Abstract
Allogeneic stem cell transplantation with umbilical cord blood (UCB) cells is limited by the cell dose a single unit provides recipients. Ex vivo expansion is one strategy to increase the number of cells available for transplantation. Aastrom Biosciences developed an automated continuous perfusion culture device for expansion of hematopoietic stem cells (HSCs). Cells are expanded in media supplemented with fetal bovine serum, horse serum, PIXY321, flt-3 ligand, and erythropoietin. We performed a phase 1 trial augmenting conventional UCB transplants with ex vivo–expanded cells. The 28 patients were enrolled on the trial between October 8, 1997 and September 30, 1998. UCB cells were expanded in the device, then administered as a boost to the conventional graft on posttransplantation day 12. While expansion of total cells and colony-forming units (CFUs) occurred in all cases, the magnitude of expansion varied considerably. The median fold increase was 2.4 (range, 1.0-8.5) in nucleated cells, 82 (range, 4.6-266.4) in CFU granulocyte-macrophages, and 0.5 (range, 0.09-2.45) in CD34+ lineage negative (lin–) cells. CD3+ cells did not expand under these conditions. Clinical-scale ex vivo expansion of UCB is feasible, and the administration of ex vivo–expanded cells is well tolerated. Augmentation of UCB transplants with ex vivo–expanded cells did not alter the time to myeloid, erythroid, or platelet engraftment in 21 evaluable patients. Recipients of ex vivo–expanded cells continue to have durable engraftment with a median follow-up of 47 months (range, 41-51 months). A randomized phase 2 study will determine whether augmenting UCB transplants with ex vivo–expanded UCB cells is beneficial.
Introduction
Banked unrelated umbilical cord blood (UCB) can serve as an alternate source of hematopoietic stem cells (HSCs) and progenitor cells for patients with malignant and nonmalignant conditions who lack traditional donors.1-7 The advantage of using UCB as the source of HSCs for transplantation is that a greater degree of human leukocyte antigen (HLA) mismatching is tolerated between donor and recipient.1,3-6,8-10 UCB units are readily available and less likely than other stem cell sources to be contaminated with blood-borne viruses that could complicate the patients' clinical course after transplantation.1,11-13
The best predictor of event-free-survival (EFS) in UCB recipients is the cell dose transplanted, with a distinct survival advantage seen in patients receiving 3 × 107 nucleated cells/kg.4-6,10,14 An UCB unit contains a finite number of hematopoietic stem and progenitor cells, which may be a limiting factor when larger recipients undergo transplantation, as well as patients with diseases known to be resistant to engraftment, such as chronic myelogenous leukemia, severe aplastic anemia, and Fanconi anemia. One strategy to increase the cell dose available from a single UCB unit is ex vivo expansion. The goal of this technology is to develop a reproducible and reliable methodology to increase the number of stem and progenitor cells available from a single UCB unit for transplantation, which could make this alternate source of HSCs available to larger pediatric and adult patients who lack traditional donors.15-18
Ex vivo manipulation of HSCs dates back to the development of “Dexter-type” culture techniques, which allowed for the investigation of hematopoiesis over an extended time course and identified the benefits of stroma for the maintenance of hematopoietic colony-forming units (CFUs).19,20 The frequency of media exchange has also emerged as a key variable for maintenance of optimal proliferation during ex vivo expansion.21 These observations led to the development of an automated continuous perfusion system for clinical-scale expansion of unselected bone marrow (BM) cells.15,21,22 When BM mononuclear cells are inoculated into this device, stromal cells proliferate extensively to form a dense, adherent layer, and they produce cytokines and other factors that contribute to the survival, proliferation, and expansion of HSCs in culture.
In preclinical studies using continuous perfusion with cytokine supplements (PIXY321, flt-3 ligand, and erythropoietin [EPO]), expansion of BM resulted in an increase in nucleated cells, CFU granulocyte-macrophages (CFU-GMs), and long-term culture initiating cells (LTCICs) during the 14-day culture period.21 Autologous BM transplants were augmented with ex vivo–expanded cells and there were no adverse reactions to the infusion of expanded cells.23 A subsequent trial used ex vivo–expanded BM cells as the sole source of transplanted cells and all recipients have maintained durable grafts for longer than 6 months.24-26
The potential for UCB ex vivo expansion in this system was studied to determine whether HSCs from UCB would expand under the conditions developed for BM. It has been hypothesized that UCB cells may have a higher proliferative potential than other sources of HSCs, making them ideally suited to expansion.18,27-29 Unlike BM cultures, UCB expansion occurs despite lack of formation of an adherent stromal layer. Small-scale expansion of UCB cells for 12 days resulted in a 4.5- to 5.4-fold increase in nucleated cells, a 171- to 518-fold increase in CFU-GMs, and LTCIC numbers ranging from 4 to 35.17,21 This fold expansion was reproduced in the automated clinical-scale system. Validation trials of clinical-scale expansions of UCB resulted in a 2.6- to 5.6-fold expansion of nucleated cells, a 180- to 338-fold expansion of CFU-GMs, and LTCIC numbers ranging from 471 to 12 647.17
In an effort to increase the number of UCB HSCs available for transplantation, we conducted a phase 1 clinical trial to test the safety and feasibility of expanding UCB cells in the AastromReplicell System (Aastrom Biosciences, Ann Arbor, MI), and using these ex vivo–expanded cells to augment conventional UCB transplants 12 days later. The results of this study are reported herein.
Patients, materials, and methods
Eligibility and study objectives
This phase 1 study of the augmentation of UCB transplants with ex vivo–expanded cells for the treatment of malignant and nonmalignant disorders was approved by the institutional review board of Duke University Medical Center (DUMC). Patients were eligible for enrollment if they lacked an HLA-matched sibling donor or a matched unrelated BM donor, or if their disease status precluded waiting to identify an unrelated BM donor. Informed consent was obtained from patients or from the parents of pediatric patients. Patients with severe aplastic anemia, Fanconi anemia, and chronic myelogenous leukemia in blast crisis were not eligible for this study.
Donor selection
Formal searches for unrelated, banked UCB units were conducted by the Placental Blood Program at the New York Blood Center for 27 patients. One patient received a related UCB unit collected from a sibling born at DUMC; the unit had been cryopreserved in a compartmentalized freezing bag at the Duke Stem Cell Laboratory.
The UCB units were selected by simultaneously prioritizing degree of HLA matching and the nucleated cell count of the UCB unit. Allelic matching at HLA-DRB1 was prioritized over serologic matching at class I (A or B), and UCB units were required to provide a minimum of 2 × 107 cells/kg of recipient weight (precryopreservation count). HLA-C, -DP, and -DQ were not considered in this trial.
Transplantation procedure
Cryopreserved UCB units were transported to DUMC via overnight mail in a dry shipping container cooled by liquid nitrogen in vapor phase and transferred to the vapor phase of liquid nitrogen in a conventional freezer upon arrival in the laboratory. The UCB units were thawed on day 0, and washed with 10% dextran 40 (Baxter, Deerfield, IL) and 5% human albumin (Bayer, Elkhart, IN).9 The majority of the UCB unit was infused into recipients as the conventional unmanipulated transplant, a minimum cell dose of 1 × 107 cells/kg was required, with some patients receiving a higher dose. The remaining cells (typically, 1-2 × 108 total cells) were ex vivo expanded as described in the next section. A nucleated cell count, trypan blue test for viability, bacterial and fungal cultures, assay for hematopoietic progenitor cells (CFU-GMs, CFU granulocyte erythroid macrophages megakaryocyte [CFU-GEMMs], and burst-forming unit erythroids [BFU-Es]), and immunophenotyping (CD34+ [PROcount method; Becton Dickinson, Franklin Lakes, NJ], CD34+lin–, CD3+, and propidium iodide) were performed on a small aliquot of the thawed UCB cells.
On the day of infusion of unmanipulated UCB cells (day 0) all patients were premedicated with intravenous methyprednisolone (Solumedrol; 0.5 mg/kg every 6 hours) and diphenhydramine (1 mg/kg) 30 minutes prior to UCB infusion. Patients were already receiving intravenous methyprednisolone (2 mg/kg/d) for graft-versus-host disease (GVHD) prophylaxis on the day that the ex vivo–expanded cells were infused (day 12), so 30 minutes prior to the expanded-cell infusion patients were premedicated with diphenhydramine (1 mg/kg).
Ex vivo expansion
The AastromReplicell System uses a sterile, single-use cassette.22 The culture space is continuously perfused by growth medium beginning on day 4 of culture. The gas space in the cell-culture chamber was provided with a flow of gases to achieve 5% CO2, 20% O2, and the balance nitrogen. The medium was stored in an adjacent compartment at 4°C. The cassette was maintained at 37°C for the 12-day culture period. A computerized system manager controls and monitors the biologic and physical environment of the cells during the expansion period.
The nucleated, thawed UCB cells were suspended in base medium composed of Iscove modified Dulbecco medium supplemented with 10% fetal bovine serum (prescreened), 10% horse serum (prescreened), hydrocortisone (5 μM), glutamine (4 mM) (Aastrom Biosciences). The base medium was supplemented with PIXY321 (5 ng/mL; Immunex, Seattle, WA), EPO (0.1 U/mL; Amgen, Thousand Oaks, CA), flt-3 ligand (25 ng/mL; Immunex), gentamicin sulfate (5 μg/mL), and vancomycin (20 μg/mL). The UCB cells were inoculated on day 0 and continuous media perfusion began on day 3. The cells were cultured in the cassette for 12 days, with an increasing rate of perfusion to accommodate the increasing number of expanding cells. The culture medium was sampled via sampling port 48 hours prior to harvest to test for bacterial and fungal contaminants.
Following expansion, the ex vivo cells were harvested in an automated fashion as follows. The nonadherent expanded cells were drained into the harvest bag. The chamber was then rinsed with harvest reagent (trypsin [0.04%], Aastrom Biosciences) and agitated mechanically, to collect the cell suspension into the harvest bag. Following harvest, the ex vivo–expanded cell product was washed free of medium components on a COBE 2991 blood cell processor (Gambro BCT, Lakewood, CO), with a 4-L wash procedure (5 wash cycles each lasting 5 minutes with a 600-mL volume followed by centrifugation at 2000 rpm) and resuspended in Normosol (Abbott Labs, Chicago, IL) with human albumin (0.5%) for infusion into recipients. The ex vivo–expanded cell product was characterized as described for the inoculum. Fold expansion was calculated by dividing the total cells harvested by the viable cells inoculated.
Ex vivo infusion
The patients were monitored closely during the infusion of the ex vivo–expanded cells for infusion toxicity according to the Common Toxicity Criteria guidelines. The patients were assessed and vital signs recorded every 15 minutes for 2 hours following infusion of the ex vivo–expanded cells.
Preparative regimens and supportive care
For patients enrolled in the study, there were 3 possible preparative regimens. Patients with hematologic malignancies were conditioned with total body irradiation (TBI), melphalan, and antithymocyte globulin (ATG). Patients with hematologic malignancies who were not candidates for TBI were conditioned with busulfan, melphalan, and ATG.1 Patients with inherited disorders were conditioned with busulfan, cyclophosphamide (Cytoxan), and ATG.1 Busulfan levels were followed and the dose was adjusted to a target steady-state level of 600 to 900 ng/mL.
All patients received supportive care measures as previously reported for our institution with the exception of the GVHD prophylaxis, which was modified to include conventional dose cyclosporine beginning on day – 2 (targeting through levels of 100-200 ng/mL) and methylprednisolone intravenously1 . Cyclosporine therapy was changed to an oral preparation when tolerated and continued through posttransplantation day 270, at which time the dose was tapered by 10% per week if there was no evidence of GVHD. The dose of methylprednisolone was 1 mg/kg/d (day 0 to day 4), then 2 mg/kg/d (day 5 to day 20). Beginning on day 21 the dose was tapered by 0.2 mg/kg weekly as long as there was no evidence of GVHD.
Study end points
The primary end points for this study were (1) the feasibility of expanding UCB ex vivo in a clinical setting and (2) to determine the safety of infusing ex vivo–expanded cells into recipients 12 days after conventional UCB transplantation. The secondary end points for the study were to determine whether the infusion of expanded cells altered (1) the time to hematopoietic engraftment and (2) EFS. We defined engraftment as achieving an absolute neutrophil count (ANC) higher than .5 × 109/L for 3 consecutive days by day 42. Platelet transfusion independence was the day when patients maintained an untransfused platelet count higher than 20 × 109/L, red blood cell (RBC) independence was 7 days following the date of the last RBC transfusion. EFS was defined as the time from transplantation to the day of the first event. Events were defined as graft failure, autologous reconstitution, relapse, or death. Relapse in leukemic patients was determined by standard criteria. Tertiary end points included description of the incidence of acute GVHD, and other measures of nonrelapse mortality. GVHD was scored according to standard criteria.30
Statistical analysis
The data were submitted for analysis on February 8, 2002, and all survival statistics reflect this date. The probability of EFS was estimated using the Kaplan-Meier method. The cumulative incidences of neutrophil recovery and platelet recovery were calculated to account for competing risks according to published methods.31 All statistical analyses were performed with the SAS (Statistical Analysis System, Release 8.2) software (Cary, NC).
Results
Patient characteristics
The patients were enrolled in the study between October 8, 1997, and September 30, 1998, with 27 patients receiving unrelated UCB units and 1 patient receiving a related UCB graft. On day 0, 26 patients received unmanipulated UCB cells, and the ex vivo–expanded cells 12 days later. One patient received the expanded cells with the conventional graft on day 0. One patient did not receive expanded cells because of fungal contamination of the expanded cells.
Patient demographics are shown in Table 1. The median weight of the patients was 17 kg and the median age was 4.5 years. Of the 28 patients, 11 were female. There were 19 of 28 patients who had malignant conditions, all with high-risk features such as transplantation in relapse, refractory disease, or second or greater complete remission. Of the 19 patients with malignancies, 7 underwent transplantation in relapse. There were 14 patients who had evidence of prior infection with cytomegalovirus (CMV), as documented by seropositivity of immunoglobulin G (IgG).
Demographics and outcomes for ex vivo expansion patients
. | . | . | . | . | . | Acute . | . | . | . | . | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Diagnosis . | Age, y . | Weight, kg . | HLA match . | Conditioning regimen . | GVHD grade . | GVHD locations . | Days of EFS . | Event . | Outcome . | |
| 1 | CID | 1.27 | 6.0 | 4/6 | Bu/Cy/ATG | 0 | NA | 80 | Death | Pulmonary failure | |
| 2 | Lesch-Nyhan syndrome | 5.00 | 13.7 | 4/6 | Bu/Mel/ATG | IV | Skin, gut | 158 | Death | Sepsis after dental procedure | |
| 3 | Infant ALL, CR2 | 3.92 | 17.1 | 5/6 | Bu/Mel/ATG | 1 | Skin, gut | > 1587 | NA | Alive and well | |
| 4 | ALL, CR3 | 5.67 | 19.4 | 4/6 | TBI/Mel*45/ATG | 0 | NA | 153 | Relapse | Died of relapse | |
| 5 | ANLL, relapse | 11.50 | 34.2 | 5/6 | TBI/Mel*45/ATG | 0 | NA | > 1566 | NA | Alive and well | |
| 6 | CD7+ acute leukemia | 15.25 | 43.4 | 3/6 | TBI/Mel*45/ATG | II | Skin, gut | 196 | Relapse | Died of relapse | |
| 7 | WAS | 4.00 | 15.9 | 4/6 | Bu/Cy/ATG | 0 | NA | > 1509 | NA | Alive and well | |
| 8 | WAS, MDS | 1.00 | 8.8 | 4/6 | Bu/Cy/ATG | 0 | NA | > 1501 | NA | Alive and well | |
| 9 | MDS/ANLL | 1.00 | 8.6 | 5/6 | Bu/Mel/ATG | 0 | NA | > 1482 | NA | Alive and well | |
| 10 | ALL, relapse | 7.75 | 24.1 | 4/6 | Bu/Mel/ATG | IV | Liver | 94 | Graft failure | Died of GVHD | |
| 11 | HD, relapse | 23.58 | 77.8 | 4/6 | Bu/Mel/ATG | 0 | NA | 41 | Death | Hemorrhagic stroke | |
| 12 | WAS | 3.92 | 17.1 | 4/6 | Bu/Cy/ATG | 1 | Skin | > 1453 | NA | Alive and well | |
| 13 | WAS | 0.58 | 6.0 | 4/6 | Bu/Cy/ATG | IV | Skin, gut | 99 | Death | GVHD | |
| 14 | T-ALL, relapse | 14.25 | 64.7 | 4/6 | TBI/Mel*45/ATG | 0 | NA | 17 | Death | Sepsis, respiratory failure | |
| 15 | WAS | 2.00 | 11.1 | 5/6 | Bu/Cy/ATG | 0 | NA | 42 | Graft failure | Alive and well after 2nd UCB | |
| 16 | MDS | 11.75 | 52.2 | 4/6 | TBI/Mel*45/ATG | 0 | NA | 43 | Death | Toxoplasmosis | |
| 17 | CML, accelerating | 8.00 | 31.6 | 4/6 | TBI/Mel*45/ATG | 0 | NA | 121 | Autoreconstitution | Died undergoing 2nd UCB | |
| 18 | MDS | 9.83 | 32.8 | 4/6 | TBI/Mel*45/ATG | III | Gut | > 1419 | NA | Alive and well | |
| 19 | ALL, Ph+, relapse | 11.92 | 30.7 | 4/6 | TBI/Mel*45/ATG | I | Skin | > 1405 | NA | Alive and well | |
| 20 | JCML | 0.58 | 8.1 | 4/6 | Bu/Mel/ATG | 0 | NA | 220 | Relapse | Alive with relapse JCML | |
| 21 | Osteopetrosis | 0.66 | 7.3 | 4/6 | Bu/Cy/ATG | I | NA | 123 | Death | Stroke | |
| 22 | MDS, monosomy 7 | 3.25 | 16.0 | 5/6 | Bu/Mel/ATG | 0 | NA | > 1383 | NA | Alive and well | |
| 23 | ANLL, relapse | 3.75 | 13.1 | 5/6 | TBI/Mel*45/ATG | 0 | NA | 42 | Graft failure | Died undergoing 2nd UCB | |
| 24 | ANLL | 15.83 | 47.4 | 3/6 | TBI/Mel*45/ATG | 0 | NA | 191 | Death | Relapsed ALL | |
| 25 | WAS | 2.00 | 12.0 | 4/6 | Bu/Cy/ATG | IV | Skin, gut, liver | 42 | Death | Adenovirus | |
| 26 | WAS | 2.33 | 9.9 | 4/6 | Bu/Cy/ATG | IV | Liver | 287 | Death | Sepsis, EBV lymphoma | |
| 27 | ALL, CR2 | 6.00 | 20.5 | 5/6 | TBI/Mel*45/ATG | I | Skin | > 1299 | NA | Alive and well | |
| 28 | AML, relapse | 36.20 | 69.3 | 4/6 | TBI/Mel*45/ATG | II | Skin | 462 | Death | Died of relapse | |
| Median | 4.50 | 17.10 | |||||||||
| Mean | 7.60 | 25.67 | |||||||||
| SD | 8.05 | 20.34 | |||||||||
. | . | . | . | . | . | Acute . | . | . | . | . | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Diagnosis . | Age, y . | Weight, kg . | HLA match . | Conditioning regimen . | GVHD grade . | GVHD locations . | Days of EFS . | Event . | Outcome . | |
| 1 | CID | 1.27 | 6.0 | 4/6 | Bu/Cy/ATG | 0 | NA | 80 | Death | Pulmonary failure | |
| 2 | Lesch-Nyhan syndrome | 5.00 | 13.7 | 4/6 | Bu/Mel/ATG | IV | Skin, gut | 158 | Death | Sepsis after dental procedure | |
| 3 | Infant ALL, CR2 | 3.92 | 17.1 | 5/6 | Bu/Mel/ATG | 1 | Skin, gut | > 1587 | NA | Alive and well | |
| 4 | ALL, CR3 | 5.67 | 19.4 | 4/6 | TBI/Mel*45/ATG | 0 | NA | 153 | Relapse | Died of relapse | |
| 5 | ANLL, relapse | 11.50 | 34.2 | 5/6 | TBI/Mel*45/ATG | 0 | NA | > 1566 | NA | Alive and well | |
| 6 | CD7+ acute leukemia | 15.25 | 43.4 | 3/6 | TBI/Mel*45/ATG | II | Skin, gut | 196 | Relapse | Died of relapse | |
| 7 | WAS | 4.00 | 15.9 | 4/6 | Bu/Cy/ATG | 0 | NA | > 1509 | NA | Alive and well | |
| 8 | WAS, MDS | 1.00 | 8.8 | 4/6 | Bu/Cy/ATG | 0 | NA | > 1501 | NA | Alive and well | |
| 9 | MDS/ANLL | 1.00 | 8.6 | 5/6 | Bu/Mel/ATG | 0 | NA | > 1482 | NA | Alive and well | |
| 10 | ALL, relapse | 7.75 | 24.1 | 4/6 | Bu/Mel/ATG | IV | Liver | 94 | Graft failure | Died of GVHD | |
| 11 | HD, relapse | 23.58 | 77.8 | 4/6 | Bu/Mel/ATG | 0 | NA | 41 | Death | Hemorrhagic stroke | |
| 12 | WAS | 3.92 | 17.1 | 4/6 | Bu/Cy/ATG | 1 | Skin | > 1453 | NA | Alive and well | |
| 13 | WAS | 0.58 | 6.0 | 4/6 | Bu/Cy/ATG | IV | Skin, gut | 99 | Death | GVHD | |
| 14 | T-ALL, relapse | 14.25 | 64.7 | 4/6 | TBI/Mel*45/ATG | 0 | NA | 17 | Death | Sepsis, respiratory failure | |
| 15 | WAS | 2.00 | 11.1 | 5/6 | Bu/Cy/ATG | 0 | NA | 42 | Graft failure | Alive and well after 2nd UCB | |
| 16 | MDS | 11.75 | 52.2 | 4/6 | TBI/Mel*45/ATG | 0 | NA | 43 | Death | Toxoplasmosis | |
| 17 | CML, accelerating | 8.00 | 31.6 | 4/6 | TBI/Mel*45/ATG | 0 | NA | 121 | Autoreconstitution | Died undergoing 2nd UCB | |
| 18 | MDS | 9.83 | 32.8 | 4/6 | TBI/Mel*45/ATG | III | Gut | > 1419 | NA | Alive and well | |
| 19 | ALL, Ph+, relapse | 11.92 | 30.7 | 4/6 | TBI/Mel*45/ATG | I | Skin | > 1405 | NA | Alive and well | |
| 20 | JCML | 0.58 | 8.1 | 4/6 | Bu/Mel/ATG | 0 | NA | 220 | Relapse | Alive with relapse JCML | |
| 21 | Osteopetrosis | 0.66 | 7.3 | 4/6 | Bu/Cy/ATG | I | NA | 123 | Death | Stroke | |
| 22 | MDS, monosomy 7 | 3.25 | 16.0 | 5/6 | Bu/Mel/ATG | 0 | NA | > 1383 | NA | Alive and well | |
| 23 | ANLL, relapse | 3.75 | 13.1 | 5/6 | TBI/Mel*45/ATG | 0 | NA | 42 | Graft failure | Died undergoing 2nd UCB | |
| 24 | ANLL | 15.83 | 47.4 | 3/6 | TBI/Mel*45/ATG | 0 | NA | 191 | Death | Relapsed ALL | |
| 25 | WAS | 2.00 | 12.0 | 4/6 | Bu/Cy/ATG | IV | Skin, gut, liver | 42 | Death | Adenovirus | |
| 26 | WAS | 2.33 | 9.9 | 4/6 | Bu/Cy/ATG | IV | Liver | 287 | Death | Sepsis, EBV lymphoma | |
| 27 | ALL, CR2 | 6.00 | 20.5 | 5/6 | TBI/Mel*45/ATG | I | Skin | > 1299 | NA | Alive and well | |
| 28 | AML, relapse | 36.20 | 69.3 | 4/6 | TBI/Mel*45/ATG | II | Skin | 462 | Death | Died of relapse | |
| Median | 4.50 | 17.10 | |||||||||
| Mean | 7.60 | 25.67 | |||||||||
| SD | 8.05 | 20.34 | |||||||||
CID indicates combined innumodefieciency; Bu, busulfan; Cy, cyclophosphamide; NA, not applicable; Mel, melphalan; ALL, acute lymphoblastic leukemia; CR2, second complete remission; CR3, third CR; ANLL, acute nonlymphocytic leukemia; WAS, Wiskott-Aldrich; WAS, MDS, Wiskott-Aldrich with evidence of myelodyspalsia; HD, Hodgkin disease; T-ALL, T-cell phenotype of acute lymphoblastic leukemia; CML, chronic myelogenous leukemia; Ph+, Philadelphia chromosome-positive; and JCML, juvenile CML.
Graft characteristics
The doses of unmanipulated and ex vivo–expanded cells administered to each patient are shown in Table 2. The median cell dose measured on the unit prior to cryopreservation was 6.74 × 107 cells/kg of recipient weight (range, 2.31-18.34 × 107 cells/kg). After thawing, on average approximately 67% of the cryopreserved cells were recovered resulting in a median cell dose of 2.05 × 107 cells/kg (range, 1.10-5.53 × 107 cells/kg) from the unmanipulated graft on day 0. The degree of HLA matching is listed for each patient in Table 1. There were 7 patients who received UCB units matching at 5 of 6 loci; 19, at 4 of 6 loci; and 2, at 3 of 6 loci.
Graft characteristics for the study patients
Parameter . | N . | Median (range) . | Mean ± SD . |
|---|---|---|---|
| Unmanipulated UCB infusion | |||
| Cryopreserved MNCs, × 107/kg | 28 | 6.74 (2.31-18.34) | 7.90 ± 4.31 |
| UCB MNCs, × 107/kg | 28 | 2.05 (1.10-5.53) | 2.63 ± 1.21 |
| CD 34+ cells, × 105/kg | 26 | 0.78 (0.02-15.99) | 1.60 ± 3.12 |
| CD 3+ cells, × 106/kg | 26 | 5.27 (1.32-16.63) | 5.76 ± 3.48 |
| CFU-GMs, × 104/kg | 28 | 0.67 (0.10-9.50) | 1.25 ± 1.79 |
| Ex vivo-expanded UCB infusion | |||
| UCB MNCs, × 107/kg | 28 | 2.05 (0.06-10.19) | 2.86 ± 2.73 |
| CD 34+ cells, × 105/kg | 28 | 0.22 (0.001-2.59) | 0.49 ± 0.59 |
| CFU-GMs, × 104/kg | 26 | 35.56 (0.11-184.32) | 61.52 ± 63.51 |
Parameter . | N . | Median (range) . | Mean ± SD . |
|---|---|---|---|
| Unmanipulated UCB infusion | |||
| Cryopreserved MNCs, × 107/kg | 28 | 6.74 (2.31-18.34) | 7.90 ± 4.31 |
| UCB MNCs, × 107/kg | 28 | 2.05 (1.10-5.53) | 2.63 ± 1.21 |
| CD 34+ cells, × 105/kg | 26 | 0.78 (0.02-15.99) | 1.60 ± 3.12 |
| CD 3+ cells, × 106/kg | 26 | 5.27 (1.32-16.63) | 5.76 ± 3.48 |
| CFU-GMs, × 104/kg | 28 | 0.67 (0.10-9.50) | 1.25 ± 1.79 |
| Ex vivo-expanded UCB infusion | |||
| UCB MNCs, × 107/kg | 28 | 2.05 (0.06-10.19) | 2.86 ± 2.73 |
| CD 34+ cells, × 105/kg | 28 | 0.22 (0.001-2.59) | 0.49 ± 0.59 |
| CFU-GMs, × 104/kg | 26 | 35.56 (0.11-184.32) | 61.52 ± 63.51 |
MNC indicates mononuclear cell.
Ex vivo expansion
There were 27 patients who received ex vivo–expanded cells (Table 3). The median number of viable cells inoculated into cassettes was 159.5 × 106 (range, 40.5-928 × 106). The median number of cells harvested from cassettes was 407.2 × 106 (range, 64.5-1919.0 × 106) resulting in a median fold expansion of 2.4 (range, 1.0-8.5). The median fold expansion for CFU-GMs was 82.7 (range, 4.6-266.4). The median fold increase for CD34+ cells was 0.5 (range, 0.09-2.45).
Ex vivo expansion characteristics for study patients
Patient no. . | Inoculum viable cells, × 106 . | Cell expansion harvested cells, × 106 . | Fold increase . | Inoculum CFU-GM, × 105 . | Harvested CFU-GM, × 105 . | Fold increase . | Inoculum CD 34+lin-, × 106 . | Harvested CD 34+ lin-, × 106 . | Fold increase . |
|---|---|---|---|---|---|---|---|---|---|
| 1* | 98.0 | 418.1 | 4.3 | 0.65 | 102.65 | 157.7 | 1.14 | 0.71 | 0.63 |
| 2 | 168.7 | 431.3 | 2.6 | 0.49 | 54.22 | 111.1 | 1.57 | 2.50 | 1.59 |
| 3 | 151.8 | 608.8 | 4.0 | 0.76 | 170.70 | 224.0 | 0.56 | 1.28 | 2.28 |
| 4 | 146.7 | 1124.7 | 8.5 | 2.75 | 260.27 | 94.6 | 2.69 | 1.75 | 0.65 |
| 5 | 120.2 | 177.3 | 1.5 | 0.23 | 14.02 | 60.1 | 0.32 | 0.59 | 1.80 |
| 6* | 99.1 | 255 | 2.6 | 0.43 | 51.54 | 120.1 | 0.98 | 0.54 | 0.55 |
| 7 | 409.8 | 1290 | 3.1 | 2.87 | 264.47 | 92.3 | 2.91 | 0.90 | 0.31 |
| 8 | 282.2 | 378.9 | 1.3 | 0.58 | 106.00 | 184.0 | 2.00 | 0.87 | 0.43 |
| 9 | 243.9 | 250.7 | 1.0 | 0.81 | 98.73 | 121.6 | 0.85 | 1.76 | 2.06 |
| 10 | 928.0 | 1919 | 2.1 | 2.50 | 97.06 | 38.8 | 16.89 | 8.06 | 0.48 |
| 11* | 48.7 | 64.5 | 1.3 | 0.21 | 0.99 | 4.6 | NA | 0.32 | NA |
| 12 | 348.7 | 1019.3 | 2.9 | 1.35 | 107.37 | 79.8 | 2.09 | 1.12 | 0.54 |
| 13 | 206.6 | 435.5 | 2.1 | 1.07 | 30.72 | 28.7 | 1.22 | 1.44 | 1.18 |
| 14* | 40.5 | 131.1 | 3.2 | 0.23 | 3.41 | 15.1 | 0.21 | 0.07 | 0.32 |
| 15* | 73.1 | 171.7 | 2.3 | 0.32 | 27.74 | 85.7 | 0.45 | 0.09 | 0.19 |
| 16* | 61.4 | 99.9 | 1.6 | 0.10 | 1.57 | 16.4 | 1.55 | 0.45 | 0.29 |
| 17* | 96.7 | 213.8 | 2.2 | 0.05 | 13.14 | 266.4 | 0.45 | 0.04 | 0.09 |
| 18 | 391.6 | 938.5 | 2.4 | 0.62 | 15.87 | 25.4 | 3.80 | 3.10 | 0.82 |
| 19 | 124.4 | 572.3 | 4.6 | 0.70 | 37.95 | 54.6 | 1.94 | 1.78 | 0.92 |
| 20 | 533.6 | 1016.4 | 1.9 | 0.88 | NA | NA | 6.83 | 0.81 | 0.12 |
| 21 | 278.3 | 800.4 | 2.9 | 0.85 | 84.26 | 98.9 | 1.00 | 0.48 | 0.48 |
| 22 | 84.4 | 146.8 | 1.7 | 0.54 | 9.59 | 17.9 | 0.74 | 0.19 | 0.26 |
| 23 | 216.4 | 378.9 | 1.8 | 0.19 | 24.47 | 125.6 | 1.36 | 0.68 | 0.50 |
| 24* | 78.7 | 396.3 | 5.0 | 0.58 | 18.79 | 32.4 | 0.44 | 0.75 | 1.71 |
| 25 | 225.8 | 1174.6 | 5.2 | 10.40 | 203.62 | 19.6 | 4.88 | 7.05 | 1.44 |
| 26 | 104.1 | 177.1 | 1.7 | 0.51 | 19.58 | 38.4 | 1.06 | NA | NA |
| 27 | 307.1 | 1391 | 4.5 | 1.95 | 356.38 | 182.8 | 1.87 | 4.59 | 2.45 |
| 28 | 167.1 | 354.2 | 2.1 | 1.92 | NA | NA | 2.46 | NA | NA |
| Mean | 215.6 | 587.8 | 2.9 | 1.23 | 84 | 88.3 | 2.3 | 1.6 | 0.9 |
| Median | 159.5 | 407.2 | 2.4 | 0.64 | 45 | 82.7 | 1.4 | 0.8 | 0.5 |
| SD | 186.2 | 482.0 | 1.6 | 1.96 | 95 | 69.9 | 3.3 | 2.0 | 0.7 |
Patient no. . | Inoculum viable cells, × 106 . | Cell expansion harvested cells, × 106 . | Fold increase . | Inoculum CFU-GM, × 105 . | Harvested CFU-GM, × 105 . | Fold increase . | Inoculum CD 34+lin-, × 106 . | Harvested CD 34+ lin-, × 106 . | Fold increase . |
|---|---|---|---|---|---|---|---|---|---|
| 1* | 98.0 | 418.1 | 4.3 | 0.65 | 102.65 | 157.7 | 1.14 | 0.71 | 0.63 |
| 2 | 168.7 | 431.3 | 2.6 | 0.49 | 54.22 | 111.1 | 1.57 | 2.50 | 1.59 |
| 3 | 151.8 | 608.8 | 4.0 | 0.76 | 170.70 | 224.0 | 0.56 | 1.28 | 2.28 |
| 4 | 146.7 | 1124.7 | 8.5 | 2.75 | 260.27 | 94.6 | 2.69 | 1.75 | 0.65 |
| 5 | 120.2 | 177.3 | 1.5 | 0.23 | 14.02 | 60.1 | 0.32 | 0.59 | 1.80 |
| 6* | 99.1 | 255 | 2.6 | 0.43 | 51.54 | 120.1 | 0.98 | 0.54 | 0.55 |
| 7 | 409.8 | 1290 | 3.1 | 2.87 | 264.47 | 92.3 | 2.91 | 0.90 | 0.31 |
| 8 | 282.2 | 378.9 | 1.3 | 0.58 | 106.00 | 184.0 | 2.00 | 0.87 | 0.43 |
| 9 | 243.9 | 250.7 | 1.0 | 0.81 | 98.73 | 121.6 | 0.85 | 1.76 | 2.06 |
| 10 | 928.0 | 1919 | 2.1 | 2.50 | 97.06 | 38.8 | 16.89 | 8.06 | 0.48 |
| 11* | 48.7 | 64.5 | 1.3 | 0.21 | 0.99 | 4.6 | NA | 0.32 | NA |
| 12 | 348.7 | 1019.3 | 2.9 | 1.35 | 107.37 | 79.8 | 2.09 | 1.12 | 0.54 |
| 13 | 206.6 | 435.5 | 2.1 | 1.07 | 30.72 | 28.7 | 1.22 | 1.44 | 1.18 |
| 14* | 40.5 | 131.1 | 3.2 | 0.23 | 3.41 | 15.1 | 0.21 | 0.07 | 0.32 |
| 15* | 73.1 | 171.7 | 2.3 | 0.32 | 27.74 | 85.7 | 0.45 | 0.09 | 0.19 |
| 16* | 61.4 | 99.9 | 1.6 | 0.10 | 1.57 | 16.4 | 1.55 | 0.45 | 0.29 |
| 17* | 96.7 | 213.8 | 2.2 | 0.05 | 13.14 | 266.4 | 0.45 | 0.04 | 0.09 |
| 18 | 391.6 | 938.5 | 2.4 | 0.62 | 15.87 | 25.4 | 3.80 | 3.10 | 0.82 |
| 19 | 124.4 | 572.3 | 4.6 | 0.70 | 37.95 | 54.6 | 1.94 | 1.78 | 0.92 |
| 20 | 533.6 | 1016.4 | 1.9 | 0.88 | NA | NA | 6.83 | 0.81 | 0.12 |
| 21 | 278.3 | 800.4 | 2.9 | 0.85 | 84.26 | 98.9 | 1.00 | 0.48 | 0.48 |
| 22 | 84.4 | 146.8 | 1.7 | 0.54 | 9.59 | 17.9 | 0.74 | 0.19 | 0.26 |
| 23 | 216.4 | 378.9 | 1.8 | 0.19 | 24.47 | 125.6 | 1.36 | 0.68 | 0.50 |
| 24* | 78.7 | 396.3 | 5.0 | 0.58 | 18.79 | 32.4 | 0.44 | 0.75 | 1.71 |
| 25 | 225.8 | 1174.6 | 5.2 | 10.40 | 203.62 | 19.6 | 4.88 | 7.05 | 1.44 |
| 26 | 104.1 | 177.1 | 1.7 | 0.51 | 19.58 | 38.4 | 1.06 | NA | NA |
| 27 | 307.1 | 1391 | 4.5 | 1.95 | 356.38 | 182.8 | 1.87 | 4.59 | 2.45 |
| 28 | 167.1 | 354.2 | 2.1 | 1.92 | NA | NA | 2.46 | NA | NA |
| Mean | 215.6 | 587.8 | 2.9 | 1.23 | 84 | 88.3 | 2.3 | 1.6 | 0.9 |
| Median | 159.5 | 407.2 | 2.4 | 0.64 | 45 | 82.7 | 1.4 | 0.8 | 0.5 |
| SD | 186.2 | 482.0 | 1.6 | 1.96 | 95 | 69.9 | 3.3 | 2.0 | 0.7 |
NA indicates not assessed.
Indicated cassettes inoculated with fewer than 100 million viable cells
For recipients of ex vivo–expanded cells the median unmanipulated cell dose infused on day 0 was 2.05 × 107 cells/kg (range, 1.1-5.5 × 107 cells/kg), and the ex vivo–expanded cell dose infused on posttransplantation day 12 was 2.05 × 107 cells/kg (range, 0.06-10.19 × 107 cells/kg). A median CD34+ dose of 0.78 × 105 cells/kg (range, 0.02-15.99 × 105 cells/kg) was delivered from the unmanipulated graft, and was supplemented with a median ex vivo–expanded CD34+ cell dose of 0.10 × 105 cells/kg (range, 0.01-1.66 × 105 cells/kg). The median unmanipulated CFU-GM dose infused was 0.67 × 105/kg (range, 0.10-9.50 × 105/kg) and was supplemented with a median ex vivo–expanded CFU-GM dose of 35.56 × 105/kg (range, 0.11-184.32 × 105/kg). The unmanipulated median CD3+ cell dose that patients received was 5.27 × 106 cells/kg. CD3+ cells decreased under these ex vivo–expansion conditions. There was a wide variation in the number of ex vivo–expanded UCB cells transplanted into patients enrolled in this study.
Although the intent of the study was to inoculate the expansion chamber with at least 1 × 108 cells, this was not achieved in 8 patients after thawing the UCB graft (Table 3). These expansions tested the ability of the device to expand cells at lower starting inoculum densities. Although the cells expanded to the same extent as higher density cultures, the absolute number of cells harvested was lower, resulting in an ex vivo cell dose of less than 1 × 107 cells/kg in 6 of these patients.
Infusion of ex vivo–expanded cells
The infusion of expanded cells was well tolerated by recipients. Although all patients were monitored every 15 minutes for the 2 hours following the ex vivo–expanded cell infusion, and closely monitored for the remaining 24 hours after infusion, none of the recipients experienced any adverse events from the infusion. One patient had an elevated creatinine level at the time of infusion, and his creatinine level continued to rise following infusion of expanded cells. This patient was found to have disseminated adenovirus.
Engraftment
There were 21 patients engrafted with myeloid cells; the median day to achieve an ANC higher than 0.5 × 109/L was 22 days (range, 13-40 days). By day 42, 3 patients failed to engraft; 2 patients died prior to engraftment; 1 patient received unmanipulated and ex vivo–expanded cells on the same day (patient 17); and 1 patient did not receive ex vivo–expanded cells because of fungal contamination (patient 20).
Prior to becoming platelet and RBC transfusion independent, 5 additional patients died. For the 16 patients who engrafted platelets, the median day to platelet transfusion independence and RBC transfusion independence was 71 (range, 39-139 days) and 58 (range, 39-131 days), respectively. The median day to a platelet count higher than 50 × 109/L was 94 (range, 41-370 days) and was achieved by all of the patients who became transfusion independent.
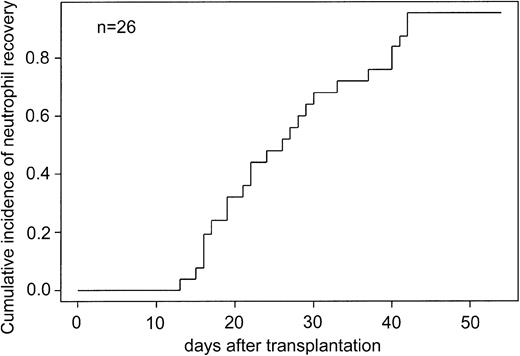
The cumulative incidence of neutrophil recovery and platelet transfusion independence was determined for 26 study patients who received the ex vivo–expanded cells on posttransplantation day 12. The cumulative incidence of neutrophil recovery in study patients was 0.95 at 42 days after transplantation (Figure 1).
Cumulative incidence of neutrophil recovery in recipients of ex vivo–expanded UCB cells.
Cumulative incidence of neutrophil recovery in recipients of ex vivo–expanded UCB cells.
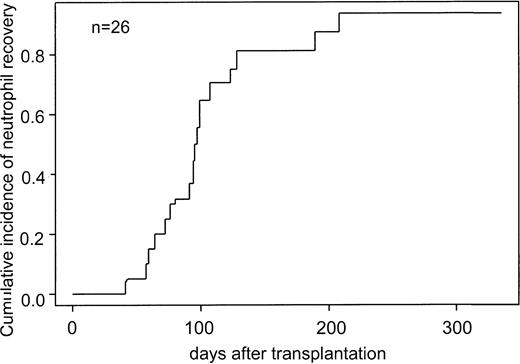
The cumulative incidence of platelet recovery was 0.64 at 100 days and increased to 0.87 at 200 days after transplantation (Figure 2).
Cumulative incidence of platelet engraftment for recipients of ex vivo–expanded UCB cells.
Cumulative incidence of platelet engraftment for recipients of ex vivo–expanded UCB cells.
Of the 27 evaluable patients, 3 developed graft failure, which was defined as failure to recover neutrophils by day 42 after transplantation. The first patient underwent a second unrelated UCB transplantation and engrafted but subsequently died of pulmonary failure. The second patient with graft failure seized with the second dose of busulfan did not complete his conditioning regimen. He experienced autologous reconstitution on day 70 and underwent a second unrelated UCB transplantation. He engrafted with donor cells and is surviving event-free 53 months after the second transplantation. The third patient eventually engrafted with donor myeloid cell on day 74 but later died of hepatic failure secondary to GVHD.
GVHD
Of the 28 patients, 22 were evaluable for acute GVHD (Table 1); 6 patients were considered nonevaluable for the previously mentioned reasons. The patient who showed myeloid engraftment on day 74 developed biopsy-proven GVHD of the gut on day 22. Of the 22 evaluable patients, 14 experienced low-grade (0-I) or no GVHD; 8 patients (36%) experienced moderate-to-severe–grade (II-IV) GVHD (Table 1).
EFS
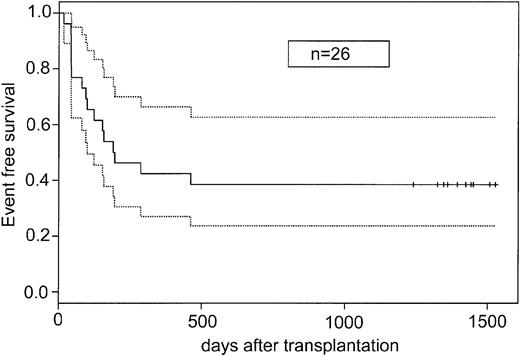
The probability of EFS for all ex vivo recipient patients (n = 26) was 0.39 (95% confidence interval [CI], 0.20-0.57) (Figure 3), with a minimum follow up of 41 months. At day 100 after transplantation, the probability of survival was 0.65 (95% CI, 0.47-0.84). Patients with malignant disorders who underwent transplantation had a 0.42 probability of EFS, and patients with nonmalignant disorders who underwent transplantation had a 0.22 probability of EFS (data not shown).
EFS for recipients of ex vivo–expanded UCB cells. Solid line indicates EFS; dotted lines, 95% confidence interval; and vertical marks, censoring time.
EFS for recipients of ex vivo–expanded UCB cells. Solid line indicates EFS; dotted lines, 95% confidence interval; and vertical marks, censoring time.
Transplantation-related mortality, relapse, and death
The events individual patients experienced as well as causes of death are listed in Table 1. Of the 19 patients with malignant diseases, 7 experienced relapse of their original disease after transplantation; 5 of these patients have died because of progressive disease, and an additional patient died of GVHD and at autopsy was found to have relapsed. Of the 27 evaluable patients, 5 experienced fatal infections: 3 infections occurred before day 50 after transplantation and the other 2 on day 100. There were 2 patients who died of pulmonary failure, 2 who died of stroke, and 1 who died of Epstein-Barr virus (EBV) lymphoproliferative disease.
Discussion
In this trial we established the safety and feasibility of infusing ex vivo–expanded UCB cells expanded in the AastromReplicell into recipients of UCB transplants. We have shown that UCB cells can be expanded in 12 days, although the magnitude of expansion varied from sample to sample. Under these conditions, CFU-GM expansion is maximal, but expansion of CD34+ lin– cells is poor. The study patients did not experience any adverse effects from infusion of the expanded cells. Supplementation of the conventional transplants with these ex vivo–expanded cells administered on posttransplantation day 12 did not impact the times to achieve myeloid, erythroid, or platelet engraftment.
Time to engraft neutrophils and red cells was similar between the study patients and times reported in the literature for adult and pediatric UCB recipients. Since the ex vivo cells were infused on day 12 and the median time to ANC higher than 0.5 × 109/L was 24 days, the contribution of the ex vivo–expanded cells to engraftment may have been masked. The cell dose that a single UCB unit can provide recipients continues to concern transplant physicians and results in underutilization of this stem cell source. Other groups have been investigating ex vivo expansion methods and have reported results of CD34+ selected expansion and stromal based expansion, confirming the ability to increase the cell dose available for recipients and to modestly increase the number of committed progenitors.32,33 Preliminary clinical reports also confirm the feasibility of the ex vivo expansion approach.34 This approach warrants further evaluation and has the potential for making this stem cell source available for larger pediatric and adult recipients. To better understand the effect of ex vivo–expanded cells on engraftment, a study including simultaneous infusion of both the unmanipulated and expanded cells on day 0 will be necessary.
A concern regarding ex vivo expansion technology is the ability to maintain functional hematopoietic repopulating cells. One potential drawback to ex vivo expansion of UCB cells is that increasing the number of committed progenitor cells may actually be depleting the more primitive stem cells from the unmanipulated inoculum. This could be true in this study because the actual number of CD34+lin– cells was decreased from that present in the original graft. Optimization of the cytokine cocktail used for expansion could improve both recovery and expansion of CD34+lin– cells. The addition of early-acting cytokines, stem cell factor, and thrombopoietin may result in significant improvement in overall nucleated cell and CD34+ cell expansion.27,35-37 The addition of stromal cells may augment expansion of the CD34+lin– compartment21,26,33 and is currently being evaluated.
In summary, we conclude that UCB cells can be expanded ex vivo, yielding large numbers of CFU-GMs in the AastromReplicell System in 12 days, and that the infusion of these ex vivo–expanded cells is safe. The infusion of cells expanded in this fashion on day 12 did not significantly alter myeloid, erythroid, or platelet engraftment in our study. We have demonstrated the safety and feasibility of this ex vivo expansion approach, and a randomized phase 2 trial will determine whether this approach is beneficial.
Prepublished online as Blood First Edition Paper, February 20, 2003; DOI 10.1182/blood-2001-12-0290.
Supported by NIDDK grant R44 DK53112.
K.G., A.S., J.D., and S.B. were employed by Aastrom Biosciences, which manufactures the AastromReplicell System studied in this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Dr Fu of Case Western Reserve University Department of Biostatistics for his expert statistical analysis. We would also like to thank the Duke Stem Cell Transplant Program staff for their excellent care of the patients.
J.J. is the recipient of a Doris Duke Charitable Foundation Clinical Scientist Development Award.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal