Abstract
Two major immunologic barriers, the host-versus-graft (HVG) and graft-versus-host (GVH) reactions, have to be overcome for successful allogeneic hematopoietic cell transplantation. T cells were shown to be primarily involved in these barriers in the major histocompatibility complex identical setting. We hypothesized that selective ablation of T cells using radioimmunotherapy together with postgrafting immunosuppression would suffice to ensure stable allogeneic engraftment. We had described a canine model of nonmyeloablative marrow transplantation in which host immune reactions were impaired by a single dose of 200 cGy total body irradiation (TBI), and both GVH and residual HVG reactions were controlled by postgrafting immunosuppression with mycophenolate mofetil (MMF) and cyclosporine (CSP). Here, we substituted the α-emitter bismuth-213 (213Bi) linked to a monoclonal antibody (mAb) against T-cell receptor (TCR) αβ, using the metal-binding chelate diethylenetriaminepentaacetic acid (DTPA) derivative cyclohexyl–(CHX)-A″, for 200 cGy TBI. Biodistribution studies using a γ-emitting indium-111–labeled anti-TCRαβ mAb showed uptake primarily in blood, marrow, lymph nodes, spleen, and liver. Four dogs were treated with 0.13 to 0.46 mg/kg TCRαβ mAb labeled with 3.7 to 5.6 mCi/kg (137-207 MBq/kg) 213Bi. The treatment was administered in 6 injections on days –3 and –2 followed by transplantation of dog leukocyte antigen-identical marrow on day 0 and postgrafting immunosuppression with MMF/CSP. The therapy was well tolerated except for elevations of transaminases that were transient in all but one of the dogs. No other organ toxicities or signs of graft-versus-host disease were noted. The dogs had prompt allogeneic hematopoietic engraftment and achieved stable mixed donor-host hematopoietic chimerism with donor contributions ranging from 5% to 55% after more than 30 weeks of follow up.
Introduction
A nonmyeloablative conditioning regimen was developed in a canine model of dog leukocyte antigen (DLA)–identical marrow transplantation using 200 cGy total body irradiation (TBI) before and immunosuppression with mycophenolate mofetil (MMF) and cyclosporine (CSP) after transplantation.1 The results of these preclinical studies have already been successfully translated into clinical trials involving elderly or medically infirm patients with hematologic malignancies who were not eligible for conventional myeloablative transplantations.2 To further reduce the risk of potential late toxic effects of external beam γ-irradiation, strategies to precisely target radiation to the marrow using radioimmunotherapy have been investigated.3,4 Using this strategy in the canine model, we previously demonstrated that it was feasible to replace external beam TBI by radioimmunotherapy with the α-emitter bismuth-213 (213Bi) linked to a panhematopoietic monoclonal antibody (mAb) against CD45.5 Other studies in this model have shown that 200 cGy external beam TBI can be replaced by 450 cGy radiation to cervical, thoracic, and upper abdominal lymph nodes with subsequent stable donor engraftment even in unirradiated marrow and lymph node sites.6 Given that finding and the fact that cytolytic T cells,7-11 especially those expressing T-cell receptor (TCR) αβ,12 are thought to be involved in marrow graft rejection, we hypothesized that it would be possible to perform marrow transplantation after treating recipients with targeted radioimmunotherapy directed at T cells. If successful, this treatment would result in nonmyeloablative conditioning with minimal toxicity. Such an approach would be especially suitable for patients with nonmalignant hematologic diseases.
Here, we conjugated the α-emitting radionuclide 213Bi to a mAb against TCRαβ using the diethylenetriaminepentaacetic acid (DTPA) derivative cyclohexyl (CHX)–A″.13 213Bi has a half-life (t1/2) of 46 minutes, is available from an actinium-225 (225Ac) generator system, and delivers short-range but high-energy radiation leading to a high relative biologic effectiveness (RBE).14 We show that selective ablation of T cells with the 213Bi–anti-TCRαβ conjugate before transplantation allows stable engraftment of DLA-identical marrow grafts when combined with postgrafting immunosuppression with MMF and CSP.
Materials and methods
Monoclonal antibodies
The anti-TCRαβ mAb 15.9D5 (immunoglobulin G1 [IgG1])15 was used for the radioimmunoconjugate. mAbs against canine CD45 (CA12.10C12, IgG1),16 CD4 (CA13.1.E4, IgG1), CD8 (CA9.JD3, IgG2a),17 and TCRαβ (CA15.9D5, IgG1) were used for flow cytometry. The anti-CD3 mAb CA17.6B3 (IgG2b) was kindly provided by Dr Peter Moore (School of Veterinary Medicine, University of California, Davis). Additionally, we used antibodies against canine CD44 (S5, IgG1)18 and canine myeloid cells (DM5, IgG1).19 As isotype control, we used mAb 31A (IgG1) directed at the mouse Thy-1 receptor that does not cross-react with canine cells.20 All mAbs were produced and purified at the Biologics Production Facilities of the Fred Hutchinson Cancer Research Center (Seattle, WA). In addition, the commercially available antibodies goat antimouse (Fab)2-fluorescein isothiocyanate (FITC) (Biosource, Camarillo, CA) and antihuman CD14 (Biosource) cross-reacting with canine CD1421 were used. The mAbs were either biotinylated or FITC-conjugated according to standard protocols.
Flow cytometry
Flow cytometry was used to quantify the leukocyte subsets and to assess antigen saturation with the 213Bi-labeled antibody. The respective FITC-conjugated or biotinylated mAbs (10 μg/mL) were added to 50 μL whole blood and incubated at 4°C for 30 minutes. Subsequently, red blood cells were lysed with a hemolytic buffer containing EDTA (ethylenediaminetetraacetic acid). For biotinylated mAbs, 2 μg phycoprobe R-phycoerythrin-streptavidin (Biomeda, Foster City, CA) was added after one wash with cold Hanks buffered salt solution (HBSS) supplemented with 2% heat inactivated horse serum (HBSS/2% HS). The mixture was incubated at 4°C for 30 minutes, and subsequently cells were washed twice with HBSS/2% HS and once with HBSS alone. After resuspension in 1% paraformaldehyde, the cells were analyzed on a fluorescence-activated cell sorter (FACScan) Flow Cytometer (Becton Dickinson, San Jose, CA).
Conjugation of the anti-TCRαβ antibody with isothiocyanatobenzyl–CHX-A″–DTPA
For demetallation, the anti-TCRαβ mAb (45 mg) was dialyzed against 2 L metal-free HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer with a minimum of 5 buffer changes over 3 days at 4°C using a Slide-A-Lyzer 10K cassette (Pierce, Rockford, IL). All buffers used were prepared with metal-free (18 MΩ) water passed over a column of Chelex-100 resin (250 g/12 L). We added 10 g Chelex-100 resin to each buffer change. The demetallated mAb was removed from dialysis and placed in an acid-washed 15-mL conical vial, and care was taken to assure that no metals were introduced. After this rigorous demetallation of the antibody, glassware, and solvents, the anti-TCRαβ mAb (15.9D5) was conjugated with the isothiocyanate form of the metal-binding chelate CHX-A″–DTPA. We added 100 μL (4.29 μmol) isothiocyanatobenzyl–CHX-A″ solution (25 mg/mL in dimethyl sulfoxide [DMSO]) to 13 mL (0.217 μmol) of the metal-free mAb (2.5 mg/mL), and the reaction mixture was stirred at room temperature for 18 hours. Following this step, the reaction mixture was placed in a dialysis cassette and dialyzed against 3 × 2 L metal-free citrate buffer (50 mM sodium citrate, 150 mM NaCl, 0.05% NaN3 adjusted to pH 5.5) over 2 days, dialyzed against 4 × 2 L 150 mM NaCl for 2 days, and stored at 4°C until used. An isoelectric-focusing gel was run to determine if complete conjugation was achieved. Complete conjugation (ie, at least 1 CHX-A″–DTPA on each mAb molecule) was evidenced by a change in the isoelectric point of the mAb from 6-7.4 to 4.5-6.0. The number of chelates attached to the mAb was analyzed by a spectrophotometric assay using yttrium-arsenazo III complex at 652 nm.22 A single batch of CHX-A″–DTPA conjugated antibody was prepared for all of the studies in dogs, and the estimated number of chelates per mAb for that batch was 2.4. To determine if the binding affinity of the mAb had been affected by the conjugation procedure, we compared the mAb–CHX-A″ with the unconjugated antibody by flow cytometry.
Radiolabeling of the anti-TCRαβ mAb–CHX-A″ conjugate with indium-111 (111In)
A volume of 500 μL metal-free sodium acetate (50 mM, pH 5.5) was added to 500 μL of a 2.4 mg/mL anti-TCRαβ mAb–CHX-A″ in an acid-washed microcentrifuge tube. To that solution 5.5 mCi (204 MBq) 111InCl3 in 30 μL 0.05 N HCl was added. The mixture was vortexed lightly and allowed to react at room temperature for 5 minutes. The reaction was then quenched with 100 μL of 100 mM EDTA solution in water. The entire mixture was placed on a Sephadex G-25 column (PD-10) that had been pre-equilibrated with 0.9% saline. The column was eluted with 0.9% saline, and the 111In-labeled mAb fractions were combined. The labeling procedure provided 4.8 mCi (178 MBq; 87% yield) of the mAb (1.2 mg), which had a 98% radiochemical purity.
Radiolabeling of the mAb–CHX-A″ conjugate with 213Bi
225Ac nitrate was purchased on a column from the Department of Energy (Oak Ridge, TN). 213Bi was obtained from 225Ac (t1/2 = 10 days) by elution of the generator column with 1 M HCl using a dual syringe pump. As the elution proceeded, the 213Bi/HCl solution was mixed with water (in a plastic chamber) and run across an MP-50 cation exchange column. The 213Bi was trapped on the ion exchange column, and the column was removed from the generator system. The column was then eluted with 0.1 N hydrogen iodide, then the pH of the eluant was adjusted to 4.2 to 4.5 using 3 M NH3OAc (Ultrex grade). A 200-μg quantity of the mAb–CHX-A″ conjugate in saline was then mixed with the eluted 213Bi. The labeled antibody was purified after 2 to 5 minutes on a size exclusion column (PD-10). A decay-corrected yield of 70% to 87% 213Bi labeling of the mAb–CHX-A″ was achieved.
To determine the radiochemical purity, a small drop of the 213Bi-labeled mAb–CHX-A″ conjugate was analyzed using instant thin-layer chromatography (ITLC) on an ITLC-SG strip (Gelman, Ann Arbor, MI) and allowed to air dry. The dry ITLC strip was placed in a development chamber containing a small amount of 80% methanol/20% 10 mM DTPA in water. When the solvent had nearly reached the top of the strip, it was air dried, cut in sections, and counted in a γ-counter. The percentage of bound activity was obtained by dividing the activity in the first half of the strip (from point of spotting) by the total activity on the ITLC strip (× 100).
Dogs
Litters of beagles and minimongrel/beagle crossbreeds were either raised at the Fred Hutchinson Cancer Research Center or purchased from other commercial kennels in the United States. The dogs were quarantined for 1 month and judged to be disease free before study. They were immunized against distemper, leptospirosis, hepatitis, papilloma virus, and parvovirus. Their median age was 10 months (range, 8-22 months), and their median weight was 9.6 kg (range, 6-13.7 kg). The experimental protocol was approved by the Institutional Animal Care and Use Committee of the Fred Hutchinson Cancer Research Center. The study was performed in accordance with the principles outlined in the Guide for Laboratory Animal of Sciences, National Research Council. The kennels were certified by the American Association for Accreditation of Laboratory Animal Care. DLA-identical littermates were selected on the basis of identity for highly polymorphic major histocompatibility complex (MHC) class I and class II microsatellite markers and identity for DLA DRBI alleles as determined by direct sequencing.23,24
Pharmacokinetic studies
Flow cytometry was used to assess the saturation of TCRαβ with the injected mAb. Whole blood samples of the study dogs were obtained before and at various intervals after mAb infusion and stained with anti-TCRαβ mAb directly conjugated to FITC or a goat antimouse (Fab′)2-FITC. Saturation was evaluated by comparing the fluorescence intensity of cells incubated with anti-TCRαβ–FITC with that of goat antimouse (Fab′)2-FITC.
An enzyme-linked immunosorbent assay (ELISA) was used to measure plasma levels of the mAb as described previously.25 Briefly, 96-well polyvinyl plates were coated with goat antimouse IgG, blocked with 5% nonfat milk in phosphate-buffered saline (PBS), and incubated with plasma from the treated dogs. Goat antimouse IgG horseradish peroxidase (HRP) was used as the secondary antibody and 2,2′ azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) as the color reagent. Plates were read with a Vmax microtiter plate reader (Molecular Devices, Menlo Park, CA) at 405 nm. Standard curves were established with known concentrations of mAb, and plasma from dogs before infusion served as controls.
Natural killer (NK) cell cytotoxicity assay
To evaluate NK cell activity before and after transplantation, chromium release assays were performed.26 Effector cells were peripheral blood mononuclear cells (PBMCs) prepared by Ficoll-Hypaque density-gradient centrifugation (density, 1.074), and targets were cells from a canine thyroid adenocarcinoma (CTAC) cell line. Effector-target ratios of 60:1, 30:1, and 15:1 in triplicate wells were used. The percentage of cytotoxicity (% specific lysis) was calculated using the mean value of triplicate cultures: % specific lysis = [(experimental release – spontaneous release)/(maximum release – spontaneous release)] × 100.
Maximum release was determined in wells with target cells and 2% Triton X. Spontaneous release was determined in wells with target cells and medium alone.
Mixed leukocyte cultures (MLCs)
Mixed leukocyte cultures were used to assess the dogs' cellular immune function before and after transplantation.27 PBMCs from the respective dogs were resuspended in Waymouth medium supplemented with 1% nonessential amino acids, 1% sodium pyruvate, 1% L-glutamine, and 10% heat-inactivated, pooled, normal dog serum. Both 1 × 105 responder cells/well and 1 × 105 irradiated (2200 cGy) stimulator cells/well were cocultured in triplicate in round-bottom 96-well plates for 6 days at 37°C in a humidified 5% CO2 air atmosphere. On day 3, concanavalin A (ConA; 4 μg) was added to responder cell triplicates used as positive control. On day 6, cultures were pulsed with 1 μCi (0.037 MBq) 3H-thymidine for 18 hours before harvesting. 3H-thymidine uptake was measured as the mean counts per minute of the 3 replicates using a β-scintillation counter (Packard BioScience, Meriden, CT).
Tissue dose estimates
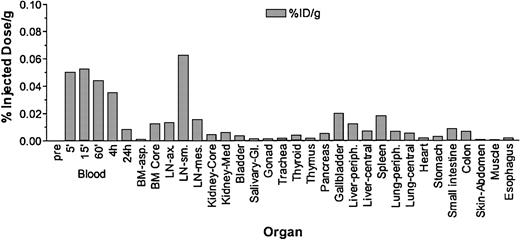
Radiation-absorbed doses to the major organs and the whole body of the dog were estimated by administering 111In-labeled mAb as a tracer and by tracking the biodistribution of the labeled antibody over time. A correction procedure was used to calculate the hypothetical absorbed doses for 213Bi-labeled mAb as though 213Bi had been substituted for 111In on the antibody. The biodistribution of 111In-labeled mAb as a function of time from injection was performed by obtaining region-of-interest (ROI) measurements on a gamma camera (Maxxus, General Electric, WI). A 10.4-kg dog (E886) was injected intravenously with 4.41 mCi (163 MBq) 111In labeled to 1.1 mg/kg anti-TCRαβ. Measurements of the radioactivity in the organ ROIs on the gamma camera provided time-activity curves for dose estimates. The gamma camera was calibrated using a 1-L standard containing a known amount of 111In (87 μCi [3.2 MBq]). Corrections were made for background and attenuation. Counts were obtained in the ROIs that imaged above tissue background to determine the uptake and retention of 111In-labeled mAb in lungs, liver, spleen, heart, blood, and whole body at 16 time points after injection (30 minutes, 45 minutes, 1 hour, etc, to 4 hours, and finally at 24 hours). The kidneys and other major organs did not show measurable uptake. These time-activity data were corrected for decay of 111In, and the biologic time-activity data for the organs and whole body were further corrected to project the effective retention of 213Bi-labeled mAb. The 213Bi-labeled mAb time-activity data were integrated over time from injection to complete decay to determine the cumulated activity. These values were then multiplied by an equilibrium dose constant for 213Bi and decay products (19.16 g-cGy/μCi-hours [517.84 g-cGy/MBq-hours]) and divided by the organ masses (grams) to yield the absorbed dose to the organs and whole body per millicurie (per 37 MBq) 213Bi-labeled mAb administered (Table 1). The absorbed dose to marrow was calculated in a similar manner from measurements of the concentrations of 111In-labeled mAb in blood samples obtained at 10 time points after injection. We assumed that the marrow contained about 5% T lymphocytes of all nucleated cells compared with 15% in circulating blood, and, therefore, that the concentration of radiolabeled mAb in the marrow was approximately one third of that in circulating blood. After 24 hours, the dog was euthanized, and concentrations of 111In in tissues were obtained by necropsy. The percentages of injected dose per gram (%ID/g) in the tissues at 24 hours after injection are shown in Figure 1. Count rates were correlated to the actual tissue concentrations obtained on necropsy.
Radiation-absorbed doses projected for 213Bi—anti-TCRαβ mAb from the 111In—anti-TCRαβ mAb biodistribution data in dog E886 (weight 10.4 kg)
Organ or tissue . | Absorbed dose, cGy/mCi 213Bi administered . |
|---|---|
| Lungs | 11.6 |
| Liver | 8.24 |
| Spleen | 30.4 |
| Heart* | 14.6 |
| Blood | 9.12 |
| Marrow | 13.7 |
| Total body | 1.67 |
Organ or tissue . | Absorbed dose, cGy/mCi 213Bi administered . |
|---|---|
| Lungs | 11.6 |
| Liver | 8.24 |
| Spleen | 30.4 |
| Heart* | 14.6 |
| Blood | 9.12 |
| Marrow | 13.7 |
| Total body | 1.67 |
Dose estimate includes activity in blood pool.
Biodistribution of 111In–anti-TCRαβ Blood clearance and tissue concentration of 111In–anti-TCRαβ after injection in E886 measured as percentage of injected dose (ID) per gram of tissue.
Biodistribution of 111In–anti-TCRαβ Blood clearance and tissue concentration of 111In–anti-TCRαβ after injection in E886 measured as percentage of injected dose (ID) per gram of tissue.
DLA-identical marrow grafts
On the basis of the experience with a 213Bi–anti-CD45 radioimmunoconjugate,5 we administered the 213Bi–anti-TCRαβ radioimmunoconjugate in 6 injections on days –3 to –2 (Figure 2). A dose of unlabeled antibody was given before injection of the radioimmunoconjugate to prevent nonspecific tissue binding of radiolabeled mAb.28 Similar to previous experiments with 213Bi–anti-CD45,5 a dose of 0.5 mg/kg mAb was targeted in the first dog, but antigen saturation occurred already after the injection of unlabeled antibody presumably because of the lower amount of target antigen. Therefore, the dose was decreased in the subsequent dogs from 0.5 mg/kg to 0.13 to 0.17 mg/kg 213Bi–anti-TCRαβ. In addition, a dose of the irrelevant isotype control antibody 31A was used instead of unlabeled anti-TCRαβ to prevent nonspecific tissue binding in all subsequent dogs. Four dogs received total doses of 3.7 (137), 4.5 (167), 4.7 (174), and 5.6 (207) mCi (MBq)/kg 213Bi-labeled anti-TCRαβ (Table 2). On day 0, the dogs were given intravenous marrow grafts (median, 5.2 × 108 [range, 3.5-7.6 × 108] mononuclear cells/kg) from their DLA-identical littermates. As postgrafting immunosuppression, MMF (10 mg/kg subcutaneously twice daily on days 0 to 27) and CSP (15 mg/kg twice daily by mouth on days –1 to 35), were administered.1 Supportive care after transplantation was given as previously described.29 Hematopoietic engraftment was assessed by recoveries of peripheral blood granulocyte and platelet counts. At the end of the study, dogs were euthanized, and autopsies, including histologic examinations, were performed to assess marrow engraftment, graft-versus-host disease (GVHD), hematopoietic recovery, and potential toxicities.
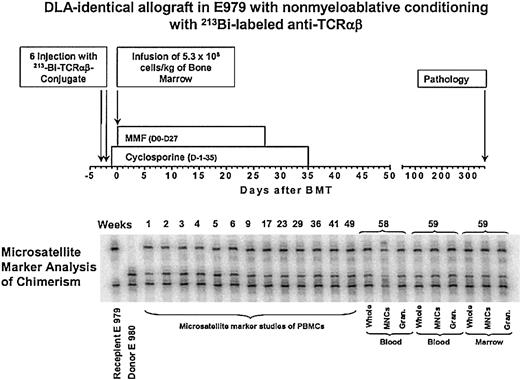
Nonmyeloablative conditioning with 213Bi–anti-TCRαβ Treatment scheme and microsatellite marker analysis of chimerism in dog E979.
Nonmyeloablative conditioning with 213Bi–anti-TCRαβ Treatment scheme and microsatellite marker analysis of chimerism in dog E979.
DLA-identical marrow grafts after conditioning with 213Bi-anti-TCRαβ
Dog no. . | Dog weight, kg . | Anti-TCRαβ mAb mg/kg . | 213Bi mCi/kg (MBq/kg) . | Marrow MNCs × 108/kg . | GVHD . | % Donor chimerism in MNCs . | Survival, wks* . |
|---|---|---|---|---|---|---|---|
| E974 | 9.6 | 0.46 | 4.5 (167) | 5.0 | No | 3-15 | > 40 |
| E986 | 9.0 | 0.17 | 5.6 (207) | 3.5 | No | 10-40 | > 40 |
| E979 | 10.7 | 0.13 | 4.7 (174) | 5.3 | No | 40-60 | > 40 |
| G110 | 6.0 | 0.13 | 3.7 (137) | 7.6 | No | 20-45 | > 35 |
Dog no. . | Dog weight, kg . | Anti-TCRαβ mAb mg/kg . | 213Bi mCi/kg (MBq/kg) . | Marrow MNCs × 108/kg . | GVHD . | % Donor chimerism in MNCs . | Survival, wks* . |
|---|---|---|---|---|---|---|---|
| E974 | 9.6 | 0.46 | 4.5 (167) | 5.0 | No | 3-15 | > 40 |
| E986 | 9.0 | 0.17 | 5.6 (207) | 3.5 | No | 10-40 | > 40 |
| E979 | 10.7 | 0.13 | 4.7 (174) | 5.3 | No | 40-60 | > 40 |
| G110 | 6.0 | 0.13 | 3.7 (137) | 7.6 | No | 20-45 | > 35 |
MNC indicates mononuclear cells.
All dogs were euthanized on completion of the study.
Chimerism analysis
Donor and host cell chimerism were evaluated using a polymerase chain reaction (PCR)–based assay of polymorphic (CA)n dinucleotide repeats with primers specific for informative microsatellite markers.30 Genomic DNA of the cells of interest was extracted, and PCR was performed under conditions described previously.31 To quantify mixed hematopoietic chimerism, digitalized PCR gel pictures were obtained using the storage phosphor imaging technique and evaluated with an image analyzing software (ImageQuant; Molecular Dynamics, Sunnyvale, CA).32 The percentage of donor origin DNA was calculated as %D = (volume integration\density of donor specific band)/(volume integration\density of recipient specific band) × 100. This technique enables detection between 2.5% and 97.5% donor cell chimerism.1
To assess lineage-specific chimerism, T cells, monocytes, and granulocytes were isolated by fluorescence-activated cell sorting. PBMCs were stained with FITC-conjugated CA15.9D5, anti-CD14, and DM5, respectively, and then purified by flow cytometric sorting (FACS Vantage; Becton Dickinson, San Jose, CA). Purity of the sorted cells was determined to be more than 95%. DNA was extracted from the purified cell suspension, and chimerism analysis was performed.
Results
Studies of biodistribution, imaging, and dose estimation using 111In–anti-TCRαβ
To assess biodistribution and organ dose of the radioimmunoconjugate, dog E886 was injected with 4.41 mCi (163 MBq) 111In on 1.1 mg anti-TCRαβ mAb, and blood samples were obtained. More than 90% of the activity was cleared in the first 24 hours after injection (Figure 1). The dog was euthanized after 24 hours, and samples from organs were obtained and counted in a γ-counter. Consistent with the results of the imaging studies, the highest uptake was measured in blood, marrow, spleen, lymph nodes, and liver (Figure 1). The results of dose estimates projected for 213Bi-labeled anti-TCRαβ mAb suggested that the spleen would receive the greatest radiation-absorbed dose (30.4 cGy/mCi [0.822 cGy/MBq] administered), followed by the marrow, lungs, blood, heart, and liver, respectively (Table 1).
DLA-identical marrow grafts after radioimmunotherapy
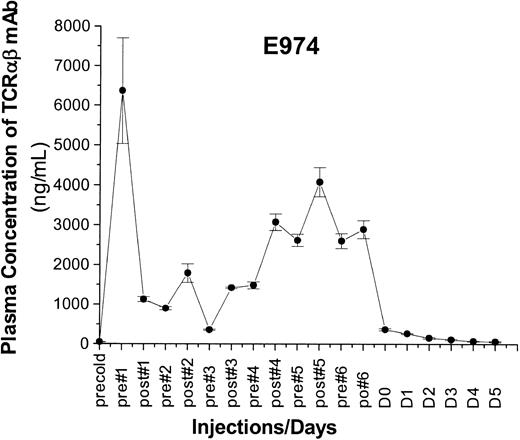
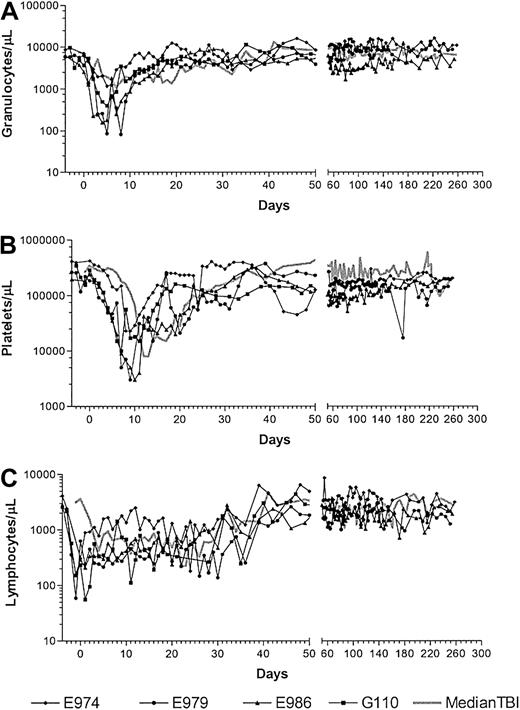
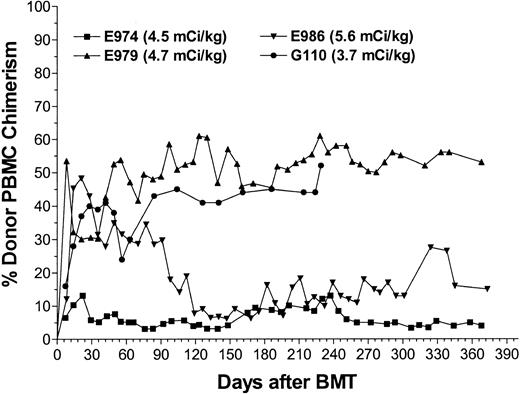
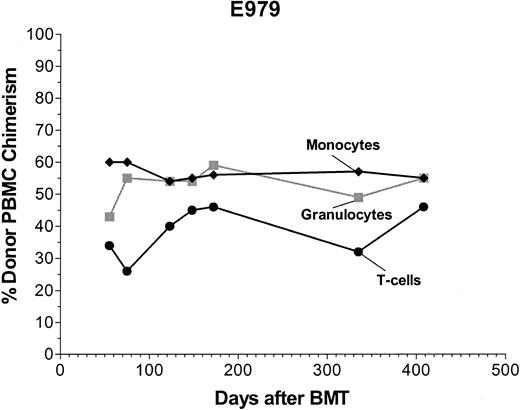
Four dogs received marrow grafts after radioimmunotherapy with 213Bi–anti-TCRαβ. Starting with a dose of 0.46 mg/kg anti-TCRαβ mAb 15.9D5 in E974, we de-escalated the dose because of early antigen saturation to 0.17 mg/kg in E979 and finally to 0.13 mg/kg in E986 and G110. Figure 2 illustrates the treatment regimen and the results of microsatellite marker studies in one representative dog. Figure 3 shows the levels of free anti-TCRαβ as determined by ELISA in E974. Saturation of the TCRαβ antigen was observed by the fourth of the 6 injections. In the subsequent dogs, the level of antigen saturation had to be estimated by flow cytometry as the dogs were injected with the irrelevant isotype control mAb 31A that was also detected by the goat-antimouse mAb used in the ELISA and has a half-life of several days.33 There was still a low level of antigen saturation detectable by flow cytometry at a dose of 0.13 mg/kg mAb but to a much smaller extent than with 0.46 mg/kg mAb (data not shown), but the quantity injected could not be decreased further, as the labeling technique used requires at least 100 μg mAb per injection. No immediate toxic effects were observed during injection of the radioimmunoconjugate. The dogs received 5.6 mCi/kg (207 MBq/kg), 4.7 mCi/kg (174 MBq/kg), 4.5 mCi/kg (167 MBq/kg), and 3.7 mCi/kg (137 MBq/kg) 213Bi-labeled anti-TCRαβ, respectively (Table 2). Granulocytopenia was seen in all dogs between days 2 and 10 with nadirs of 86 to 1178 granulocytes/μL on days 4 to 5. Thrombocytopenia (< 20 × 109/L platelets) occurred between days 6 to 14 with nadirs of 0 to 23 000 platelets/μL without the need for platelet transfusions. Lymphocyte nadirs of 56 to 304 lymphocytes/μL occurred between days –1 and +1 (Figure 4). The declines in peripheral blood counts, especially in the lymphocytes, were steeper, more profound, and occurred earlier than in historical controls receiving transplants after conditioning with 200 cGy TBI.1 All 4 dogs had fast hematopoietic recoveries and showed mixed hematopoietic chimerism in peripheral blood as early as 1 week after transplantation. Mixed chimerism was stable in all dogs and ranged from 5% to 55% through the end of study (Figure 5). Evaluation of chimerism in T cells (TCRαβ+), monocytes (CD14+), and granulocytes (DM5+) showed slightly lower chimerism among T cells compared with the 2 other cell types (Figure 6). Flow cytometric monitoring of the absolute numbers of peripheral blood T cells, granulocytes, and monocytes showed return to pretransplantation levels by day 50 (data not shown). T-cell and NK-cell functions as measured by NK assays and MLC were normal within 1 month of transplantation (Figure 7).
Plasma concentration of 213Bi–anti-TCRαβ Plasma concentrations determined by ELISA of the 213Bi–anti-TCRαβ conjugate before and after each injection in E974 receiving 0.46 mg/kg mAb.
Plasma concentration of 213Bi–anti-TCRαβ Plasma concentrations determined by ELISA of the 213Bi–anti-TCRαβ conjugate before and after each injection in E974 receiving 0.46 mg/kg mAb.
Peripheral blood granulocyte, platelet, and lymphocyte counts of the dogs after nonmyeloablative marrow transplantation. Counts of granulocytes (A), platelets (B), and lymphocytes (C) in the 4 dogs treated with 3.7 to 5.6 mCi/kg (137-207 MBq/kg) of the radioimmunoconjugate 213Bi–anti-TCRαβ and compared with the median of historic controls receiving transplants after 200 cGy TBI.
Peripheral blood granulocyte, platelet, and lymphocyte counts of the dogs after nonmyeloablative marrow transplantation. Counts of granulocytes (A), platelets (B), and lymphocytes (C) in the 4 dogs treated with 3.7 to 5.6 mCi/kg (137-207 MBq/kg) of the radioimmunoconjugate 213Bi–anti-TCRαβ and compared with the median of historic controls receiving transplants after 200 cGy TBI.
Donor PBMC chimerism in the dogs after nonmyeloablative marrow transplantation. Results of microsatellite marker studies of hematopoietic donor chimerism in the 4 dogs receiving transplants after conditioning with the radioimmunoconjugate 213Bi–anti-TCRαβ.
Donor PBMC chimerism in the dogs after nonmyeloablative marrow transplantation. Results of microsatellite marker studies of hematopoietic donor chimerism in the 4 dogs receiving transplants after conditioning with the radioimmunoconjugate 213Bi–anti-TCRαβ.
Donor chimerism of T cells, monocytes, and granulocytes. Results of microsatellite marker studies to evaluate donor chimerism in sorted TCR+ (T cells), CD14+ (monocytes), and DM5+ cells (granulocytes) in dog E979.
Donor chimerism of T cells, monocytes, and granulocytes. Results of microsatellite marker studies to evaluate donor chimerism in sorted TCR+ (T cells), CD14+ (monocytes), and DM5+ cells (granulocytes) in dog E979.
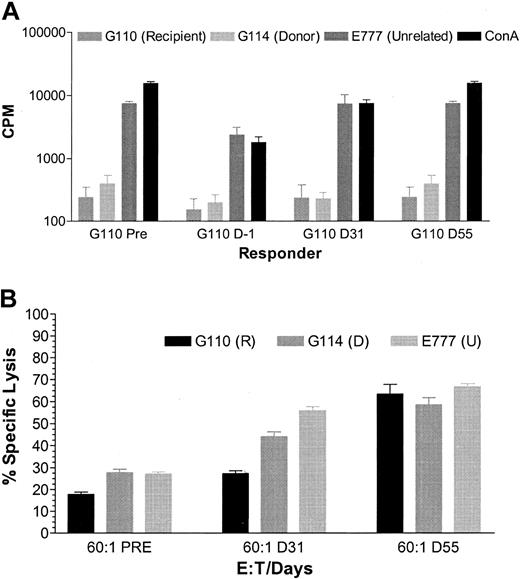
MLC and NK assays of G110. In vitro lymphocyte function of one representative dog, G110, compared with the donor dog and an unrelated control dog measured by MLC and NK assay. In the MLC (A), 3H thymidine uptake was measured as the mean counts per minute of triplicates, pretransplantation, on day –1, 31, and 55. In the NK assay (B) cytotoxicity is expressed as percentage of specific lysis pretransplantation, on day 31 and 55.
MLC and NK assays of G110. In vitro lymphocyte function of one representative dog, G110, compared with the donor dog and an unrelated control dog measured by MLC and NK assay. In the MLC (A), 3H thymidine uptake was measured as the mean counts per minute of triplicates, pretransplantation, on day –1, 31, and 55. In the NK assay (B) cytotoxicity is expressed as percentage of specific lysis pretransplantation, on day 31 and 55.
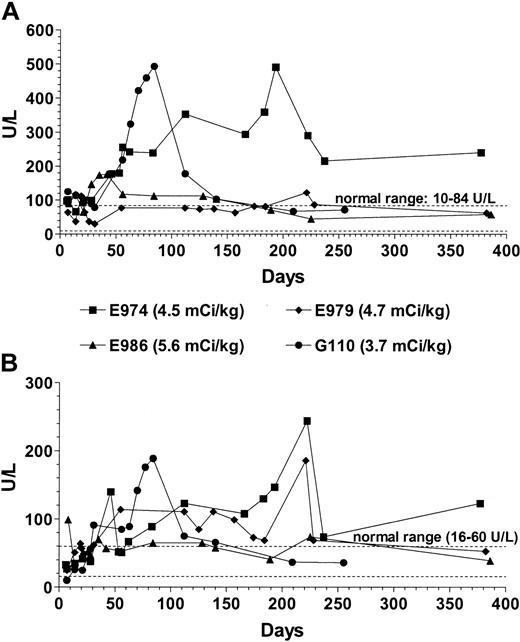
Clinically, the transplantations were well tolerated, and none of the dogs developed any signs of infection or GVHD. Elevations of liver enzymes without clinically detectable signs of liver dysfunction developed that were transient in all but one of the dogs (Figure 8). E974 had more sustained elevations of transaminases with an alkaline phosphatase of 240 U/L and an aspartate aminotransferase (AST) of 123 U/L at the end of study. All dogs were euthanized on completion of the study, and extensive autopsies were performed. In dog E974, histologic liver changes in the form of pericellular and periportal fibrosis without signs of frank cirrhosis were found. Apart from this finding, the gross and histologic autopsy findings in all dogs were normal; especially there were no signs of GVHD.
Liver enzymes in dogs conditioned with 213Bi–anti-TCRαβ Values of alkaline phosphatase (A) and AST (B) in the 4 dogs receiving transplants after conditioning with the radioimmunoconjugate 213Bi–anti-TCRαβ.
Liver enzymes in dogs conditioned with 213Bi–anti-TCRαβ Values of alkaline phosphatase (A) and AST (B) in the 4 dogs receiving transplants after conditioning with the radioimmunoconjugate 213Bi–anti-TCRαβ.
Discussion
In this study we investigated whether selective ablation of T cells with radioimmunotherapy in conjunction with postgrafting immunosuppression was sufficient to achieve stable engraftment in a canine model of DLA-identical marrow transplantation. Selective targeting of T cells would entail minimal toxicity and make the procedure suitable for the treatment of patients with nonmalignant hematologic diseases.
The use of radioimmunotherapy in the conditioning for marrow transplantation has been increasingly explored. Studies using β-emitting isotopes have demonstrated that it is feasible to selectively target the hematopoietic system with radioimmunotherapy.3,34,35 Successful clinical trials using radioimmunotherapy in addition to TBI in autologous or allogeneic marrow transplantation with the β-emitting radionuclides iodine-131 or rhenium-188 followed.36,37 These studies have demonstrated that it was feasible to replace part of, but not all of, the TBI used in myeloablative transplantation.
To target hematopoietic cells in the setting of marrow transplantation, α-emitting radionuclides possess several physical characteristics, making them more suitable than β-emitters. α-Emitters are short-ranging (40-80 μm), high-energy (5-8 MeV) particles with a high linear energy transfer (LET). The high LET and the limited ability of cells to repair DNA damage caused by α-radiation result in a high RBE.38 Compared with β-particles, α-emitters have about 400 times greater LET and are 5 to 100 times more effective in killing target cells.14,39,40 Studies in mice comparing the β-emitter yttrium-90 (90Y) to the α-emitter 213Bi showed that at equivalent doses the α-emitter had significantly higher therapeutic efficacy.41 The short range limits the cytotoxic effect to several cell diameters and spares surrounding tissues from the toxic effect of radiation. For our study, we chose the α-emitting radionuclide 213Bi that has a half-life of 46 minutes, a particle range of 81 μm, an energy of 8.35 MeV with a LET of 102 keV/μm.39 The labeling technique used for production of the radioimmunoconjugate highly influences its toxicity and efficacy. The conjugates have to be stable and kinetically inert to avoid deposition of 213Bi in the kidney and other nontarget organs. DTPA chelates incorporating a CHX group produce conjugates that label rapidly and are resistant to the release of 213Bi.42,43 Using 213Bi labeled to a panhematopoietic anti-CD45 mAb, we were able to completely replace external beam TBI by radioimmunotherapy in our canine model of marrow transplantation.5 The applicability of this approach to the clinic in the nontransplantation setting has recently been shown in patients with relapsed or refractory acute myelogenous leukemia using 213Bi-HuM195, a radioimmunoconjugate targeting the myeloid antigen CD33.44
Alloreactive T cells have been shown to be the major cell type mediating resistance to the engraftment of allogeneic donor marrow.7-9,11,12 As cells expressing TCRαβ12 are thought to be the T-cell subset involved in marrow graft rejection, we based our radioimmunoconjugate on the mAb 15.9D5 directed against canine TCRαβ. The mouse mAb 15.9D5 (IgG1) recognizes the α/β chain of the canine TCRαβ/CD3 complex and has a serum half-life of 24 hours. In vitro, 15.9D5 inhibits MLC but is not mitogenic. Used alone in a canine model of DLA-identical marrow transplantation, it significantly lowered the incidence of rejection in dogs conditioned with only 450 cGy TBI.15 However, it was ineffective when given with 200 cGy TBI.
The data on biodistribution using a 111In-TCRαβ radioimmunoconjugate showed the specific targeting to hematopoietic organs with highest uptake in blood, lymph nodes, bone marrow, and spleen. Among nontarget organs, the liver received the highest dose, most likely because of nonspecific uptake.
We performed marrow transplantation studies to determine whether radioimmunotherapy with 213Bi–anti-TCRαβ together with postgrafting immunosuppression with MMF and CSP would be sufficient to achieve engraftment. Rapid and stable engraftment occurred in all 4 dogs, resulting in mixed hematopoietic chimerism as early as 1 week after transplantation. The mixed chimerism in T cells, myeloid cells, monocytes, and marrow was sustained through the end of study in all dogs. The nadirs of the peripheral blood counts were more pronounced than those observed in dogs treated with 200 cGy TBI,1 indicating that a lower dose of radioimmunotherapy might be sufficient to achieve an irradiation dose equivalent to 200 cGy. Of note is the very early and steep decline of peripheral blood lymphocytes, reflecting the specific targeting by the radioimmunoconjugate. Compared with animals treated with 213Bi–anti-CD45,5 the timing of the decline was similar, but the nadirs after 213Bi–anti-TCRαβ were less profound. Generally, the decline in peripheral blood counts in dogs conditioned with radioimmunotherapy occurred earlier than in the historical controls treated with TBI, most likely because of immediate elimination of targeted cells while already committed marrow progenitor cells may still be able to mature after exposure to low-dose external beam γ-radiation. There are 2 possible explanations for the observation of profound nadirs of the peripheral blood counts, although only T cells have been targeted. First, the crossfire effect is certainly of importance. Although the path length of 213Bi is only 81 μm, this is still several cell diameters wide, and, given the high RBE of α-emitters, damage of progenitor cells residing close to targeted T cells could occur. In experiments targeting myeloid cells using a 213Bi–anti-CD33 radioimmunoconjugate at up to 1 mCi/kg (37 MBq/kg) in patients with myeloid leukemia, one dose-limiting toxicity was pancytopenia, including platelets, although only myeloid cells have been targeted.44 Second, the extent of the nadir in peripheral blood counts does not only reflect the direct cytotoxic effect of the radioimmunoconjugate, but there is also a graft-versus-host effect that eliminates host hematopoietic cells while establishing engraftment of the incoming donor cells.
Rapid immune reconstitution was indicated by pretransplantation levels of TCR+, CD4+, CD8+, granulocyte, and monocyte counts and normal T-cell and NK-cell functions as measured by MLCs and NK assays within 1 month after transplantation. The treatment with the radioimmunotherapy was well tolerated except for transient elevations of transaminases without clinical consequences in all but one of these dogs with more sustained elevations but no clinical signs of liver failure. That dog, E974, had received the highest amount of free radioimmunoconjugate because of early antigen saturation. On autopsy this dog showed histologic liver abnormalities without the signs of frank cirrhosis. The autopsies in all other dogs were normal without any signs of liver or renal toxicity or GVHD. In studies in mice, renal toxicity was dose limiting, as free radiobismuth accumulates in the kidney.41 The fact that we did not see any signs of renal toxicity reflects the high in vivo stability of our radioimmunoconjugate. The liver is the nontarget organ that receives the highest dose of radiation, suggesting that the elevation of transaminases is due to radiation hepatitis. This situation could be the result of nonspecific uptake of radioimmunoconjugate by endothelial cells and Kupffer cells and of the fact that lymphocytes comprise up to 20% of nonparenchymal cells in the liver.45 In our previous study using a 213Bi–anti-CD45 we observed more pronounced liver toxicity than in the current study.5 The toxicity seemed dependent on the amount of free radioimmunoconjugate. The one published clinical trial using 213Bi as radioisotope used much lower activities, 0.28 to 1.0 mCi/kg (10-37 MBq/kg),44 as compared with the 4 to 6 mCi/kg (148-222 MBq/kg) in this study. They found signs of hepatic toxicity in 22% of the patients that were mild and transient, suggesting that, with lower doses of 213Bi, toxicity can be reduced. The favorable characteristics of α-emitters compared with β-emitters and external beam irradiation may allow lowering the administered radiation dose even below levels equivalent to external beam TBI. It is, therefore, reasonable to explore de-escalation of the radiation dose and perhaps different schedules of administration of the radioimmunoconjugate, thereby decreasing the amount of unbound 213Bi–anti-TCRαβ.
In summary, this study demonstrates for the first time, in a large animal model, that selective ablation of TCRαβ-positive T cells using radioimmunotherapy together with postgrafting immunosuppression is sufficient to achieve stable engraftment of allogeneic DLA-identical littermate marrow.
Prepublished online as Blood First Edition Paper, February 27, 2003; DOI 10.1182/blood-2002-12-3867.
Supported in part by grants HL36444, CA78902, and CA15704 awarded by the National Institutes of Health, Department of Health and Human Services, Bethesda, MD; by a grant from the Gabrielle Rich Leukemia Foundation; and by a fellowship from Deutsche Krebshilfe, Dr Mildred-Scheel-Stiftung für Krebsforschung (W.A.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Howard Shulman, MD, for performing the pathology studies; Michele Spector, DVM, and the technicians in the canine facilities of the Fred Hutchinson Cancer Research Center; Drs Nash, Kiem, Yunusov, Georges, Hogan, Mielcarek, Dell'Agnola, Diaconescu, and Little who participated in the weekend treatments; Marie-Terese Little, PhD, Stacy Zellmer, and Serina Gisburne for the DLA-typing; the technicians of the hematology and pathology laboratories of the Fred Hutchinson Cancer Research Center; Dr Elizabeth Squires, Sangstat Medical Corporation, Menlo Park, CA, for the gift of oral cyclosporine; Dr Sabine Hadulco, Roche Bioscience, Nutley, NJ, for the gift of mycophenolate mofetil; and Peter F. Moore, PhD, DVM, for providing us with the CA17.6B3 antibody.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal