A cytokine-screening assay of cultured peripheral blood cells obtained using immune rosetting and separation of progenitors was developed to identify determinants of fetal hemoglobin (HbF) modulation during adult erythropoiesis. Among the 12 erythroid growth-promoting cytokines tested, stem cell factor (SCF) at a concentration of 50 ng/mL resulted in the most significant increase in cell proliferation and HbF content. The average HbF/hemoglobin A (HbA) ratio was 30.9% ± 18.7% in cultures containing SCF compared with 4.1% ± 2.2% in those grown with erythropoietin (EPO) alone (P = 8.5E-8). To further investigate the hemoglobin-modulating effects of SCF, we examined the surface expression pattern of the SCF receptor, CD117, among maturing erythroblasts. CD117 expression increased during the first week of culture and peaked on culture days 7 to 9. After culture day 9, the level of CD117 declined to lower levels. The rise in CD117 expression to high levels mirrored that of the transferrin receptor (CD71), and the subsequent reduction in CD117 was inversely related to increases in expression of glycophorin A. SCF-related increases in the HbF/HbA ratio correlated with the expression pattern of CD117. SCF added during days 7 to 14 resulted in a more pancellular distribution of HbF on day 14 compared with the heterocellular distribution present in cultures supplemented with SCF on days 0 to 7. A significant SCF-mediated increase in HbF was also measured using progenitors derived from cord blood. These results suggest that the HbF response to SCF is greatest at the late progenitor stage as a function of surface CD117 expression.
Introduction
A complete understanding of fetal hemoglobin (HbF) production among adult erythroblasts is critical for the treatment of sickle cell anemia and β thalassemia. HbF prevents polymerization of hemoglobin S (HbS) in all newborns until HbS becomes the dominant hemoglobin in circulating erythrocytes.1 HbF levels of more than 25% also prevent sickling in a subset of patients with S-deletion form of hereditary persistence of fetal hemoglobin (HPFH). A “benign” form of sickle cell anemia among adults in eastern Saudi Arabia has further been attributed to elevated levels of HbF.2,3 Pharmacologic induction of HbF has favorably influenced the course of disease in patients with sickle cell diseases and β thalassemia.4,5 For these reasons, efforts aimed at the clinical augmentation of HbF during adult erythropoiesis continue to be an active focus of research.6
“Switching” of the dominant hemoglobin in circulating erythrocytes from the fetal to adult type is usually completed during infancy. However, some individuals continue to have increased expression of HbF later in their lives.7 In many cases, those individuals possess elevations in HbF content due to large deletions involving the β-globin locus on chromosome 11. Other individuals possess defined mutations within regulatory regions of that locus.8 Those mutations have led to the general conclusion that DNA-binding proteins and chromatin structure are important determinants of γ-globin gene transcription during adult life. Other families have elevations in HbF that are linked to separate genomic regions.9-11Recognition that certain forms of leukemia are associated with higher levels of HbF suggests nonglobin genes involved in erythroid growth or leukemogenesis may regulate globin gene transcription.12 In addition to malignant transformation, it has long been recognized that conditions of acute erythroid stress are associated with elevated HbF levels.13 Kinetic models have been proposed to explain this phenomenon,14 but a molecular mechanism has not yet been defined. Several investigators have attempted to reproduce this stress response in vitro using growth-related cytokines. Although strong correlations between cytokine supplementation and HbF production have been reported,15those results are challenged by others.16 Stem cell factor (SCF) has been most well studied and is thought to influence HbF production through signaling at the very early stages of erythropoiesis.17-19
In this report, we compare a dozen hematopoietic growth-promoting cytokines for their HbF-modulating effects by screening matched cultures of primary human erythroblasts. SCF produced the most significant and consistent increase in the HbF content in the cells of several donors. Although an SCF response was consistently demonstrated, the absolute increase in HbF- hemoglobin A (HbA) varied considerably among donors. SCF was further examined to determine whether effects on HbF were varied according to erythroid development and maturation. SCF effects on HbF appeared to be greater at later stages of erythroblast maturation and correlated well with a highly regulated pattern of CD117 (c-kit; SCF receptor) expression. A significant SCF effect on HbF was also detected using progenitor cells derived from cord blood.
Materials and methods
Culture and analysis of primary cells from human blood
Primary hematopoietic cells were obtained from donated blood. All blood samples were collected under approved protocols at the National Institutes of Health, Clinical Center, and the National Navy Medical Center. Mononuclear cells and enucleated erythrocytes were isolated using RosetteSep human progenitor enrichment cocktail (Stem Cell Technologies, Vancouver, BC, Canada) followed by lymphocyte separation media (ICN Pharmaceutical, Costa Mesa, CA) according to the manufacturer's protocols. Red blood cells were collected and frozen for future high-performance liquid chromatography (HPLC) analyses. Mononuclear cells were washed and cultured for 14 consecutive days in the Dulbecco modified Eagle medium (DMEM) containing 30% fetal bovine serum (FBS; Intergen, Purchase, NY), 2 mM glutamine (Biofluids, Rockville, MD), 1% bovine serum albumin (BSA), 10−5 M β-mercaptoethanol, 10−6 M dexamethasone, 0.3 mg/mL hollo-transferrin, antibiotics (penicillin/streptomycin), and cytokines (all cytokines added separately). The erythropoietin (EPO; Amgen, Thousand Oaks, CA) and SCF (R & D Systems, Minneapolis, MN) final concentrations for all studies were 4 U/mL and 50 ng/mL, respectively. The other cytokines used for the initial screening assay were all obtained commercially from the same supplier (R & D Systems) with the exception of basic fibroblast growth factor (bFGF; Sigma-Aldrich, St Louis, MO). The Dulbecco phosphate-buffered saline (PBS) used for washing the cells was purchased from Mediatech (Herdon, VA). All other reagents were purchased from Sigma, unless otherwise stated in the text. The cells were enumerated using an electronic cell counter (Coulter, Hialeah, FL). An aliquot was also collected for HPLC analysis after washing twice in Dulbecco PBS.
Immunostaining and flow cytometry
Immunostaining with monoclonal antibodies labeled with fluorescein isothiocyanate (FITC) or phycoerythrin (PE) and directed against CD117, CD71, glycophorin A (GPA; Beckman Coulter, Miami, FL), HbF (Caltag Laboratories, Burlingame, CA), HbA (Perkin Elmer Wallac, Norton, OH), and with appropriate isotypic controls was performed according to each manufacturer's procedure and analyzed by flow cytometry. Flow cytometric analyses were performed using an EPICS ELITE ESP flow cytometer (Beckman Coulter, Hialeah, FL). In each experiment, at least 10 000 cells were analyzed using argon laser excitation and bandpass emission filters: 525 nm for FITC and 575 nm for PE.
HPLC of hemoglobin
Cells were lysed in deionized sterile water, by repeated freezing and thawing. Cell debris was removed by filtration through Ultrafree-MC devices (Millipore, Bedford, MA) before cation-exchange chromatography. Hemoglobin species from cell lysates were separated on a 20 × 4-mm POLYCATA column (Poly LC, Columbia, MD) fitted to a Gilson HPLC system (Gilson, Middleton, WI). The hemoglobin was eluted during 4 minutes 8% to 40% gradient of buffer B (20 mM Bis-Tris [tris(hydroxymethyl)aminomethane], 2 mM KCN, 200 mM NaCl, pH 6.55 ) in buffer A (20 mM Bis-Tris, 2 mM KCN, pH 6.96 ) according to the manufacturer's protocol. Hemoglobin proteins were detected by absorbance measurements at 415 nm. Ratios of HbF/HbA were calculated by integration of the areas under the HbF and HbA peaks using software supplied by the manufacturer and expressed as percentage values. Two-tailed, paired t tests were used for all statistical analyses. Confirmation of HbF and HbA retention times in experimental samples was performed by electrospray mass spectroscopy (Hewlett-Packard Series 1100). Purified HbF and HbA (Perkin-Elmer Wallac) were used for reference.
Results
Screening assay of growth-associated cytokines
For the purpose of screening cytokines and other biologically active molecules for possible effects on hemoglobin modulation during erythropoiesis, we used a single-phase culture system of progenitor cells from the buffy coats of healthy blood donors. This single-phase culture system used negative selection as the method for obtaining progenitor cells, and the cells were obtained from the buffy coats of blood donations rather than mobilized apheresis products as we have previously described.20 Once isolated, the cell populations were cultured at an initial concentration of approximately 104 cells/mL for 14 days in EPO-containing (4 U/mL) medium. After 14 days, the cells were counted and the ratio of fetal and adult hemoglobin (HbF/HbA) was determined on the basis of HPLC analyses. In cultures supplemented with EPO alone and no additional cytokines, an average of 8 million erythroid precursor cells per unit of donated blood (3.8 ± 0.2 E05 cells/mL culture medium) were generated after the 14-day culture period. Overall, the HbF/HbA ratio averaged 3.9% ± 0.2% after the 14-day culture period in EPO without additional cytokines.
Based on the desired effects of EPO on erythroblast proliferation and hemoglobin content, cells from 35 additional individuals were used in an initial cytokine screening assay. From each individual, the cells were initially divided into matched sets to ensure proper control of patient-specific results. In one flask, the cells were cultured in EPO alone as a control. The paired flask from each donor was grown in the presence of EPO plus an additional cytokine. After 14 days, results from duplicate or triplicate cultures were examined to quantitate differences in cell growth or HbF (Table1). For this study, a dozen cytokines were screened on the basis of previous reports suggesting a possible association with erythroid growth using other culture systems. In cases where previous reports were unavailable, the cytokines were added at low and high concentrations for measurement of a possible dose effect (higher concentrations shown in Table 1). No significant erythroid growth was detected in control cells grown in the cytokines alone in the absence of EPO (not shown). In the presence of EPO, cytokine-specific effects on proliferation and hemoglobin content were detected. The majority of cytokines tested had no significant effects on cell growth or HbF/HbA in this culture system. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 3 (IL-3) caused no significant increase in HbF/HbA, but did stimulate cell division. Supplementing IL-6 or SCF to the EPO-containing medium resulted in a significant change (P ≤ .05) in HbF/HbA and cell proliferation.
Cytokine effects on erythroid proliferation and hemoglobin modulation
| Cytokine (concentration) . | EPO . | EPO + cytokine . | ||
|---|---|---|---|---|
| Cell count, ×106 . | HbF/HbA, % . | Cell count, ×106 (P) . | HbF/HbA, % (P) . | |
| SCF (50 ng/mL) | 0.60 ± 0.40 | 2.5 ± 0.68 | 2.65 ± 0.40 (.02) | 21 ± 1.4 (.0004) |
| IL-6 (200 ng/mL) | 0.53 ± 0.15 | 5.1 ± 1.7 | 1.8 ± 2.0 (.20) | 8.7 ± 2.9 (.05) |
| LIF (500 ng/mL) | 0.28 ± 0.1 | 6.3 ± 3.6 | 0.28 ± 0.11 (.46) | 6.1 ± 3.4 (.40) |
| Activin (100 ng/mL) | 0.33 ± 0.17 | 4.8 ± 3.1 | 0.22 ± 0.11 (.05) | 5.7 ± 2.2 (.31) |
| IGF-II (50 ng/mL) | 0.22 ± 0.10 | 7 ± 1.41 | 0.45 ± 0.20 (.24) | 5.5 ± 2.1 (.31) |
| BMP 4 (1 μg/mL) | 0.26 ± 0.11 | 3.2 ± 5.3 | 0.25 ± 0.24 (.48) | 3.9 ± 5.0 (.18) |
| IL-3 (10 ng/mL) | 0.63 ± 0.22 | 3.9 ± 1.7 | 3.5 ± 0.95 (.0003) | 4.6 ± 1.4 (.14) |
| GM-CSF (10 ng/mL) | 0.63 ± 0.22 | 3.1 ± 1.7 | 2.0 ± 1.5 (.02) | 3.9 ± 1.3 (.50) |
| bFGF (200 ng/mL) | 0.29 ± 0.02 | 4.0 ± 3.4 | 0.28 ± 0.01 (.29) | 3.9 ± 3.1 (.35) |
| HGF (100 ng/mL) | 0.72 ± 0.52 | 2.5 ± 2.3 | 0.73 ± 0.53 (.34) | 3.7 ± 2.9 (.12) |
| IGF-I (50 ng/mL) | 0.19 ± 0.07 | 4.2 ± 2.6 | 0.83 ± 0.66 (.33) | 3.5 ± 0.78 (.33) |
| OSM (200 ng/mL) | 0.49 ± 0.30 | 4.1 ± 2.8 | 1.5 ± 1.7 (.23) | 3.2 ± 1.1 (.30) |
| Cytokine (concentration) . | EPO . | EPO + cytokine . | ||
|---|---|---|---|---|
| Cell count, ×106 . | HbF/HbA, % . | Cell count, ×106 (P) . | HbF/HbA, % (P) . | |
| SCF (50 ng/mL) | 0.60 ± 0.40 | 2.5 ± 0.68 | 2.65 ± 0.40 (.02) | 21 ± 1.4 (.0004) |
| IL-6 (200 ng/mL) | 0.53 ± 0.15 | 5.1 ± 1.7 | 1.8 ± 2.0 (.20) | 8.7 ± 2.9 (.05) |
| LIF (500 ng/mL) | 0.28 ± 0.1 | 6.3 ± 3.6 | 0.28 ± 0.11 (.46) | 6.1 ± 3.4 (.40) |
| Activin (100 ng/mL) | 0.33 ± 0.17 | 4.8 ± 3.1 | 0.22 ± 0.11 (.05) | 5.7 ± 2.2 (.31) |
| IGF-II (50 ng/mL) | 0.22 ± 0.10 | 7 ± 1.41 | 0.45 ± 0.20 (.24) | 5.5 ± 2.1 (.31) |
| BMP 4 (1 μg/mL) | 0.26 ± 0.11 | 3.2 ± 5.3 | 0.25 ± 0.24 (.48) | 3.9 ± 5.0 (.18) |
| IL-3 (10 ng/mL) | 0.63 ± 0.22 | 3.9 ± 1.7 | 3.5 ± 0.95 (.0003) | 4.6 ± 1.4 (.14) |
| GM-CSF (10 ng/mL) | 0.63 ± 0.22 | 3.1 ± 1.7 | 2.0 ± 1.5 (.02) | 3.9 ± 1.3 (.50) |
| bFGF (200 ng/mL) | 0.29 ± 0.02 | 4.0 ± 3.4 | 0.28 ± 0.01 (.29) | 3.9 ± 3.1 (.35) |
| HGF (100 ng/mL) | 0.72 ± 0.52 | 2.5 ± 2.3 | 0.73 ± 0.53 (.34) | 3.7 ± 2.9 (.12) |
| IGF-I (50 ng/mL) | 0.19 ± 0.07 | 4.2 ± 2.6 | 0.83 ± 0.66 (.33) | 3.5 ± 0.78 (.33) |
| OSM (200 ng/mL) | 0.49 ± 0.30 | 4.1 ± 2.8 | 1.5 ± 1.7 (.23) | 3.2 ± 1.1 (.30) |
Erythroid progenitors from the buffy coats of blood donations were cultured for 2 weeks in matched pairs, in the presence of 4 U/mL erythropoietin alone (EPO) or EPO plus an additional cytokine as indicated. Cell counts per milliliter culture medium and HbF/HbA ratios expressed as percentages are shown with SDs. Cells collected from 35 blood donations were cultured separately for duplicate or triplicate analysis of each cytokine. Two-tailed, pairedt test analyses were preformed to determine the significance of the cytokines. The cytokines are listed in descending order according to their effect on the HbF/HbA ratio.
LIF indicates leukemia inhibitory factor; activin, activin A; IGF-II, insulinlike growth factor II; BMP 4, bone morphogenetic protein 4; HGF, hepatocyte growth factor; IGF-I, insulinlike growth factor I; and OSM, oncostatin M.
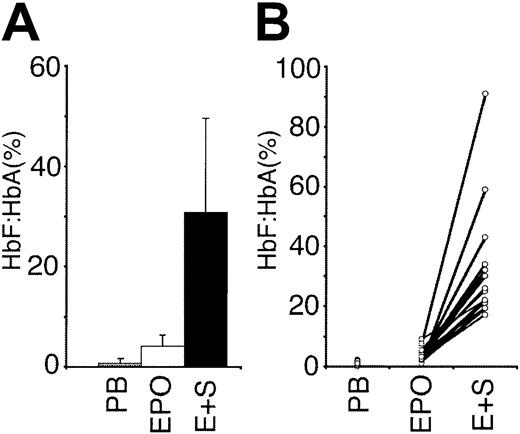
Due to the dramatic effect of SCF on growth and HbF/HbA, this cytokine was studied further. First, we tested several additional donors to determine the reproducibility and variability of SCF effects on HbF/HbA (Figure 1). Buffy coats from a total of 19 blood donors were studied as matched culture pairs. Consistent with the screening studies, the HbF/HbA ratios for cells grown in EPO plus SCF were significantly higher than in EPO alone (EPO alone, 4.1% ± 2.2%; EPO + SCF, 30.9% ± 18.7%;P = 8.5E-08). Whereas an SCF-related increase in HbF/HbA was measured in cultures from every donor, the range of response was broad with HbF/HbA ratios from 17% to 91% (Figure 1B). To determine if the donors with a large response had inherently high HbF values in vivo, HPLC analyses were performed on their peripheral blood erythrocytes. No donor-specific correlation was identified, and the erythrocytes had a lower distribution of HbF than the cells cultured in either EPO or EPO plus SCF (erythrocyte HbF/HbA, 0.8% ± 0.8%; P = 1.9E-06).
Effect of SCF on the HbF/HbA ratios from cultures of cells from 19 different blood donors.
For each donor, the HbF/HbA ratios were obtained from HPLC analyses of circulating erythrocytes in the peripheral blood (PB) of the donors, erythroid progenitors cultured for 2 weeks in the presence of erythropoietin (EPO; 4 U/mL) alone, and in EPO (4 U/mL) plus SCF (50 ng/mL; E+S). (A) Mean values with SD bars. (B) Values from each donor's matched cultures attached by lines.
Effect of SCF on the HbF/HbA ratios from cultures of cells from 19 different blood donors.
For each donor, the HbF/HbA ratios were obtained from HPLC analyses of circulating erythrocytes in the peripheral blood (PB) of the donors, erythroid progenitors cultured for 2 weeks in the presence of erythropoietin (EPO; 4 U/mL) alone, and in EPO (4 U/mL) plus SCF (50 ng/mL; E+S). (A) Mean values with SD bars. (B) Values from each donor's matched cultures attached by lines.
SCF receptor (CD117) expression is regulated during erythropoiesis
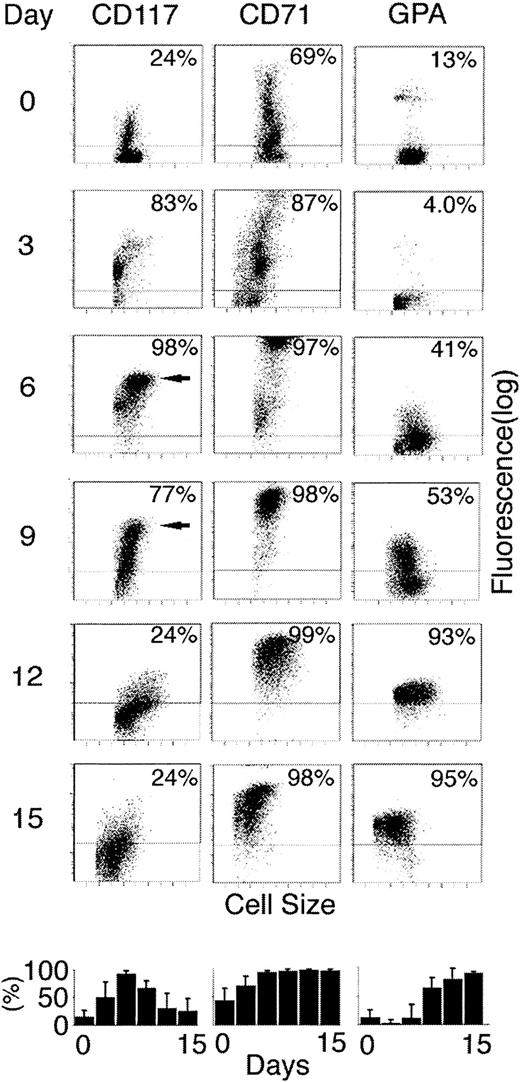
SCF exerts its effects on eukaryotic cells through dimerization of the membrane protein CD117.21 Therefore, to determine the level of surface CD117 expression on differentiating erythroblasts, cells grown in EPO alone were sampled every 3 days and stained with labeled anti-CD117 monoclonal antibody (Figure2). As shown, the percentage of cells expressing CD117 at significant levels increased during the first week in culture. On day 6, 98% had detectable CD117 expression. Thereafter, the percentage of cells with detectable levels gradually declined with less than 25% positive by day 12. In addition to the total percentage of cells expressing CD117, it is important to also note changes in the level of CD117 expression among those positive cells. By day 6, a distinct population of larger cells was clearly detected with CD117 expressed at relatively high levels (third decade of fluorescence). Although cells expressing high-level CD117 persisted on day 9, they were no longer detected by day 12. When EPO and SCF were added on day 0, the CD117 signal remained low for the first 48 hours followed by a delayed increase during the first week and lower peak levels compared to cells cultured in EPO alone. In triplicate analyses, the mean CD117 fluorescence on culture day 6 in EPO alone was 13.3 fluorescence units compared with 3.8 fluorescent units in matched cultures containing EPO plus SCF.
Expression of SCF receptor (CD117), transferrin receptor (CD71), and GPA during the 14-day culture period.
On every third day in erythroid culture (indicated at left), cells were sampled and stained with fluorescent-labeled antibodies and analyzed by flow cytometry. Each panel shows distribution of antibody binding (fluorescence; y-axis) versus the forward scatter (cell size; x-axis). The horizontal lines demonstrate the upper level of cellular autofluorescence plus the signals from isotypic control antibodies. The percentage of cells with fluorescence above the isotypic controls is shown in the upper right corner of each panel. Arrows show populations with high-level CD117 expression on days 6 and 9. Bottom panels show mean percentages of positive cells with SE bars from separate experiments performed on the cells of 3 donors.
Expression of SCF receptor (CD117), transferrin receptor (CD71), and GPA during the 14-day culture period.
On every third day in erythroid culture (indicated at left), cells were sampled and stained with fluorescent-labeled antibodies and analyzed by flow cytometry. Each panel shows distribution of antibody binding (fluorescence; y-axis) versus the forward scatter (cell size; x-axis). The horizontal lines demonstrate the upper level of cellular autofluorescence plus the signals from isotypic control antibodies. The percentage of cells with fluorescence above the isotypic controls is shown in the upper right corner of each panel. Arrows show populations with high-level CD117 expression on days 6 and 9. Bottom panels show mean percentages of positive cells with SE bars from separate experiments performed on the cells of 3 donors.
To place the rise and subsequent fall of CD117 into the context of other markers of erythroid development, the cells were also stained for transferrin receptor (CD71) and GPA expression. These surface proteins have been demonstrated to provide flow cytometric correlates of the developmental stage of the erythroblasts.20 CD71 expression at very high levels is the hallmark of the onset of hemoglobin production at the preproerythroblast-proerythroblast stage of differentiation. GPA is expressed later relative to CD71 and marks the transition toward terminal maturation. Among the cells studied here, the transition from mid- to high-level CD117 mirrored that of CD71 on days 3 to 6. However, unlike CD71, a decline in the expression of CD117 coincided with the onset of GPA expression at higher levels. Dual staining demonstrated that GPA expression at high levels directly coincided with the loss of CD117 (data not shown). Hence, CD117 expression appears to be highly regulated in adult erythroid cells with an initial rise to very high levels in proerythroblasts followed by a rapid decline associated with terminal differentiation.
SCF modulation of HbF correlates with CD117 expression
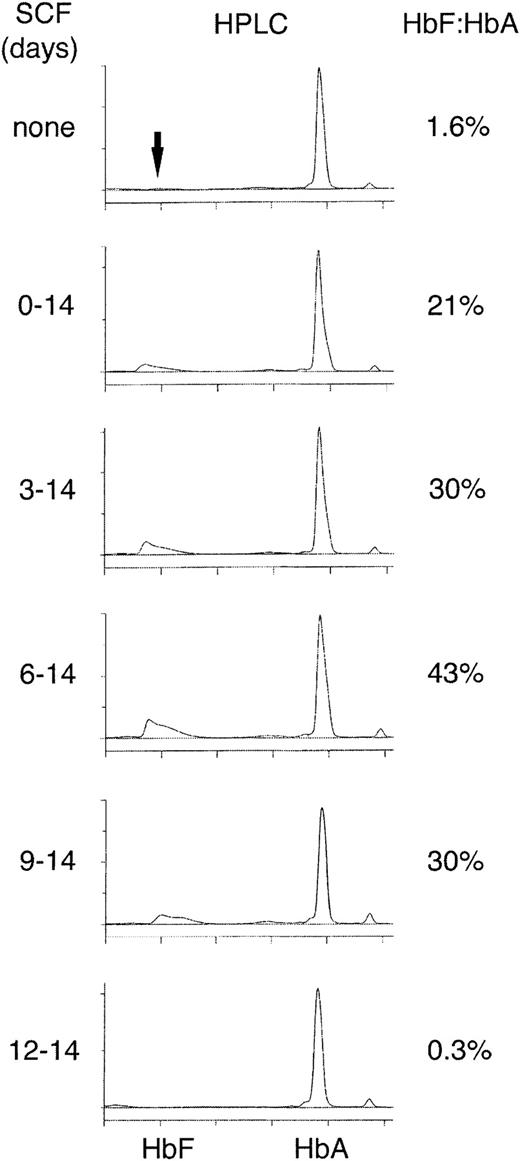
Based on the highly regulated pattern of SCF expression on the erythroblast membrane, the temporal pattern of SCF effects on HbF according to the developmental stage of the cells was studied. For this purpose, SCF was added at delayed time intervals in the culture period and HPLC was performed to determine the HbF/HbA ratio (Figure3). Cells from each donor were divided into 6 matched aliquots in separate flasks and cultured for 2 weeks. SCF was then added to individual flasks after 3-day intervals as shown. On day 14, the cells from all the flasks were harvested and the HbF/HbA ratios were calculated from HPLC profiles. Cell counts at that time revealed a progressive decline from 3.0 × 106 cells/mL in the presence of SCF on days 0 to 14 to 2.0 × 105cells/mL in the absence of SCF. In contrast to the gradual reduction in cell counts, a significant reduction in the SCF effect on HbF was not noted until day 12 when the HbF/HbA ratio returned to levels similar to that in the control flask supplemented with EPO alone. Notably, high-level expression of CD117 expression was no longer present by day 12. The maximum effect on the HbF/HbA ratio occurred when SCF was added at the end of 1 week, that is, when CD117 was expressed at its highest level at the proerythroblast stage of development. This pattern was reproduced in cultured cells from 3 separate donors (HbF/HbA ratio, EPO alone on days 0-14, 3.7% ± 1.3%; EPO days 0-14 plus SCF on days 0-14, 23% ± 12%; SCF days 3-14, 33% ± 21%; SCF days 6-14, 48.3% ± 18.6%; SCF days 9-14, 38% ± 9.8%; SCF days 12-14, 6.2% ± 1.4%).
HPLC analyses on day 14 after addition of SCF progressively later during the culture period.
EPO (4 U/mL) was present in the culture medium of all cultures and SCF (50 ng/mL) was added on the days indicated on the left in matched cultures. HPLC measurements performed on day 14 for all the samples. The HbF and HbA elution times were determined with standard proteins and by mass spectroscopy. The HbF/HbA ratio, expressed as a percentage, is shown on the right. The panels shown are representative of cultures from 3 separate blood donations demonstrating similar results.
HPLC analyses on day 14 after addition of SCF progressively later during the culture period.
EPO (4 U/mL) was present in the culture medium of all cultures and SCF (50 ng/mL) was added on the days indicated on the left in matched cultures. HPLC measurements performed on day 14 for all the samples. The HbF and HbA elution times were determined with standard proteins and by mass spectroscopy. The HbF/HbA ratio, expressed as a percentage, is shown on the right. The panels shown are representative of cultures from 3 separate blood donations demonstrating similar results.
Early versus late effects of SCF on HbF modulation
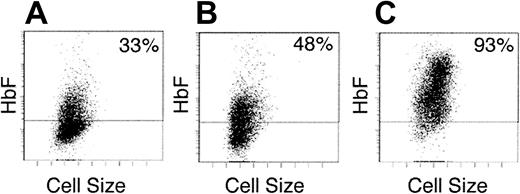
Based on the unexpectedly high level of HbF/HbA on delayed addition of SCF, we next compared the SCF added during the first week (early) versus the second week (late) in culture. This approach was used to differentiate SCF effects in cells with rising levels of CD117 and the onset of hemoglobin production (week 1) versus those populations with falling levels of CD117 and established hemoglobin production (week 2). On day 14, the HbF/HbA ratios were calculated in matched cultures under these conditions and the distribution of HbF was measured by flow cytometry. As shown, the distribution of HbF+ cells on culture day 14 was heterocellular in the presence of EPO alone (Figure 4, left panel). Similarly, a heterocellular pattern of HbF-based fluorescence was detected when SCF was present on days 0 to 7 (48% compared with 33% in EPO alone). This increase in HbF+ cells correlated with a small increase in relative HbF content (HbF/HbA, 5.0% ± 1.7% compared to EPO alone, 2.2% ± 1.1%). When SCF addition was delayed until the second week, a pancellular distribution of HbF+ cells (93% over background fluorescence) was detected and the HbF/HbA ratio increased significantly (20% ± 6.1%). The flow cytometric analysis also demonstrated that the population with higher HbF levels included the larger cells. Cytospin preparations confirmed that those larger cells had a proerythroblast morphology consistent with the reported ability of SCF to partially inhibit erythroid maturation.22
Early versus late effects of SCF on HbF distribution.
Cells were analyzed after 2-week erythroid culture in (A) EPO alone on days 0 to 14, (B) EPO plus SCF on days 0 to 7 followed by EPO alone on days 7 to 14, and (C) EPO alone on days 0 to 7 followed by EPO plus SCF on days 7 to 14. Each panel shows the flow cytometric distribution of HbF after staining with fluorescent antibodies (y-axis) versus cell size (x-axis). A negative fluorescence level (below horizontal bar) was determined using isotypic control antibodies. The percentage of positive cells is shown on the upper right corner of each panel. The panels shown are representative of cultures from 3 separate blood donors demonstrating similar results.
Early versus late effects of SCF on HbF distribution.
Cells were analyzed after 2-week erythroid culture in (A) EPO alone on days 0 to 14, (B) EPO plus SCF on days 0 to 7 followed by EPO alone on days 7 to 14, and (C) EPO alone on days 0 to 7 followed by EPO plus SCF on days 7 to 14. Each panel shows the flow cytometric distribution of HbF after staining with fluorescent antibodies (y-axis) versus cell size (x-axis). A negative fluorescence level (below horizontal bar) was determined using isotypic control antibodies. The percentage of positive cells is shown on the upper right corner of each panel. The panels shown are representative of cultures from 3 separate blood donors demonstrating similar results.
SCF effect in cord blood–derived progenitors
Progenitor cells derived from cord blood were also studied using this culture model in the presence or absence of SCF. Cord blood–derived erythroblasts demonstrate the same pattern of an initial rise followed by a reduction of CD117 expression as adult peripheral blood–derived cells (data not shown). In the absence of SCF, erythroid differentiation of cultured cord blood progenitor cells expressed both HbF and HbA in a pancellular fashion (Figure5). When SCF (50 ng/mL) was added on days 7 to 14 to cord blood cultures, a pancellular distribution of HbF and HbA was still present. However, the HPLC profiles showed that SCF caused a pronounced rise in the HbF content of the cells to dominant levels with the mean HbF/HbA ratio increasing from 136% ± 25% in EPO alone to 295% ± 34% in EPO plus SCF (P = .004). This novel result demonstrates that SCF has a significant effect on the HbF content of cultured erythroid progenitor cells circulating at the time of birth. In addition, this result demonstrates SCF-mediated increases in HbF among populations already expressing this hemoglobin with a pancellular distribution.
SCF-mediated modulation of HbF in cord blood–derived erythroblasts.
The cells were grown for 14 days in EPO with or without SCF added on days 7 to 14 using identical culture conditions as those for the adult cells. (A) Hemoglobin distribution from flow cytometric analyses of HbF and HbA after staining with fluorescent antibodies (y-axis) versus cell size (x-axis) is shown for each culture condition. A negative fluorescence level (below horizontal bar) was determined using isotypic control antibodies. The percentage of positive cells is shown on the upper right corner of each panel. (B) Representative HPLC profiles from cells cultured in EPO alone (left) versus EPO plus SCF are shown for comparison. The HbF/HbA ratios from 3 separate donors are shown next to each HPLC profile (mean ratios ± SDs;P = .004).
SCF-mediated modulation of HbF in cord blood–derived erythroblasts.
The cells were grown for 14 days in EPO with or without SCF added on days 7 to 14 using identical culture conditions as those for the adult cells. (A) Hemoglobin distribution from flow cytometric analyses of HbF and HbA after staining with fluorescent antibodies (y-axis) versus cell size (x-axis) is shown for each culture condition. A negative fluorescence level (below horizontal bar) was determined using isotypic control antibodies. The percentage of positive cells is shown on the upper right corner of each panel. (B) Representative HPLC profiles from cells cultured in EPO alone (left) versus EPO plus SCF are shown for comparison. The HbF/HbA ratios from 3 separate donors are shown next to each HPLC profile (mean ratios ± SDs;P = .004).
Discussion
In this report, we have begun to explore the hypothesis that modulation of HbF may result from signal transduction involving fully committed erythroblasts. This hypothesis arose from the demonstration that HbF and HbA share a coordinated rather than “switched” expression pattern during adult human erythropoiesis.20Our matched-culture strategy using primary cells from peripheral blood was developed to screen several growth-related cytokines for possible effects on hemoglobin modulation. In the absence of additional cytokines, the progenitor cells from those healthy blood donors proliferated and differentiated into populations containing 95% or more GPA+ erythroblasts with a HbF/HbA of less than 5% after 14 days in EPO-supplemented culture medium. Of note, these results using mononuclear cells from a relatively large number of blood donations demonstrated a nearly identical in vitro pattern of erythroid development to that of mobilized CD34+cells.20 Several of the tested cytokines did not significantly affect hemoglobin modulation with the screening assay described here, but formal dose titration and cytokine combinations must be performed before their effects on erythropoiesis are fully understood. As an initial screen, this primary cell culture system appears to be well suited for testing other cytokines, pharmacologic agents, or mixtures thereof. Among the cytokines demonstrating significant effects on hemoglobin modulation, SCF was studied further based on its dramatic effects on HbF and cell proliferation as shown here and elsewhere.17-19 SCF caused a consistent rise in HbF/HbA among 19 healthy blood donors, but the magnitude of the SCF response was highly variable. Host response variability has similarly been identified in the response of HbF-modulating agents used in patients with hemoglobinopathies.23 24
The flow cytometric analyses of the SCF receptor (CD117) expression demonstrated a highly regulated pattern during human erythropoiesis. Of particular interest was the demonstration of increased CD117 expression to higher levels coincidental with the rise in CD71 expression. Initial increases in CD117 expression were followed several days later by a decline to lower levels associated with the expression of GPA at high levels on the erythroblast surface. Hence, the highest level of CD117 expression was present in cells having a CD71hi, GPAlow cellular phenotype. This phenotype has been previously shown to identify committed, rapidly proliferating erythroblasts.20 The increased expression of CD71 also correlates with the onset of hemoglobin production at low levels in each cell.20 Consistent with the increased level of CD117, cells at this developmental stage also appear to be the most sensitive to SCF (compare Figures 4 and 5). Furthermore, the decline of CD117 from the cell surface toward the end of the culture period corresponded to a loss of SCF effect. We conclude that CD117 expression is highly regulated during erythroid differentiation and that the magnitude of SCF-mediated effects is proportionate to the extent of CD117 expression on the cell surface. Although not studied here, others have shown that HbF-modulating effects of SCF on erythroblasts are maintained in circulating erythrocytes in vivo.25
While designing these studies, we predicted that increases in the HbF/HbA ratio would be greatest after continuous exposure to SCF on days 0 to 14. However, cells cultured in the same concentration of SCF only during the second week resulted in higher HbF/HbA ratios at the end of the 14-day period. This unexpected result may be related to the initial loss of SCF-bound CD117 from the cell surface. After SCF dimerizes CD117 on the cell surface, the receptor-ligand complexes are internalized and degraded.21 Unlike the recycling of transferrin receptor to the cell surface after transferrin internalization, CD117 is not recycled and receptor levels on the cell surface are down-regulated until new CD117 is produced. We speculate that the internalization SCF-CD117 complexes from the surface of immature cells provide an autoregulatory mechanism for SCF effects in more mature human erythroblasts. This type of ligand-dependent regulation of receptor expression in erythroblasts could provide the basis for differences in the HbF production associated with acute and chronic states of stress or other transitions in hemoglobin production.14 Whether the high serum SCF levels associated with stress erythropoiesis in humans27 correlates with lower CD117 levels in bone marrow erythroblasts is unknown. HbF modulation during erythroid stress in vivo will likely involve additional factors including signaling from both soluble and transmembranous forms of SCF, local concentrations of additional cytokines, and interactions between erythroblasts and other cells in the marrow.
We were unable to identify any special population of erythroid progenitors that were recruited by the addition of SCF during the earliest period of erythroid development. The HbF distribution in the presence of SCF during the first week was nearly identical to that of cells cultured in EPO alone (Figure 4). This result challenges the assumption that hemoglobin-modulating effects of SCF are greatest at the earliest stages of erythropoiesis. In this study, the most significant effect of SCF on HbF modulation occurred in late progenitors as CD117 increased just prior to the terminal stages of differentiation. This “late” effect of SCF in human cells was not predicted from nonhuman models because EPO down-regulates the expression of c-kit among murine progenitor cells within 3 hours.26 SCF altered the erythroid program of the adult cells as reflected by the significant increase in the HbF/HbA ratio and the increased distribution in HbF. In cord blood–derived populations, the HbF/HbA ratio rose to HbF-dominant levels. In addition, the response in cord blood–derived progenitor cells suggests that SCF modulates hemoglobin by increasing its expression in cells already producing the protein in addition to the “reactivation” of HbF in adult cells.
The developmental program of committed human erythroblasts is neither fixed nor determined exclusively by EPO. Germline mutations in either murine SCF (Steel factor) or CD117 (c-kit) result in severe anemia in the mouse.28 Multiple intracellular signaling pathways are involved in the SCF response primarily through the control of gene transcription.29,30 In erythroid cells, integration of EPO and SCF signaling may occur at the level of their membrane receptors31 or within downstream pathways.32The first evidence that SCF possessed hemoglobin-modulating properties in addition to its effect on erythroid growth was derived from in vitro assays using the cells of patients with sickle cell disease.17 Although questioned by some,27,33these results have been reproduced here and by others.18,25 Recently, an elegant unicellular system has been reported to demonstrate clonal SCF-mediated HbF modulation at very early stages of erythropoiesis.19 Our data suggest that SCF effects on HbF are not restricted to the earliest erythroblasts, but may be even more dramatic among more mature erythroblast populations as a function of increased CD117 expression on their membranes. We propose that consecutive generations of human erythroblasts possess hemoglobin programs that are dynamic and determined, at least in part, by the environment in which those cells arise.
We thank the blood donors and the National Institutes of Health Department of Transfusion Medicine for kindly providing the cells used in this study. Dr Alan Schechter is recognized for helpful discussions and critical reading of the manuscript.
Prepublished online as Blood First Edition Paper, August 22, 2002; DOI 10.1182/blood-2002-03-0756.
U.W. and K.R.L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jeffery L. Miller, Laboratory of Chemical Biology, Bldg 10, Rm 9B17, National Institutes of Health, Bethesda, MD 20892; e-mail: jm7f@nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal