The use of retroviral gene transfer into hematopoietic stem cells for human gene therapy has been hampered by the absence of retroviral vectors that can generate long-lasting, lineage-specific gene expression. We developed self-inactivating retroviral vectors that incorporate gene-regulatory elements from the macrophage-restricted human CD68 gene. Through the transplantation of transduced murine hematopoietic stem cells (HSCs), we show that a vector incorporating a 342–base pair (bp) fragment of 5′ flanking sequence from the CD68 gene, in addition to the CD68 first intron, was able to direct macrophage-specific expression of an enhanced green fluorescent protein (EGFP) reporter gene in inflammatory cell exudates and lymphoid organs in vivo. Levels of EGFP expression generated by this vector were greater than those generated by a standard Moloney murine leukemia retroviral vector, and they were stable for at least a year after transplantation of transduced HSCs. To evaluate the ability of this vector to generate therapeutically useful levels of gene expression, we transplanted apolipoprotein E (ApoE)–deficient HSCs transduced with a virus encoding ApoE into ApoE-deficient mice. Macrophages from these mice expressed levels of ApoE that were comparable to those from wild-type mice, and vector-driven expression of ApoE in macrophages was sufficient to reverse both hypercholesterolemia and atherosclerotic lesion development. The future application of this retroviral vector should provide a powerful tool to further elucidate macrophage function and for human gene therapy.
Introduction
Stable gene transfer into hematopoietic stem cells (HSCs) has great potential for treating inherited and acquired human diseases, as shown by the recent success in treating 2 patients with severe combined immunodeficiency.1,2 Such an approach requires the efficient transduction of HSCs with a vector that is capable of generating long-term, high-level gene expression. At present, retroviral (including oncoretroviral and lentiviral) vectors are the only option for meeting these criteria.3 However, expression generated by promoter elements in the viral long terminal repeat (LTR) of these vectors is often only short-lived in vivo, a problem that has hampered the clinical application of retroviral gene therapy.4-6 In addition, these vectors direct expression in all progeny lineages derived from transduced HSCs, which may be detrimental in many circumstances. Thus, much work has been performed with the aim of developing vectors that can generate long-lasting transgene expression in specific hematopoietic lineages.
Attempts to create oncoretroviral vectors (RVs) for lineage-specific or cell type–specific gene expression have focused on the incorporation of lineage-specific promoter elements into RVs. Work on generating RVs for targeting β-globin expression to red blood cells has exemplified the problems with such an approach. The insertion of an additional promoter into an RV often results in “promoter interference,” reducing the activity of the internal promoter and/or the LTR.7-9 One potential solution to this problem is the use of self-inactivating (SIN) RVs, in which promoter and enhancer elements from the viral LTR are deleted during viral replication.10 However, incorporation of a cell type–specific promoter into SIN-RVs often results in severe (100-fold) reduction of viral titers.3 More recently, attention has focused on generating vectors derived from lentiviruses such as human immunodeficiency virus-1 (HIV-1). Unlike SIN-RVs, SIN-modified lentiviral vectors containing an internal promoter do not appear to have problems with reduced viral titers.11-13 This has led to the generation of lentiviral vectors for restricted gene expression in multiple lineages derived from transduced HSCs, including red blood cells and T cells.14-17 Despite these advances, the generation of RVs that direct lineage-restricted expression is still an important goal, as at present only RVs have been approved for use in human gene therapy.
Macrophages (mφs) are an attractive target for gene therapy. They play important roles in the pathogenesis of chronic inflammatory diseases such as atherosclerosis and arthritis, are involved in the growth and metastasis of tumors, and are pivotal to both innate and acquired immune responses.18-20 In addition, the high intrinsic biosynthetic capacity of the mφ makes it suitable for delivery of therapeutic gene products at physiologically useful concentrations. The study of mφ biology has been hampered by the absence of suitable gene-regulatory sequences for the overexpression of heterologous genes in mφs in transgenic mice. However, the recent description of transcriptional regulatory elements from the mφ-restricted, human CD68 gene offers a promising new tool for analyzing mφ function.21 22
In this study we describe the generation and characterization of 2 novel SIN-RVs containing transcriptional regulatory elements from the human CD68 gene. We show that a vector incorporating a 342–base pair (bp) fragment of 5′ flanking sequence from theCD68 gene, in addition to the CD68 first intron, was able to direct macrophage-specific expression of an enhanced green fluorescent protein (EGFP) reporter gene in vivo. Levels of EGFP expression generated by this vector were greater than those generated by a standard Moloney murine leukemia retroviral vector and were stable for at least a year after transplantation of transduced HSCs. By using this RV to drive expression of apolipoprotein E, we were able to reverse hypercholesterolemia and atherosclerotic lesion development in ApoE-deficient (ApoE−/−) mice.
Materials and methods
Retroviral vector generation
All constructs were generated by means of standard molecular biology techniques and were verified by DNA sequencing. Retroviral expression plasmids pBM-I-EGFP,23pBM-SIN-lacZ,24 and LZRS-pBM-lacZ25 were generously provided by G. Nolan (Stanford University, Stanford, CA). A version of the enhanced green fluorescent protein with a hemagglutinin (HA) epitope tag at the C terminus of the protein (HA-EGFP) was generated by polymerase chain reaction (PCR) and cloned in the sense orientation into pCR2.1-TOPO (Invitrogen, Carlsbad, CA). The HA-EGFP cDNA fragment was excised via digest withSpeI and XbaI and cloned into plasmid pBSCD68hSR-AI22 that had been digested withXbaI, generating plasmid pBSCD68HA-EGFP. DNA fragments containing either 2940 bp (CD68L; generated by digest of pBSCD68HA-EGFP with ClaI and BsaAI) or 342 bp (CD68S; generated by digest of pBSCD68HA-EGFP with PvuII) of sequence 5′ to the ATG initiation codon of the CD68 gene, in addition to the CD68first intron (IVS-1), HA-EGFP cDNA and bovine growth hormone polyadenylation signal (pA) were excised, rendered blunt-ended, and cloned into plasmid pBM-SIN-lacZ that had been digested withEcoRI and NotI and similarly blunt-ended. Restriction digest analyses were used to obtain clones in which the viral 5′ LTR and CD68 gene promoters are in opposite transcriptional orientations (Figure 1A). The resulting plasmids were used as templates for multiple rounds of PCR-based mutagenesis to eliminate a cryptic polyadenylation signal (−726 to −721 relative to CD68 gene ATG) and a BspHI site (−2277 relative to the CD68 gene ATG) in the reversed CD68L promoter, in addition to inserting HindIII andNotI restriction sites upstream of the ATG of HA-EGFP to allow the future cloning of other cDNAs. The vector backbone of all plasmids was subsequently replaced with that from the plasmid LZRS-pBM-lacZ utilizing common BspHI sites, to allow the episomal maintenance of plasmids in transfected retroviral packaging cell lines. A cDNA encoding murine ApoE was generated by PCR, using murine liver cDNA as a template, and cloned into the CD68S containing retroviral vector that had been digested with NotI to remove the HA-EGFP cDNA. High-titer retroviral supernatants were generated by selecting transfected Phoenix amphotropic and ecotropic packaging cell lines in medium containing 2 μg/mL puromycin, as described previously.23 25 Further details of the sequences of oligonucleotides and plasmids used in the generation of the retroviral expression vectors are available on request.
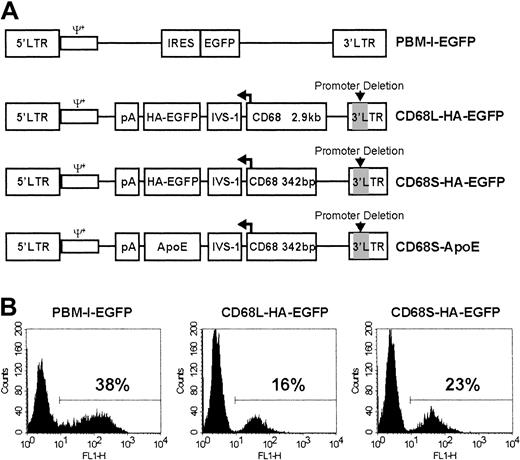
Design and in vitro testing of macrophage-specific retroviral vectors.
(A) Schematic representation of RVs used in this study. All RVs contain LTRs and packaging signals (ψ+) derived from the Moloney murine leukemia virus. PBM-I-EGFP is a standard, non–cell type–specific RV in which expression is driven by the viral 5′ LTR.23 This vector contains an internal ribosomal entry site (IRES) in addition to the cDNA encoding EGFP. The other RVs have a deletion of essential promoter and enhancer elements (indicated by gray shading) in the 3′ LTR, making them self-inactivating (SIN). In these vectors, gene expression is solely driven by a promoter containing either 2.9 kb (CD68L) or 342 bp (CD68S) of sequence 5′ to the ATG initiation codon of the CD68 gene, in addition to the CD68 first intron (IVS-1). The direction of transcription, indicated by the bent arrow, is opposite that of the viral 5′ LTR. In addition to cDNAs encoding either an HA epitope–tagged version of EGFP or ApoE, the RVs contain a bovine growth hormone polyadenylation signal (pA) to terminate RNA transcription. All of the RVs are in plasmids that contain expression cassettes for the puromycin resistance gene and the Epstein-Barr virus nuclear antigen (EBNA) in addition to the EBNA origin of replication (not shown). This allows selection of packaging cell lines with episomal copies of the plasmid.25 (B) Bone marrow–derived mφs (BMDMs) were transduced with each of the different retroviral constructs, and EGFP expression was analyzed by fluorescence-activated cell sorter (FACS). The percentage of EGFP+ cells was determined using a gate set to exclude nontransduced control cells. Data are from a single experiment performed in duplicate and are representative of 3 similar experiments.
Design and in vitro testing of macrophage-specific retroviral vectors.
(A) Schematic representation of RVs used in this study. All RVs contain LTRs and packaging signals (ψ+) derived from the Moloney murine leukemia virus. PBM-I-EGFP is a standard, non–cell type–specific RV in which expression is driven by the viral 5′ LTR.23 This vector contains an internal ribosomal entry site (IRES) in addition to the cDNA encoding EGFP. The other RVs have a deletion of essential promoter and enhancer elements (indicated by gray shading) in the 3′ LTR, making them self-inactivating (SIN). In these vectors, gene expression is solely driven by a promoter containing either 2.9 kb (CD68L) or 342 bp (CD68S) of sequence 5′ to the ATG initiation codon of the CD68 gene, in addition to the CD68 first intron (IVS-1). The direction of transcription, indicated by the bent arrow, is opposite that of the viral 5′ LTR. In addition to cDNAs encoding either an HA epitope–tagged version of EGFP or ApoE, the RVs contain a bovine growth hormone polyadenylation signal (pA) to terminate RNA transcription. All of the RVs are in plasmids that contain expression cassettes for the puromycin resistance gene and the Epstein-Barr virus nuclear antigen (EBNA) in addition to the EBNA origin of replication (not shown). This allows selection of packaging cell lines with episomal copies of the plasmid.25 (B) Bone marrow–derived mφs (BMDMs) were transduced with each of the different retroviral constructs, and EGFP expression was analyzed by fluorescence-activated cell sorter (FACS). The percentage of EGFP+ cells was determined using a gate set to exclude nontransduced control cells. Data are from a single experiment performed in duplicate and are representative of 3 similar experiments.
Animals
C57BL/6J (Ly-5.2), B6.SJL-Ptprca/Pep3b/Boyj(Ly5.1), and ApoE−/− (backcrossed 10 times to C57BL/6J) mice were all obtained from the Jackson Laboratory (Bar Harbor, ME) and were maintained under specific-pathogen–free conditions and fed a normal chow diet. Thioglycollate-elicited peritoneal cells and bone marrow–derived mφs (BMDMs) were generated as previously described.22 All experimental procedures were performed under approval of the Animal Care Committee of the University of Washington.
Bone marrow transduction and transplantation
Transduced BMDMs were generated by 2 consecutive 24-hour incubations of isolated bone marrow cells in amphotropic retroviral supernatant containing 5 μg/mL polybrene and 2000 U/mL recombinant human macrophage-colony stimulating factor (rhM-CSF; R&D Systems, Minneapolis, MN) and subsequent culture for 4 days in growth medium containing 2000 U/mL rhM-CSF. For stem cell transplants, bone marrow cells were isolated from mice injected 3 days previously with 5-fluorouracil (150 mg/kg administered intraperitoneally) and were prestimulated in Dulbecco Modified Eagle Medium (DMEM) containing 15% fetal calf serum (Gibco BRL, Gaithersburg, MD), 100 ng/mL mouse stem cell factor, 20 ng/mL human interleukin 6 (IL-6), and 10 ng/mL mouse IL-3 (all recombinant; Kirin, Tokyo, Japan). After 48 hours cells were resuspended in fresh ecotropic retroviral supernatant containing 50 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 4 μg/mL polybrene and stem-cell factor, IL-6 and IL-3 at the above concentrations, and transferred to 6-well plates that had been previously coated with human fibronectin (Sigma, St Louis, MO) by a 4-hour incubation with a 25-μg/mL fibronectin solution in phosphate-buffered saline (PBS). Plates were centrifuged at 1700g for 2 hours at 37°C and incubated for a further 22 hours at 37°C. Nonadherent cells were subsequently harvested, resuspended in fresh transduction mix, and returned to the 6-well plate for a second round of spin transduction and incubation. After a total of 48 hours of transduction, adherent and nonadherent cells were harvested, washed twice in PBS, and 1 × 106 transduced cells were injected into lethally irradiated (10.5-Gy single dose) recipient mice via the lateral tail vein.
Analysis of HA-EGFP expression
EGFP expression by isolated macrophage populations was measured by FACS, and data analysis was performed with CellQuest software (Becton Dickinson, San Jose, CA). The degree of chimerism was assessed by staining with biotinylated Ly-5.1– and Ly-5.2–specific antibodies and phycoerythrin (PE)–conjugated streptavidin (BD Pharmingen, San Diego, CA). Equal amounts of protein from tissue lysates, prepared by homogenizing snap-frozen mouse organs in lysis buffer (150 mM NaCl, 10 mM EDTA [ethylenediaminetetraacetic acid], 10 mM NaN3, 10 mM Tris [tris(hydroxymethyl)aminomethane, pH 8.0], 2 μg/mL aprotinin, 2 μg/mL leupeptin, 1 μg/mL pepstatin, 100 μg/mL phenylmethylsulphonyl fluoride, and 1% Nonidet P-40), were separated by sodium dodeclysulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions prior to transfer to Immobilon membrane (Millipore, Bedford, MA). Membranes were probed with antibodies recognizing the HA epitope (Zymed Laboratories, San Francisco, CA) or macrosialin (Serotec, Raleigh, NC) and appropriate peroxidase-conjugated secondary antibodies prior to visualization by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, Piscataway, NJ). Tissues from mice perfused with PBS containing 4% paraformaldehyde were embedded in paraffin, and 6-μm sections were stained with antibodies recognizing the HA epitope, F4/80 (Serotec), and MAC-2 (Cedarlane, Hornby, Ontario, Canada), using a 3-step protocol with 3,3′-diaminobenzidine as chromagen, as described previously.26
Analysis of ApoE−/−mice
Eight-week-old male ApoE−/−mice received transplants of ApoE−/− HSCs transduced with CD68S-based retroviruses expressing either HA-EGFP or ApoE. Cholesterol measurements were performed by Northwest Lipid Research Laboratories (Seattle, WA), using sera obtained by tail-vein bleeds performed 6 weeks after transplantation or at the time the mice were killed. Measurements of ApoE in serum and macrophage lysates and supernatants were obtained by analyzing immunoblots performed with an anti-murine ApoE antibody (Biodesign, Saco, ME) with LabWorks software (Media Cybernetics, Silver Spring, MD). Mice were injected with thioglycollate 4 days before being killed (12 weeks after transplantation), and elicited cells were collected prior to perfusion via the left ventricle with 10 mL PBS containing 1 mM EDTA and 30 mL fixative (PBS, 4% paraformaldehyde, and 5% sucrose). The heart and aortic root were dissected, fixed overnight, and frozen in optimal cutting temperature (OCT) compound. Cryosections of the proximal aorta (10-μm thick) were stained with Oil Red O and counterstained with hematoxylin, and images were captured with a Spot Insight digital camera (Diagnostic Instruments, Sterling Heights, MI). Lesion area was quantified by measuring Oil Red O staining, using a color threshold and Image Pro Plus 4.5 software (Media Cybernetics). Measurements were made on 8 sections, 50 μm apart, and data are expressed as an average lesion area per mouse in square microns.
Results
Generation and in vitro analysis of CD68-based retroviral vectors
To develop an RV for directing macrophage-restricted transgene expression, we generated SIN-RVs containing promoter fragments from the human CD68 gene (Figure 1A). Two constructs were created, containing either a long, 2.9-kilobase (kb) (CD68L) or a short, 342-bp (CD68S) fragment of sequence 5′ to the ATG initiation codon of theCD68 gene, in addition to the CD68 first intron (IVS-1). Because the IVS-1 sequence plays an important role in regulating the levels and specificity of CD68 transcriptional activity,27 the CD68 expression cassette was cloned in the reverse transcriptional orientation, ensuring that the intron would not be removed during viral RNA processing. Both constructs contained a cDNA encoding HA epitope–tagged version of the HA-EGFP as a reporter gene.
The constructs were first tested by using retrovirus-containing supernatants to transduce BMDMs. FACS analysis of adherent cells revealed significant levels of EGFP expression directed by both CD68-containing RVs (Figure 1B). Transduction efficiencies were 30% to 50% lower than with the pBM-I-EGFP control retrovirus, presumably reflecting lower retroviral titers, as similar results were obtained when the murine macrophage cell lines RAW-264 and J774 were transduced (data not shown). These data showed that CD68 promoter elements do not have a prohibitively negative impact on viral titers, as is often seen when cell type–specific promoter elements are incorporated into RVs.28
CD68S-based retroviral vectors generate long-lasting gene expression in vivo
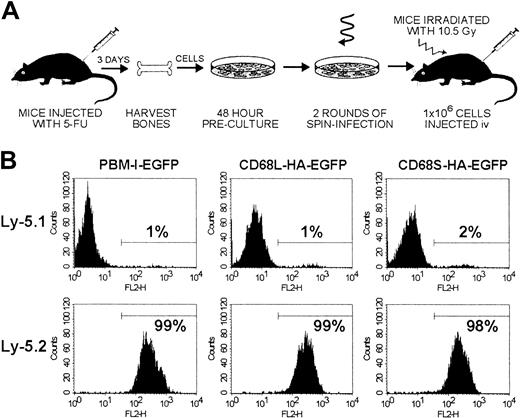
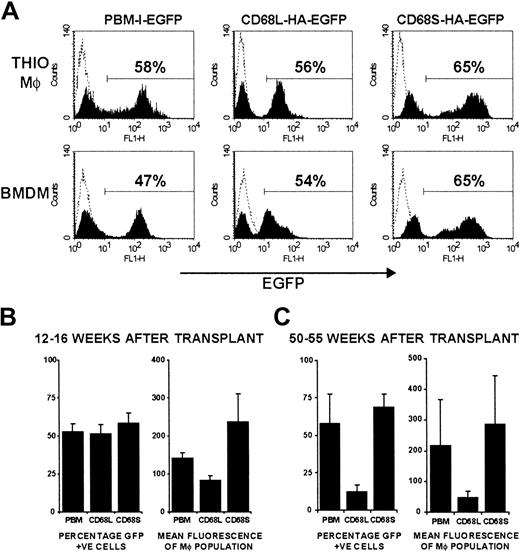
To examine the utility of CD68-based RVs for in vivo gene expression, we performed HSC transplantations using a modified version of published protocols (Figure 2A). Initial experiments were performed by transplanting transduced HSCs from Ly-5.2 donors into 5 Ly-5.1 recipient mice for each retroviral construct. Mice were killed 12 to 16 weeks after transplantation, and FACS analysis of thioglycollate-elicited peritoneal macrophages (Thio-mφs) revealed that more than 98% of cells were of donor origin (Ly-5.2+), regardless of the RV used to transduce the HSCs (Figure 2B). Expression of EGFP by both Thio-mφs and BMDMs from mice that had received transplants was also measured by FACS (Figure3A). For each of the 3 retroviral constructs, distinct EGFP+ and -negative populations can be observed, with positive cells expressing relatively homogenous levels of EGFP. Expression profiles were comparable for Thio-mφs and BMDMs generated from the same mouse. The efficiency of HSC transduction, as measured by the percentage of EGFP+ Thio-mφs, was approximately 50% for each of the 3 retroviral constructs, with little variation between mice of the same group (Figure 3B). Measurements of mean EGFP fluorescence intensity showed that the control RV, pBM-I-EGFP, generated a consistent level of EGFP expression. Levels of expression from the CD68L-based RV were equally consistent but were lower than those generated by the control retrovirus. The CD68S-containing retrovirus directed higher levels of EGFP expression than both the control and CD68L retroviruses, although these differences did not quite reach statistical significance (P = .15 and P = .06, respectively). However, the expression of EGFP by the CD68S retrovirus did show higher levels of variability, suggesting that this RV is more influenced by the site of chromosomal integration.
Experimental procedure and measurements of chimerism.
(A) Schematic representation of the HSC transplantation protocol. Bone marrow cells were isolated from donor mice injected 3 days previously with 5-fluorouracil (5-FU) and were cultured for 48 hours in medium containing stem-cell factor, IL-3, and IL-6 to stimulate proliferation of HSCs. Cells were subsequently transduced for 48 hours, using ecotropic retroviral supernatants and culture dishes coated with fibronectin. The transduction procedure incorporated replacement of the retroviral supernatant after 24 hours and 2 rounds of centrifugation for 2 hours to increase the effective viral titer. After transduction, cells were harvested and injected intravenously into recipient mice that had been lethally irradiated (10.5 Gy) 24 hours previously. (B) Levels of chimerism were measured by FACS analysis of thioglycollate-elicited cells from Ly-5.1 recipient mice that had received transplants of Ly-5.2 HSCs. Cells were stained with biotinylated antibodies recognizing Ly-5.1 or Ly-5.2 and PE-conjugated streptavidin. The percentage of PE-positive cells was determined by use of a gate set to exclude unstained control cells. Data for each RV are from a single mouse but are representative of results from 5 other mice.
Experimental procedure and measurements of chimerism.
(A) Schematic representation of the HSC transplantation protocol. Bone marrow cells were isolated from donor mice injected 3 days previously with 5-fluorouracil (5-FU) and were cultured for 48 hours in medium containing stem-cell factor, IL-3, and IL-6 to stimulate proliferation of HSCs. Cells were subsequently transduced for 48 hours, using ecotropic retroviral supernatants and culture dishes coated with fibronectin. The transduction procedure incorporated replacement of the retroviral supernatant after 24 hours and 2 rounds of centrifugation for 2 hours to increase the effective viral titer. After transduction, cells were harvested and injected intravenously into recipient mice that had been lethally irradiated (10.5 Gy) 24 hours previously. (B) Levels of chimerism were measured by FACS analysis of thioglycollate-elicited cells from Ly-5.1 recipient mice that had received transplants of Ly-5.2 HSCs. Cells were stained with biotinylated antibodies recognizing Ly-5.1 or Ly-5.2 and PE-conjugated streptavidin. The percentage of PE-positive cells was determined by use of a gate set to exclude unstained control cells. Data for each RV are from a single mouse but are representative of results from 5 other mice.
CD68S-based retroviral vectors generate long-lasting gene expression in vivo.
(A) FACS analysis of EGFP expression by thioglycollate-elicited and bone marrow–derived macrophages from a single mouse. Macrophages in thioglycollate-elicited peritoneal cells were gated on the basis of forward- and side-scatter properties. The percentage of EGFP+ cells was determined by use of a gate set to exclude control cells from mice that had not received transplants (dotted line). The plots are representative of data from at least 5 mice per retroviral construct. (B-C) Analysis of the percentage of EGFP+ cells and the mean fluorescence intensity of thioglycollate-elicited macrophages from mice killed either 12 to 16 weeks (B) or 50 to 55 weeks (C) after HSC transplantation. Graphs represent mean ± SEM from groups of 5 (12-16 weeks) or 4 (50-55 weeks) mice per retroviral construct.
CD68S-based retroviral vectors generate long-lasting gene expression in vivo.
(A) FACS analysis of EGFP expression by thioglycollate-elicited and bone marrow–derived macrophages from a single mouse. Macrophages in thioglycollate-elicited peritoneal cells were gated on the basis of forward- and side-scatter properties. The percentage of EGFP+ cells was determined by use of a gate set to exclude control cells from mice that had not received transplants (dotted line). The plots are representative of data from at least 5 mice per retroviral construct. (B-C) Analysis of the percentage of EGFP+ cells and the mean fluorescence intensity of thioglycollate-elicited macrophages from mice killed either 12 to 16 weeks (B) or 50 to 55 weeks (C) after HSC transplantation. Graphs represent mean ± SEM from groups of 5 (12-16 weeks) or 4 (50-55 weeks) mice per retroviral construct.
To assess the ability of CD68-based retroviruses to produce persistent gene expression, we measured EGFP levels in cells from mice killed 50 to 55 weeks after transplantation. Thio-mφs from mice that received HSCs transduced with either pBM-I-EGFP or CD68S-HA-EGFP had comparable percentages of EGFP+ cells and similar mean fluorescence intensities to cells from mice killed 12 to 16 weeks after transplantation (Figure 3C). In contrast, the CD68L-based RV showed a significant reduction in the levels of EGFP and the percentage of cells expressing EGFP.
CD68-based retroviral vectors direct expression specifically to mφs in vivo
In addition to measuring levels of reporter gene expression in mφs, we analyzed the mice described above for the cell-type specificity of EGFP expression generated by each of the RVs. Thioglycollate-elicited peritoneal cells contain neutrophils and lymphocytes as well as mφs. These 3 populations of cells can be distinguished by FACS analysis on the basis of their forward-scatter (FSC) and side-scatter (SSC) properties. The relative proportions of the 3 cell types were similar, independent of the RV (Figure4A). To examine the cell-type specificity of each of the 3 RVs, we generated FSC versus SSC plots after gating for EGFP+ cells. The pBM-I-EGFP RV was able to direct expression to lymphocytes (green), neutrophils (blue), and mφs (red), as predicted for a viral LTR promoter with no cell-type specificity. In contrast, both CD68-based RVs generated expression of EGFP almost exclusively in the mφ population. These specificities were seen in all mice examined and were not affected by the length of time after HSC transplantation.
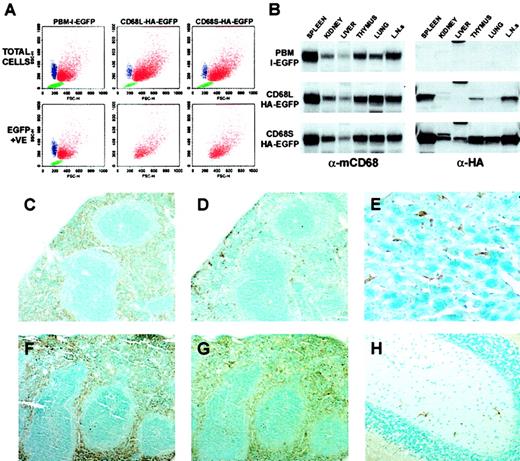
CD68-based retroviral vectors generate expression specifically in mφs in vivo.
(A) Peritoneal cells elicited 4 days after injection with thioglycollate were analyzed by FACS. Three distinct cellular populations could be distinguished on the basis of forward- and side-scatter properties: neutrophils (blue), macrophages (red), and lymphocytes (green). These designations were confirmed by staining with cell type–specific antibodies (data not shown). By using a gate for EGFP-positive cells, which was established using samples from mice that had not received transplants, the cell-type specificity of EGFP expression could be established. The data are from cells isolated from a single mouse and are representative of data from 10 mice per retroviral construct. (B) Immunoblot analysis of tissue lysates (50 μg total protein per lane) from mice that had received HSC transplants (16 weeks after transplantation), using antibodies recognizing macrosialin (α-mCD68) or HA epitope–tagged EGFP (α-HA). Blots are from identical exposures to allow comparisons of the relative levels of HA-EGFP expression. Data are from a single mouse per retroviral construct, and are representative of results from 2 similar mice. L.N.s indicate lymph nodes. (C-H) Immunohistochemical staining of tissues from mice that had received transplants of HSCs transduced with CD68S–HA-EGFP retrovirus. Tissue sections from mice killed 16 weeks (C-D) or 55 weeks (E-H) after transplantation were stained with antibodies recognizing F4/80 (C,F) or HA-EGFP (D-E, G-H). Serial sections of spleen (C-D, F-G) show expression of HA-EGFP predominantly in the macrophages of the red pulp, a pattern that is identical to that of the F4/80 antigen. The differences in the extent of HA-EGFP staining between tissues taken at 16 weeks and at 55 weeks after transplantation represent the relatively slow kinetics of repopulation of the spleen with macrophages of donor HSC origin. Staining of sections of liver (E) and brain (H) reveal expression of HA-EGFP in Küpffer cells and microglia, respectively. Sections stained with species-matched antibody controls showed no specific staining pattern. Original magnifications: × 10, C-D, F-G; × 40, E-H.
CD68-based retroviral vectors generate expression specifically in mφs in vivo.
(A) Peritoneal cells elicited 4 days after injection with thioglycollate were analyzed by FACS. Three distinct cellular populations could be distinguished on the basis of forward- and side-scatter properties: neutrophils (blue), macrophages (red), and lymphocytes (green). These designations were confirmed by staining with cell type–specific antibodies (data not shown). By using a gate for EGFP-positive cells, which was established using samples from mice that had not received transplants, the cell-type specificity of EGFP expression could be established. The data are from cells isolated from a single mouse and are representative of data from 10 mice per retroviral construct. (B) Immunoblot analysis of tissue lysates (50 μg total protein per lane) from mice that had received HSC transplants (16 weeks after transplantation), using antibodies recognizing macrosialin (α-mCD68) or HA epitope–tagged EGFP (α-HA). Blots are from identical exposures to allow comparisons of the relative levels of HA-EGFP expression. Data are from a single mouse per retroviral construct, and are representative of results from 2 similar mice. L.N.s indicate lymph nodes. (C-H) Immunohistochemical staining of tissues from mice that had received transplants of HSCs transduced with CD68S–HA-EGFP retrovirus. Tissue sections from mice killed 16 weeks (C-D) or 55 weeks (E-H) after transplantation were stained with antibodies recognizing F4/80 (C,F) or HA-EGFP (D-E, G-H). Serial sections of spleen (C-D, F-G) show expression of HA-EGFP predominantly in the macrophages of the red pulp, a pattern that is identical to that of the F4/80 antigen. The differences in the extent of HA-EGFP staining between tissues taken at 16 weeks and at 55 weeks after transplantation represent the relatively slow kinetics of repopulation of the spleen with macrophages of donor HSC origin. Staining of sections of liver (E) and brain (H) reveal expression of HA-EGFP in Küpffer cells and microglia, respectively. Sections stained with species-matched antibody controls showed no specific staining pattern. Original magnifications: × 10, C-D, F-G; × 40, E-H.
The results presented above were based on the analysis of EGFP expression by isolated, inflammatory mφs. We next examined expression of EGFP by resident mφ populations within tissues of mice that had received HSC transplants. Immunoblots of protein lysates from organs of mice that had received transplants of HSCs transduced with each of the 3 retroviral constructs showed similar banding patterns when probed with an antibody recognizing the murine homolog of CD68 (mCD68; Figure4B). Because all tissue mφs express mCD68, the intensities of the bands are representative of the relative abundance of mφs, with mφ-rich organs such as the spleen and lymph nodes giving the highest signals. Identical immunoblots probed with an antibody raised against the HA epitope showed that the pattern of HA-EGFP expression driven by both of the CD68-based RVs was highly similar to that of the mφ-restricted mCD68 gene. Comparisons of the relative intensities of bands from matching tissues showed that expression levels generated by the CD68S retrovirus were significantly higher than those generated by the CD68L vector, consistent with the data from the analysis of EGFP expression in Thio-mφs (Figure 3B).
To further examine the cell-type specificity of CD68-based retroviruses, we performed immunohistochemical analyses of tissue sections from mice that had received HSC transplants. Staining of serial sections of spleen, taken from mice that had received HSCs transduced with the CD68S-HA-EGFP retrovirus, with antibodies recognizing the mφ-restricted F4/80 antigen (Figure 4C,F) or the HA epitope (Figure 4D-G) showed virtually identical staining patterns. Both antibodies predominantly stained mφs within the splenic red pulp, with very little staining in the T- and B-cell–rich white pulp regions, indicating mφ-specific expression from the CD68S-based retrovirus. The difference in the percentage of HA-EGFP–positive red pulp mφs in sections taken from mice 16 and 55 weeks after transplantation (compare Figure 4D and Figure 4G) highlights the relatively slow kinetics of turnover of the resident splenic mφ population.29 Similar staining of other tissues showed expression of HA-EGFP exclusively in mφ populations, including Küpffer cells in the liver (Figure 4E) and microglia in the brain (Figure 4H).
Gene therapy of ApoE−/−mice using a CD68S retroviral vector reverses hypercholesterolemia and lesion formation
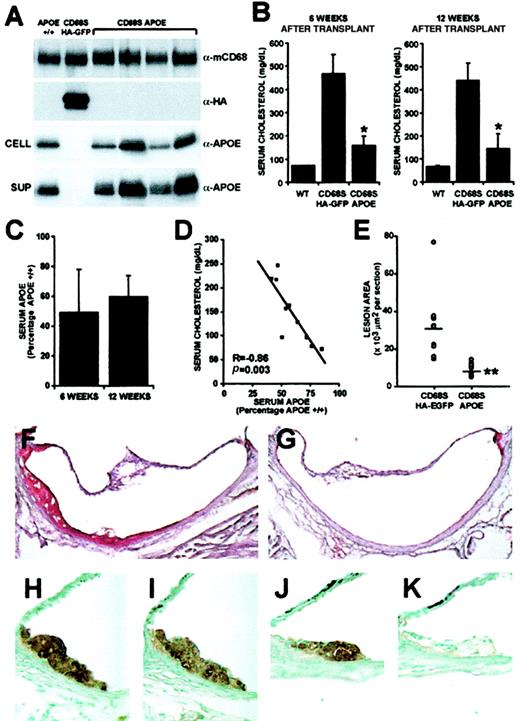
The data presented so far have shown that the CD68S-containing RV is able to deliver long-lasting, mφ-specific gene expression at levels comparable to a standard RV. We next wanted to examine whether the CD68S-based RV was capable of generating physiologically relevant levels of gene expression. Previous studies have shown that hypercholesterolemia and atherosclerotic lesion development in ApoE−/− mice can be corrected through transplantation of ApoE+/+ bone marrow.30 We therefore sought to determine whether similar results could be achieved through gene therapy using a CD68S RV encoding the ApoE gene (CD68S-ApoE; Figure 1A). Groups of 10ApoE−/− mice received transplants ofApoE−/− HSCs transduced with either CD68S-ApoE or CD68S–HA-EGFP retroviruses and were maintained on a normal chow diet until being killed 12 weeks after transplantation. Expression of ApoE in transplant recipients was examined by immunoblotting of cell lysates and supernatants from Thio-mφs cultured ex vivo for 24 hours, with an antibody recognizing murine ApoE (Figure5A). The levels of ApoE generated by the CD68S-ApoE RV were variable between mice, but in all cases the levels were comparable to, if not significantly greater than, levels of ApoE produced by mφs from an ApoE+/+ control. No detectable expression of ApoE was seen in mφs from mice receiving HSCs transduced with CD68S–HA-EGFP retrovirus, but these cells did express high levels of HA-EGFP, with 84% (SEM ± 9%; n = 10) of cells being positive for EGFP (Figure 5A and data not shown).
Gene therapy of ApoE−/− mice using a CD68S retroviral vector.
(A) ApoE expression by thioglycollate-elicited macrophages from wild-type mice (ApoE+/+) orApoE−/− mice that had received transplants of HSCs transduced with either CD68S–HA-EGFP or CD68s-ApoE retroviruses was assessed by immunoblotting of lysates (CELL) or supernatants (SUP) from macrophages cultured ex vivo for 24 hours using an antibody recognizing ApoE (α-ApoE). A similar blot probed with an antibody against macrosialin (α-mCD68) served as loading control, and blotting with an antibody recognizing the HA epitope (α-HA) showed expression of HA-EGFP only in macrophages fromApoE−/− mice that had received transplants of HSCs transduced with CD68S–HA-EGFP retrovirus. (B) Serum cholesterol was measured in samples obtained from bleeds performed 6 and 12 weeks after HSC transplantation. Graphs show the mean serum cholesterol ± SD (n = 10). *P < .0001 as measured by the Mann-Whitney nonparametric test. (C) ApoE levels in sera fromApoE−/− mice that had received transplants of HSCs transduced with CD68S-ApoE retrovirus were measured by densitometric analyses of immunoblots performed with an antibody against ApoE. The graphs show mean ApoE expression ± SD (n = 10) expressed as a percentage of ApoE present in serum from anApoE+/+ control mouse. (D) A plot of serum cholesterol and ApoE values from individual mice reveals an inverse, linear relationship (R = −0.86, p = .003, by Spearman rank correlation test). (E) Quantitative measurement of atherosclerosis in the aortic root inApoE−/− mice 12 weeks after transplantation. Each point (open circle) represents the mean lesional area (μm2 × 103 per section) from an individual mouse. A horizontal line indicates the average lesion area for each group of mice. **P < .0001 as measured by the Mann-Whitney nonparametric test (n = 10). (F-G) Representative Oil Red O–stained sections from the aortic root ofApoE−/− mice that had received transplants of HSCs transduced with CD68S–HA-EGFP (F) or CD68S-ApoE (G) retroviruses. (H-K) Immunohistochemical analysis of similar lesions fromApoE−/− mice that had received transplants of HSCs transduced with CD68S–HA-EGFP (H-I) or CD68S-ApoE (J-K) retroviruses reveals comparable levels of macrophage staining with an antibody recognizing MAC-2 (H,J), but only macrophages in lesions from mice receiving CD68S–HA-EGFP–transduced HSCs show reactivity with an antibody against the HA epitope (I,K). Original magnifications: × 10, F-G; × 40, H-K.
Gene therapy of ApoE−/− mice using a CD68S retroviral vector.
(A) ApoE expression by thioglycollate-elicited macrophages from wild-type mice (ApoE+/+) orApoE−/− mice that had received transplants of HSCs transduced with either CD68S–HA-EGFP or CD68s-ApoE retroviruses was assessed by immunoblotting of lysates (CELL) or supernatants (SUP) from macrophages cultured ex vivo for 24 hours using an antibody recognizing ApoE (α-ApoE). A similar blot probed with an antibody against macrosialin (α-mCD68) served as loading control, and blotting with an antibody recognizing the HA epitope (α-HA) showed expression of HA-EGFP only in macrophages fromApoE−/− mice that had received transplants of HSCs transduced with CD68S–HA-EGFP retrovirus. (B) Serum cholesterol was measured in samples obtained from bleeds performed 6 and 12 weeks after HSC transplantation. Graphs show the mean serum cholesterol ± SD (n = 10). *P < .0001 as measured by the Mann-Whitney nonparametric test. (C) ApoE levels in sera fromApoE−/− mice that had received transplants of HSCs transduced with CD68S-ApoE retrovirus were measured by densitometric analyses of immunoblots performed with an antibody against ApoE. The graphs show mean ApoE expression ± SD (n = 10) expressed as a percentage of ApoE present in serum from anApoE+/+ control mouse. (D) A plot of serum cholesterol and ApoE values from individual mice reveals an inverse, linear relationship (R = −0.86, p = .003, by Spearman rank correlation test). (E) Quantitative measurement of atherosclerosis in the aortic root inApoE−/− mice 12 weeks after transplantation. Each point (open circle) represents the mean lesional area (μm2 × 103 per section) from an individual mouse. A horizontal line indicates the average lesion area for each group of mice. **P < .0001 as measured by the Mann-Whitney nonparametric test (n = 10). (F-G) Representative Oil Red O–stained sections from the aortic root ofApoE−/− mice that had received transplants of HSCs transduced with CD68S–HA-EGFP (F) or CD68S-ApoE (G) retroviruses. (H-K) Immunohistochemical analysis of similar lesions fromApoE−/− mice that had received transplants of HSCs transduced with CD68S–HA-EGFP (H-I) or CD68S-ApoE (J-K) retroviruses reveals comparable levels of macrophage staining with an antibody recognizing MAC-2 (H,J), but only macrophages in lesions from mice receiving CD68S–HA-EGFP–transduced HSCs show reactivity with an antibody against the HA epitope (I,K). Original magnifications: × 10, F-G; × 40, H-K.
Analysis of serum samples taken 6 and 12 weeks after transplantation showed that mφ-specific expression of ApoE driven by the CD68S-ApoE retrovirus caused a significant reduction in cholesterol levels (66% and 67%, respectively; P < .0001, n = 10 at both time points) compared with control mice that had received transplants with the CD68S–HA-EGFP retrovirus (Figure 5B). Levels of ApoE reached 59% (SD ± 14%) the levels of anApoE+/+ control at the time of death (Figure5C), and there was a strong inverse correlation between levels of serum ApoE and cholesterol (Figure 5D).
The effect of gene therapy with the CD68S-ApoE retrovirus on atherosclerotic lesion development was examined by quantitative morphometry of Oil Red O–stained sections taken from the root of the aorta (Figure 5E-G). ApoE−/− mice receiving HSCs transduced with the CD68S-ApoE retrovirus showed a 74% reduction in lesion area compared with mice receiving CD68S–HA-EGFP retrovirus (mean lesion areas per section ± SEM, 8027 ± 1158 μm2 vs 31 353 ± 6213 μm2;P < .0001, n = 9). Immunohistochemical staining of lesions from both groups of mice with the mφ-specific antibody MAC-2 showed that they contained predominantly mφ-derived foam cells (Figure 5H,J). Owing to problems with high background, no specific staining could be seen when an antibody against ApoE was used (data not shown); however, sections from mice that had received HSCs transduced with CD68S–HA-EGFP showed staining with an antibody recognizing the HA epitope, indicating that CD68S-based RVs can direct gene expression to mφs in atherosclerotic lesions (Figure 5I,K).
Discussion
The data presented in this paper represent advances in several areas associated with the use of retroviral gene delivery to HSCs as a tool for gene therapy. The most significant aspect of this study is that it is the first time that a nonlentiviral, retroviral vector incorporating lineage-specific gene-regulatory sequences has been successfully used to treat a mouse model of a single-gene disorder, theApoE−/− mouse. What makes these results particularly encouraging is the absolute levels of gene expression generated by the CD68S-ApoE RV. ApoE is a very abundant gene product in mφs, representing 1% to 2% of total mRNA production, as measured by serial analysis of gene expression (SAGE).31 The CD68S-ApoE RV was able to generate levels of ApoE expression that were comparable to, and in some cases greater than, those seen for the endogenous gene in an ApoE+/+ mouse. Indeed, the levels of ApoE expression were approximately 50- to 100-fold greater than those obtained using a standard, LTR promoter–driven RV used in an analogous experimental approach.32 One unexpected aspect of our data was the relatively modest reductions in serum cholesterol levels that were seen in mice expressing 30% to 50% of normal serum ApoE levels. Previous studies using total bone marrow transplantation have shown that serum ApoE levels that are 10% of normal levels are sufficient to produce a significant reduction in serum cholesterol.30,33 Although this discrepancy is most likely due to differences in experimental procedure, the recent discovery that hepatocytes can be derived from transplanted bone marrow suggests that the cellular source of ApoE may also play a role.34
Another important aspect of our data is the demonstration of restricted gene expression in cells of a specific lineage derived from retrovirally transduced HSCs. Both of the SIN-RVs we generated, containing either a long (2.9 kb; CD68L) or a short (342 bp; CD68S) fragment of transcriptional regulatory sequence 5′ to the ATG initiation codon of the human CD68 gene, were able to direct gene expression specifically to mφs. These results provide a further example of the utility of CD68 regulatory sequences to specifically target gene expression to mφs in vivo.21,22 Comparison of the levels and longevity of gene expression generated by the CD68L- and CD68S-containing SIN-RVs revealed some differences. The CD68L-based RV showed lower but more consistent levels of gene expression in cells taken 12 to 16 weeks after transplantation; however, expression was reduced at the later time point analyzed. In contrast, the CD68S-containing RV generated higher levels of long-lasting gene expression, but showed more variability between mice. These differences may be the result of the unusual genomic location of theCD68 gene locus, with the ATG initiation codon of theCD68 gene lying 670 bp downstream of the ubiquitously expressed eukaryotic Initiation Factor 4AI (eIF4AI) gene on chromosome 17p13.35 This means that the genetic elements required to restrict gene expression to mφs are likely to be condensed in the intergenic region, while the 3′ end of the eIF4AI gene, which makes up most of the CD68L-promoter sequence, may contain negative regulatory sequences for high-level expression in mφs.
The high-efficiency retroviral transduction of HSCs in this study was achieved without the ex vivo selection of transduced cells. Several reports have suggested that preselection of transduced HSCs, often through FACS sorting of EGFP+ cells, is a prerequisite for generating long-lasting gene expression in vivo.36-38 Our results were achieved by incorporating several modifications to previously published protocols. We generated very-high-titer retroviral supernatants by incorporating elements from the Epstein-Barr virus into our RV plasmid backbone, allowing the episomal maintenance of the plasmid in stable retrovirus-producing packaging cell lines (Figure1A).25 In addition, retroviral transduction of HSCs was performed with fibronectin-coated culture dishes and incorporated a period of centrifugation, 2 modifications that have previously been shown to enhance the efficiency of retroviral transduction (Figure2A).39 40 This approach allows high-efficiency transduction of long-term repopulating HSCs, as shown by long-lasting gene expression in primary HSC recipients (Figure 3C) and mice receiving bone marrow from primary recipients of retrovirally transduced HSCs (data not shown).
The ability to use CD68S-based SIN-RVs to overexpress genes at high levels in mφs in atherosclerotic lesions, without the time-consuming process of generating and breeding transgenic lines, will greatly facilitate the elucidation of mφ functions in atherogenesis. The CD68S-based SIN-RVs also provide the opportunity to evaluate the impact of altered mφ gene expression at distinct stages of disease progression. For example, by transplanting transduced HSCs into 40-week-old ApoE−/− mice, we have been able to express genes in mφs in pre-established, advanced atherosclerotic lesions (data not shown). Indeed, the application of these mφ-specific RVs to murine models of other chronic inflammatory or infectious diseases should be a powerful tool in establishing a better understanding of the roles played by mφs, and in identifying novel therapeutic targets. In addition to providing a potent experimental resource, the future clinical use of these high-efficiency, mφ-specific RVs may offer genetic therapies for many human diseases.
We are grateful to Gary Nolan for providing retroviral expression plasmids, to Glaxo Wellcome and David Greaves for providing the CD68-promoter DNA, and to Kirin Brewing for providing cytokines. We also thank Karen Honey, Kelli McIntyre, and Paul Martin for help with stem cell transplants; Roderick Browne for expert technical assistance; and David Greaves, Karen Honey, and Kyle Garton for many helpful comments and suggestions.
Prepublished online as Blood First Edition Paper, September 5, 2002; DOI 10.1182/blood-2002-07-2131.
Supported by National Institutes of Health grant HL18645, a postdoctoral fellowship from the American Heart Association, and a University of Washington Royalty Research Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter J. Gough, Department of Pathology, Harborview Medical Center, 325 9th Ave, Box 359675, Seattle, WA 98104-2499; e-mail: pgough@u.washington.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal