Abstract
HOX11 encodes a homeodomain protein that is aberrantly expressed in T-cell acute lymphoblastic leukemia as a consequence of the t(10;14) and t(7;10) chromosomal translocations. We previously reported that HOX11 immortalizes murine hematopoietic progenitors and induces pre–T-cell tumors in mice after long latency. It has been demonstrated in a number of studies that HOX11, similar to other homeodomain proteins, binds DNA and transactivates transcription. These findings suggest that translocation-activated HOX11 functions as an oncogenic transcription factor. Here we report that HOX11 represses transcription through both TATA-containing and TATA-less promoters. Interestingly, transcriptional repression by HOX11 is independent of its DNA binding capability. Moreover, a systematic mutational analysis indicated that repressor activity was separable from immortalizing function, which requires certain residues within the HOX11 homeodomain that make base-specific or phosphate-backbone contacts with DNA. We further showed that the pathologic action of HOX11 involves DNA binding-dependent transcriptional pathways that are distinct from those controlling expression of a chromosomal target gene (Aldh-1). We conclude that dysregulated expression of a particular set of downstream target genes by DNA binding via the homeodomain is of central importance for leukemia initiation mediated by HOX11.
Introduction
The involvement of homeobox genes in hematologic malignancies is becoming increasingly recognized.1 HOX11 (TCL-3) is a homeobox gene identified on chromosome 10 at the t(10;14)(q24;q11) and t(7;10)(q35;q24) chromosomal translocations associated with pediatric T-cell acute lymphoblastic leukemia (T-ALL).2-5 As a result of translocation, the T-cell receptor δ or β regulatory region is juxtaposed upstream of the HOX11 coding region, resulting in high-level synthesis of a structurally intact HOX11 homeodomain protein. During murine embryo-genesis, Hox11 expression has been detected in the bronchial arches, the hindbrain, and the splenic anlage arising from splanchnic mesoderm.6-8 Because HOX11 is not normally expressed in T cells, dysregulated HOX11 expression as a consequence of aberrant recombinational events during T-cell receptor rearrangement is believed to be an early step in the etiology of this subtype of T-ALL.2-5 The transforming potential of HOX11 has been confirmed by both in vitro and in vivo studies. We have previously shown that retroviral vector–mediated expression of HOX11 in primary murine bone marrow (BM) cells gives rise to immortalized progenitor lines at high frequency and promotes T-cell tumorigenesis in mice,9,10 whereas others have reported that targeted expression of HOX11 in thymocytes of transgenic mice resulted in cell-cycle aberration and progression to malignancy.11
Several possible mechanisms through which inappropriate HOX11 expression might lead to malignant transformation have been proposed. In particular, HOX11 is believed to function as a transcriptional regulator on the basis of its nuclear localization, the DNA binding activity of its homeodomain, and its ability to transactivate transcription of reporter genes.12-15 In support of this view, HOX11 induces the up-regulation of an endogenous gene, Aldh-1, in stably transfected NIH3T3 cells; interestingly, an inverse relationship between Hox11 expression and Aldh-1 expression is observed during spleen organogenesis, suggesting that HOX11 may exert its oncogenic effects through up- or down-regulation of target genes.16,17 Besides the homeodomain, HOX11 contains a Gly/Pro-rich NH2-terminus and a Gln-rich COOH-terminus, both of which are required for efficient transcriptional transactivation.13 HOX11 also contains a “PBX interaction motif” (PIM; FPWME). Although the PIM is not required for induction of Aldh-1 expression, it is essential for HOX11 association with PBX proteins— members of the TALE (three amino acid loop extension) superclass of homeodomain proteins—in vitro.12 Thus, cooperative DNA binding with PBX cofactors is postulated to contribute to some mechanisms of HOX11 action.
HOX11 has also been shown to interact with CTF1, a ubiquitous transcription factor that associates with TFIIB and the basal transcription machinery.18 In addition, interaction of many HOX proteins with the histone acetyltransferase transcriptional coregulator, Ca++-response element binding protein (CREB; CBP/p300), has been described.19 Finally, association of HOX11 with the phosphatases PP2A and PP1 results in disruption of the G2/M cell-cycle checkpoint.20 Collectively, these observations suggest that physiologic or pathologic functions of HOX11 may be mediated by multiple mechanisms, possibly DNA binding–dependent or –independent, and may be influenced by cellular context and availability of cofactors. In the present study, we performed a comprehensive structure-function analysis and examined the potential of HOX11 mutants to regulate transcription. Both transcriptional repression and activation were demonstrated that involved overlapping regions of the HOX11 protein near the NH2- and COOH-termini and within the homeodomain. We concurrently investigated which domains of HOX11 are critical for arrest of hematopoietic cell differentiation and immortalization of BM progenitors. We show that, although HOX11 can act as a potent transcriptional repressor via indirect mechanisms involving protein-protein interactions, transforming activity is consistent with dysregulated expression of HOX11 target genes mediated by the binding of the homeodomain to DNA in a sequence-specific manner.
Materials and methods
Oligonucleotide-directed mutagenesis of HOX11 and construction of expression plasmids
The HOX11 expression plasmid (pcDNA3-HOX11) was constructed by insertion of an EcoRI/XhoI fragment from pMSCV-HOX11 containing a full-length HOX11 cDNA9 into the pcDNA3 expression vector (Invitrogen, Carlsbad, CA). FLAG-tagged wild-type (WT) and mutant HOX11 proteins were generated by subcloning polymerase chain reaction (PCR)–amplified fragments, corresponding to the following HOX11 regions in frame with the sequence encoding a FLAG peptide into EcoRI/EcoRV restriction sites of pcDNA3: HOX11 D1 (amino acids 51-330), HOX11 D2 (amino acids 98-330), HOX11 D3 (amino acids 120-330), HOX11 D4 (amino acids 164-330), HOX11 D5 (amino acids 201-330), HOX11 D6 (amino acids 2-260), and HOX11 D7 (amino acids 2-197). Mutations in the PBX binding site (FPWME; amino acids 199-203) of HOX11 (M1, FPWIE; M2, FAWME; and M5, NGSSR) or internal deletions of HOX11 (M3, M4, H3d, and HD) were generated using a PCR method as described previously.21 HOX11 M3 has an internal deletion from amino acid 150 to amino acid 164, HOX11 M4 has an internal deletion from amino acid 160 to amino acid 200, HOX11 H3d has an internal deletion from amino acid 246 to amino acid 251, and HOX11 HD has an internal deletion from amino acid 211 to amino acid 260. HOX11 Thr47Ile, HOX11 Asn51Ala, and HOX11 Lys55Gln were generated using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). All constructs were confirmed by DNA sequencing. The plasmid expressing S-tagged HOX11 was generated by subcloning a SmaI/XhoI fragment containing HOX11 cDNA from pMSCV-HOX11 into blunted EcoRI/XhoI sites of pET-29a+ (Novagen, Madison, WI). Reporter plasmids used include pSV2CAT, pCMVCAT, pBLCAT2, and pneuCAT, containing the simian virus 40 (SV40) early, cytomegalovirus (CMV), herpes simplex thymidine kinase (HSV tk), and rat neu gene promoters,22 respectively, driving chloramphenicol acetyltransferase (CAT) gene expression, and pCMV-β-gal expressing β-galactosidase. Mutants of pSV2CAT were generated by making deletions using convenient restriction sites: pSV2ΔEn1 was generated by deleting an SphI/SphI fragment, pSV2ΔEn2 was generated by deleting the SphI/AccI fragment, pSV2ΔGC was generated by deleting the SphI/NcoI fragment, and pSV2ΔTATA was generated by deleting the StuI/NcoI fragment.
CAT and β-galactosidase assays
Cells were seeded in 100-mm dishes 16 hours prior to transfection. Duplicate dishes for each construct were set up to test CAT activity and protein expression level. A modified calcium phosphate precipitation method was used for transfection as described previously.23 Unless otherwise stated, cells were transfected with 1 μg CMVβ-gal, 8 μg pcDNA3-HOX11, and 2 μg CAT reporter plasmid DNA. In concentration-dependent assays, the total amount of DNA in each transfection was kept constant by the addition of an appropriate amount of the pcDNA3 plasmid. Thirty-six hours after transfection, cells were harvested, washed, and lysed by the freeze/thaw cycle method. One fifth of cell lysate was used for β-galactosidase (β-gal) assays using ONPG (O-nitrophenyl-β-D-galactopyranosidase) as a substrate; the results were used to adjust the amount of lysate for the CAT assay. Thin-layer chromatography CAT assays were performed as previously described, except that 1-deoxy (dichloroacetyl-1-14C) chloramphenicol (Amersham, Piscataway, NJ) was used instead of standard chloramphenicol. Because only one acetyl group could be transferred to this substrate, a single product was seen. Repression assays using the pCMV-β-gal reporter plasmid were carried out as earlier, and β-galactosidase activity was determined using a commercial assay kit (Gene Therapy Systems, San Diego, CA) according to the manufacturer's instructions.
In vitro transcription assays
In vitro transcription reactions (25 μL) were carried out as described previously.24 Briefly, 300 ng plasmid DNA template pML(C2AT)-50 containing a G-less cassette downstream of adenovirus major later promoter (–50 to +10) was used for the transcription assays. The RNA polymerase II holoenzyme used in the in vitro transcription assay was purified by affinity chromatography.25 The protein-affinity column was prepared by immobilizing GST-TFIIS fusion protein on glutathione-Sepharose 4B beads. After washing with 0.05 M affinity chromatography buffer (ACB), the column was loaded with HeLa whole-cell extract. Bound proteins were eluted with 0.3 M ACB. Eluates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and the presence of components of the RNA polymerase II holoenzyme was verified by Western blotting and silver staining. Purified RNA polymerase II holoenzyme (5 μg) was added to each reaction. S-tagged HOX11 proteins were purified from Escherichia coli cell lysates using S-Tag purification kit (Novagen). Purified HOX11 (100 ng) was added to each reaction. pET control proteins from pET-29a+ transformed E coli were prepared in the same way as S-tagged HOX11.
Immunoprecipitation and Western blotting
For the detection of WT and mutant FLAG-tagged HOX11 proteins, transiently transfected cells were harvested and lysed in 1% NP-40 lysis buffer 36 hours after transfection.26 After centrifugation, cleared cell lysates were incubated with 2 μg anti–FLAG-M5 antibody (Kodak/Sigma-Aldrich, St Louis, MO) overnight at 4°C. Anti-mouse immunoglobulin G (IgG) agarose was added, and lysates were rotated for 1 hour at 4 °C. Immunoprecipitates were separated by 10% SDS-PAGE. Proteins were transferred to polyvinylidene diflouride (PVDF) membranes, immunoblotted with the anti–FLAG-M5 antibody, and detected using the enhanced chemiluminescence (ECL) technique (Amersham). For detection of HOX11 proteins in stably transduced NIH3T3 cells and BM cultures, cells were lysed in RIPA buffer for 30 minutes on ice, and 20 μg whole-cell lysates were separated by 12% SDS-PAGE, transferred to PVDF membranes, and probed with either an anti-HOX11 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or anti–FLAG-M5 antibody. Blots were developed using the ECL kit and analyzed on a Storm 860 instrument (Molecular Dynamics, Piscataway, NJ) equipped with ImageQuant software.
Nucleic acid analysis
Northern analysis for expression of Aldh-1, HOX11, and β-actin RNA transcripts was carried out according to standard procedures.27 Briefly, 20 μg total RNA was separated on a 1% agarose-formaldehyde gel and transferred to nylon membranes. Probes used were a 1.8-kilobase (kb) Aldh-1 fragment from pBSK/ahd-2 (kindly provided by K. Bunting, Hematopoiesis Department, American Red Cross, Rockville, MD), an EcoRI/NotI fragment from pMSCV-HOX11, and a β-actin PCR product. Southern blot analysis was performed as previously described.28 Genomic DNA (10 μg) from each sample was digested with EcoRI, separated on 1% agarose gels, and transferred to nylon membranes. Blots were hybridized with a neo probe isolated as a BamHI/StuI fragment from pMSCV. Northern and Southern blots were exposed to phosphor screens and analyzed on a Storm 860 instrument using ImageQuant software.
Generation of stably transduced retroviral producer and NIH3T3 cell lines
HOX11 mutant constructs expressed in the MSCV retroviral vector were used to generate stably transduced GP+E-86 retroviral producers and NIH3T3 cells. Briefly, human embryonic kidney (HEK) 293T cells (4 × 106/100-mm dish) were transiently transfected with each MSCV-HOX11 construct (10 μg), the pEQPAM3-E gag-pol packaging plasmid (10 μg), and a vesicular stomatitis virus G protein envelope plasmid (6.7 μg) using the calcium phosphate precipitation technique.28 Supernatants were harvested from transfected cells 48 hours later and filtered through 0.45-μm membranes. Retroviral supernatants routinely had titers of more than 107 vector particles/mL. Supernatants were added to GP+E-86 or NIH3T3 cultures, and transduced cells were selected in 400 μg/mL G418 (Invitrogen).
In vitro hematopoietic progenitor immortalization assay
BM cells were harvested from 6- to 8-week-old female BALB/c mice (National Cancer Institute, Frederick, MD) following 4-day 5-fluorouracil treatment. BM cells (2 × 106 cells/mL) were prestimulated for 48 hours in Iscoves modified Dulbecco medium containing 10% heat-inactivated fetal bovine serum (FBS), and 10% conditioned media from X630-rIL-3 (interleukin 3, IL-3), CHO-KLS (stem cell factor), and Sp2/IL-6 (IL-6) cells. Following 48 hours of prestimulation, BM cells were cocultured with irradiated (1500 rad) GP+E-86 producer cell lines containing the complete series of MSCV-HOX11 mutants or the MSCV control vector only in the presence of 6 μg/mL polybrene and cytokines. After 48 hours of transduction, BM cells were harvested from coculture, maintained in Iscove modified Dulbecco medium containing 10% FBS and 10% IL-3–conditioned media, and selected in 0.75 μg/mL G418. BM cultures were maintained until immortalized cell lines could be established, typically for more than 3 months.9
Flow cytometry
Immortalized BM cell lines were immunophenotyped as previously described.9 Cells (5 × 105) were incubated with 200 μL supernatant from the 2.4G2 hybridoma to block Fc receptor binding. Surface expression of the following markers was analyzed with fluorescein isothiocyanate (FITC)–or R-phycoerythrin (PE)–conjugated antibodies: CD18-FITC, CD24-PE, CD44-PE, CD45-FITC, Mac-1–PE (CD11b), Gr-1–FITC (all from BD PharMingen, San Diego, CA). Flow cytometry data were acquired on a BD LSR instrument and analyzed using CellQuest software.
Immunohistochemistry
Transiently transfected HEK 293T cells were cultured on glass coverslips coated with fibronectin. Cultured cells were washed with phosphate-buffered saline (PBS) + 1% bovine serum albumin (BSA) and fixed in 3.7% formaldehyde in PBS for 5 minutes. After washing with PBS, cells were treated with 0.5% Triton X-100 for 5 minutes. Cells were blocked with 10% normal blocking serum (NBS; Santa Cruz Biotechnology), and incubated with anti-HOX11 polyclonal antibody (10 μg/mL in 1.5% NBS). After washing in PBS (1 × 10 minutes and 2 × 5 minutes) coverslips were incubated with FITC-conjugated goat antirabbit polyclonal antibody (Santa Cruz Biotechnology; 5 μg/mL in 1.5% NBS). All blocks and antibody incubations were carried out at 37°C for 1 hour. Coverslips were mounted onto glass slides with FluorSave (Calbiochem, San Diego, CA) and visualized using a × 100 oil immersion lens on a fluorescence microscope (Nikon, Melville, NY).
Results
HOX11 represses transcription from multiple promoters
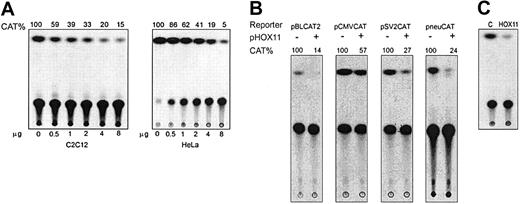
HOX11-dependent transcriptional repression was first detected by cotransfection of a HOX11 expression plasmid (pcDNA3-HOX11) and a reporter plasmid (pSV2CAT) into C2C12 and HeLa cells. Cotransfection of pcDNA3-HOX11 and pSV2CAT into these cell lines resulted in a concentration-dependent repression of the SV40 early promoter, with CAT activity reduced to 15% and 5% of control in C2C12 and HeLa cells, respectively (Figure 1A). To determine whether HOX11 suppressed transcription from other natural promoters, we tested additional reporter plasmids in C2C12 cells. We found that HOX11 also inhibited transcription from 2 other TATA-containing promoters, the HSV tk (pBLCAT2) and CMV promoters, and a TATA-less promoter from the rat neu gene (Figure 1B). HOX11 was previously reported to activate gene expression in yeast and in mammalian (C3H10T1/2, NIH3T3, COS7) cell lines by various mechanisms.13,14,17 Therefore, we examined whether HOX11-dependent transcriptional repression was specific to the cells tested or may also occur in the cell lines used previously. The pcDNA3-HOX11 and pSV2CAT plasmids were cotransfected into NIH3T3 cells, and significant repression of the SV40 early promoter was observed (Figure 1C), demonstrating that HOX11 is a potent repressor of transcription from a wide range of natural promoters in cells of human and murine origin.
HOX11 represses basal transcription of TATA-containing and TATA-less promoters in HeLa cells and C2C12 cells. (A) Transcriptional repression by HOX11 is concentration dependent. Transient transfection and CAT assays were performed in C2C12 cells and HeLa cells using pSV2CAT (2 μg) and indicated amounts of pcDNA3-HOX11. Relative CAT activity, expressed as percentage of control, is indicated. Data shown are representative of 3 independent experiments. (B) HOX11 represses transcription from different promoters. pcDNA3-HOX11 (8 μg) and reporter plasmids (2 μg) containing HSV tk (pBLCAT2), CMV (pCMVCAT), SV40 early, and rat neu (pneuCAT) promoters were cotransfected into C2C12 cells. CAT activity expressed as percentage of control. (C) HOX11 represses SV40 promoter activity in murine cells. pcDNA3-HOX11 (8 μg) and pSV2CAT (2 μg) were cotransfected into NIH3T3 cells and CAT activity measured.
HOX11 represses basal transcription of TATA-containing and TATA-less promoters in HeLa cells and C2C12 cells. (A) Transcriptional repression by HOX11 is concentration dependent. Transient transfection and CAT assays were performed in C2C12 cells and HeLa cells using pSV2CAT (2 μg) and indicated amounts of pcDNA3-HOX11. Relative CAT activity, expressed as percentage of control, is indicated. Data shown are representative of 3 independent experiments. (B) HOX11 represses transcription from different promoters. pcDNA3-HOX11 (8 μg) and reporter plasmids (2 μg) containing HSV tk (pBLCAT2), CMV (pCMVCAT), SV40 early, and rat neu (pneuCAT) promoters were cotransfected into C2C12 cells. CAT activity expressed as percentage of control. (C) HOX11 represses SV40 promoter activity in murine cells. pcDNA3-HOX11 (8 μg) and pSV2CAT (2 μg) were cotransfected into NIH3T3 cells and CAT activity measured.
Repressor function of HOX11 is mediated by interaction with the basal transcriptional machinery
To further investigate the mechanisms by which HOX11 represses transcription, we examined whether cis-regulatory elements in the SV40 early promoter contribute to HOX11-induced repression. The SV40 early promoter is composed of 2 copies of an enhancer sequence, 6 copies of a GC-box sequence, and one TATA-like sequence. A series of deletions within each of these domains was generated (Figure 2A). Mutant constructs were cotransfected with pFLAG-HOX11 WT into HeLa and C2C12 cells, and CAT activities were determined (Figure 2B). Deletion of any one of the 3 regions from the SV40 early promoter decreased basal transcriptional activity but failed to abolish HOX11-mediated transcriptional repression, suggesting that repression was unlikely to be due to interference with specific transcription factors bound to cis-regulatory elements within the promoter. Rather, the observations were most likely explained by interaction of HOX11 with components of the basal transcriptional machinery. To address this issue, we performed an in vitro transcription assay in which GST-TFIIS affinity-purified RNA polymerase II holoenzyme from HeLa cells and S-tagged HOX11 recombinant proteins were used. We have previously shown that the RNA polymerase holoenzyme purified by GST-TFIIS affinity chromatography contains RNA polymerase II and associated general transcription factors.25,29 In the presence of the holoenzyme, initiation of basal transcription from the adenovirus major late promoter was observed, and transcription could be activated by addition of Gal4-VP16 (Figure 2C, lanes 1 and 5). Addition of S-tagged HOX11 repressed basal transcription from the template DNA (Figure 2C, lanes 3-4), but it failed to reduce transcription activated by Gal4-VP16 (Figure 2C, lane 6). Because the adenovirus major late promoter does not contain any cognate DNA binding sites for HOX11, these results indicated that HOX11 exerts its repressor function by interacting with one or more components of the RNA polymerase II holoenzyme.
HOX11-dependent repression may be mediated by interactions with components of the basal general transcription machinery. (A) Schematic diagram of the SV40 promoter in control and mutant reporter plasmids: enhancer region (En); GC BOX (GC); TATA-like motif (TATA). (B) Deletion (Δ) of cis-regulatory elements, including the enhancer, GC box, and TATA-like motif from the SV40 promoter, failed to abolish HOX11-mediated repression. Relative CAT activities from cotransfection of pFLAG-HOX11 and parental or mutant SV40 reporter plasmids into C2C12 cells (▪) and HeLa cells (□). Transfection assays were repeated at least 3 times. Variability between assays was not more than 15%. (C) HOX11 represses in vitro transcription driven by purified RNA polymerase II holoenzyme. In vitro transcription assays were performed using 0.3 μg reporter plasmid containing the adenovirus major late promoter driving a G-less cassette (G-less) and 5 tandem copies of the Gal4 DNA-binding site.21 Reaction conditions consisted of 5 μg purified polymerase II holoenzyme and 100 ng purified S-tagged HOX11 or PET control protein. Transcription was measured in the absence or presence of purified Gal4-VP16 proteins (50 ng). Radiolabeled RNA was resolved on a 4.5% acrylamide-6M urea denaturing gel and visualized by autoradiography.
HOX11-dependent repression may be mediated by interactions with components of the basal general transcription machinery. (A) Schematic diagram of the SV40 promoter in control and mutant reporter plasmids: enhancer region (En); GC BOX (GC); TATA-like motif (TATA). (B) Deletion (Δ) of cis-regulatory elements, including the enhancer, GC box, and TATA-like motif from the SV40 promoter, failed to abolish HOX11-mediated repression. Relative CAT activities from cotransfection of pFLAG-HOX11 and parental or mutant SV40 reporter plasmids into C2C12 cells (▪) and HeLa cells (□). Transfection assays were repeated at least 3 times. Variability between assays was not more than 15%. (C) HOX11 represses in vitro transcription driven by purified RNA polymerase II holoenzyme. In vitro transcription assays were performed using 0.3 μg reporter plasmid containing the adenovirus major late promoter driving a G-less cassette (G-less) and 5 tandem copies of the Gal4 DNA-binding site.21 Reaction conditions consisted of 5 μg purified polymerase II holoenzyme and 100 ng purified S-tagged HOX11 or PET control protein. Transcription was measured in the absence or presence of purified Gal4-VP16 proteins (50 ng). Radiolabeled RNA was resolved on a 4.5% acrylamide-6M urea denaturing gel and visualized by autoradiography.
Repression of basal transcription requires multiple domains of HOX11
HOX11 contains structural domains with previously characterized functional activities (eg, those involved in transactivation, PBX interaction, and DNA binding). To identify specific structural features critical for transcriptional repression, a series of FLAG-tagged HOX11 mutant constructs were generated (Figure 3A). Comparable expression levels of FLAG-HOX11 proteins were detected for most constructs with the exception of HOX11 D4 and HOX11 D5 (Figure 3B). The ability of HOX11 mutant proteins to repress reporter gene expression from the SV40 early promoter (as measured by normalized CAT and β-gal activities) was investigated (Figure 4A-B). Compared with the empty vector, HOX11 WT resulted in more than a 20-fold reduction in reporter activity in HeLa cells, comparable with initial observations (Figures 4A and 1A). Analysis of HOX11 mutants revealed that multiple regions of HOX11 contributed to its repressor function. Removal of the first 50 amino acids of HOX11 had no significant effect on repression (D1 mutant). However, further truncation of the Gly/Pro domain (mutants D2-D4) resulted in a gradual reduction in repression, which was abolished when the entire Gly/Pro domain was removed (D5 mutant). Because protein expression levels of HOX11 D4 and HOX11 D5 were lower than that of HOX11 WT in transient transfections (Figure 3B), lower repression levels may be a result of poor protein expression in these instances. Deletion of amino acids 160 to 200 of the NH2-terminus region (M4 mutant), resulted in a 5-fold reduction in repression, suggesting this domain is required for efficient repression.
HOX11 constructs and protein expression. (A) Schematic diagram of expression constructs for FLAG-tagged HOX11 WT and mutant proteins. FLAG peptide, Gly/Pro-rich region (Gly/Pro), homeodomain (HD), Gln-rich region (Gln), and PBX interaction motif (PIM; FPWME) are indicated. (B) FLAG-tagged HOX11 proteins were detected in lysates from HeLa cells transiently transfected with pFLAG-HOX11 constructs using immunoprecipitation and Western blotting with an anti–FLAG-M5 antibody (top row, middle row) or by direct Western blotting of lysates from stably transduced NIH3T3 cells using an anti-HOX11 antibody (bottom row). C indicates control.
HOX11 constructs and protein expression. (A) Schematic diagram of expression constructs for FLAG-tagged HOX11 WT and mutant proteins. FLAG peptide, Gly/Pro-rich region (Gly/Pro), homeodomain (HD), Gln-rich region (Gln), and PBX interaction motif (PIM; FPWME) are indicated. (B) FLAG-tagged HOX11 proteins were detected in lysates from HeLa cells transiently transfected with pFLAG-HOX11 constructs using immunoprecipitation and Western blotting with an anti–FLAG-M5 antibody (top row, middle row) or by direct Western blotting of lysates from stably transduced NIH3T3 cells using an anti-HOX11 antibody (bottom row). C indicates control.
Multiple domains of HOX11 contribute to HOX11-mediated transcriptional regulation. (A) CAT assays were performed to evaluate the contributions of each domain to HOX11 repressor activity. Transient transfections were performed in HeLa cells using pcDNA3-HOX11 (8 μg) and pSV2CAT (2 μg). Thirty-six hours after transfection, CAT activities were determined and normalized to protein expression. The data represent mean values from 3 independent experiments. (B) Point mutations within the homeodomain do not inhibit HOX11 repressor function. HOX11 constructs with point mutations within helix 3 of the homeodomain (Thr47Ile and Lys55Gln) were transiently transfected into HEK 293T cells with a CMV-β-gal reporter plasmid. Data represent 3 independent experiments. (C) Specific HOX11 domains and DNA binding are required for induction of endogenous gene expression. Full-length HOX11 (HOX11) or FLAG-tagged HOX11 (HOX11 WT) and mutant constructs were stably transduced into NIH3T3 cells, and up-regulation of Aldh-1 was measured by Northern blot analysis. β-Actin mRNA served as an internal loading control.
Multiple domains of HOX11 contribute to HOX11-mediated transcriptional regulation. (A) CAT assays were performed to evaluate the contributions of each domain to HOX11 repressor activity. Transient transfections were performed in HeLa cells using pcDNA3-HOX11 (8 μg) and pSV2CAT (2 μg). Thirty-six hours after transfection, CAT activities were determined and normalized to protein expression. The data represent mean values from 3 independent experiments. (B) Point mutations within the homeodomain do not inhibit HOX11 repressor function. HOX11 constructs with point mutations within helix 3 of the homeodomain (Thr47Ile and Lys55Gln) were transiently transfected into HEK 293T cells with a CMV-β-gal reporter plasmid. Data represent 3 independent experiments. (C) Specific HOX11 domains and DNA binding are required for induction of endogenous gene expression. Full-length HOX11 (HOX11) or FLAG-tagged HOX11 (HOX11 WT) and mutant constructs were stably transduced into NIH3T3 cells, and up-regulation of Aldh-1 was measured by Northern blot analysis. β-Actin mRNA served as an internal loading control.
Because the NH2-terminus contains several sites for potential protein-protein interactions, specific mutations were introduced into this region of the HOX11 protein. Three point mutants (M1, M2, and M5) were generated within the PBX interaction motif FPWME (amino acids 174-178). Neither the M1 mutation, which has been shown previously to disrupt in vitro PBX interactions,12 nor the M2 mutation altered repression activities (Figure 4A). HOX11 M5 also repressed transcription to similar levels as HOX11 WT protein (Figure 4B). These results indicate that interaction with PBX proteins is unlikely to be required for repressor activity. Another potential interaction domain consisting of 3 copies of a PXXP motif, reported previously as the minimal consensus site for Src homology region 3 (SH3)–mediated protein-protein interactions,30 was identified in the NH2-terminus.31 Deletion of these sequences (amino acids 155-166; M3 mutant) had no effect on repression activity. In contrast, the Gln-rich domain near the COOH-terminus was shown to possess modest repressor function, because deletion of this region (D6 mutant) caused a 5-fold reduction in repression (Figure 4A).
Several homeodomain mutants (H3d, HD, D7, Thr47Ile, Asn51Ala, and Lys55Gln) were evaluated next. Deletions of the homeodomain completely abrogated HOX11-mediated repression (Figure 4A; mutants D7 and HD). Although deletion of the third helix of the homeodomain (H3d mutant), which is essential for DNA binding,3,15 abolished HOX11-mediated repressor function (Figure 4A-B), the HOX11 Thr47Ile and HOX11 Lys55Gln mutants containing point mutations within helix 3 were capable of inhibiting transcription as efficiently as HOX11 WT (20% and 13% of control, respectively; Figure 4B). Similarly, the HOX11 Asn51Ala mutant was capable of repression to equivalent levels as HOX11 WT (data not shown). These latter results demonstrate that, although a structurally intact homeodomain is essential for repression, alteration of the DNA recognition surface (ie, DNA binding specificity) had no significant effect on repressor activity.
Specific functional domains are required for HOX11-dependent transcriptional activation of Aldh-1
We next investigated the ability of HOX11 mutant constructs to activate expression of a known endogenous HOX11 target gene, Aldh-1. Northern blot analysis using total cellular RNA isolated from stably transduced NIH3T3 cells indicated that, in agreement with a prior report,16 HOX11 up-regulates Aldh-1 in this cellular context. The FLAG epitope did not inhibit HOX11 function (Figure 4C, compare native HOX11 and FLAG-tagged HOX11 WT). Deletion of the Gly/Pro transactivation domain significantly abrogated the up-regulation of Aldh-1 gene expression (D1 mutant) as did deletion of the COOH-terminus transactivation domain (D6 mutant) or the homeodomain (HD and H3d mutants). However, disruption of the PBX interaction motif (M1, M2, and M5 mutants) did not affect HOX11 transcriptional activation nor did deletion of the PXXP motif (M3 mutant). To further determine the role of specific DNA binding in the up-regulation of Aldh-1, several homeodomain point mutants were tested. Surprisingly, the Thr47Ile mutation, which changes the in vitro HOX11 DNA target sequence from TAAC/TAAT to TAAT only,13 was unable to induce Aldh-1 gene expression (Figure 4C). Gene induction was restored on stable introduction of HOX11 WT into the same cells (Figure 4C), implying that up-regulation of Aldh-1 in NIH3T3 cells may depend on HOX11-dependent transcriptional regulatory mechanisms involving “TAAC-type” DNA target sequences. Mutation of the invariant asparagine residue at position 51 of the homeodomain (Asn51Ala mutant), which is absolutely required for DNA binding,32 also abrogated Aldh-1 up-regulation (Figure 4C).33 Finally, the HOX11 Lys55Gln mutant was unable to up-regulate Aldh-1 gene expression (Figure 4C). The mutated residue corresponds to Lys55 in helix 3 of the homeodomain, which forms a salt bridge between the protein and the phosphate groups of DNA.34 Lys55 has also been shown to be part of an interaction region through which many homeodomain proteins associate with and repress the activity of CBP/p300 coregulators.19 Although lack of transcriptional activation of Aldh-1 by HOX11 Lys55Gln because of abrogation of CBP/p300 binding remains a possibility, repeated attempts to coimmunoprecipitate CBP with HOX11 were unsuccessful. Consequently, it is likely that DNA-binding interactions are also disrupted by the Lys255Gln mutation.
COOH-terminal domain of HOX11 is dispensable for immortalization but essential for early block of hematopoietic cell differentiation
We have previously shown that retroviral gene transfer of HOX11 into murine hematopoietic progenitors efficiently gives rise to IL-3–dependent cell lines having an immature myeloid phenotype.9,10 Although these cell lines are not malignant, immortalization involves pathways contributing to oncogenic conversion and, therefore, serves as a useful surrogate assay for fundamental aspects of neoplastic transformation. A priori, HOX11-mediated transcriptional activation and/or repression of downstream targets may negatively regulate hematopoietic differentiation programs, promoting progenitor cell immortalization and development of leukemia. Therefore, to gain insight into the functional domains and molecular events that are responsible for initiation of leukemo-genesis, BM progenitors from 4-day 5-fluorouracil–pretreated mice were transduced with each of the HOX11 mutants (with the exception of HOX11 M4) expressed in the MSCV retroviral vector. STable vector expression was obtained by selection for G418 resistance, and the cultures were supplemented with IL-3 until permanent factor–dependent cell lines were established or only slowly dividing differentiated mast cells remained.9,10
Seven HOX11 mutants retained immortalizing function in several independent experiments (Table 1). HOX11 proteins of expected size were detected in each case (Figure 5D). As reported previously,10 deletion of the first 50 amino acids (D1 mutant) important for transcriptional up-regulation of Aldh-1 did not abrogate the immortalizing potential of HOX11, but further deletion of the NH2-terminus indicated that the Gly/Pro-rich transactivation domain was required for efficient differentiation block. Morphologic (Figure 5A) and immunophenotypic (Table 1) features of HOX11 WT and HOX11 D1 cells were comparable to those previously described.9,10 In contrast to these findings, deletion of the entire COOH-terminus (D6 mutant) resulted in maturation arrest at a later stage of myeloid differentiation. Morphologically, HOX11 D6 cell lines had distinctly ruffled membranes (Figure 5B), and immunophenotyping revealed that the cells expressed high levels of the monocytic marker Mac-1 (Table 1). We speculated that the difference in phenotype may be due to differences in copy number and thus levels of gene expression. However, Southern blot analysis performed on 3 independently derived HOX11 WT and HOX11 D6 cell lines revealed 2 to 3 proviral copies per line (Figure 5E). Both HOX11 WT and HOX11 D6 cell lines were thus similarly oligoclonal in nature, suggesting that the phenotypic differences between these lines are due to deletion of the COOH-terminal transactivation domain of HOX11. The biologic response of 3 independently derived HOX11 WT and HOX11 D6 lines to phorbol myristate acetate, a chemical agent that induces differentiation of a variety of hematopoietic progenitor cell lines, was subsequently investigated. Although HOX11 WT cells could be induced to express both monocytic and granulocytic differentiation markers (Gr-1 and Mac-1, respectively), HOX11 D6 cell lines did not exhibit any increase in either marker (Figure 5F). The combined data indicate that in the BM progenitor immortalization assay HOX11 WT blocks hematopoietic differentiation at the bipotential monocyte-granulocyte precursor stage, whereas the HOX11 D6 mutant immortalizes more differentiated monocytic cells.
Surface immunophenotype of HOX11-immortalized cell lines
. | HOX11 mutant, % positive . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker . | WT . | D1 . | D6 . | M1 . | M2 . | M3 . | Thr47Ile . | ||||||
| CD18 | 99 | 96 | 100 | 95 | 98 | 100 | 99 | ||||||
| CD24 | 100 | 100 | 100 | 96 | 100 | 100 | 98 | ||||||
| CD45 | 92 | 100 | 66 | 81 | 87 | 80 | 85 | ||||||
| CD44 | 100 | 86 | 100 | 99 | 100 | 100 | 94 | ||||||
| Gr-1 | 2 | 2 | 3 | 5 | 0 | 0 | 2 | ||||||
| Mac-1 | 2 | 1 | 100 | 16 | 1 | 1 | 14 | ||||||
. | HOX11 mutant, % positive . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker . | WT . | D1 . | D6 . | M1 . | M2 . | M3 . | Thr47Ile . | ||||||
| CD18 | 99 | 96 | 100 | 95 | 98 | 100 | 99 | ||||||
| CD24 | 100 | 100 | 100 | 96 | 100 | 100 | 98 | ||||||
| CD45 | 92 | 100 | 66 | 81 | 87 | 80 | 85 | ||||||
| CD44 | 100 | 86 | 100 | 99 | 100 | 100 | 94 | ||||||
| Gr-1 | 2 | 2 | 3 | 5 | 0 | 0 | 2 | ||||||
| Mac-1 | 2 | 1 | 100 | 16 | 1 | 1 | 14 | ||||||
Representative cell lines.
Establishment and characterization of HOX11-immortalized hematopoietic cell lines. HOX11 constructs were transduced into murine BM progenitors, and cells were maintained in IL-3–containing media until lines were established (> 3 months in culture). Transduction with each construct was performed at least 3 times, with the exception of D1 and M5 (n = 2). Wright-Giemsa–stained cells generated with HOX11 WT (A), D6 (B), and Thr47Ile (C). Original magnification, × 100. (D) Western blot analysis of HOX11 protein expression in representative BM progenitor-derived cell lines using either anti-HOX11 polyclonal antibody (WT, D1, M1, M2, M3, and Thr47Ile mutants) or anti-FLAG antibody (D6 mutant). (E) Southern blot analysis of proviral copy number in 3 independently derived HOX11 WT and HOX11 D6 cell lines. Genomic DNA (10 μg), isolated from 107 cells and digested with EcoRI, was resolved on a 1% agarose gel and hybridized with a neo probe. (F) HOX11 WT cells represent a bipotential monocytic-granulocytic precursor. Three HOX11 WT and HOX11 D6 cell lines were treated with phorbol myristate acetate (PMA; 100 ng/mL). After 72 hours, surface expression of the granulocytic marker Gr-1 and the macrophage marker Mac-1 was determined by immunofluorescence flow cytometric analysis.
Establishment and characterization of HOX11-immortalized hematopoietic cell lines. HOX11 constructs were transduced into murine BM progenitors, and cells were maintained in IL-3–containing media until lines were established (> 3 months in culture). Transduction with each construct was performed at least 3 times, with the exception of D1 and M5 (n = 2). Wright-Giemsa–stained cells generated with HOX11 WT (A), D6 (B), and Thr47Ile (C). Original magnification, × 100. (D) Western blot analysis of HOX11 protein expression in representative BM progenitor-derived cell lines using either anti-HOX11 polyclonal antibody (WT, D1, M1, M2, M3, and Thr47Ile mutants) or anti-FLAG antibody (D6 mutant). (E) Southern blot analysis of proviral copy number in 3 independently derived HOX11 WT and HOX11 D6 cell lines. Genomic DNA (10 μg), isolated from 107 cells and digested with EcoRI, was resolved on a 1% agarose gel and hybridized with a neo probe. (F) HOX11 WT cells represent a bipotential monocytic-granulocytic precursor. Three HOX11 WT and HOX11 D6 cell lines were treated with phorbol myristate acetate (PMA; 100 ng/mL). After 72 hours, surface expression of the granulocytic marker Gr-1 and the macrophage marker Mac-1 was determined by immunofluorescence flow cytometric analysis.
HOX11 PBX interaction motif but not the PXXP sequence is required for immortalization
Several mutations within the PIM were tested in the BM progenitor immortalization assay. We previously documented that HOX11 M1 does not interact with purified PBX proteins in electrophoretic mobility shift assays (EMSAs).12 However, it remains uncertain how closely the in vitro HOX11-PBX interactions and cooperative DNA-binding specificity observed in EMSAs mimic the in vivo situation on natural DNA targets in the presence of additional protein cofactors. Because the HOX11 M1 and HOX11 M2 mutants retain the tryptophan or the tryptophan and methionine residues of the PIM motif that other studies have indicated are critical for formation of some HOX-PBX complexes,35 we generated mutant HOX11 M5 in which the FPWME PIM motif was completely eliminated (modified to NGSSR). Notably, although both HOX11 M1 and HOX11 M2 mediated transcriptional repression, activated Aldh-1 expression, and immortalized BM progenitors as efficiently as HOX11 WT (Table 1 and data not shown), HOX11 M5 failed to induce immortalization (n = 2) even though it was still fully capable of repressing basal transcription and up-regulating Aldh-1 expression. These data distinguish the pathways operating in progenitor cell immortalization from those involved in the other processes. The findings are congruent with observations obtained with the HoxB8 homeodomain protein, in which a similar requirement for the PIM in myeloid progenitor cell arrest was reported.36 Whereas the immortalizing potential of HOX11 appeared to depend on an interaction with PBX proteins, association with other proteins via the putative SH3-interacting motif PXXP was not essential as the HOX11 M3 mutant minus this motif was capable of readily generating myeloid progenitor lines (Table 1).
Homeodomain is essential for immortalization
On the basis of its structural characteristics, HOX11 likely functions primarily as a DNA-binding transcription factor, but DNA binding-independent actions had previously been suggested by the observation that HOX11 interacts with the cell cycle proteins PP2A/PP1 in the absence of the homeodomain.20 In the present study, neither HOX11 HD nor HOX11 H3d gave rise to cell lines. In contrast, expression of HOX11 Thr47Ile in hematopoietic progenitors resulted in cell lines with phenotype and morphology similar to HOX11 WT (Figure 5C and Table 1). This ability to immortalize hematopoietic progenitors contrasts with the inability of HOX11 Thr47Ile to up-regulate expression of Aldh-1 (Figure 4B), providing further evidence in support of the notion that the genetic mechanisms involved in HOX11-mediated up-regulation of Aldh-1 and BM progenitor immortalization are distinct. Finally, HOX11 Asn51Ala and HOX11 Lys55Gln failed to establish hematopoietic progenitor cell lines (n = 3) despite the ability to enter the nucleus of HEK 293T and NIH3T3 cells (Figure 6F and data not shown). Because the invariant asparagine residue at position 51 of the homeodomain has been shown to be indispensable for efficient DNA binding32,33 and, as indicated above, the lysine at position 55 of the homeodomain most probably forms a salt bridge with the phosphate backbone of the DNA binding site,34 we interpret the findings with the helix 3 point mutants to indicate that HOX11 induces hematopoietic progenitor immortalization by transcriptional dysregulation of specific target genes.
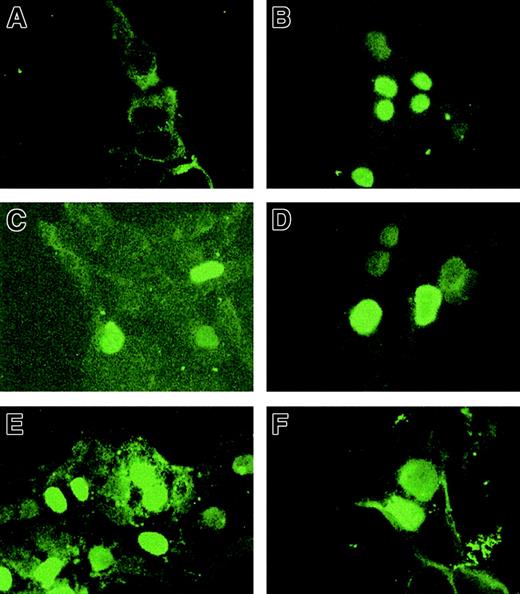
HOX11 mutants are expressed in nuclei of transfected cells. Transiently transfected HEK 293T cells were cultured on fibronectin-coated coverslips and fixed in 3.7% formaldehyde. Immunohistochemical analysis was performed using 10 μg/mL anti-HOX11 polyclonal antibody and 5 μg/mL fluorescein-linked goat–anti-rabbit secondary antibody and visualized using a fluorescence microscope. Original magnification, × 100. (A) pcDNA3 vector, (B) HOX11 WT, (C) HOX11 H3d, (D) HOX11 M5, (E) HOX11 Thr47Ile, (F) HOX11 Lys55Gln.
HOX11 mutants are expressed in nuclei of transfected cells. Transiently transfected HEK 293T cells were cultured on fibronectin-coated coverslips and fixed in 3.7% formaldehyde. Immunohistochemical analysis was performed using 10 μg/mL anti-HOX11 polyclonal antibody and 5 μg/mL fluorescein-linked goat–anti-rabbit secondary antibody and visualized using a fluorescence microscope. Original magnification, × 100. (A) pcDNA3 vector, (B) HOX11 WT, (C) HOX11 H3d, (D) HOX11 M5, (E) HOX11 Thr47Ile, (F) HOX11 Lys55Gln.
Discussion
Several previous studies have focused on the ability of HOX11 to activate transcription in yeast and in nonhematopoietic mammalian cells, based on the hypothesis that dysregulated gene expression of downstream targets is the initiating event in leukemia development.13,14,16,17 In this paper, we demonstrated that HOX11 is also capable of repressing transcription in a DNA binding-independent manner. We subsequently performed a comprehensive structure-function analysis and showed that the NH2- and COOH-terminal domains of HOX11 previously found to transactivate transcription and contribute to the up-regulated expression of Aldh-1 were likewise necessary for maximal transcriptional repression. Whereas previous in vitro transactivation studies used either Gal4-HOX11 fusion proteins or reporter genes driven by a minimal promoter linked to multiple tandem repeats of synthetic HOX11 target sequences, transcriptional repression was observed in this study with the full-length HOX11 protein and was detected in the context of a number of natural cellular and viral gene promoters. Additionally, transcriptional repression was demonstrated in several cell lines of both human and murine origin, suggesting that the repression phenomenon is not narrowly restricted to a particular cellular milieu.
A number of homeodomain proteins are known to possess both activation and repressor activities.37 For example, the pancreatic homeodomain-containing factor PDX1 represses or activates transcription, depending on the PBX isoform with which it associates.38 By comparison, our data indicate that HOX11 does not depend on PBX cofactors to inhibit promoter activity but does require an intact homeodomain. Thus, deletion of helix 3 abolished repressor function (HOX11 H3d). However, homeodomain point mutants previously shown to alter or abrogate DNA-binding activity were found to repress as effectively as HOX11 WT, suggesting that repression is mediated through protein-protein interactions rather than homeodomain binding to specific DNA sequences. Furthermore, there are no clear HOX11 binding sites in the SV40 early promoter or in the other promoters we tested and, in gel retardation assays with in vitro–translated HOX11 protein or nuclear lysates from transfected cells, no HOX11 DNA binding was detected (Y.-X.Z., unpublished results, 1998). A trivial explanation would be if the HOX11 H3d protein failed to enter the nucleus. Although examination of cytoplasmic and nuclear lysates prepared from transiently transfected HeLa cells has indicated that the H3d deletion impairs the nuclear localization of HOX11 somewhat (Y.-X.Z., unpublished results, 1998), the HOX11 H3d protein still accumulates in the nucleus (Figure 6C) in accord with k-NN predictions of subcellular distribution (70% probability of nuclear retention; PSORT, http://psort.nibb.ac.jp/).
Transcriptional repression by several other homeodomain proteins has been shown to require multiple domains, including the homeodomain, and to occur in the absence of DNA binding.39,40 In this context, transcriptional repressors are known to function by altering chromatin and nucleosomal structure,41 by competing with factors for the same binding site,42,43 by interacting with the transcriptional machinery,44 or by quenching, whereby the DNA-bound repressor directly interferes with the activity of an activator bound nearby.40,45-47 Because HOX11-dependent repression was not affected by deletion of different regions of the SV40 early promoter, HOX11 probably acts as a repressor by contacting components of the basal transcription machinery. Consistent with this notion, we found that purified S-tagged HOX11 was able to inhibit basal transcription in vitro directed by purified RNA polymerase II holoenzymes. At present, it is not clear which factors HOX11 interacts with, although it is tempting to draw parallels to the Msx-1 and Msx-2 homeodomain proteins. Both proteins repress transcription from TATA-containing and TATA-less promoters. In each case, maximum repression requires multiple protein domains and was shown to be dependent on interactions between the homeodomain and components (TBP, TFIIA and TFIIB; and TFIIF, respectively) of the basal transcription machinery.46
The extensive mutational analysis we conducted distinguishes HOX11 repressor activity from the mechanisms responsible for up-regulated expression of Aldh-1 and for hematopoietic progenitor immortalization, the latter modes of action both requiring DNA-binding activity. Furthermore, a distinction can be made between the transcriptional pathways resulting in induction of Aldh-1 expression in NIH3T3 cells and those responsible for the leukemogenic action of HOX11 (Table 2). Deletion of the first 50 amino acids of HOX11 resulted in significantly reduced Aldh-1 gene up-regulation but had little or no effect on immortalization. Furthermore, deletion of the Gln-rich domain near the COOH terminus, although required for robust Aldh-1 gene activation, was still compatible with hematopoietic cell line establishment. Of note, cells in the HOX11 D6 cultures were more differentiated than HOX11 WT cells, consistently exhibiting a monocytic phenotype based on cell surface expression of Mac-1. A possible explanation for this observation is that deletion of the COOH-terminus results in a less potent transcriptional effector, as indicated by our current studies and those of others.14,17 Dysregulation of target gene expression would therefore occur to a lesser degree, allowing “leaky” differentiation further along the monocytic pathway. Previously, we identified a spontaneous mutant of HOX11 in a BM transplantation model in which 40 amino acids at the carboxyl terminus were deleted.9,10 Permanent cell lines that were established from hematopoietic tissues of transplant recipients engrafted with BM precursors ectopically expressing the truncated HOX11 mutant displayed a phenotype intermediate between HOX11 WT and HOX11 D6 cells, providing corroborative evidence for this hypothesis.
Summary of activities of HOX11 mutants
Construct . | Mutation . | Repression* . | Aldh-1 up-regulation . | Immortalization† . |
|---|---|---|---|---|
| HOX11 WT | ++++ | + | + (6/6) | |
| HOX11 D1 | Δ1-50 | ++++ | ± | + (3/3) |
| HOX11 D2 | Δ1-97 | +++ | - | - (0/3) |
| HOX11 D3 | Δ1-119 | ++ | - | - (0/3) |
| HOX11 D4 | Δ1-163 | + | - | - (0/3) |
| HOX11 D5 | Δ1-300 | - | - | - (0/3) |
| HOX11 D6 | Δ261-330 | + | ± | + (4/4) |
| HOX11 D7 | Δ198-330 | - | - | - (0/3) |
| HOX11 M1 | Met202Ile | ++++ | + | + (3/3) |
| HOX11 M2 | Pro200Ala | ++++ | + | + (3/3) |
| HOX11 M3 | Δ150-164 | ++++ | + | + (3/3) |
| HOX11 M4 | Δ160-200 | ++ | ND | ND |
| HOX11 M5 | FPWME→NGSSR | ++++ | + | - (0/2) |
| HOX11 H3d | Δ246-251 | - | - | - (0/3) |
| HOX11 HD | Δ211-260 | - | - | - (0/3) |
| HOX11 Thr47Ile | Thr247Ile | ++++ | - | + (4/4) |
| HOX11 Asn51Ala | Asn251Ala | ++++ | - | - (0/3) |
| HOX11 Lys55Gln | Lys255Gln | +++ | - | - (0/3) |
Construct . | Mutation . | Repression* . | Aldh-1 up-regulation . | Immortalization† . |
|---|---|---|---|---|
| HOX11 WT | ++++ | + | + (6/6) | |
| HOX11 D1 | Δ1-50 | ++++ | ± | + (3/3) |
| HOX11 D2 | Δ1-97 | +++ | - | - (0/3) |
| HOX11 D3 | Δ1-119 | ++ | - | - (0/3) |
| HOX11 D4 | Δ1-163 | + | - | - (0/3) |
| HOX11 D5 | Δ1-300 | - | - | - (0/3) |
| HOX11 D6 | Δ261-330 | + | ± | + (4/4) |
| HOX11 D7 | Δ198-330 | - | - | - (0/3) |
| HOX11 M1 | Met202Ile | ++++ | + | + (3/3) |
| HOX11 M2 | Pro200Ala | ++++ | + | + (3/3) |
| HOX11 M3 | Δ150-164 | ++++ | + | + (3/3) |
| HOX11 M4 | Δ160-200 | ++ | ND | ND |
| HOX11 M5 | FPWME→NGSSR | ++++ | + | - (0/2) |
| HOX11 H3d | Δ246-251 | - | - | - (0/3) |
| HOX11 HD | Δ211-260 | - | - | - (0/3) |
| HOX11 Thr47Ile | Thr247Ile | ++++ | - | + (4/4) |
| HOX11 Asn51Ala | Asn251Ala | ++++ | - | - (0/3) |
| HOX11 Lys55Gln | Lys255Gln | +++ | - | - (0/3) |
ND indicates not done; +, effect observed; -, no effect observed; and ±, weak effect observed.
Relative repression levels.
Cell lines obtained/experiments performed.
Point mutations within the third helix of the homeodomain provided further support for a mechanism of transformation involving DNA-dependent transcriptional dysregulation distinct from transcriptional control of Aldh-1. Notably, whereas the HOX11 Asn51Ala and the HOX11 Lys55Gln mutants were unable to immortalize hematopoietic progenitors, the HOX11 Thr47Ile mutant displayed immortalizing activity. Nonetheless, all 3 constructs were incapable of up-regulating expression of Aldh-1. Considered together with our earlier finding,10 which was confirmed and extended in this study, that the NH2-terminal 50 amino acids of HOX11 required for optimal activation of Aldh-1 are dispensable for HOX11 immortalizing function, we conclude that the transcriptional pathways converging on Aldh-1 are distinct from those mediating HOX11 oncogenicity. We operationally designate the former “TAAC-type” pathways and the latter “TAAT-type” pathways reflecting the in vitro DNA binding specificity of HOX11 WT and HOX11 Thr47Ile, respectively.6
Cofactor interactions are frequently required for DNA binding activity of homeodomain proteins, and cooperative activation of HOX genes and cofactor genes belonging to the TALE superclass of homeobox-containing genes has been observed in both murine and human leukemias.48-50 Of relevance in this regard, HOX11 possesses a functional FPWME PIM motif, and high levels of expression of the TALE family homeobox gene PBX2 were detected in 2 HOX11-positive human T-ALL cell lines, K3P and ALL-SIL.12 By mutating the tryptophan-methionine residues of the PIM shown to be necessary to completely disrupt PBX interactions (HOX11 M5),35 we demonstrated that PBX cooperativity is crucial to HOX11-transforming ability. However, in concurrence with an earlier report, we found that PBX interactions were not required for up-regulation of Aldh-1,17 nor were they needed for HOX11-mediated transcriptional repression. These observations again highlight the multiple activities of HOX11 and suggest that different protein-protein interactions occur between the HOX11 NH2-terminal domain in the context of progenitor immortalization than are involved in the regulation of target genes such as Aldh-1.
In summary, the HOX11 homeodomain protein can function as a transcriptional repressor as well as a transcriptional activator. Repression by HOX11 appears to be independent of DNA binding, as indicated by the homeodomain point mutants. However, the oncogenic properties of HOX11 are most likely due to direct interactions between the homeodomain and the regulatory regions of specific genes. At present, these key downstream HOX11 target genes are unknown. It will thus be of interest to compare gene expression patterns in the panel of HOX11-immortalized cell lines derived in this study to gene expression patterns in HOX11-positive T-ALL51 to try to identify the TAAT-type genes regulated by this oncoprotein that are relevant to leukemia development.
Prepublished online as Blood First Edition Paper, February 13, 2003; DOI 10.1182/blood-2002-09-2857.
Supported in part by grant HL66305 from the National Institutes of Health (R.G.H.), grants from the Medical Research Council of Canada/Canadian Institutes of Health Research and the National Cancer Institute of Canada (R.G.H., J.F.G.), and funds from The Toronto Hospital/Princess Margaret Hospital (R.G.H., P.E.G.).
B.M.O., Y-X.Z., and T-C.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ming Lu for providing HOX11 reagents and Don Branch and Teresa Hawley for helpful discussions.
Portions of this study were performed by B.M.O. in partial fulfillment of the requirements for the PhD degree in Molecular and Cellular Oncology from the Institute for Biomedical Sciences, The George Washington University, Washington, D.C. J.F.G. is an International Research Scholar of the Howard Hughes Medical Institute.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal