We have investigated the role of Smad family proteins, known to be important cytoplasmic mediators of signals from the transforming growth factor–β (TGF-β) receptor serine/threonine kinases, in TGF-β–dependent differentiation of hematopoietic cells, using as a model the human promyelocytic leukemia cell line, HL-60. TGF-β–dependent differentiation of these cells to monocytes, but not retinoic acid–dependent differentiation to granulocytes, was accompanied by rapid phosphorylation and nuclear translocation of Smad2 and Smad3. Vitamin D3 also induced phosphorylation of Smad2/3 and monocytic differentiation; however the effects were indirect, dependent on its ability to induce expression of TGF-β1. Simultaneous treatment of these cells with TGF-β1 and all-trans-retinoic acid (ATRA), which leads to almost equal numbers of granulocytes and monocytes, significantly reduced the level of phospho–Smad2/3 and its nuclear accumulation, compared with that in cells treated with TGF-β1 alone. TGF-β1 and ATRA activate P42/44 mitogen-activated protein (MAP) kinase with nearly identical kinetics, ruling out its involvement in these effects on Smad phosphorylation. Addition of the inhibitor-of-protein serine/threonine phosphatases, okadaic acid, blocks the ATRA-mediated reduction in TGF-β–induced phospho-Smad2 and shifts the differentiation toward monocytic end points. In HL-60R mutant cells, which harbor a defective retinoic acid receptor–α (RAR-α), ATRA is unable to reduce levels of TGF-β–induced phospho-Smad2/3, coincident with its inability to differentiate these cells along granulocytic pathways. Together, these data suggest a new level of cross-talk between ATRA and TGF-β, whereby a putative RAR-α–dependent phosphatase activity limits the levels of phospho-Smad2/3 induced by TGF-β, ultimately reducing the levels of nuclear Smad complexes mediating the TGF-β–dependent differentiation of the cells to monocytic end points.
Introduction
The growth and differentiation of hematopoietic cells are regulated by a number of cytokines, in vitro and in vivo. HL-60, a human promyelocytic cell line, has been extensively used as an in vitro model for studying the effects of factors that regulate growth and differentiation of hematopoietic cells in general, and of myeloid leukemia cells in particular.1These cells proliferate as promyelocytes, yet retain the capacity to undergo terminal myeloid or monocytic differentiation in response to various inducing agents. In the presence of all-trans-retinoic acid (ATRA), HL-60 cells undergo differentiation to granulocytes, whereas 1α,25(OH)2–vitamin D3 (Vit D3) and phorbol ester induce differentiation into monocytes/macrophages.2 Transforming growth factor–β (TGF-β), known to be a negative regulator of growth at all stages of hematopoiesis,3,4 induces differentiation of HL-60 cells to promonocytes, and has been shown to act synergistically with Vit D3, tumor necrosis factor (TNF), or the combination of ATRA plus TNF to induce monocytic differentiation of several other myeloid leukemic cell lines.6,7 Other studies showing that induction of terminal differentiation by retinoids and Vit D3 requires TGF-β1 as an autocrine mediator suggest that endogenous TGF-β1 plays a critical role in the differentiation of leukemia cells.8-10
TGF-β binds to a heteromeric cell-surface complex consisting of 2 type II and 2 type I transmembrane-receptor serine/threonine kinases in which ligand binding induces phosphorylation and activation of the type I receptors by the type II receptor kinases.11,12Signaling is mediated in part by Smad proteins, which are activated directly by the TGF-β type I receptor kinase by phosphorylation on their C-terminus and then translocate to the nucleus in complex with the common mediator Smad4 to regulate transcription of target genes. The principal receptor-activated Smads involved in signaling from TGF-β receptors are Smad2 and Smad3. In the nucleus, Smad proteins can bind directly to their cognate DNA-binding sites and/or interact with an increasing number of transcription factors, transcriptional coactivators, or transcriptional repressors.13,14 The TGF-β1 response can also be regulated by Smad6 and Smad7, which inhibit the TGF-β–induced activation of Smad2 and Smad315,16 and which target the receptor complex for proteasomal degradation.17 The complexity of the TGF-β responses and their dependence on the physiological context and cell type may relate to the relative levels of different Smad proteins and their cooperativity with specific transcription factors, which themselves may be regulated by different signaling pathways.18 Ras, extracellular signal–regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), or p38 mitogen-activated protein kinase (MAPK) may also be regulated by TGF-β in different cell lines.19 Yet other studies show the involvement of S6 kinase in inhibition of growth20,21 and phosphatidylinositol 3 (PI-3) kinase in epithelial-to-mesenchymal transdifferentiation mediated by TGF-β.22
In HL-60 cells, Vit D3 and ATRA have each been shown to stimulate MAP/ERK kinase (MEK)–dependent activation of ERK2,23,24 causing subsequent hypophosphorylation of p53 and retinoblastoma protein (pRb), cell differentiation, and G0 arrest. The MAPK inhibitor PD98059 has been shown to block ATRA-induced granulocytic differentiation.24 In this study, we have investigated the role of Smad proteins in TGF-β1–induced monocytic differentiation in HL-60 cells and in the interplay between MAPK pathways and Smad-signaling pathways. We have found that whereas ATRA, Vit D3, or TGF-β1 each activates ERK1/2 in HL-60 cells, only TGF-β1 or Vit D3, each of which results in monocytic differentiation, results in phosphorylation of Smad2 and Smad3. Moreover, in cells treated simultaneously with TGF-β1 and ATRA, leading to differentiation of cells along both granulocytic and monocytic pathways, ATRA decreases the levels of phosphorylated Smad2/3, possibly by retinoic acid receptor–α (RAR-α)–dependent activation of a putative phosphatase activity, as suggested by experiments using the inhibitor-of-protein serine/threonine phosphatases, okadaic acid. Together, our data suggest that levels of phosphorylated Smad2/3 are sensors of the interplay between ATRA and TGF-β or Vit D3 on HL-60 cells and contribute to the balance achieved between granulocytic and monocytic pathways of differentiation.
Materials and methods
Cell culture and induction of differentiation
The HL-60 cells (American Type Culture Collection, Rockville, MD) and HL-60R cells resistant to retinoic acid–induced differentiation (a gift of Steve Collins, Fred Hutchinson Cancer Center, Seattle, WA) were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 4.0 mM glutamine, nonessential amino acids, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (Life Technologies, Gaithersburg, MD). Experiments were carried out in the same medium supplemented with 5% fetal bovine serum. Differentiation was induced by exposing 3 to 5 × 105 cells per milliliter to 10 nM ATRA (Sigma, St Louis, MO), 10 ng/mL TGF-β1 (a kind gift of R&D Systems, Minneapolis, MN), or 10 nM ATRA plus 10 ng/mL TGF-β1 or 100 nM Vit D3(Roche, Basel, Switzerland) for the indicated time periods. For determination of cell morphology, cytospins were made following 4 days of culture with the various treatments, and slides were stained with Wright Giemsa and photographed at 400 × magnification. PD98059 (Cell Signaling Technology, Beverly, MA) was diluted from a 2-mM stock in dimethyl sulfoxide (DMSO) and added to cells 2 hours prior to addition of TGF-β or ATRA. TGF-β control (12H5) or neutralizing (1D11) antibodies (Genzyme Diagnostics, Cambridge, MA) were used at 30 μg/mL. In the experiments in Table 2, a murine immunoglobulin G1κ (IgG1κ) (BD Pharmingen, San Diego, CA) served as the isotype control. For effects of okadaic acid (LC Laboratories, Woburn, MA) on cellular differentiation, cells were cultured in 2.5 nM okadaic acid for 48 hours (cells showed greater than 60% viability cultured in 2.5 nM okadaic acid for 72 hours).
Flow cytometry analyses
Aliquots of 1 × 106 cells were harvested, washed twice with phophate-buffered saline (PBS), and incubated for 45 minutes at room temperature with 0.5 μL fluorescein isothiocyanate (FITC)–antihuman CD66b (Becton Dickinson, Mountain View, CA), 0.5 μL FITC–antihuman CD15 (Becton Dickinson), or 0.5 μL phycoerythrin (PE)–antihuman CD14 (Becton Dickinson) to analyze the expression of these surface markers. Cells were then washed 3 times with ice-cold PBS, and resuspended in 1 mL PBS. Two-parameter analysis was performed by means of a FACS Calibur Flow Cytometer (Becton Dickinson, Franklin Lakes, NJ). Isotypic mouse IgG1 or IgM was used to set threshold parameters. Functional activity was assessed by cytochemical determination of monocytic nonspecific serine esterase (NSE)5 or nitroblue tetrazolium (NBT) reduction,25 as previously described.
Immunohistochemical staining
For intracellular localization of Smad proteins, cytospins collected at the different treatment time points were fixed in 10% neutral-buffered formalin for 5 to 10 minutes. Staining was performed by means of the Optimax Plus 2.0 Automated Cell Staining System with research software from BioGenex (San Ramon, CA). Following blocking of endogenous peroxidase, nonspecific protein binding was blocked with a solution containing 1% bovine serum albumin (BSA) and 5% goat serum for 30 minutes. Sections were incubated for 2 hours with rabbit anti-Smad2 IgG (Santa Cruz Biotechnology, CA), rabbit anti-Smad3 IgG (Zymed Laboratories, South San Francisco, CA), or normal rabbit IgG at 4 μg/mL in tris-buffered saline (TBS)/1% BSA. Antigen-antibody complexes were detected by means of the Vectastain Elite ABC peroxidase kit from Vector Laboratories (Burlingame, CA) according to the manufacturer's instructions. After a 30-minute incubation with biotinylated secondary antibody and a 30-minute incubation with ABC reagent, a 5-minute reaction with 3,3′diamino-benzidine (DAB)/H2O2 (BioGenex) was used to detect the bound peroxidase. Carazzi hematoxylin was used as the counterstain. Cells were assessed for the presence of nuclear staining of the Smad proteins by means of a × 200 microscope.
Immunofluorescence staining and confocal microscopy
HL-60 cells were treated for the indicated times, and the cells were attached to slides by cytospin, fixed in cold 3.5% paraformaldehyde for 5 minutes, washed with PBS, and permeabilized in methanol at −20°C for 2 minutes as described previously.26 After blocking with 10% normal rabbit serum, the cells were incubated overnight with rabbit anti-Smad2 (1:50) (Santa Cruz Biotechnology) or Smad3 antibodies (1:50) (Zymed Laboratories) in PBS containing 5% normal rabbit serum. Cells were washed with PBS; incubated with antirabbit FITC secondary antibody (1:500) (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hour at room temperature; washed; mounted with medium containing DAPI (4,6-diamidino-2-phenylindole) (Vectashield, Vector Laboratories); and examined by means of a confocal immunofluorescence microscope.
Reverse transcriptase (RT)–PCR assays
Total RNA was extracted from HL-60 cells cultured with various inducing agents for different periods of time with the use of TRIzol Reagent (Life Technologies); cDNA was synthesized with the use of 1 μg total RNA primed with oligo d(T) (deoxy-thymidine) in 50-μL reactions. To test for contamination by genomic DNA, additional reactions were done without adding reverse transcriptase. The resulting total cDNA was then used in the polymerase chain reaction (PCR) to measure the mRNA levels of Smads with the use of primers as described below; the mRNA level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control. Linear amplification cycles were determined separately for each Smad as published elsewhere.27 Smad2, Smad3, Smad4, and Smad7 primers were ordered from BioServe Biotechnologies (Laurel, MD) with the following sequences: Smad2 forward, 5′-TCAAGCTTGAGTGTAAACCCTTACCACTATC-3′; Smad2 reverse, 5′-TAGCGGCCGCGAAAGCTATGATTAACAGGGG-3′; Smad3 forward, 5′-TCAAGCTTGAACACCAGTTCTACCTCCTG-3′; Smad3 reverse, 5′-TAGCGGCCGCGAAATGTCTCCCCGACGCGCTG-3′; Smad4 forward, 5′-TCAAGCTTGATGATCTCTCAGGATTAACAC-3′; Smad4 reverse, 5′-TAGCGGCCGCGAACACCAATACTCAGGAGCAG-3′; Smad7, forward, 5′-GGCTGTGTTGCTGTGAATCTTACG-3′; Smad7 reverse, 5′-CAGTGTGGCGGACTTGATGAAG-3′; GAPDH forward, 5′-CGTTCCCAAAGTCCTCCTGTTTC-3′; and GAPDH, reverse-5′-TTTTTTTCCGCAGCCGCCTG-3′.
As negative controls, tubes without cDNA were included. The PCR products were checked on a 1.5% agrose gel with DNA molecular weight markers (Life Technologies).
P44/42 MAPK kinase assays
Cell lysates (200 μL containing 200 μg total protein) were added to 15 μL (15 μg) resuspended immobilized phospho-p44/42 MAP kinase (Thr202/Tyr204) monoclonal antibody (Cell Signaling Technology), and incubated with gentle rocking overnight at 4°C. Samples were then microcentrifuged for 30 seconds at 4°C, and the pellet was washed twice with 500 μL 1 × lysis buffer and twice with 500 μL 1 × kinase buffer before being resuspended in 50 μL 1 × kinase buffer supplemented with 200 μM adenosine triphosphate (ATP) and 2 μg Ets-like transcription factor–1 (Elk-1) fusion protein and incubated for 30 minutes at 30°C. The reaction was terminated with 25 μL 3 × sodium dodecyl sulfate (SDS) sample buffer, boiled for 5 minutes, vortexed, and micocentrifuged for 2 minutes. The sample (30 μL) was loaded on SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blotting with the use of the phospho–Elk-1 antibody (1:100 dilution) (Cell Signaling Technology).
Western blot analysis
Western blot analysis was performed as described previously.26 Briefly, cells were lysed in 0.5 mL Triton X-100 lysis buffer (25 mM HEPES at pH 7.5, 150 mM NaCl, 10% glycerol, 5 mM EDTA (ethylenediaminetetraacetic acid), 1% Triton X-100) in the presence of phosphatase and protease inhibitors. Whole cell lysates (40 μL) were separated by SDS-PAGE and transferred onto Immobilon-P membranes (Millipore, Bedford, MA). The membrane was incubated for 1 hour in blocking buffer PBS containing 0.05% polysorbate 20 and 5% nonfat dry milk) followed by a 2-hour incubation with anti–phospho-Smad2 antibody (Upstate Biotech, Lake Placid, NY) or with anti-Smad2 antibody (Santa Cruz Biotechnology) in blocking buffer. After extensive washing, the blot was incubated with secondary antibody for 1 hour and processed with the use of Chemiluminescence Reagent according to the manufacturer's directions (Pierce, Rockford, IL). For experiments with okadaic acid and MG-132 (Calbiochem, San Diego, CA), the cells were shifted to medium containing the inhibitors and 0.2% serum for 3 hours, then washed 3 times with 5% serum-containing medium, and incubated in the presence of TGF-β, ATRA, or the combination for another 24 hours in the absence of inhibitors.
Results
Induction of differentiation of HL-60 cells by TGF-β1 and ATRA
It has previously been shown that TGF-β1 and ATRA inhibit the growth of HL-60 cells in a dose-dependent fashion.5Morphological studies revealed that whereas treatment with TGF-β1 alone induced differentiation of HL-60 cells to promonocytes (68%), treatment with both TGF-β1 and ATRA induced both monocytic (54%) and granulocytic (46%) differentiation.5 We obtained similar results from assessment of the morphology of the cells treated with these 2 agents (data not shown) and confirmed the nature and extent of differentiation induced by ATRA or TGF-β1 by examining the expression of lineage-specific markers on HL-60 cells by fluorescence-activated cell sorter (FACS) analysis (Table1). Addition of ATRA to the culture medium induced the expression of the granulocyte marker CD66b in about 90% of the cells, whereas addition of TGF-β induced expression of the monocyte-specific marker CD14 in about 23% of the cells (Table 1). When treated with both TGF-β1 and ATRA, a mixed cell population appeared in which approximately half of the cells expressed CD14 and half of them CD66b; only 5% of the cells exhibited both monocytic and granulocytic characteristics (not shown). Similarly to previously reported results,5 TGF-β treatment induced expression of the cytoplasmic enzyme, NSE5 in about 22% of the cells, whereas about 26% of the cells expressed NSE following treatment with both ATRA and TGF-β1 (Table 1). The increased expression of CD14 and NSE following induction of differentiation in these cells by TGF-β1 confirms previous observations that TGF-β1 stimulates monocytic differentiation of HL-60 cells. It is noteworthy that while TGF-β1 enhances the commitment of HL-60 cells to the monocytic lineage, it can induce differentiation only to promonocytes, which variably express either no CD14 or low CD14.5 ATRA, in combination with TGF-β, acts both to drive the differentiation of these committed cells all the way to mature, CD14+ monocytes, and to enhance the differentiation of cells committed to the granulocytic lineage to CD66b+ granulocytes.
Effect of TGF-β1 and ATRA on HL-60 cell differentiation
| Treatment . | CD14+, % . | CD66b+, % . | NSE+, % . |
|---|---|---|---|
| Control | 4 ± 2 | 4 ± 1 | 1 ± 1 |
| TGF-β1 | 23 ± 3 | 3 ± 1 | 22 ± 5 |
| ATRA | 5 ± 2 | 90 ± 7 | 2 ± 1 |
| TGF-β1 + ATRA | 46 ± 4 | 45 ± 9 | 26 ± 4 |
| Treatment . | CD14+, % . | CD66b+, % . | NSE+, % . |
|---|---|---|---|
| Control | 4 ± 2 | 4 ± 1 | 1 ± 1 |
| TGF-β1 | 23 ± 3 | 3 ± 1 | 22 ± 5 |
| ATRA | 5 ± 2 | 90 ± 7 | 2 ± 1 |
| TGF-β1 + ATRA | 46 ± 4 | 45 ± 9 | 26 ± 4 |
Cells were treated with 10 ng/mL TGF-β, 100 nM ATRA, or both for 4 days. Antibodies and FACS analysis and assay for NSE are as described in “Materials and methods.”
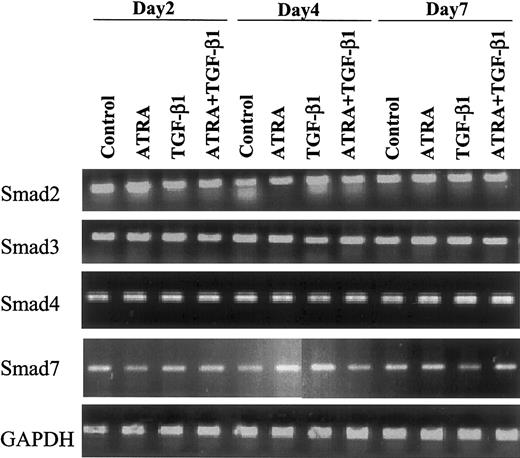
Expression of Smad mRNAs in HL-60 cells is not modulated by TGF-β or ATRA
To ascertain whether treatment with TGF-β or ATRA might alter the expression of Smads in the cells, we examined the expression of Smad2, 3, 4, and 7 mRNAs in HL-60 cells by semiquantitative RT-PCR. Each Smad was expressed in the cells, and the levels of expression were invariant whether cells were treated with 10 nM ATRA, 10 ng/mL TGF-β1, or 10 nM ATRA plus 10 ng/mL TGF-β1 and whether they were treated for 2, 4, or 7 days (Figure1).
Effect of ATRA versus TGF-β1 on expression of Smad mRNAs.
Expression of Smad mRNAs is not changed following induction of differentiation of HL-60 cells to monocytes or granulocytes by 10 nM ATRA or 10 ng/mL TGF-β1. Human Smad-specific primer pairs were selected from the corresponding cDNA sequence information obtained from the National Institutes of Health (NIH) database as indicated in “Materials and methods.” Primer pairs were used to amplify Smad-specific fragments from reverse-transcribed total RNA isolated from the indicated samples as template. In all cases, 1 μg total RNA, quantified by spectrophotometry and agarose gel analysis, was used for reverse transcription. Smad2, Smad3, Smad4, Smad7, and GAPDH (as an internal control) were amplified by PCR and analyzed on an agarose gel.
Effect of ATRA versus TGF-β1 on expression of Smad mRNAs.
Expression of Smad mRNAs is not changed following induction of differentiation of HL-60 cells to monocytes or granulocytes by 10 nM ATRA or 10 ng/mL TGF-β1. Human Smad-specific primer pairs were selected from the corresponding cDNA sequence information obtained from the National Institutes of Health (NIH) database as indicated in “Materials and methods.” Primer pairs were used to amplify Smad-specific fragments from reverse-transcribed total RNA isolated from the indicated samples as template. In all cases, 1 μg total RNA, quantified by spectrophotometry and agarose gel analysis, was used for reverse transcription. Smad2, Smad3, Smad4, Smad7, and GAPDH (as an internal control) were amplified by PCR and analyzed on an agarose gel.
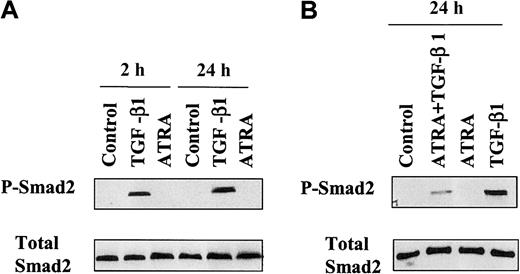
TGF-β1 induces the phosphorylation and nuclear accumulation of endogenous Smad2/3
The phosphorylation of Smad2 on Ser465/467 and subsequent dimerization with Smad4 and translocation to the nucleus are known to be required for signal transduction by the TGF-β receptor, leading to the transcriptional activation of target genes.13 To investigate if Smad2 is phosphorylated following treatment of HL-60 cells with ATRA, TGF-β1, or the combination of ATRA plus TGF-β1, the levels of C–terminally phosphorylated Smad2 in cell lysates were measured by Western blot. Immunoblotting with an antibody that specifically recognizes Ser465/467–phosphorylated Smad2 showed that whereas ATRA alone had no detectable effect on the phosphorylation of Smad2 at 2 hours or 24 hours, treatment with TGF-β1 strongly induced Smad2 phosphorylation (Figure 2A). Combined treatment with ATRA plus TGF-β1 significantly reduced the level of phospho-Smad2 in the total cell population compared with that seen in cells treated with TGF-β alone (Figure 2B). The expression level of total Smad2 remained unchanged, as shown by reblotting the membranes with an anti-Smad2 antibody (Figure 2A-B). Although an antibody for phospho-Smad3 is not available, assessment of the binding of Smad3 to a biotinylated CAGA Smad-binding oligonucleotide28 29 showed that it was activated in a pattern similar to that of Smad2 (data not shown).
Western blot analysis of activation of endogenous Smad2 in HL-60 cells.
Protein prepared from HL-60 cells untreated or treated with 10 ng/mL TGF-β1 or 10 nM ATRA (A) or treated with both (B) for the indicated times, as described in “Materials and methods,” were directly subjected to immunoblotting with antibodies against Smad2 (Total Smad2) or C–terminally phosphorylated Smad2 (P-Smad2).
Western blot analysis of activation of endogenous Smad2 in HL-60 cells.
Protein prepared from HL-60 cells untreated or treated with 10 ng/mL TGF-β1 or 10 nM ATRA (A) or treated with both (B) for the indicated times, as described in “Materials and methods,” were directly subjected to immunoblotting with antibodies against Smad2 (Total Smad2) or C–terminally phosphorylated Smad2 (P-Smad2).
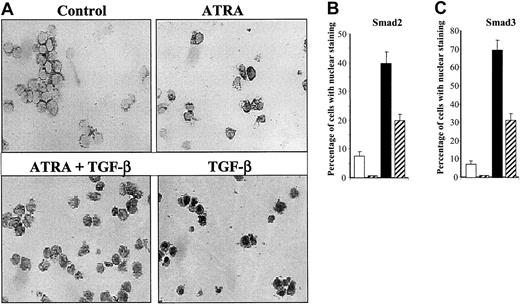
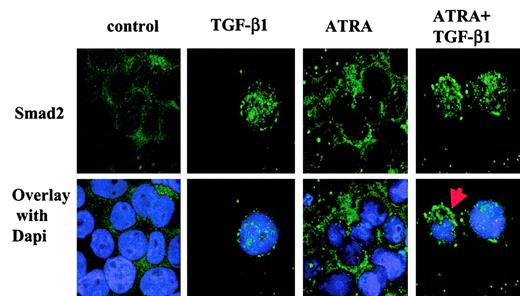
To ascertain whether these observed changes in the phosphorylation of Smad2 and Smad3 were accompanied by changes in the intracellular distribution of these Smad proteins in HL-60 cells, we used specific antibodies30 to assess the intracellular localization of endogenous Smad2 and Smad3 after treatment of the cells with 10 ng/mL TGF-β1, 10 nM ATRA, or a combination of 10 nM ATRA plus 10 ng/mL TGF-β1 for 18 hours. In the control group, endogenous Smad2 and Smad3 are found predominantly in the cytoplasm (Figure3A). TGF-β1 stimulation resulted in the translocation of Smad2/Smad3 to the nucleus. Quantitation of the percentage of cells with nuclear staining showed that at 18 hours about 75% and 41% of the cells showed staining for Smad3 or Smad2, respectively, in the nucleus (Figure 3B-C). Significantly, treatment with ATRA alone reduced the basal number of cells showing Smad2/Smad3 nuclear staining, and treatment with both ATRA and TGF-β1 strongly reduced the number of cells exhibiting nuclear Smad2/3, compared with that seen following treatment with TGF-β1 alone. These changes in the subcellular localization of Smad2/3 in particular subpopulations of cells were confirmed by confocal microscopy, which clearly demonstrated the nuclear translocation of Smad2 induced by treatment with TGF-β1 alone, and the presence of a mixed population of cells with either nuclear or cytoplasmic localization following treatment with both ATRA and TGF-β1 (Figure 4). Together, these results demonstrate that TGF-β1 induces phosphorylation and nuclear translocation of Smad2/Smad3, which are likely to play a critical role in activation of the transcriptome responsible for TGF-β1–induced monocytic differentiation of HL-60 cells. Moreover, the data show that treatment with ATRA decreases the number of cells in which there is detectable nuclear Smad2/3 staining compared with cells treated with TGF-β1 alone, consistent with the hypothesis that the subset of cells lacking nuclear Smad2/3 may then be committed to differentiate to granulocytes.
Immunohistochemical staining of Smad3/Smad2 in HL-60 cells.
(A) Expression of Smad3 as detected by anti-Smad3 antibody in HL-60 cells untreated (white) or treated with 10 nM ATRA (gray striped), with 10 ng/mL TGF-β1 (black), or with both (black and white striped) for 18 hours as described. Original magnification × 200. (B-C) Following immunohistochemical staining, the percentage of cells with Smad2 (B) or Smad3 (C) staining predominantly or exclusively in the nucleus was determined among 1000 cells in 5 different fields. Values are expressed as the means ± SEMs of 3 experiments.
Immunohistochemical staining of Smad3/Smad2 in HL-60 cells.
(A) Expression of Smad3 as detected by anti-Smad3 antibody in HL-60 cells untreated (white) or treated with 10 nM ATRA (gray striped), with 10 ng/mL TGF-β1 (black), or with both (black and white striped) for 18 hours as described. Original magnification × 200. (B-C) Following immunohistochemical staining, the percentage of cells with Smad2 (B) or Smad3 (C) staining predominantly or exclusively in the nucleus was determined among 1000 cells in 5 different fields. Values are expressed as the means ± SEMs of 3 experiments.
Localization of endogenous Smad2 in HL-60 cells by confocal microscopy.
After 18-hour incubation with 10 ng/mL TGF-β1, 10 nM ATRA, or both, the cells were fixed, subjected to immunochemistry with primary antibodies against Smad2 and secondary antibodies linked to fluorescein isothiocyanate, and mounted with medium containing 4,6- DAPI. The red arrow indicates that Smad2 is localized both in the nucleus and in the cytoplasm. Original magnification for all panels, × 630. The result is representative of 3 similar experiments.
Localization of endogenous Smad2 in HL-60 cells by confocal microscopy.
After 18-hour incubation with 10 ng/mL TGF-β1, 10 nM ATRA, or both, the cells were fixed, subjected to immunochemistry with primary antibodies against Smad2 and secondary antibodies linked to fluorescein isothiocyanate, and mounted with medium containing 4,6- DAPI. The red arrow indicates that Smad2 is localized both in the nucleus and in the cytoplasm. Original magnification for all panels, × 630. The result is representative of 3 similar experiments.
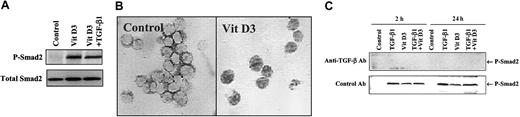
Vitamin D3 induces phosphorylation of Smad proteins indirectly through a mechanism dependent on TGF-β
It has been reported that the induction of terminal differentiation by Vit D3 requires TGF-β1 as an autocrine mediator in U937 cells.6 We have shown that treatment of HL-60 cells with 100 nM Vit D3 induces monocytic differentiation to CD14+ cells (not shown) characterized by NSE activity (Table 2). To investigate if Smad2 and Smad3 are also activated in Vit D3–induced monocytic differentiation, we examined the level of phosphorylated Smad2. Strong phosphorylation of Smad2 can be seen after 24 hours' incubation with 100 nM Vit D3 (Figure5A) coincident with nuclear translocation of Smad2/Smad3 (Figure 5B). After incubation with 100 nM Vit D3 for 18 hours, about 74% and 39% of the cells showed nuclear staining for Smad3 or Smad2, respectively, similar to the pattern seen in cells treated with TGF-β (compare with Figure 3B-C). To test whether these effects of Vit D3 on Smad phosphorylation were dependent on induction of TGF-β activity, we treated cells with either control antibodies or antibodies to TGF-β. As shown in Figure 5C, the TGF-β1–neutralizing antibody 1D11 blocked both Vit D3– and TGF-β–induced phosphorylation of Smad2, whereas the control antibody (IgG1κ) had no effect. To ascertain that the effects of the blocking antibody on Smad2 phosphorylation paralleled effects on differentiation, we assessed the functional activity of HL-60 cells 6 days after treatment with Vit D3 or TGF-β, using the NSE assay for monocytic differentiation and the reduction of NBT as a measure of granulocytic differentiation. As shown in Table 2, addition of the 1D11 TGF-β blocking antibody to cells treated with Vit D3 reduced the proportion of NSE+ cells from 66% to 23%, whereas an isotype control antibody had no effect. For comparison, addition of the antibody to cells treated with TGF-β reduced the number of NSE+ cells from 16% to 1%. Together, these results strongly suggest that Vit D3 induces monocytic differentiation of HL-60 cells, at least in part, through expression of TGF-β and subsequent activation of the Smad-signaling pathway.
Antibodies to TGF-β interfere with the induction of monocytic differentiation by vitamin D3
| Treatment . | NSE+, %* . | NBT+, %* . |
|---|---|---|
| Control | 3 ± 1 | 5 ± 3 |
| Vit D3 | 66 ± 5 | 3 ± 2 |
| 1D11 | 0 | 0 |
| Vit D3/1D11 | 23 ± 4 | 1 ± 1 |
| Vit D3/isotype control | 61, 57 | 7, 5 |
| TGF-β | 16 ± 7 | 0 |
| TGF-β/1D11 | 1 ± 1 | 0 |
| Treatment . | NSE+, %* . | NBT+, %* . |
|---|---|---|
| Control | 3 ± 1 | 5 ± 3 |
| Vit D3 | 66 ± 5 | 3 ± 2 |
| 1D11 | 0 | 0 |
| Vit D3/1D11 | 23 ± 4 | 1 ± 1 |
| Vit D3/isotype control | 61, 57 | 7, 5 |
| TGF-β | 16 ± 7 | 0 |
| TGF-β/1D11 | 1 ± 1 | 0 |
Cells were cultured for 6 days in 5% serum plus the following additions as indicated: Vit D3, 100 nM, TGF-β, 5 ng/mL; 1D11, 30 μg/mL, IgG1κ (isotype control, 30 μg/mL). NSE and NBT were assayed as described in “Materials and methods.” Data are the averaged values of 3 separate experiments ± standard error (SE), with the exception of the isotype control, which was performed twice.
Five hundred cells were counted.
Vitamin D3induction of activation of Smad2/Smad3 in HL-60 cells.
Vitamin D3 induces activation of Smad2/Smad3 in HL-60 cells in a TGF-β–dependent manner. (A) Western blot analysis of phosphorylation of endogenous Smad2 in HL-60 cells untreated or treated with 100 nM Vit D3 for 24 hours. (B) Immunohistochemical staining of Smad3 in HL-60 cells untreated (Control) or treated with 100 nM Vit D3 at 18 hours. Original magnification × 400. (C) TGF-β1–neutralizing antibodies block the phosphorylation of Smad2 induced by treatment of Hl-60 cells with either 10 ng/mL TGF-β or 100 nM Vit D3. Lysates from cells incubated in either the presence or absence of 12.5 μg/mL TGF-β1 control (12H5) or neutralizing (1D11) antibody at 2 hours or 24 hours were directly subjected to immunoblotting with antibodies against Smad2 (not shown) and phosphorylated Smad2.
Vitamin D3induction of activation of Smad2/Smad3 in HL-60 cells.
Vitamin D3 induces activation of Smad2/Smad3 in HL-60 cells in a TGF-β–dependent manner. (A) Western blot analysis of phosphorylation of endogenous Smad2 in HL-60 cells untreated or treated with 100 nM Vit D3 for 24 hours. (B) Immunohistochemical staining of Smad3 in HL-60 cells untreated (Control) or treated with 100 nM Vit D3 at 18 hours. Original magnification × 400. (C) TGF-β1–neutralizing antibodies block the phosphorylation of Smad2 induced by treatment of Hl-60 cells with either 10 ng/mL TGF-β or 100 nM Vit D3. Lysates from cells incubated in either the presence or absence of 12.5 μg/mL TGF-β1 control (12H5) or neutralizing (1D11) antibody at 2 hours or 24 hours were directly subjected to immunoblotting with antibodies against Smad2 (not shown) and phosphorylated Smad2.
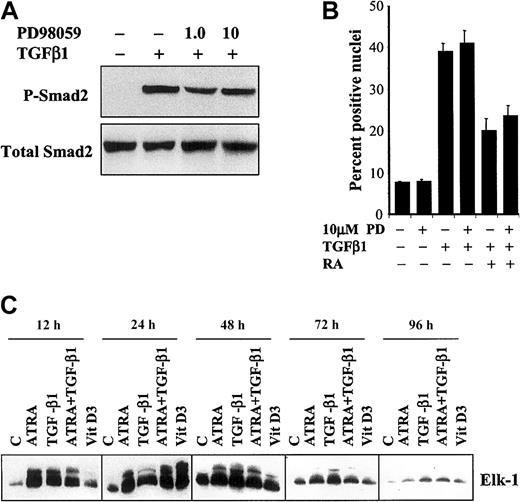
Induction of both monocytic and granulocytic differentiation of HL-60 cells is accompanied by activation of ERK1/2
Activation of the ERK1/2 MAPK pathway is essential for granulocytic differentiation of HL-60 cells by retinoic acid.24 Since this same pathway has been shown to inhibit nuclear translocation of Smad2/Smad3 induced by TGF-β,31we investigated whether cross-talk between the ERK1/2 MAPK pathway and the Smad pathway could alter the pattern of phosphorylation or nuclear accumulation of Smads in HL-60 cells treated with either TGF-β1 alone or the combination of ATRA and TGF-β1. A specific inhibitor of ERK1/2, PD98059 (1.0 and 10 μM), inhibited phosphorylation of ERK1/2 in a dose-dependent manner (data not shown), but had no effect on either TGF-β–induced phosphorylation of Smad2 (Figure6A) or nuclear translocation of Smad3 in HL-60 cells treated with either TGF-β1 alone or the combination of TGF-β1 and ATRA (Figure 6A). Moreover, when we examined the effects of ATRA, TGF-β1, ATRA plus TGF-β1, or Vit D3 on the activation of the ERK1/2 MAPK pathway in HL-60 cells, each of these treatments increased activation of this pathway with a similar time course, as measured by phosphorylation of the substrate Elk-1 in an in vitro kinase assay (Figure 6C). The somewhat delayed induction of ERK activation by Vit D3 may suggest that it, like Vit D3–induced Smad phosphorylation, is mediated indirectly by TGF-β1. Together, these data show that TGF-β activates both the Smad and ERK1/2 pathways in HL-60 cells, but that these pathways appear to act independently, since activation of ERK1/2 occurs at an early stage of differentiation independently of the effects of the various differentiating agents on activation of Smad2/Smad3 or on commitment to specific lineages.
The effect of inhibition of MAPK on phosphorylation and nuclear translocation of Smad2 induced by TGF-β1.
(A) Phosphorylation of Smad2 was not affected by treatment with 10 μM PD98059 for 2 hours followed by addition of 10 ng/mL TGF-β1 for an additional 24 hours. (B) There was no significant difference observed in the percentage of nuclei positive for Smad2 immunostaining (of 1000 cells counted in 5 different fields) 18 hours after treatment with 10 ng/mL TGF-β1 and 10 nM ATRA with or without the addition of 10 μM PD98059 (10 μM PD) at 2 hours prior to the other treatments (means ± SEMs, n = 3). (C) The p44/42 MAP kinase activity is activated independently of the particular pathway of differentiation. Proteins were immunoprecipitated with anti–phospho-p44/42 MAP kinase antibody as described in “Materials and methods,” and the immunoprecipitates were subjected to an in vitro kinase assay with the use of the Elk-1 fusion protein. Reaction mixtures were separated by SDS-PAGE and immunoblotted with anti–phospho–Elk-1 antibody. The data shown are representative of 3 experiments with similar results.
The effect of inhibition of MAPK on phosphorylation and nuclear translocation of Smad2 induced by TGF-β1.
(A) Phosphorylation of Smad2 was not affected by treatment with 10 μM PD98059 for 2 hours followed by addition of 10 ng/mL TGF-β1 for an additional 24 hours. (B) There was no significant difference observed in the percentage of nuclei positive for Smad2 immunostaining (of 1000 cells counted in 5 different fields) 18 hours after treatment with 10 ng/mL TGF-β1 and 10 nM ATRA with or without the addition of 10 μM PD98059 (10 μM PD) at 2 hours prior to the other treatments (means ± SEMs, n = 3). (C) The p44/42 MAP kinase activity is activated independently of the particular pathway of differentiation. Proteins were immunoprecipitated with anti–phospho-p44/42 MAP kinase antibody as described in “Materials and methods,” and the immunoprecipitates were subjected to an in vitro kinase assay with the use of the Elk-1 fusion protein. Reaction mixtures were separated by SDS-PAGE and immunoblotted with anti–phospho–Elk-1 antibody. The data shown are representative of 3 experiments with similar results.
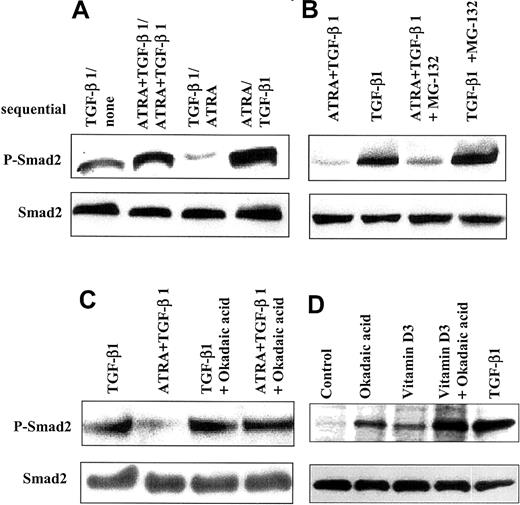
ATRA increases dephosphorylation of Smad2
Since ATRA decreases levels of phospho-Smad2/Smad3 and reduces the number of cells with nuclear staining for Smad2/Smad3 in cells treated with TGF-β1, we investigated whether pre-exposure of HL-60 cells to ATRA or TGF-β1 would affect the subsequent ability of these agents to activate Smad2. HL-60 cells were incubated for 2 days in the presence of either TGF-β1, ATRA, or the combination; washed to remove these effectors; and then incubated with a different factor for another 24 hours prior to analysis of the levels of phospho-Smad2 (Figure7A). These experiments showed that the level of phospho-Smad2 in cells that had been treated with TGF-β1 for 2 days was lower when treatment was followed by addition of ATRA for 24 hours than when cells were treated for the final 24 hours with only control medium. As before, there was no change in the expression level of total Smad2. We interpreted these data to suggest that treatment with ATRA actively reduced the level of existing phospho-Smad2 in cells previously treated with TGF-β1.
Effect of ATRA on dephosphorylation of Smad2.
ATRA increases dephosphorylation of Smad2. (A) ATRA reduced the levels of phospho-Smad2 induced by TGF-β1 in HL-60 cells. HL-60 cells treated sequentially showed a lower level of phospho-Smad2 when cells were incubated with 10 ng/mL TGF-β1 for 48 hours, washed, and then treated with 10 nM ATRA for 24 hours, as compared with cells similarly pretreated with 10 ng/mL TGF-β1, washed, and treated with control medium. (B-D) Cells were changed to 0.2% serum, and okadaic acid or MG-132 was added. After 3 hours, cells were washed 3 times with medium containing 5% serum and incubated for an additional 24 hours with addition of TGF-β, Vit D3, ATRA, or TGF-β plus ATRA, in the absence of the inhibitors. Lysates were analyzed by immunoblotting with an antibody specific to C–terminally phosphorylated Smad2. (B) The proteasome inhibitor MG-132 (50 μM) did not have specific effects on cells treated with ATRA. (C) Treatment with okadaic acid (100 nM) blocks the ability of ATRA (10 nM) to decrease levels of phospho-Smad2 induced by TGF-β1 (10 ng/mL). (D) Treatment with okadaic acid (100 nM) alone enhances detection of phospho-Smad2, in addition to its ability to augment levels induced by Vit D3.
Effect of ATRA on dephosphorylation of Smad2.
ATRA increases dephosphorylation of Smad2. (A) ATRA reduced the levels of phospho-Smad2 induced by TGF-β1 in HL-60 cells. HL-60 cells treated sequentially showed a lower level of phospho-Smad2 when cells were incubated with 10 ng/mL TGF-β1 for 48 hours, washed, and then treated with 10 nM ATRA for 24 hours, as compared with cells similarly pretreated with 10 ng/mL TGF-β1, washed, and treated with control medium. (B-D) Cells were changed to 0.2% serum, and okadaic acid or MG-132 was added. After 3 hours, cells were washed 3 times with medium containing 5% serum and incubated for an additional 24 hours with addition of TGF-β, Vit D3, ATRA, or TGF-β plus ATRA, in the absence of the inhibitors. Lysates were analyzed by immunoblotting with an antibody specific to C–terminally phosphorylated Smad2. (B) The proteasome inhibitor MG-132 (50 μM) did not have specific effects on cells treated with ATRA. (C) Treatment with okadaic acid (100 nM) blocks the ability of ATRA (10 nM) to decrease levels of phospho-Smad2 induced by TGF-β1 (10 ng/mL). (D) Treatment with okadaic acid (100 nM) alone enhances detection of phospho-Smad2, in addition to its ability to augment levels induced by Vit D3.
To address possible mechanisms whereby ATRA might decrease levels of phospho-Smad2/3 in cells treated with TGF-β1, we investigated whether either proteasome-mediated degradation of phospho-Smad32or phosphatase-mediated dephosphorylation might be involved. Addition of the proteasome inhibitors MG-132 or lactacystin (not shown) had only a small effect, slightly increasing the levels of phospho-Smad2 seen in cells treated with either TGF-β1 alone or the combination of TGF-β1 and ATRA (Figure 7B). In contrast, preincubation of HL-60 cells with okadaic acid, an inhibitor of protein serine/threonine phosphatases,33 blocked the ability of ATRA to reduce the level of phospho-Smad2 in cells treated with both TGF-β and ATRA (Figure 7C). Surprisingly, treatment of the cells with okadaic acid alone resulted in detectable phospho-Smad2, although at levels lower than that observed by treatment of the cells with TGF-β (Figure7D). Together, these data suggest that endogenous protein serine-threonine phosphatases probably control the basal level of phospho-Smad2 and that ATRA reduces levels of phospho-Smad2 through induction of such phosphatases.
We then assessed the effect of okadaic acid on the differentiation of HL-60 cells treated with ATRA, TGF-β, or both to test the hypothesis that induction of the putative serine/threonine phosphatase would favor granulocytic differentiation and that inhibition of serine/threonine phosphatase activity would shift the balance toward monocytes. As shown in Table 3, treatment of HL-60 cells with ATRA for 2 days followed by incubation for an additional 2 days with 2.5 nM okadaic acid increased expression of both CD14 and NSE activity and decreased expression of CD15, compared with cells treated only with ATRA. Okadaic acid also strongly enhanced the ability of TGF-β to induce differentiation to functional monocytes and, when added for the second 2 days to cells treated with the combination of ATRA and TGF-β, reduced the expression of CD15 and enhanced expression of both CD14 and NSE activity. These data show that okadaic acid enhances the proportion of HL-60 cells expressing monocytic features, independently of the presence of other differentiating agents, and consistent with its effects on phospho-Smad2 (Figure 7C-D). When cells are treated with both ATRA and TGF-β, which results in reduced nuclear phospho-Smad2/3 compared with cells treated with TGF-β alone (Figures 2-3), the addition of okadaic acid inhibits the ATRA-dependent suppression of Smad2/3 phosphorylation (Figure 7C) simultaneously with its skewing the differentiation toward monocytes (Table 3).
Okadaic acid enhances monocytic differentiation of HL-60 cells
| Treatment . | CD14+CD15−, % . | CD15+CD14−, % . | NSE+, %3-150 . |
|---|---|---|---|
| 2d C, 2d C | 1, 1 | 84, 87 | 0, 0 |
| 2d C, 2d OA | 39, 32 | 7, 11 | 35, 32 |
| 2d ATRA, 2d C | 1, 2 | 79, 84 | 0, 3 |
| 2d ATRA, 2d OA | 29, 27 | 8, 11 | 62, 47 |
| 2d ATRA, 2d ATRA | 1, 3 | 84, 91 | 0, 0 |
| 2d ATRA, 2d ATRA + OA | 23, 24 | 11, 19 | 47, 38 |
| 2d TGF-β, 2dC | 6, 8 | 75, 71 | 37, 39 |
| 2d TGF-β, 2d OA | 43, 37 | 9, 14 | 74, 69 |
| 2d TGF-β + ATRA, 2d TGF-β + ATRA | 31, 35 | 63, 55 | 65, 57 |
| 2d TGF-β + ATRA, 2d TGF-β + ATRA + OA | 51, 52 | 22, 28 | 81, 91 |
| Treatment . | CD14+CD15−, % . | CD15+CD14−, % . | NSE+, %3-150 . |
|---|---|---|---|
| 2d C, 2d C | 1, 1 | 84, 87 | 0, 0 |
| 2d C, 2d OA | 39, 32 | 7, 11 | 35, 32 |
| 2d ATRA, 2d C | 1, 2 | 79, 84 | 0, 3 |
| 2d ATRA, 2d OA | 29, 27 | 8, 11 | 62, 47 |
| 2d ATRA, 2d ATRA | 1, 3 | 84, 91 | 0, 0 |
| 2d ATRA, 2d ATRA + OA | 23, 24 | 11, 19 | 47, 38 |
| 2d TGF-β, 2dC | 6, 8 | 75, 71 | 37, 39 |
| 2d TGF-β, 2d OA | 43, 37 | 9, 14 | 74, 69 |
| 2d TGF-β + ATRA, 2d TGF-β + ATRA | 31, 35 | 63, 55 | 65, 57 |
| 2d TGF-β + ATRA, 2d TGF-β + ATRA + OA | 51, 52 | 22, 28 | 81, 91 |
Okadaic acid concentration is 2.5 nM; TGF-β is 10 ng/mL; ATRA is 100 nM for the first 2 days of incubation and 10 nM for the second 2 days of incubation. Experiments are done in 5% serum. Individual values are shown for 2 separate experiments. 2d indicates second 2 days of incubation; C, control medium; and OA, okadaic acid.
500 cells were counted.
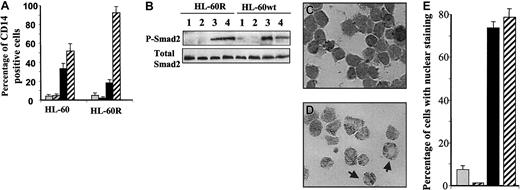
The ability of ATRA to decrease levels of phospho-Smad2/3 is dependent on RAR-α
To further address the mechanism by which ATRA modulates the levels of phospho-Smad2 and to link these effects to the ability of ATRA to induce granulocytic differentiation of HL-60 cells, we used retinoic acid–resistant cells (HL-60R), which have a point mutation in the RAR-α ligand–binding domain that confers dominant-negative activity.34 ATRA is unable to induce granulocytic differentiation of HL-60R cells (Robertson et al,34 and data not shown), whereas the ability of TGF-β1 to induce monocytic differentiation, as assessed by expression of CD14, is unimpaired (Figure 8A). The ability of ATRA, when added with TGF-β, to stimulate maturation of promonocytic HL-60R cells stands in strong contrast to its inability to stimulate granulocytic differentiation, suggesting different roles of RAR-α in these 2 processes. Immunoblotting with the anti–phospo-Smad2 antibody demonstrated that ATRA is unable to reduce levels of phospho-Smad2 in HL-60R cells treated with ATRA and TGF-β1, with the result that the level of phosphorylated Smad2 is similar in cells treated with TGF-β1 alone or with the combination of ATRA and TGF-β1 (Figure 8B). Consistent with this, immunohistochemical staining showed that the percentage of cells with nuclear staining for Smad2/Smad3 was also similar in cells treated with either TGF-β1 alone or the combination of ATRA and TGF-β1 (Figure 8C,E).
The effect of RAR-α on the ability of ATRA to decrease levels of phospho-Smad2/Smad3.
The ability of ATRA to decrease levels of phospho-Smad2/Smad3 is dependent on RAR-α. (A) Treatment for 4 days with ATRA together with TGF-β1 enhances the expression of CD14 by HL-60R compared with cells treated with TGF-β1 alone (means ± SEMs). (B) In contrast to wild-type HL-60, ATRA is unable to reduce levels of phospho-Smad2 induced by TGF-β1 in RAR-α–mutant HL-60 cells (HL-60R) treated simultaneously with ATRA and TGF-β1 (lane 3 compared with lane 4). Treatments were for 24 hours. (C-E) Immunohistochemical staining showed that ATRA is unable to reduce the TGF-β1–dependent Smad3 nuclear localization in RAR-α–mutant HL-60 cells treated for 18 hours with ATRA and TGF-β1 (C) as seen in wild-type HL-60 cells (D). Original magnification × 400. The arrow on the left shows a cell with both nuclear and cytoplasmic staining, and the arrow on the right shows a cell with only cytoplasmic staining. (E) Quantitation of the data in panel C was obtained by assessment of the staining patterns in 1000 cells in 5 different fields of treated HL-60R cells. For panels A, B, and E, treatments were (1) vehicle (gray); (2) ATRA, 10 nM (black and gray striped); (3) TGF-β1, 10 ng/mL (black); or (4) the combination of ATRA and TGF-β1 (black and white striped).
The effect of RAR-α on the ability of ATRA to decrease levels of phospho-Smad2/Smad3.
The ability of ATRA to decrease levels of phospho-Smad2/Smad3 is dependent on RAR-α. (A) Treatment for 4 days with ATRA together with TGF-β1 enhances the expression of CD14 by HL-60R compared with cells treated with TGF-β1 alone (means ± SEMs). (B) In contrast to wild-type HL-60, ATRA is unable to reduce levels of phospho-Smad2 induced by TGF-β1 in RAR-α–mutant HL-60 cells (HL-60R) treated simultaneously with ATRA and TGF-β1 (lane 3 compared with lane 4). Treatments were for 24 hours. (C-E) Immunohistochemical staining showed that ATRA is unable to reduce the TGF-β1–dependent Smad3 nuclear localization in RAR-α–mutant HL-60 cells treated for 18 hours with ATRA and TGF-β1 (C) as seen in wild-type HL-60 cells (D). Original magnification × 400. The arrow on the left shows a cell with both nuclear and cytoplasmic staining, and the arrow on the right shows a cell with only cytoplasmic staining. (E) Quantitation of the data in panel C was obtained by assessment of the staining patterns in 1000 cells in 5 different fields of treated HL-60R cells. For panels A, B, and E, treatments were (1) vehicle (gray); (2) ATRA, 10 nM (black and gray striped); (3) TGF-β1, 10 ng/mL (black); or (4) the combination of ATRA and TGF-β1 (black and white striped).
Discussion
This study provides new insights into mechanisms by which hematopoietic cells interpret and integrate the multiplicity of extracellular signals that ultimately specify distinct lineage decisions. Using the model system of HL-60 cells, a human myeloblastic leukemia with promyelocytic features, we have shown that the interplay of signals from ATRA, which specifies differentiation to granulocytes, or TGF-β1/Vit D3, which specify commitment to monocytic differentiation, is mediated, in part, through a balance between protein serine/threonine phosphatase activity and levels of phosphorylated Smad2 and Smad3. Thus, we have shown that TGF-β1 induces phosphorylation of Smad2/3 and that addition of ATRA together with TGF-β1 reduces the level of phospho-Smad2/3 and increases the extent of commitment to the granulocytic lineage at the expense of the monocytic lineage. Conversely, okadaic acid, which inhibits protein serine/threonine phosphatases and which enhances the level of phospho-Smad2/3 in cells, pushes the balance toward monocytic differentiation. Together, these data suggest that monocytic differentiation is favored by lower protein phosphatase activity and/or increased levels of nuclear Smad2/3 (if the inducing agents are TGF-β or Vit D3) and that granulocytic differentiation is favored by higher protein phosphatase activity and/or reduced nuclear Smad2/3. In the specific case in which ATRA and either TGF-β or Vit D3 are acting on the cell simultaneously, the data suggest that the induction of protein serine/threonine phosphatase activity by ATRA can modulate the levels of phospho-Smad2/3 induced by TGF-β and thereby alter the partitioning between the granulocytic and monocytic pathways.
Signal transduction pathways are regulated by dynamic interplay between protein kinases and phosphatases. Numerous reports have examined the ability of ATRA to alter phosphatase activity in HL-60 cells during the process of differentiation to granulocytes.35-38 Thus, Src homology 2 (SH2)–containing protein tyrosine phosphatase–1 (SHP-1), a protein tyrosine phosphatase, was shown to be induced by ATRA in HL-60 cells,35 but another report showing it to be also elevated by phorbol ester–induced monocytic differentiation of these same cells39 raises questions regarding the lineage specificity of this effect. Most germane to our findings is the report that ATRA elicits a transient and reversible interconversion of the protein phosphatase 2A (PP2A) holoenzyme at the G1/S boundary during ATRA-induced granulocytic differentiation of HL-60 cells.36 PP2A accounts for the majority of the serine/threonine phosphatase activity in most cells and is inhibited by okadaic acid.40 Although several other studies show down-regulation of the catalytic subunit of PP2A beginning about 48 hours after treatment with ATRA and continuing for 3 to 5 days,37,38 it is probably the transient changes in the regulatory subunit at 18 at 24 hours,36 which are predicted to result in a change in substrate specificity, that are most likely to affect levels of phospho-Smads at these times, as observed (Figures 2B and 3). While effects of TGF-β on activation of serine-threonine phosphatase activity have been reported,41,42 our data are the first to implicate serine-threonine phosphatase activity in control of the phosphorylation state of receptor-activated Smad proteins in cells. In HL-60R cells, which are resistant to effects of ATRA on granulocytic differentiation, cytosolic PP2A but not PP1 activity is reduced by almost 50% compared with wild-type HL-60 cells.43 Consistent with our hypothesis that the ability of ATRA to reduce levels of phospho-Smad2/3 induced by TGF-β1 treatment may depend, in part, on alterations in phosphatase activity, ATRA is unable to decrease levels of TGF-β–induced phospho-Smad2/3 in either mutant HL-60R cells (Figure8B) or wild-type HL-60 cells treated with okadiac acid (Figure 7B). The ability of okadaic acid to induce both phenotypic and functional attributes of monocytes (Table 3) and to enhance basal levels of phospho-Smad2/3 (Figure 7D), even in the absence of other inducing agents, further suggests that modulation of the levels of phosphatases can alter lineage commitment (Table 3).
Vit D3 has been shown to induce an autocrine TGF-β pathway in several different cell types. Thus in NRP-152 rat prostatic epithelial cells, the ability of Vit D3 to induce expression of fibronectin and thrombospondin, 2 target genes of TGF-β, was shown to be mediated indirectly by its activation of TGF-β.44 U937 cells treated with Vit D3differentiate to CD14+ cells with phagocytic capacity, and this has been shown to result from activation of an autocrine TGF-β pathway.8 In HL-60 cells, the Vit D3 analog EB1089 has been shown to induce expression of both TGF-β receptors and TGF-β ligand, and its antiproliferative activity is blocked by a TGF-β–neutralizing antibody.45 Our data now extend these studies and show that in HL-60 cells, the ability of Vit D3 to phosphorylate Smad2/3 (Figure 5C) and to stimulate monocytic differentiation can be blocked by neutralizing antibodies to TGF-β (Table 2), suggesting that Vit D3acts indirectly by activating signaling from either autocrine or paracrine (exogenous) TGF-β in these cells. Moreover, these data also show that reduction in levels of Smad2/3 phosphorylation is sufficient to reduce the commitment of these cells to differentiate to monocytes, even in the absence of changes in the levels of phosphatases. Whereas treatment with TGF-β alone results in arrest of differentiation at the CD14− promonocyte stage, Vit D3 induces HL-60 cells to express CD14 and differentiate to mature monocytes, an effect that has been shown to be dependent on induction of phosphatidylinositol 3–kinase activity.46 These additional effects of Vit D3 are probably independent of TGF-β, or possibly dependent on synergistic interaction of the vitamin D receptor with Smad3 to regulate expression of certain target genes containing both Vit D3–response elements and Smad-binding elements, as previously reported.47 48
Previous studies suggested that Smad5 is involved in the signaling pathway by which TGF-β inhibits proliferation of primitive human hematopoietic progenitor cells, since suppression of Smad5 expression by antisense oligonucleotides reversed the inhibitory effects of TGF-β on hematopoietic colony formation.49 While this study was one of the first to suggest that Smad 5, typically activated by bone morphogenetic proteins (BMPs), might be activated by TGF-β, more recent studies have shown this to occur in other cells as well, including intestinal epithelial cells50 and endothelial cells.51,52 It has also been shown that BMPs can regulate the development of human hematopoietic stem cells, presumably through the BMP-activated Smad proteins, Smad1 and Smad5, which they express.53 In contrast to these observations, our results suggest that in the more differentiated HL-60 cells, it is the canonical TGF-β–activated Smad proteins, Smad2 and Smad3, that mediate the effects of TGF-β.
Granulocytic differentiation of HL-60 cells has been shown to be mediated by the RAR-α receptor, consistent with the observations that it is the retinoid receptor most commonly expressed in hematopoietic cells and that the retinoid-resistant HL-60R cells have been shown to have a point mutation in the ligand-binding domain of RAR-α.34 Using similar reasoning, we have concluded that the ability of ATRA to reduce the levels of phospho-Smad2/3 induced by TGF-β is either directly or indirectly dependent on RAR-α. Whereas it has been suggested that the different retinoid receptors may control the expression of specific target genes in different cell types and thus may be functionally distinct, studies in HL-60 cells in which either RAR-α, RAR-β, RAR-γ, or RXR-α have been transduced into the retinoid-resistant HL-60R cells show that each of these distinct receptors has the capacity to mediate granulocytic differentiation.54 Thus, the possibility must be considered that in nonhematopoietic cells, other retinoid receptors may play a role similar to that of RAR-α in modulating effects of TGF-β on differentiation. Moreover, certain effects of ATRA may also be independent of RAR-α or compatible with the reduced ligand affinity of the point-mutated receptor in HL-60R cells,34since ATRA cooperates with TGF-β in induction of fully mature CD14+ monocytes in HL-60R cells where its ability to induce granulocytic differentiation is impaired (Figure 8A).
In summary, our data provide new insights into the mechanisms whereby HL-60 cells can integrate a multiplicity of differentiation signals impinging on the cell simultaneously. We demonstrate for the first time that cellular levels of phosphatase activity and of phosphorylated Smad2/3 induced by TGF-β can each affect the commitment to differentiation. However, in the particular context of treatment of cells simultaneously with ATRA and TGF-β, these 2 mechanisms are interrelated in that elevation of phosphatase activity by ATRA appears to underlie the decrease in the level of Smad2/3 phosphorylation. It remains to be demonstrated whether this unique mode of integration of signals from ATRA and TGF-β will also be relevant for other myeloid leukemia cells or for other cell systems regulated by these 2 agents.
We thank Xinle Cui and Carl Sadowski for many helpful discussions and technical assistance, and we also thank Jim McNally for excellent help on the use of the confocal microscopy facility.
Prepublished online as Blood First Edition Paper, September 12, 2002; DOI 10.1182/blood-2002-05-1549.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anita B. Roberts, Chief, Laboratory of Cell Regulation and Carcinogenesis, National Cancer Institute, Bldg 41, Rm C629, 41 Library Dr, MSC 5055, Bethesda, MD 20892-5055; e-mail:robertsa@dce41.nci.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal