mice have proved pivotal in defining the stages of commitment toward hematopoiesis and within the hematopoietic system. Such analyses have identified major regulatory molecules that direct blood cell development.40 SCL/Tal1, a bHLH transcription factor whose expression is deregulated by chromosomal translocations in T-cell leukemia,41 is essential for initiation of the hematopoietic program at the putative hemangioblast stage.3 In comparison, the absence of runx1/AML1, also known as core-binding factor (CBF) A2, leads to the loss of definitive hematopoietic lineages, whereas primitive erythroid development is relatively unaffected.39 42
Our aim here was to study events downstream of SCL and AML1 to identify the phenotype of the first hematopoietic progenitors arising during development. On differentiation of wild-type and SCL-deficient embryoid bodies, we identified CD41 (GpIIb) as the earliest surface marker missing from SCL−/− EBs. Although commonly referred to as the megakaryocyte-specific marker during adult hematopoiesis, CD41+ cells derived from wild-type EBs displayed relatively homogeneous blast cell morphology with no characteristics of megakaryocytes. On culture in hematopoietic growth conditions, CD41+ cells gave rise to various definitive colony types, including mixed colonies, demonstrating multilineage potential within the CD41+ compartment. In contrast, CD41−cells gave rise to definitive hematopoietic progeny only rarely and did not demonstrate multilineage potential. These results strongly suggest that during hematopoietic commitment within EBs, the expression of CD41 correlates with the initiation of definitive hematopoiesis. Lack of CD41 expression and all hematopoietic activity in SCL−/−EBs not only suggests that CD41 is an authentic marker for hematopoietic development, it also suggests that CD41 may be a target gene for SCL during hematopoietic commitment. Our recent data from conditionally targeted SCL mice and cell lines demonstrate that CD41 expression is dependent on SCL in adult hematopoietic cells, suggesting that CD41 may be a direct target of SCL function (H.K.A.M and S.H.O., unpublished data, May 2002).
Comparison of CD41 expression in other transcription factor mutants with blocks in various stages of hematopoietic development demonstrates the sustained expression of CD41 in the absence of runx1/AML1, GATA-1, and GATA-2 (Figure 1; Y.F. and S.H.O., unpublished data, January 2001). The failure of runx1/AML1-deficient EBs to develop CD41+c-kit+ double-positive cells suggests that CD41 and c-kit mark definitive hematopoietic progenitors in the embryo. This hypothesis is supported by progenitor assays of sorted subpopulations in which definitive hematopoietic colonies in the d6 EBs and E9.5 yolk sac are highly enriched in the CD41+c-kit+ double-positive fraction.
Expression of CD41 appears after the hemangioblast stage and development of primitive hematopoietic and endothelial cell lineages
The earliest hematopoietic activity detected in vitro from the EBs is derived from Flk1+ cells, which, in response to VEGF, give rise to blast cell colonies that exhibit definitive and primitive hematopoietic and endothelial cell potential, filling the formal criteria for the hemangioblast. Because most of the daughter cells in blast cell colonies also express CD41, we considered the possibility that CD41 might be a marker for the hemangioblast. However, expression of CD41 in the EBs appears after the detection of hemangioblast and primitive erythroid activity (d3.25-d3.5 and d4, respectively)31 and endothelial marker expression. Although early CD41+ cells from the EBs occasionally gave rise to primitive erythroid colonies, as did CD41− cells, our data do not support a consistent expression pattern for CD41 with respect to primitive erythroid development in the embryoid bodies. In contrast, the kinetics and abundant expression of CD41 in the EBs correlated with the development of definitive hematopoietic progenitors. Likewise, in the yolk sac, the appearance of CD41 is delayed from the peak of primitive erythroid progenitor activity (E7-E8).13 Furthermore, FACS analysis with CD41 and Ter119 in the yolk sac demonstrate distinct expression patterns of these markers, suggesting that maturing primitive erythroid cells do not express CD41.
The endothelial cell markers endoglin, PECAM, and MECA32 appear in EBs approximately 1 day earlier than CD41, suggesting that the endothelial cell lineage develops before the development of CD41+cells. Furthermore, adherent cells expressing Flk1 and PECAM developed from the CD41− fraction from EBs, suggesting that CD41+ cells are not precursors of endothelial cells. Hematopoietic commitment of CD41+ cells is supported by wholemount staining of mouse embryos, where endothelial cells in the E9.5 yolk sac and embryo proper do not express detectable levels of CD41. Nevertheless, it cannot be excluded that in other culture conditions or in specific sites in vivo, CD41+ cells might give rise to endothelial cell progeny or that CD41 might be expressed in a specified subset of endothelium, the hemogenic endothelium. It is possible that early CD41 expression marks a transition from hemogenic endothelium to definitive hematopoietic progenitors. This hypothesis is supported by shared marker gene expression because the first CD41+ cells in the EBs and blast cell colonies express PECAM and, to a lesser degree, MECA32 and Flk1. CD41+ cells form a subpopulation within the PECAM/MECA32+ cells in the EBs, suggesting that CD41 expression divides the endothelial cell population into hemogenic and nonhemogenic compartments. Interestingly, runx1/AML1−/− knockout EBs did express CD41, albeit at lower levels; these CD41+ cells failed to coexpress c-kit or CD45 and continued to express the endothelial cell marker MECA32. It is possible that in runx1/AML1−/− EBs, CD41/PECAM/MECA32 expression defines the stage at which the block in definitive hematopoietic commitment occurs.
All CD41+ cells in EBs and blast colonies also expressed α4-integrin (CD49e), a marker previously implicated in commitment to hematopoiesis.43 In EBs, however, this CD41+fraction represented only a minor subfraction of all α4-integrin+ cells. Most important, abundant expression of α4-integrin was observed in SCL−/− EBs, suggesting that early expression of α4-integrin in EBs marks a heterogenous cell population with minor hematopoietic and major nonhematopoietic compartment. Furthermore, lack of correlation of α4-integrin expression and Flk1 expression/blast colony-forming activity also indicates that α4-integrin is not a specific marker for the hemangioblast.
CD41+ cells in EBs and yolk sac coexpress markers of definitive hematopoiesis (CD45) and neonatal reconstituting stem cells (c-kit and CD34)
In adult hematopoiesis, CD45 is generally considered a panhematopoietic marker. Our data show that in the EBs and in the yolk sac, the expression of CD45 appears exclusively in CD41+cells approximately 1 to 2 days later than CD41. Interestingly, in EBs and in the yolk sac, a significant proportion of colony-forming activity was detected in the CD45− CD41+population. Furthermore, the expression of CD45 did not correlate with a gain in differentiation potential in culture because CD45+ and CD45− fractions gave rise to mixed colonies and to single-lineage definitive erythroid, megakaryocyte, and myeloid colonies. These data suggest that in vitro in EBs and in vivo in the yolk sac, the expression of CD41 spans the development from a Flk1+ hemangioblast to definitive hematopoietic progenitors that later express CD45 (Figure 7). One obvious caveat is that our data are derived from progenitor assays, whereas the development and surface phenotype of HSCs remains to be studied by in vivo assays. Nevertheless, newborn reconstituting stem cells within the yolk sac have been shown to express c-kit and CD34,23 markers that we found to be coexpressed with CD41 in the yolk sac and in EBs. During mouse development, it has been proposed that the earliest adult repopulating HSCs arise from the walls of dorsal aorta in the AGM region and vitelline and umbilical vessels.17-21 Wholemount staining and FACS analyses showed some expression of CD41 in the vitelline and umbilical vessels and in the AGM region. In situ immunohistochemistry analysis of CD41 expression during mouse embryonic development demonstrates that CD41 is expressed in hematopoietic clusters in the walls of dorsal aorta and umbilical artery and in the lumen of yolk sac blood islands.44 The developmental potential of the early CD41+ cells in the mouse remains to be addressed by in vivo assay. Interestingly, North et al45 recently reported the presence of CD45− HSCs in E10.5 AGM and E12.5 fetal liver, a phenomenon that was accentuated by runx1/AML1 haploinsufficiency. Whether these early HSCs express CD41 remains to be studied further.
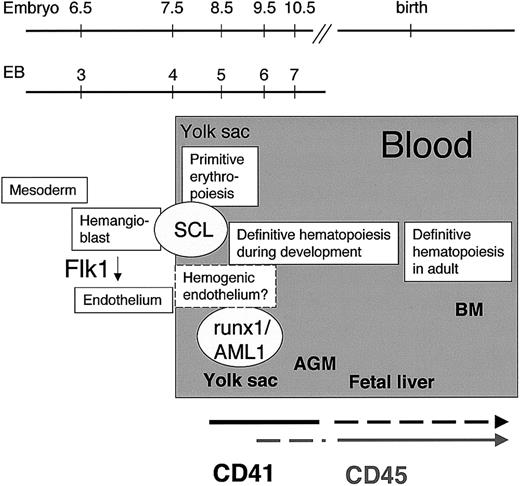
Initiation of hematopoiesis in the mouse embryo.
CD41, a candidate target gene of SCL, marks the initiation of definitive hematopoiesis during embryoid body development in vitro and in the yolk sac in vivo. Expression of the panhematopoietic marker CD45 appears later in a subpopulation of hematopoietic progenitors in the yolk sac. By the fetal liver stage, the expression of CD41 is down-regulated, whereas CD45 is expressed in all definitive hematopoietic progenitors.
Initiation of hematopoiesis in the mouse embryo.
CD41, a candidate target gene of SCL, marks the initiation of definitive hematopoiesis during embryoid body development in vitro and in the yolk sac in vivo. Expression of the panhematopoietic marker CD45 appears later in a subpopulation of hematopoietic progenitors in the yolk sac. By the fetal liver stage, the expression of CD41 is down-regulated, whereas CD45 is expressed in all definitive hematopoietic progenitors.
Expression of CD41 is down-regulated in hematopoietic progenitors by the fetal liver stage
The fetal liver serves as the site of definitive hematopoietic development and HSC maturation from E11.5 to early postnatal life. Abundant adult reconstituting HSCs are found in the fetal liver from E12.517,20 46 We extended the sorting analysis with CD45 and CD41 expression to E14.5 fetal liver and performed colony-forming unit assays from sorted subpopulations. In contrast to embryoid bodies and yolk sac in which a significant fraction of hematopoietic progenitors do not express CD45, all hematopoietic activity was detected within the CD45+ population in the E14.5 fetal liver. When subfractionated with CD41 expression, most CFU-C, including mixed colonies and CFU-S8, were found in the CD45 single-positive population (H.K.A.M. and S.H.O., unpublished data, August 2001). Further long-term transplantation studies are required to determine whether CD41 is expressed in adult BM reconstituting stem cells in the fetal liver.
In humans, CD41 cells in cord blood exhibit myeloid and lymphoid potential long-term culture initiating cell (LTC-IC) and nonobese diabetic/severe combined immunodeficient (NOD-SCID) repopulating activity.47 In the avian model, CD41+CD45+ cells also develop from intra-aortic clusters, the sites of HSC emergence, and in embryonic and adult BM exhibit multilineage potential.48 These data suggest that across species, CD41 expression is not restricted to the megakaryocyte lineage. At the time of initiation of definitive hematopoiesis, CD41 is expressed in most definitive hematopoietic progenitors, whereas later in development its expression is restricted to a subset of progenitors with more restricted developmental potential.47-49Differential expression of hematopoietic surface markers during ontogeny has been described for other surface markers, such as CD34, Mac1, and AA4.20,50,51 The functional significance of such variation in relation to specific microenvironment influences has yet to be fully explored, though CD34 positivity appears to correlate with stem cell activation.52 Thus far, no markers that are predominantly associated with the yolk sac stage have been identified. Whether down-regulation of CD41 is involved in the maturation of adult BM reconstituting stem cells remains to be studied by further in vivo assays.
Role of CD41 in hematopoiesis during ontogeny is unknown
We have identified CD41 as a commitment marker for hematopoiesis between Flk1+ hemangioblasts and CD45+hematopoietic cells during mouse ontogeny (Figure 7). Sorting for CD41 and c-kit enables the isolation of cell populations from EBs or yolk sac in which early hematopoietic activity can be studied. Further in vivo studies are required to determine whether CD41+hematopoietic cells represent a transient progenitor population during development or whether they serve as precursors for the developing stem cells. A relationship between CD41 expression and the establishment of definitive hematopoiesis is supported by gene-targeting studies. Expression of Cre recombinase under the control of regulatory elements of the CD41 gene results in blood-specific recombination between loxP sites and the expression of a Cre-activatable reporter gene in all lymphoid and myeloid cells in the fetus.53 If CD41+ cells are the earliest precursors for developing HSCs in the mouse embryo and during in vitro differentiation of mouse ES cells, evaluation of the expression of CD41 during hematopoietic development in human embryos and ES cells will be of interest. In support of the relevance of CD41 as a marker for developmental hematopoiesis in humans, CD41+ cells from human cord blood have been shown to exhibit reconstitution potential.47Furthermore, in the era of stem cell plasticity and transdifferentiation studies, the possibility of existence of CD45−CD41+ hematopoietic progenitors/stem cells during the transdifferentiation process should be considered.
Besides the usefulness of CD41 as a marker of the definitive hematopoietic lineage in the embryo, the functional role of CD41 during hematopoietic development is still unknown. If CD41 is expressed and functionally important in early embryonic hematopoiesis, it is perhaps surprising that CD41 knockout mice fail to demonstrate any striking abnormalities in hematopoiesis other than a platelet function defect.54 This suggests that lack of CD41 and signaling through the CD41CD61 (GPIIbIIIa) complex are not selected against during hematopoietic development. However, it is possible that in hematopoietic progenitors, the function of CD41 can be partially replaced by an alternative dimerization partner for CD61. Recent studies with mast cells in CD41 knockout mice show that the absence of CD41 in BM mast cells resulted in a compensatory increase in αV-integrin levels and a corresponding maintenance of CD61 expression. Compensatory increase of αV-integrin level was not observed in platelets of CD41 knockout mice, in which platelet aggregation was severely impaired. Although the growth and differentiation of CD41-deficient mast cells were not affected, their binding to fibronectin in vitro was impaired, suggesting that the CD41CD61 complex functions as the major fibronectin receptor in mast cells.55 Additional functional studies in vivo and in vitro are required to reveal the role of CD41 in hematopoiesis during murine ontogeny.
S.H.O. is an Investigator of the Howard Hughes Medical Institute. We thank Gordon Keller and Marion Kennedy for help in setting up the blast cell colony assay. We also thank Nancy Speck and Gordon Keller for the runx1/AML−/− 1 ES cells and Yumi Matsuzaki and Attila Fabian for FACS sorting.
Prepublished online as Blood First Edition Paper, September 19, 2002; DOI 10.1182/blood-2002-06-1699.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stuart H. Orkin, Department of Pediatric Oncology, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA, 02115; e-mail: stuart_orkin@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal