Abstract
We analyzed lymphocyte morphology, histology, immunophenotype, immunoglobulin heavy chain (IgVH) gene mutations, and clinical course in 80 unselected patients presenting with circulating t(11;14) lymphocytes. Of the 80 patients, 43 had peripheral lymphadenopathy (nodal group), and histology confirmed mantle cell lymphoma (MCL) in all. There were 37 patients with no lymphadenopathy (nonnodal group); 13 of 37 had histology, all showing MCL. IgVH genes were unmutated in 28 (90%) of 31 nodal and 15 (44%) of 34 nonnodal cases (P = .0001); CD38 was positive in 32 (94%) of 34 nodal and 16 (48%) of 33 nonnodal cases (P < .001); 41 (95%) of 43 nodal patients required immediate treatment compared with 18 (49%) of 37 nonnodal patients who had indolent disease (P < .0001). Median survival (95% confidence interval) was 30 months (10-50) in the nodal group and 79 months (22-136) in the nonnodal group (P = .005). Mutation status did not statistically affect survival, but of 6 long-term survivors (> 90 months) all were nonnodal and 5 of 5 had mutated IgVH genes. Lymphocyte morphology was heterogeneous in both groups: typical MCL in 56 cases (34 nodal, 22 nonnodal), blastoid MCL in 8 cases (3 nodal, 5 nonnodal), and small-cell MCL in 16 cases (6 nodal, 10 nonnodal, P = .12). Matutes immunophenotyping score was 1 in 65 cases and 2 in 15 (8 nodal, 7 nonnodal). We find no evidence against a diagnosis of MCL in the nonnodal group and suggest that mutated IgVH genes may help identify patients with indolent disease.
Introduction
The t(11;14)(q13;q32) is an important translocation in B-cell malignancy. It results in the juxtaposition of the BCL1 gene and the immunoglobulin heavy chain locus with consequent overexpression of cyclin D1.1 The t(11;14) is the hallmark of mantle cell lymphoma (MCL), a disease also characterized by well-defined nodal histology and an immunophenotype typified by the strong expression of surface immunoglobulin, usually IgM and IgD, together with CD5, CD19, CD20, and CD79b. The translocation is not, however, exclusive to MCL, as it is found for example in myeloma,2,3 a disease readily differentiated by its morphology and clinical picture. It has also been described in a small percentage of other chronic lymphoproliferative diseases, but the literature is not consistent on this point, and some groups have considered the presence of the t(11;14) translocation in circulating lymphocytes to be synonymous with MCL.4 The incidence of t(11;14) in diseases other than MCL or myeloma is therefore difficult to define. Whatever the nomenclature chosen for these conditions, the clinical picture of patients with circulating atypical clonal lymphocytes carrying the t(11;14) translocation is variable: both benign and very aggressive outcomes have been described.5-8
In cases of t(11;14) lymphocytosis that have peripheral lymphadenopathy or in which splenectomy is indicated, tissue histology provides a diagnosis, which is almost invariably MCL. Cases originally diagnosed as prolymphocytic leukemia (PLL) or atypical chronic lymphocytic leukemia (CLL) have been reclassified as MCL when additional tissue such as spleen has become available during the course of the disease.9 The diagnostic problems arise in those cases without access to histology.10-12 Here the diagnosis is based on factors such as the morphology of the circulating lymphocytes, bone marrow appearance, immunophenotype, and karyotype. In the literature these cases have variously been called MCL, mantle cell leukemia, atypical CLL, PLL, and splenic lymphoma with villous lymphocytes (SLVL).13-17 The basic question as to whether all cases with t(11;14) circulating lymphocytes are MCL variants or whether they encompass other diseases is unresolved.
Sequencing the variable region of the immunoglobulin heavy chain (IgVH) genes has provided new insights into the clonal origin of the chronic B-cell malignancies. An absence of somatic mutations is consistent with origin from a pre–germinal center B cell, whereas tumors that show somatic hypermutation arise either from germinal center cells or from post–germinal center memory cells. While some B-cell malignancies typically arise from one or other of these groups, CLL and SLVL are heterogeneous with respect to IgVH gene mutations; cases lacking mutations have a more aggressive clinical course than cases with somatic hypermutation.18,19 In addition, study of IgVH genes has demonstrated biased use, which is disease related (use of V1-69 in unmutated CLL, V4-34 in mutated CLL, and V1-2 in SLVL)19,20 and may be antigen driven. The initial study of IgVH gene sequences in 6 patients with MCL found no somatic mutations, consistent with the tumor arising from either primary lymphoid follicles or the mantle zones of secondary follicles.21 However, subsequent studies have found mutations in a minority of cases.22-25 We have reviewed the lymphocyte morphology, histology, immunophenotype, IgVH gene status, and clinical course of 80 patients who presented with circulating lymphocytes characterized by the t(11;14) translocation. The aims of this study were to determine whether those cases with a more favorable course can be identified at presentation and to evaluate whether a knowledge of the IgVH gene status can help to resolve the diagnostic uncertainty surrounding those cases that present without palpable lymphadenopathy.
Patients and methods
There were 80 patients with peripheral blood lymphocytes carrying the t(11;14)(q13;q32) translocation studied from 3 sources: the Royal Bournemouth (n = 24) and Royal Marsdsen (n = 12) Hospitals, United Kingdom, and the University Hospital, Nantes, France (n = 44). Informed consent was given according to the Declaration of Helsinki, and the only selection criteria were the availability of diagnostic information, clinical follow-up, and DNA for IgVH analysis.
Histology and morphology
Lymph node and spleen histology were reviewed by a single hematopathologist in each of the 3 centers. Cases that caused diagnostic difficulty were all reviewed by an independent lymphoma expert (DW). All cases were classified according to the current World Health Organization (WHO) criteria. The morphology of circulating leukemic lymphocytes was reviewed collaboratively among the 3 groups, and the cases were classified according to the French-American-British criteria.26 Patients diagnosed with MCL were subclassified as follows27 : (1) typical MCL, the predominant lymphocyte population was of medium size with speckled chromatin and an indented nucleus; (2) blastoid MCL, more than 20% of circulating cells were large and had reticular chromatin and 1 to 3 nucleoli, or resembled lymphoblasts; and (3) small-cell MCL, the majority of lymphocytes were small with condensed chromatin, although a minority had features of typical mantle cells.
Immunophenotyping
Expression of surface immunoglobulin, CD5, CD23, CD79b or CD22, and FMC7 was routinely performed on all cases by standard multicolor flow cytometry. Each case was assigned a Matutes score, 1 point scored for each of the following: CD5 positivity, weak expression of surface immunoglobulin, CD23 positivity, weak or negative CD79b or CD22 expression, and FMC7 negativity.28,29 CD38 expression (HB7-PE, Becton Dickinson, San Jose, CA) was measured by a 3-color assay with sequential gating of CD5+CD19+ or CD5+CD20+ lymphocytes and determination of CD38 positivity within this population. Positivity threshold was set at 30% of tumor cells.30
The t(11; 14) translocation
The t(11; 14) translocation was demonstrated by karyotyping (28 patients) or interphase fluorescence in situ hybridization (FISH; 52 patients) and was present in all cases. Karyotype analysis was carried out on Giemsa, Trypsin, Leishman (GTL)–banded slides from unstimulated and/or PMA (phorbol 12 myristate 13 acetate)–stimulated peripheral blood, bone marrow, or lymph node specimens cultured for 3,4, or 5 days and processed by standard cytogenetic techniques to obtain metaphase preparations. FISH analysis was performed in accordance with previously published conditions2 with 11q13 (CCND1 probe; Vysis, Downers Grove, IL) and 14q32 (158A2 bacterial artificial chromosome (BAC) probe mapping to the JH and first constant regions of the IgH gene) probes. The cutoff level (mean, + 3 SD) for the detection of t(11;14) was set at 7%.
IgVH gene sequencing
At least 5 × 106 lymphocytes were extracted in RNAzol B (Cinna Biotecx Labs, Houston, TX) for RNA, or TRI Reagent (Sigma, Poole, Dorset, United Kingdom) for RNA and DNA following the manufacturer's instructions. Reverse transcription to cDNA was carried out from RNA (∼ 2 μg) with NotI-d(T)18 primer and first-strand cDNA Synthesis kit (Amersham Pharmacia Biotech, Uppsala, Sweden) in a final volume of 15 μL using the manufacturer's protocol. One fifth of the DNA was subjected to amplification.
Polymerase chain reaction (PCR) amplification of cDNA or DNA was carried out using primers and conditions described previously. DNA sequence analysis of inserts, and data analyses to identify tumor-derived IgVH gene use and mutation pattern were performed as reported previously.31 In all cases, PCR for IgVH gene identification was repeated for confirmation. To allow for a low degree of allelic polymorphism, homology to the germ-line configuration of 98% or higher was considered to represent unmutated IgVH gene status. Intraclonal heterogeneity in the mutated cases was analyzed by comparison of 4 to 12 tumor-derived sequences, obtained from subcloned plasmids, or by direct sequencing of PCR product from multiple reactions.
Statistical methods
Fisher exact test was used to compare the percentage of patients with small-cell morphology, splenomegaly, involvement of the gastrointestinal tract (GIT), male sex, complex karyotype, expression of CD38, and mutated IgVH genes in the nodal and nonnodal groups. Logistic regression was used to determine whether the associations of CD38 expression and IgVH gene mutations with nodal status were independent of each other. Survival times from diagnosis were summarized using Kaplan-Meier survival curves, and median survival with 95% confidence intervals (CIs). Survival curves were compared between groups defined by IgVH status, lymphocyte morphology, sex, and the presence of peripheral lymphadenopathy using the log-rank test. Data were analyzed using SPSS for Windows Version 10 (SPSS, Chicago, IL) and GraphPad Prism (GraphPad Software, San Diego, CA). P values less than .05 were considered statistically significant.
Results
Clinical features
The 80 patients had a mean (SD) age of 63.9 (11.7) years; 55 patients (69%) were male. There were 53 patients (66%) with splenomegaly, and 10 (13%) had involvement of the GIT. At presentation, 43 patients (54%) had peripheral lymphadenopathy larger than 2 cm. There were 37 patients (46%) with no palpable nodes larger than 2 cm, and, where it was performed (29 cases), computerized tomography (CT) or ultrasound scanning confirmed the absence of intra-abdominal or intrathoracic lymphadenopathy. The median lymphocyte count was 13.2 × 109/L (range, 1.2-426 × 109/L). Because the great majority of diagnostic uncertainties occurred in cases without lymphadenopathy, where nodal histology was often not available, and because these cases included almost all the patients with stable disease and long survival, we also considered results in relation to these 2 groups, hereafter designated nodal and nonnodal. Table 1 summarizes data on clinical and laboratory details, contrasting these 2 groups.
Comparison of patient characteristics in nodal and nonnodal groups
. | Nodal group . | Nonnodal group . | P . |
|---|---|---|---|
| Patients | |||
| No. | 43 | 37 | |
| M/F | 2.3/1 | 2/1 | 1.0 |
| Mean age, y (range) | 65 (42-87) | 63 (36-81) | .58 |
| Clinical (%) | |||
| Splenomegaly | 25/43 (58) | 28/37 (76) | .15 |
| GI tract | 8/43 (19) | 2/37 (5) | .1 |
| CD38, 30% or more positive (%) | 32/34 (94) | 16/33 (48) | < .001 |
| IgVH genes (%) | |||
| 98% or higher homology | 28/31 (90) | 15/34 (44) | |
| 97% homology | 3/31 (10) | 3/34 (9) | < .001 |
| Less than 97% homology | 0/31 | 16/34 (47) | |
| Karyotype (%) | |||
| Complex | 11/11 (100) | 9/17 (53) | .01 |
| Single | 0/11 | 8/17 (47) | |
| Median survival, mo (95% confidence limits) | 30 (10-50) | 79 (22-136) | .005 |
. | Nodal group . | Nonnodal group . | P . |
|---|---|---|---|
| Patients | |||
| No. | 43 | 37 | |
| M/F | 2.3/1 | 2/1 | 1.0 |
| Mean age, y (range) | 65 (42-87) | 63 (36-81) | .58 |
| Clinical (%) | |||
| Splenomegaly | 25/43 (58) | 28/37 (76) | .15 |
| GI tract | 8/43 (19) | 2/37 (5) | .1 |
| CD38, 30% or more positive (%) | 32/34 (94) | 16/33 (48) | < .001 |
| IgVH genes (%) | |||
| 98% or higher homology | 28/31 (90) | 15/34 (44) | |
| 97% homology | 3/31 (10) | 3/34 (9) | < .001 |
| Less than 97% homology | 0/31 | 16/34 (47) | |
| Karyotype (%) | |||
| Complex | 11/11 (100) | 9/17 (53) | .01 |
| Single | 0/11 | 8/17 (47) | |
| Median survival, mo (95% confidence limits) | 30 (10-50) | 79 (22-136) | .005 |
Review of histology and lymphocyte morphology
Histology was available in 51 patients for 34 lymph nodes, 19 spleens, 9 GIT biopsies, and 2 tonsils. From 13 nonnodal cases were 10 splenic, 1 hilar node, and 2 GIT biopsies. The histology in all cases was that of typical MCL, except in 4 patients exhibiting a blastoid variant. Blood lymphocyte morphology was typical MCL in 56 cases (34 nodal, 22 nonnodal), blastoid MCL in 8 cases (3 nodal, 5 nonnodal), and small-cell MCL in 16 cases (6 nodal, 10 nonnodal). Of the typical MCL cases, 3 also had some cells with features of PLL (2 cases) or lymphoplasmacytoid lymphoma (1 case). No cases had the morphology of SLVL, PLL, or typical CLL. Of the 13 nonnodal cases that also had tissue histology, lymphocyte morphology was typical in 9, blastoid in 1, and small cell in 3. There was no significant difference in numbers of patients with small-cell morphology between the nodal and nonnodal groups (P = .12).
Immunophenotyping
Displaying the typical MCL phenotype as described in “Patients and methods” and with a Matutes score of 1 were 65 patients. In the remaining patients the phenotype differed in one of these criteria: 2 patients were CD5– (both in the nodal group), 6 patients were FMC7 negative (3 nodal, 3 nonnodal), and 7 patients were weakly positive for CD23 (3 nodal, 4 nonnodal). All of these 15 cases had a Matutes score of 2 (see Table 2 for details). Expression of CD38 was measured in 67 patients and was positive in 48 (72%). As shown in Table 1, CD38 positivity was found in 94% and 48% of patients in the nodal and nonnodal groups, respectively (P < .001).
Details of the 15 patients with Matutes score of 2
Patient no. . | CD5 . | CD23 . | FMC7 . | CD38, % . | IgVH gene homology, % . | Clinical . | Outcome:survival, mo/alive (A) or dead (D) . |
|---|---|---|---|---|---|---|---|
| 5 | + | - | - | 97 | 97 | Nodal | 23/D |
| 6 | - | - | + | 99 | 97 | Nodal | 29/D |
| 8 | - | - | + | 89 | 100 | Nodal | 5/D |
| 14 | + | - | - | 92 | NR | Nodal | 8/D |
| 26 | + | Weak + | + | 0 | 99 | Nodal | 5/D |
| 37 | + | Weak + | + | 44 | 100 | Nodal | 8/D |
| 38 | + | - | - | 99 | NR | Nodal | 40/A |
| 42 | + | Weak + | + | NR | 98.6 | Nodal | 78/D |
| 47 | + | - | - | 13 | 96 | Nonnodal | 12/A |
| 49 | + | Weak + | + | 5 | 94.5 | Nonnodal | 90/A |
| 62 | + | Weak + | + | 0 | 87 | Nonnodal | 99/A |
| 65 | + | - | - | 0 | 100 | Nonnodal | 79/A |
| 68 | + | Weak + | + | 4 | 95 | Nonnodal | 30/A |
| 70 | + | Weak + | + | 0 | 98.6 | Nonnodal | 46/A |
| 75 | + | - | - | NR | 87.8 | Nonnodal | 31/D |
Patient no. . | CD5 . | CD23 . | FMC7 . | CD38, % . | IgVH gene homology, % . | Clinical . | Outcome:survival, mo/alive (A) or dead (D) . |
|---|---|---|---|---|---|---|---|
| 5 | + | - | - | 97 | 97 | Nodal | 23/D |
| 6 | - | - | + | 99 | 97 | Nodal | 29/D |
| 8 | - | - | + | 89 | 100 | Nodal | 5/D |
| 14 | + | - | - | 92 | NR | Nodal | 8/D |
| 26 | + | Weak + | + | 0 | 99 | Nodal | 5/D |
| 37 | + | Weak + | + | 44 | 100 | Nodal | 8/D |
| 38 | + | - | - | 99 | NR | Nodal | 40/A |
| 42 | + | Weak + | + | NR | 98.6 | Nodal | 78/D |
| 47 | + | - | - | 13 | 96 | Nonnodal | 12/A |
| 49 | + | Weak + | + | 5 | 94.5 | Nonnodal | 90/A |
| 62 | + | Weak + | + | 0 | 87 | Nonnodal | 99/A |
| 65 | + | - | - | 0 | 100 | Nonnodal | 79/A |
| 68 | + | Weak + | + | 4 | 95 | Nonnodal | 30/A |
| 70 | + | Weak + | + | 0 | 98.6 | Nonnodal | 46/A |
| 75 | + | - | - | NR | 87.8 | Nonnodal | 31/D |
NR indicates no result.
IgVH gene analysis
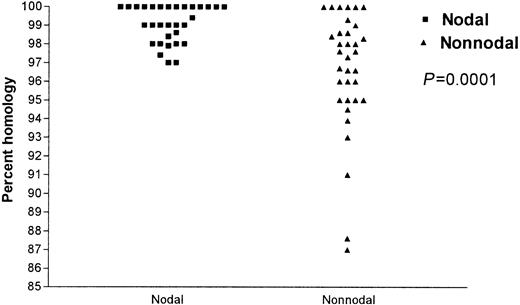
IgVH gene analysis was successful in 65 patients. IgVH genes were unmutated in 43 (66%) and mutated in 22 (34%) cases. In the nodal group, 28 (90%) of 31 patients had unmutated IgVH genes and the remaining 3 patients showed a low degree of mutation (97% germ-line homology). In contrast, in the nonnodal group, 15 (44%) patients had unmutated IgVH genes, while 19 (56%) had mutated IgVH genes, the lowest degree of homology being 87% (P = .0001, Figure 1).
Pecentage of IgVH gene somatic hypermutations: comparison of the nodal and nonnodal groups. ▪ indicates nodal and ▴ indicates nonnodal.
Pecentage of IgVH gene somatic hypermutations: comparison of the nodal and nonnodal groups. ▪ indicates nodal and ▴ indicates nonnodal.
The effects of CD38 (P = .013) and IgVH gene status (P = .018) on nodal status were statistically independent of one other. IgVH gene family use was comparable in both the unmutated and the mutated cases and similar to that of the normal B-cell repertoire (Table 3).
Distribution of IgVH and JH gene families in unmutated (UM) and mutated (MUT) mantle cell lymphoma (MCL)
. | UM normal B cells CD5+IgM+,* n = 113 (%) . | MUT normal B cells CD5+IgM+,* n = 31 (%) . | UM MCL, n = 40 (%) . | MUT MCL, n = 22 (%) . |
|---|---|---|---|---|
| VH family | ||||
| VH 1 | 24 (21.2) | 3 (9.7) | 9 (22.5) | 4 (18.2) |
| VH 2 | 2 (1.8) | 1 (3.2) | 0 | 0 |
| VH 3 | 59 (52.2) | 22 (71.0) | 19 (47.5) | 13 (59.1) |
| VH 4 | 21 (18.6) | 5 (16.1) | 8 (20.0) | 4 (18.2) |
| VH 5 | 2 (1.8) | 0 | 3 (7.5) | 0 |
| VH 6 | 2 (1.8) | 0 | 1 (2.5) | 1 (4.6) |
| VH 7 | 3 (2.7) | 0 | 0 | 0 |
| JH family | ||||
| JH1 | 0 | 1 (3.2) | 0 | 0 |
| JH2 | 4 (3.5) | 1 (3.2) | 1 (2.5) | 0 |
| JH3 | 4 (3.5) | 5 (16.1) | 5 (12.5) | 2 (9.1) |
| JH4 | 54 (47.8) | 21 (67.7) | 21 (52.5) | 11 (50.0) |
| JH5 | 13 (11.5) | 2 (6.5) | 9 (22.5) | 7 (31.8) |
| JH6 | 38 (33.6) | 1 (3.2) | 4 (10.0) | 2 (9.1) |
. | UM normal B cells CD5+IgM+,* n = 113 (%) . | MUT normal B cells CD5+IgM+,* n = 31 (%) . | UM MCL, n = 40 (%) . | MUT MCL, n = 22 (%) . |
|---|---|---|---|---|
| VH family | ||||
| VH 1 | 24 (21.2) | 3 (9.7) | 9 (22.5) | 4 (18.2) |
| VH 2 | 2 (1.8) | 1 (3.2) | 0 | 0 |
| VH 3 | 59 (52.2) | 22 (71.0) | 19 (47.5) | 13 (59.1) |
| VH 4 | 21 (18.6) | 5 (16.1) | 8 (20.0) | 4 (18.2) |
| VH 5 | 2 (1.8) | 0 | 3 (7.5) | 0 |
| VH 6 | 2 (1.8) | 0 | 1 (2.5) | 1 (4.6) |
| VH 7 | 3 (2.7) | 0 | 0 | 0 |
| JH family | ||||
| JH1 | 0 | 1 (3.2) | 0 | 0 |
| JH2 | 4 (3.5) | 1 (3.2) | 1 (2.5) | 0 |
| JH3 | 4 (3.5) | 5 (16.1) | 5 (12.5) | 2 (9.1) |
| JH4 | 54 (47.8) | 21 (67.7) | 21 (52.5) | 11 (50.0) |
| JH5 | 13 (11.5) | 2 (6.5) | 9 (22.5) | 7 (31.8) |
| JH6 | 38 (33.6) | 1 (3.2) | 4 (10.0) | 2 (9.1) |
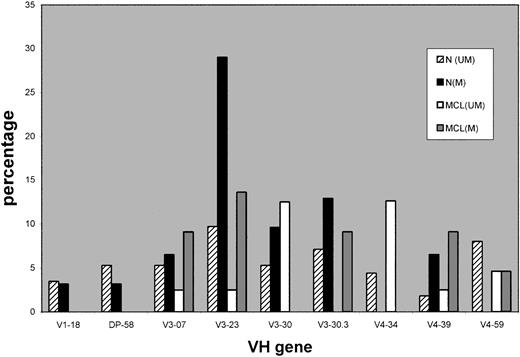
As to individual IgVH gene use, Figure 2 shows a comparison between the more frequently used IgVH genes in CD5+IgM+ normal B cells and those used in our MCL cases. V3-30 and V4-34 appear elevated in the unmutated MCL group. The mutated subset was investigated for intraclonal heterogeneity as described in “Patients and methods” and this was absent in all cases, suggesting that the mutated cases do not arise from the malignant transformation of a germinal center B cell.
A comparison of the most frequently used VH genes in CD5+IgM+ normal B cells with mantle cell lymphoma VH genes. Data on frequency of VH gene use in normal B cells from Brezinschek et al.32 n indicates normal B cells; MCL, mantle cell lymphoma cases; U, unmutated IgVH genes (≥ 98% germ-line homology); and M, mutated IgVH genes (< 98% homology).
A comparison of the most frequently used VH genes in CD5+IgM+ normal B cells with mantle cell lymphoma VH genes. Data on frequency of VH gene use in normal B cells from Brezinschek et al.32 n indicates normal B cells; MCL, mantle cell lymphoma cases; U, unmutated IgVH genes (≥ 98% germ-line homology); and M, mutated IgVH genes (< 98% homology).
Karyotyping and interphase FISH
In the majority of cases the presence of the t(11;14) translocation was established by interphase FISH. However, in 28 cases a karyotype was available (Table 1). This was complex in 11 (100%) of 11 nodal cases compared with 9 (53%) of 17 nonnodal cases (P = .01). In the latter, t(11;14) was the sole abnormality in the 8 remaining patients.
Clinical course and survival analysis
In the nodal group, 41 of 43 patients were treated immediately after diagnosis, while 1 patient had a stable clinical course of 24 months before requiring treatment and 1 elderly man died untreated within 1 month of diagnosis. A variety of treatments was used (≥ 3 in 19 patients), and the great majority of patients received combination chemotherapy (cyclophosphamide, adriamycin, vincristine, and prednisone [CHOP] or similar regimens). Splenectomy was performed in 8 patients; 11 patients received an autologous stem cell transplant and 8 received Rituximab. In the nonnodal group, treatment was delayed because of initially stable disease in 9 patients (by a mean of 29 months; range, 10-60 months), and a further 9 patients have never required treatment during a mean follow-up of 58 months (range, 8-175 months). Of the patients, 17 received combination chemotherapy, 9 were splenectomized, 3 were autografted, and 2 received Rituximab. Details of treatment are shown in Table 4.
Comparison of treatment in the nodal and nonnodal groups
Treatment details . | Nodal group . | Nonnodal group . |
|---|---|---|
| Patients never treated (%) | 1 (2) | 9 (24) |
| Patients with treatment delay (%) | 1 (2) | 9 (24) |
| Treatment delay, mo (range) | 24 | Mean, 29 (8-175) |
| Splenectomy (%) | 8 (19) | 9 (24) |
| Combination chemotherapy (%) CHOP/other | 20 (47)/11 (25) | 13 (35)/3 (8) |
| Autologous stem cell transplantation (%) | 11 (26) | 3 (8) |
| Patients receiving 3 or more treatment regimens (%) | 19 (44) | 7 (19) |
Treatment details . | Nodal group . | Nonnodal group . |
|---|---|---|
| Patients never treated (%) | 1 (2) | 9 (24) |
| Patients with treatment delay (%) | 1 (2) | 9 (24) |
| Treatment delay, mo (range) | 24 | Mean, 29 (8-175) |
| Splenectomy (%) | 8 (19) | 9 (24) |
| Combination chemotherapy (%) CHOP/other | 20 (47)/11 (25) | 13 (35)/3 (8) |
| Autologous stem cell transplantation (%) | 11 (26) | 3 (8) |
| Patients receiving 3 or more treatment regimens (%) | 19 (44) | 7 (19) |
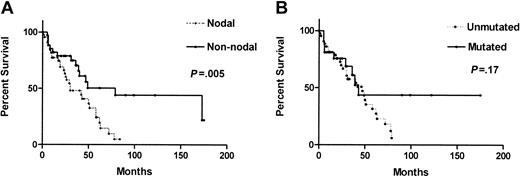
As shown in Figure 3A, the median survival of the nodal group was 30 months (95% CI, 10-50), significantly shorter than that of 79 months (95% CI, 22-136) in the nonnodal group (P = .005). Interestingly, all 6 long-surviving patients (> 90 months) were in the nonnodal group. Their lymphocyte morphology was mixed: 4 had typical MCL and 2 had small-cell MCL (1 of whom had a GIT biopsy showing MCL). Among them, 3 patients have never required treatment and 1 remained in CR for 13 years after 2 courses of attenuated CHOP. The other 2 patients had initially indolent disease with later prolonged responses to attenuated CHOP or chlorambucil.
Comparison of overall survival between the nodal and nonnodal groups and between patients with unmutated and mutated IgVH genes. (A) Nodal status had a significant adverse effect on survival (median survival: nodal, 30 months; nonnodal, 79 months). (B) The effect of IgVH gene mutation status does not attain statistical significance.
Comparison of overall survival between the nodal and nonnodal groups and between patients with unmutated and mutated IgVH genes. (A) Nodal status had a significant adverse effect on survival (median survival: nodal, 30 months; nonnodal, 79 months). (B) The effect of IgVH gene mutation status does not attain statistical significance.
CD38 positivity had no statistically significant effect on survival (median, 173 months for CD38 less than 30%, 43 months in CD38 30% or more; P = .16). Overall survival by IgVH gene status (Figure 3B) also did not attain statistical significance (median survivals and 95% CIs, 47 months [36-58] and 43 months [28-50]; P = .17) However, all 5 long-term survivors with IgVH gene analysis showed somatic mutations.
Discussion
This study evaluates lymphocyte morphology, histology, immunophenotype, IgVH gene status and clinical course of 80 patients presenting with atypical blood lymphocytes carrying the t(11;14) translocation. The cases were divided into 2 groups, with or without peripheral lymphadenopathy at presentation, based on the observation that diagnostic difficulties and heterogeneity of clinical course were confined to those cases without peripheral lymphadenopathy.
The patients who presented with lymphadenopathy formed a remarkably homogeneous group. All were diagnosed as having MCL on the basis of histology. The great majority had an aggressive clinical course, CD38 positivity, unmutated IgVH genes, and, where measured, a complex karyotype. These results are very similar to those of other studies: for example, Thorselius et al's finding that 80% of MCL cases had unmutated IgVH genes, and the finding that karyotype complexity is common in MCL and correlates with short survival.33-35,48 This group thus appears to correspond to the conventional concept of MCL as an aggressive lymphoma that commonly presents with nodal disease.
Much greater heterogeneity was found within the cases that presented without peripheral lymphadenopathy. Many of these cases also had clinically aggressive disease, but 9 patients have never required treatment and in a further 9, therapy was delayed by a mean of 29 months from presentation. The median survival of the nonnodal group was 79 months, significantly longer than that of 30 months in the nodal group. Of 33 cases, 17 (52%) were CD38–, and IgVH gene mutations were found in 19 (56%) of 34 nonnodal cases including 10 cases with extensive somatic mutations. Of the 6 longest survivors (> 90 months), all 5 in whom the analysis was successful had mutated IgVH genes.
A key issue is whether the heterogeneity in the nonnodal group represents a true variation within MCL36,37 or is due to the inclusion of other B-cell malignancies with the t(11;14) translocation. Following an extensive review of peripheral blood morphology, histology, immunophenotype, karyotype, and IgVH genes, we found no reason to exclude any of the original 80 cases of t(11;14) lymphocytosis on the grounds of a diagnosis other than MCL. Heterogeneity of peripheral blood lymphocyte morphology was found in both the nodal and the nonnodal groups: typical MCL morphology was found in 79% and 59%, blastoid MCL in 7% and 14%, and small-cell morphology in 14% and 27%, respectively. No cases had morphology suggestive of SLVL, typical CLL, or PLL. The main potential diagnostic difficulty lies in the 16 cases in which the predominant cell was a small lymphocyte. These were classified as small-cell MCL based on the presence of some typical mantle cells, but we would have been unable to be confident of a diagnosis of MCL on the basis of morphology alone. The main differential diagnosis here would be atypical CLL or possibly peripheral blood spillover from splenic marginal zone lymphoma (SMZL). However, all 6 small-cell nodal cases had lymph node histology confirming MCL, and the histologic diagnosis was consistent with MCL in all 5 small-cell nonnodal cases in which tissue was available (1 spleen, 1 colon, 1 conjunctiva, and 2 bone marrow trephines).
We recognize that the histologic diagnosis of MCL, particularly in nonnodal cases, may be difficult. Gastrointestinal appearances in MCL can resemble those of other low-grade lymphomas, and bone marrow histology is not always diagnostically conclusive38,39 However, all the biopsies in this study were, at the very least, consistent with MCL, and in particular, none showed paraimmunoblasts suggestive of CLL. Spleen histology in both MCL and follicle center cell lymphoma may sometimes have a marginal zone appearance; however, where a diagnosis of SLVL with t(11;14) has previously been entertained, this has been on the basis of peripheral blood data alone. Conversely, a recent study of spleen histology in SMZL failed to find cases with cyclin D1 positivity.40
All cases had a typical MCL immunophenotype with a Matutes score of 1 (65 cases) or differed in only one criterion, giving a score of 2. The 15 cases scoring 2 were similarly divided between nodal and nonnodal groups. There were 2 CD5– patients and both were in the nodal group with confirmatory MCL histology. A recent review of immunophenotyping in chronic lymphoid malignancies shows that only 2% of CLL have a score of 2, none having a score of 1.28 Typical CLL has a score of 4 or 5, but there is a small overlap between MCL and atypical CLL. Equally, some cases of PLL and SLVL have previously been reported as CD5+, so that although all of our 80 cases have immunophenotypes that are entirely consistent with MCL, the present panel of markers cannot definitely exclude atypical CLL, SLVL, or PLL. A recent report suggests that coexpression of CD18 and CD54 may be helpful in distinguishing MCL from atypical CLL.41
There were 28 patients who had karyotypic analysis. All 11 nodal cases had a complex karyotype, whereas there was heterogeneity in the nonnodal group (9 cases complex, 8 cases single t(11;14)). With regard to the diagnostic difficulties in the nonnodal group, there were no cases of 7q abnormalities or +3 to indicate SLVL, or +12 to suggest an alternative diagnosis of CLL. However, cytogenetic overlap between atypical CLL and MCL as evinced by +12, 13q14 deletions, and 11q loss is well reported.34,42 A recent study43 comparing nodal and leukemic MCL identifies genomic loss of 8p as much more common in the latter. We did not find this on karyotyping, but it may be that the use of the more sensitive FISH technique, as used in the study, is necessary to pick this up.
Study of IgVH gene mutational status is another parameter that demonstrates homogeneity of the nodal group (90% unmutated) and heterogeneity of the nonnodal group (44% unmutated). Sequence analysis of the t(11;14) junctions has recently shown that this chromosomal translocation arises early in B-cell ontogeny, at a stage when the cell is undergoing VDJ recombination.44 The heterogeneity of IgVH mutational status in cases with the t(11;14) translocation implies that a subsequent transforming event may occur in either an unmutated B cell or alternatively in a post–germinal center memory B cell. Similar heterogeneity is well recognized in CLL and has recently been described in SMZL. This IgVH gene heterogeneity within diseases defined by WHO criteria precludes the use of IgVH gene status as a means of elucidating whether the nonnodal t(11;14) cases represent one or more disease entities. The finding of biased IgVH gene use, an activated cell surface phenotype, and short telomere lengths strongly suggests that the unmutated subset of CLL has encountered antigen. However, recent cDNA microarray data show that the genetic signature of both groups of CLL is closer to that of a normal memory B cell than to a normal naive or germinal center B cell.45 A similar study in patients with a t(11;14) lymphocytosis may help to clarify whether there is a common genetic signature among these cases in spite of the heterogeneity of IgVH gene mutational status. In contrast to CLL and SLVL we found no significant asymmetry of IgVH gene use either at the level of gene families or individual VH gene segments, suggesting origins from diverse cells of a functional B-cell repertoire. The spread of individual IgVH genes used is wide and the numbers in each group correspondingly small. However in conjunction with 2 other recent studies46,47 we confirm that, unlike CLL, use of V3-21 and V4-34 is largely confined to the unmutated group. Thorselius et al observed the use of VH4-34 in 22% of MCL cases, but the association of mutational status was not clarified.48 Confirmation of overutilization of V4-34 in unmutated MCL would raise an interesting question as to the nature of antigen recognized since VH4-34 immunoglobulin is able to define both conventional antigens and superantigens.
In the present study there was no evidence from lymphocyte morphology, histology, immunophenotype, karyotype, or IgVH gene use that the unmutated nonnodal cases are more similar to the unmutated nodal cases than to the mutated nonnodal cases. Although IgVH gene status did not have prognostic significance within the nonnodal cases, it is of interest that the long-term survivors, all in the nonnodal group, all had mutated IgVH genes. In both CLL and SMZL, IgVH gene mutations are associated with more benign disease and a larger study is required to determine the true prognostic significance of IgVH gene mutational status in cases with a t(11;14) lymphocytosis.
In CLL, CD38 expression has been explored as a surrogate marker for IgVH gene mutations. Although it does not reliably identify the same subsets, it has been shown to be an independent prognostic factor.30 In the present much smaller MCL study, neither IgVH gene mutation nor CD38 expression was significantly associated with survival, although both were independently associated with nodal status. CD38 positivity was correlated with unmutated IgVH genes in 70% of cases, a concordance similar to that in CLL.
In conclusion, we have studied 80 patients presenting with peripheral blood lymphocytes carrying the t(11;14) translocation. Those patients with peripheral lymphadenopathy in whom histology was readily available were all confirmed as having MCL, with a high frequency of unmutated IgVH genes, CD38 positivity, and complex karyotype. Those without peripheral nodes, many of whom did not have confirmatory histology, were much more heterogeneous. Lymphocyte morphology, nonnodal histology, immunophenotype, karyotype, and IgVH genes were separately not definitive in classifying all of these cases as MCL. However, in none of these unselected t(11;14) cases did we find any evidence for the concept that the translocation is occurring in CLL, SLVL, or PLL. The nonnodal group is of clinical importance because some of these patients have indolent disease. We suggest that mutated IgVH genes may be of use in identifying those asymptomatic patients for whom early intensive treatment is not indicated. Further study of these patients and particularly of other approaches, such as array data, is required.
Prepublished online as Blood First Edition Paper, February 27, 2003; DOI 10.1182/blood-2002-06-1864.
Supported by the Bournemouth Leukaemia Research Fund and Tenovus United Kingdom.
J.O. and R.G. contributed equally to the study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Drs B. Mahé, P. Moreau, N. Morineau, M. J. Rapp (Service d'Hématologie Clinique, Centre Hospitalier Universitaire, Nantes, France); M. Hamidou, M. Brisseau (Service de Medecine Interne A, Centre Hospitalier Universitaire, Nantes, France); J. F. Ramée (Centre Catherine de Sienne, Nantes, France); A. Le Mevel (Centre René Gauducheau, Nantes, France); P. De Faucal (Clinique Viaud, Nantes, France); H. Maisonneuve (Medecine A–Hématologie, Centre Hospitalier Départemental, La Roche-Sur-Yon, France); P. Moreau (Service de Médecine Interne, Centre Hospitalier de Bretagne Sud, Lorient, France); H. Jardel (Service de Médecine Interne, Hôpital Prosper Chubert, Vannes, France); A. Milne, A. Roy (North Hampshire Hospitals, United Kingdom); C. Mattock (St Helier Hospital, Jersey, United Kingdom); M. Al-Hilali (Dorset County Hospital, United Kingdom); and Prof T. J. Hamblin (Royal Bournemouth Hospital, United Kingdom) for providing us with samples, data, and follow-up information. We thank Dr F. Gaillard (University Hospitals, Nantes, France); Dr A.Wotherspoon (Royal Marsden NHS Trust for histologic diagnosis); and Prof D. Wright (Southampton University Hospitals, United Kingdom) for expert review of difficult histology, and Dr V. Brito-Babapaulle, Mr S. Atkinson, and Dr G. Giustolisi (Royal Marsden) for technical support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal