Abstract
Multiple sclerosis (MS) is considered an autoimmune inflammatory disease of the central nervous system. Under physiologic conditions, we compared the adhesiveness of CD4+ and CD8+ lymphocytes from nontreated patients with acute, relapsing-remitting multiple sclerosis (RRMS) and from healthy donors. We show that in patients with RRMS CD8+, but not with RRMS CD4+, T cells display increased rolling and arrest in inflamed murine brain venules. Moreover, CD8+, but not CD4+, lymphocytes from MS patients show increased rolling on P-selectin in vitro. Anti–P-selectin glycoprotein ligand-1 (PSGL-1) antibodies dramatically block the recruitment of CD8+ cells in brain vessels of patients with MS, suggesting that PSGL-1 represents a novel pharmaceutical target that may be exploited to block the selective entrance of CD8+ cells during early inflammation. Vascular cell adhesion molecule-1 (VCAM-1), but not PSGL-1, is critical for the adhesion of CD4+ cells in MS patients, highlighting a fundamental dichotomy in the mechanisms governing the recruitment of lymphocyte subsets in RRMS. Importantly, 7-color fluorescence-activated cell sorter (FACS) analysis, together with functional data, indicates that a large fraction of CD8+ cells from MS patients display the characteristics of memory-effector phenotype. In conclusion, our results show that CD8+, but not CD4+, T cells from patients with RRMS in the acute phase of the disease display increased ability to be recruited in inflamed brain venules.
Introduction
Multiple sclerosis (MS) is a common, often disabling disease of the central nervous system that has initially a relapsing-remitting course (relapsing-remitting multiple sclerosis [RRMS]) in most patients.1 MS is believed to be of autoimmune origin, and until now research has focused nearly exclusively on the role of CD4+ T cells in the induction of the disease.2 However, inflammatory infiltrates from the brains and spinal cords of patients with MS and from animals with experimental autoimmune encephalomyelitis (EAE), the animal model of MS, include CD4+ and CD8+ T cells.3-5 Moreover, CD8+ T cells may also play a major role in the induction of autoimmune demyelination in EAE.6,7 Recent data have shown that in actively demyelinating lesions, CD8+ T cells are far more numerous than CD4+ T cells.8 Moreover, analysis of T-cell receptor-β (TCR-β) gene rearrangements by polymerase chain reaction (PCR) of individual cells from frozen sections of lesional tissue revealed that CD8+ T-cell population expansion is oligoclonal, whereas, in contrast, CD4+ T cells are more heterogeneous.8
Lymphocyte migration to the brain represents a crucial moment in the initiation of the inflammatory process in the central nervous system (CNS). The process leading to lymphocyte extravasation is a finely regulated sequence of steps controlled by adhesion molecules and activating factors. It involves initial contact (tethering or capture) and rolling along the vessel wall, mediated by selectins and integrins and their ligands; chemoattractant-induced heterotrimeric Gi protein–dependent intracellular biochemical changes, leading to integrin activation; integrin-dependent firm arrest; and diapedesis.9-11 We recently developed a novel intravital microscopy model to directly analyze through the skull the interactions between lymphocytes and the endothelium in cerebral venules of mice.12 In this new model we have previously shown that P-selectin glycoprotein ligand-1 (PSGL-1)/endothelial selectins are critical in the recruitment of mouse autoreactive lymphocytes in inflamed brain microvessels.
We examined for the first time the adhesive capabilities of CD4+ and CD8+ T cells derived from patients with acute MS in a physiologic experimental setting. We took advantage of the fact that human adhesion molecules expressed by leukocytes are able to efficiently interact with their endothelial ligands expressed by mouse endothelium in an intravital microscopy setting.13-15 Our results reveal an increased adhesive capacity of CD8+, but not CD4+, T cells from patients with acute MS in brain vessels, supporting a role for CD8+ T cells in early inflammation in RRMS. Importantly, we show that PSGL-1 is responsible for the preferential recruitment of CD8+ cells in brain vessels, whereas vascular cell adhesion molecule-1 (VCAM-1) has a critical role in the recruitment of CD4+ T cells. The dichotomy in the adhesion mechanisms of CD8+ versus CD4+ lymphocytes provides important information for the potential selective blocking of the recruitment of lymphocyte subpopulations in MS.
Patients, materials, and methods
Patients
Peripheral blood samples from 20 patients (16 women and 4 men; mean age, 34.4 years; range, 19-51 years) with clinically diagnosed RRMS16,17 were obtained from the Centre for Multiple Sclerosis of the Neurological Unit “Lancisi” of the San Camillo Hospital in Rome, Italy, after informed consent was obtained and approval was given by the scientific ethics committee of the hospital. Patients were in the initial phase of the disease (mean duration of disease, 2.9 years; range, 0-9 years) and scored low on the expanded disability status scale (EDSS) (mean, 0.66; range, 0-3). None of the patients had previously undergone immunomodulating therapy. Relapse was defined according to the diagnostic criteria currently recommended for multiple sclerosis.16,17 Peripheral blood samples were also obtained from 12 apparently healthy age- and sex-matched control subjects.
Isolation and immunostaining of blood-derived cells
Peripheral blood mononuclear cells (PBMCs) were isolated from blood samples according to standard procedures; 0.5 × 106 cells were used for each staining. Monoclonal antibodies (conjugated with the appropriate fluorochrome) were added to predetermined optimal concentrations, and cells were incubated for 20 minutes at 4°C. Cells were then washed and analyzed through flow cytometry.
Antibodies
The following antibodies were used: anti-CD3 phycoerythrin–Texas Red (PE-TxRed), anti-CD3 allophycocyanin (APC), anti-CD3 tricolor (TC) (PE-Cy5), anti-CD4 PE-Cy7, anti-CD4 PE, anti-CD8 APC-Cy7, and anti-CD11a fluorescein isothiocyanate (FITC) were from Caltag (Burlingame, CA); anti-CD49d FITC, anti-CD49d APC, anti-CD162/PSGL-1 (KPL1) PE, anti-CD11a APC, and anti-CD8 PE were from BD Biosciences Pharmingen (San Diego, CA); and anti-CD62L ECD (PE-TxRed) was from Beckman Coulter. Isotype controls were as follows: immunoglobulin G2a (IgG2a) PE-Tx Red, IgG2a APC, IgG2a TC, IgG2a PE-Cy7, IgG2a PE, IgG2a APC-Cy7, IgG1 FITC (all from Caltag); IgG1 FITC, IgG1 APC, and IgG1 PE (all from BD Biosciences Pharmingen); and IgG1 ECD (Beckman Coulter). For intravital microscopy studies, we used the following monoclonal antibodies (mAbs): PL1, anti–human PSGL-1,18 MK2.7, and antimouse VCAM-1 (American Type Culture Collection).
Flow cytometry
Seven-color fluorescence-activated cell sorter (FACS) analysis was performed on a Cytomation MoFlo Cytofluorimeter (Cytomation, Fort Collins, CO). Data were compensated and analyzed using FlowJo software (Tree-Star, San Carlos, CA).
Ex vivo purification of CD4+ and CD8+ T cells
Peripheral blood leukocytes (PBLs) were divided into 2 aliquots, and CD4+ or CD8+ subsets were isolated using MACS MicroBeads (Miltenyi Biotech GmbH, Germany). A purity of more than 98% was achieved for both subsets.
Intravital microscopy
Lymphocytes were labeled with either green CMFDA (5-chloromethylfluorescein diacetate; Molecular Probes) or orange CMTMR (5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine) (Molecular Probes).
SJL young females (Harlan-Nossan, Udine, Italy) were housed and used according to current European Community rules. Mice were injected intraperitoneally with 12 μg lipopolysaccharide (LPS) (Escherichia coli 026:B6; Sigma) 5 to 6 hours before the intravital experiment was started. Animals were anesthetized, and a heparinized PE-10 catheter (Intramedic Clay Adams, Sparks, MD) was inserted into the right common carotid artery toward the brain. To exclude noncerebral vessels from the analysis, the right external carotid artery and the pterygopalatine artery, a branch of the internal carotid, were ligated.12
The preparation was placed on an Olympus BX50WI microscope, and a water-immersion objective with long focal distance (Olympus Achroplan; focal distance, 3.3 mm; numeric aperture, 0.5°) was used. Blood vessels were visualized through the bone by using fluorescent dextrans.12 Fluorescence-labeled cells (2 × 106)/condition were slowly injected into the carotid artery through a digital pump. Images were visualized by using a silicon-intensified target videocamera (VE-1000 SIT; Dage MTI, Michigan City, IN) and a Sony SSM-125CE monitor and were recorded using a digital videocassette recorder (NV-DV10000; Panasonic).
Image analysis
Video analysis was performed by playback of digital videotapes. Vessel diameter (D), hemodynamic parameters, and the velocities of rolling were determined by using a personal computer-based system.12 The velocities of 20 or more consecutive freely flowing cells per venule were calculated. From the velocity of the fastest cell in each venule (Vfast), we calculated the mean blood flow velocities (Vm): Vm = Vfast/(2 — ϵ2). Wall shear rate (WSR) was calculated from WSR = 8 × Vm/D (s—1), and wall shear stress (WSS) acting on rolling cells was approximated by WSR × 0.025 (dyne/cm2), assuming a blood viscosity of 0.025 Poise. Lymphocytes that remained stationary on the venular wall for 30 seconds or longer were considered adherent. At least 140 consecutive cells per venule were examined. Rolling and firm arrest fractions were determined as the percentages of cells that rolled or firmly arrested within a given venule in the total number of cells that entered that venule during the same period.12
In vitro rolling assay under flow
One hundred-microliter microcap glass capillary tubes were coated with human P-selectin or VCAM-1 kindly provided by Dr Daniele D'Ambrosio (Bioxell S.p.A., Milan, Italy). Lymphocytes were suspended in phosphate-buffered saline (PBS), CaCl2 1 mM, MgCl2 1 mM, 10% fetal calf serum (FCS), pH 7.2, and passed through the coated capillary tubes at a flow rate of 1250 μL/min (WSS of 2 dyne/cm2). Images were recorded on S-VHS videotape and analyzed.19
Statistics
A 2-tailed Student t test was used for statistical comparison of 2 samples. Multiple comparisons were performed using the Kruskall-Wallis test with Bonferroni correction of P. Velocity histograms were compared using the Mann-Whitney U and Kolmogorov-Smirnov tests. Differences were regarded as significant at P < .05.
Results
Patients
Current international guidelines for MS therapy indicate early treatment in patients with RRMS.20,21 In this study, patients with RRMS had never previously undergone immunomodulation therapy. The group of 20 patients with RRMS was homogenous—they all were in the initial phase of the disease and scored low on the expanded disability status scale (EDSS). A group of age- and sex-matched healthy subjects was used as controls. All MS patients donated blood within 24 hours of the onset of the first clinical symptom of neurologic dysfunction. Consequently, CD4+ and CD8+ cells obtained from these patients represent a precious human material and give high relevance to the results obtained in functional assays. All new symptoms and aggravations of preexisting symptoms of neurologic dysfunction lasted more than 24 hours, in agreement with the recommended diagnostic criteria for relapse in RRMS.16,17
Seven-color FACS analysis of CD4+ and CD8+ cells
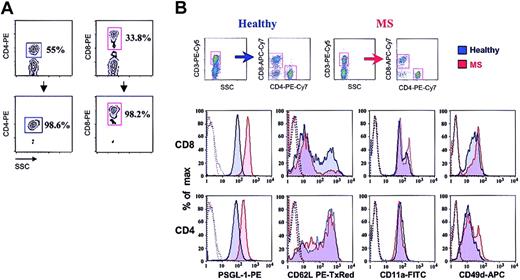
We obtained a purity of more than 98% for CD4+ and CD8+ T cells isolated from patients with acute MS and from control donors (Figure 1A). We first compared the expression of rolling and sticking receptors between CD4+ T cells obtained from healthy donors and patients with acute MS (Figure 1B; Table 1). We observed no statistically significant differences in L-selectin, LFA-1, and α4 integrin expression. Although the percentage of CD4+ cells expressing PSGL-1 was similar between the 2 populations, mean fluorescence intensity (MFI) was significantly increased in MS patients (P < .05).
Phenotype of CD4+and CD8+T cellsobtained from the blood of patients with acute MSand of healthy controls. (A) Percentage of CD4+ and CD8+ T cells before and after isolation from PBL using MACS MicroBeads. (B) Cells were phenotyped using 7-color FACS. Gates were set on either CD4+ T cells or CD8+ cells. Results shown are from 1 of 8 representative experiments. In histograms, dotted lines indicate isotype controls.
Phenotype of CD4+and CD8+T cellsobtained from the blood of patients with acute MSand of healthy controls. (A) Percentage of CD4+ and CD8+ T cells before and after isolation from PBL using MACS MicroBeads. (B) Cells were phenotyped using 7-color FACS. Gates were set on either CD4+ T cells or CD8+ cells. Results shown are from 1 of 8 representative experiments. In histograms, dotted lines indicate isotype controls.
Expression of adhesion molecules on CD4+ and CD8+ T cells isolated from healthy controls and MS patients
Lymphocyte subpopulation . | Donor . | CD162/PSGL-1, % (MFI) . | CD11a, % (MFI) . | CD62L, % (MFI) . | CD49d, % (MFI) . |
|---|---|---|---|---|---|
| CD4 | Healthy | 91 ± 4 (138 ± 42) | 98 ± 1 (73 ± 10) | 66 ± 8 (255 ± 51) | 87 ± 8 (19 ± 5) |
| CD4 | MS | 99 ± 0.5 (308 ± 130) | 98 ± 0.5 (66 ± 35) | 61 ± 19 (305 ± 217) | 90 ± 10 (31 ± 24) |
| CD8 | Healthy | 96 ± 3 (235 ± 106) | 91 ± 4 (109 ± 26) | 49 ± 12 (154 ± 10) | 91 ± 17 (31 ± 7) |
| CD8 | MS | 98 ± 2 (433 ± 125) | 99 ± 0.5 (128 ± 75) | 34 ± 19 (145 ± 119) | 92 ± 18 (41 ± 31) |
Lymphocyte subpopulation . | Donor . | CD162/PSGL-1, % (MFI) . | CD11a, % (MFI) . | CD62L, % (MFI) . | CD49d, % (MFI) . |
|---|---|---|---|---|---|
| CD4 | Healthy | 91 ± 4 (138 ± 42) | 98 ± 1 (73 ± 10) | 66 ± 8 (255 ± 51) | 87 ± 8 (19 ± 5) |
| CD4 | MS | 99 ± 0.5 (308 ± 130) | 98 ± 0.5 (66 ± 35) | 61 ± 19 (305 ± 217) | 90 ± 10 (31 ± 24) |
| CD8 | Healthy | 96 ± 3 (235 ± 106) | 91 ± 4 (109 ± 26) | 49 ± 12 (154 ± 10) | 91 ± 17 (31 ± 7) |
| CD8 | MS | 98 ± 2 (433 ± 125) | 99 ± 0.5 (128 ± 75) | 34 ± 19 (145 ± 119) | 92 ± 18 (41 ± 31) |
Shown are the percentages and MFIs of marker-positive cells in the total population of 4 to 8 experiments.
We next compared the expression of rolling and sticking receptors between CD8+ T cells obtained from healthy donors and patients with acute MS (Figure 1B; Table 1). In contrast to data obtained with CD4+ cells, we observed a significant decrease in the percentage of L-selectin–positive cells in patients with acute MS (P < .05). Moreover, a significant increase of the MFI for PSGL-1 was revealed in patients with acute MS (P < .02). We observed no statistically significant difference in LFA-1 or α4 integrin expression.
Finally, we compared the expression of various adhesion receptors between the CD4+ and CD8+ T cells from patients with acute MS. Importantly, we observed that the percentage of CD8+ T cells expressing L-selectin was statistically lower when compared with that of CD4+ cells (P < .005). MFI for L-selectin was also decreased in the CD8+ population (P < .03). Moreover, CD8+ T cells expressed statistically higher MFI for PSGL-1 (P < .0002), LFA-1 (P < .03), and α4 integrin (P < .05). We observed that CD8+ cells that were negative for both CCR7 and L-selectin were responsible for the increased MIF of PSGL-1 (data not shown). Furthermore, CD27—CD8+, but not CD27+CD8+, T cells isolated from patients with RRMS showed an increased expression of PSGL-1 (data not shown). Taken together, these results show that at the initiation of a clinical relapse in RRMS, CD8+ T cells are more activated than CD4+ T cells and display an increase in the characteristics of memory-effector phenotype.
CD4+ T cells from patients with acute MS do not show increased adhesiveness in brain venules in vivo
We investigated the rolling and arrest of CD4+ and CD8+ cells in brain venules by using a novel method of intravital microscopy in mouse brain microcirculation.12 It has been shown that human adhesion molecules expressed by leukocytes are able to efficiently interact with endothelial ligands expressed by mouse endothelium in intravital microscopy settings.13-15 Our intravital microscopy experiments allowed us to study for the first time the behavior of MS lymphocyte subpopulations under physiologic conditions. Normal brain vessels were unable to mediate rolling and arrest of CD4+ or CD8+ cells from healthy subjects or MS patients (data not shown). In fact, we have previously shown that brain endothelium requires activation to recruit lymphocytes from the blood.12 It is now widely accepted that murine and human brain endothelium express P- and E-selectin, VCAM-1, and intracellular adhesion molecule-1 (ICAM-1) during acute and subacute inflammation.12,22-25 Moreover, brain endothelium expresses endothelial selectins, VCAM-1, and ICAM-1 during EAE and MS.12,26,27 Thus, we next studied the interaction of human CD4 and CD8 T cells by using a novel, well-characterized in vivo model of subacute inflammation in which brain endothelium expresses P-selectin, E-selectin, VCAM-1, and ICAM-1.12
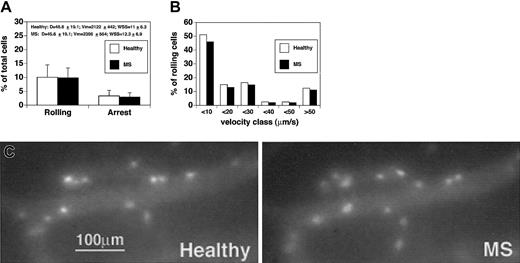
We first compared rolling and sticking of CD4+ T cells isolated from healthy subjects versus CD4+ cells obtained from patients with acute MS (Figure 2A-C). Comparison between cells derived from healthy controls and MS patients was performed in the same venules, hence under similar hemodynamic conditions and in the presence of equivalent expressions of endothelial ligands. Given that fluctuations in blood flow may occur in brain vessels, hemodynamic parameters were measured during the injection of cells derived from healthy subjects and MS patients. Notably, microvascular hemodynamics were similar during the injection of control cells or after the administration of MS-derived cells, supporting the accuracy of our in vivo results (Figure 2A). No statistically significant differences were observed between the percentage of rolling of CD4+ cells obtained from healthy donors and patients with acute MS (10.1% ± 4.4% vs 9.9% ± 3.5%; mean ± SD) (Figure 2A). To search for potential differences between healthy subjects and MS patients, we next analyzed the quality and strength of rolling by measuring the rolling velocities (Vroll) of CD4+ lymphocytes. The median rolling velocity of CD4 lymphocytes from healthy subjects was 12.6 μm/s, whereas the median rolling velocity of MS CD4 cells was 11 μm/s. Vroll from different experiments were pooled in velocity classes to better analyze potential rolling differences between CD4+ cells from healthy donors and patients with acute MS. We observed no significant differences in the velocity classes between the 2 groups (Figure 2B).
CD4+lymphocytes from patients with acute MS do not show increased rolling and sticking in brain venules. CD4+ cells were isolated from 4 patients and 4 healthy controls. Experiments were performed in 4 mice. Ten venules were examined. (A) Rolling and arrest fractions were compared between healthy controls (□) and MS patients (▪). Mean ± SD are presented. D indicates diameter. (B) Velocity histograms were generated by measuring rolling velocities. Frequency distributions were calculated after cells were assigned to velocity classes from more than 0 μm/s to 10 μm/s, 10 to 20 μm/s, 20 to 30 μm/s, and so on. Sixty-one rolling cells were examined from healthy donors (□), and 69 rolling cells were considered from patients with acute MS (▪). (C) Adherent CD4+ T cells obtained from a healthy donor (Healthy) (CMFDA-labeled) and a patient with acute MS (MS) (CMTMR-labeled) in the same cerebral venule.
CD4+lymphocytes from patients with acute MS do not show increased rolling and sticking in brain venules. CD4+ cells were isolated from 4 patients and 4 healthy controls. Experiments were performed in 4 mice. Ten venules were examined. (A) Rolling and arrest fractions were compared between healthy controls (□) and MS patients (▪). Mean ± SD are presented. D indicates diameter. (B) Velocity histograms were generated by measuring rolling velocities. Frequency distributions were calculated after cells were assigned to velocity classes from more than 0 μm/s to 10 μm/s, 10 to 20 μm/s, 20 to 30 μm/s, and so on. Sixty-one rolling cells were examined from healthy donors (□), and 69 rolling cells were considered from patients with acute MS (▪). (C) Adherent CD4+ T cells obtained from a healthy donor (Healthy) (CMFDA-labeled) and a patient with acute MS (MS) (CMTMR-labeled) in the same cerebral venule.
We also analyzed the arrest of lymphocytes in brain vessels. The percentage of CD4+ cells that firmly adhered was 3.6% ± 2% (mean ± SD) for healthy donors and 3% ± 1.5% for MS patients (Figure 2A-C). Thus, as also shown for rolling fractions, no statistically significant differences were observed between healthy donors and MS patients in the percentages of CD4+ T cells undergoing firm arrest. This suggests that CD4+ lymphocytes from patients with acute MS do not show increased adhesiveness in murine brain venules in vivo.
CD8+ T cells from patients with acute MS display increased rolling and sticking in murine brain venules
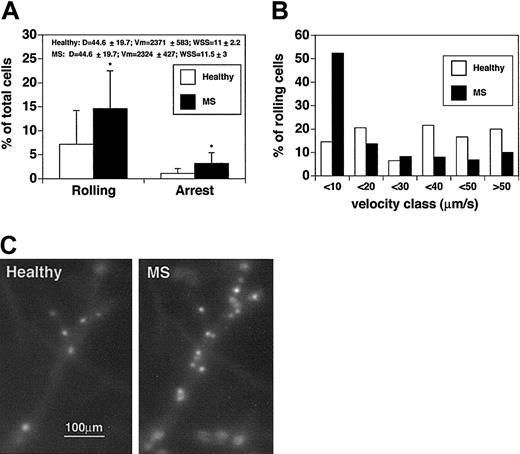
In contrast to the results obtained with CD4+ T cells, the rolling fraction of MS-derived CD8+ T cells was 2 times higher than for CD8+ T cells from healthy controls (14.6% ± 7.9% vs 7.9% ± 7%; P < .04) (Figure 3A). Comparisons were performed in the same venules, and hemodynamic parameters were measured during the injection of cells derived from healthy subjects and MS patients. We next measured and analyzed the rolling velocities of CD8+ lymphocytes (Figure 3B). The median rolling velocity for CD8+ cells from healthy controls was 15.5 μm/s, whereas the median rolling velocity of CD8+ cells from patients with acute MS was 8 μm/s, suggesting an increase in the fraction of slow-rolling cells. Vroll results from different experiments were pooled in velocity classes, as indicated in Figure 3B and on the Blood website (see the Supplemental Video link at the top of the online article). Considering the pool of cells that display a Vroll lower than 10 μm/s, the difference between CD8+ cells from healthy controls and CD8+ cells from MS patients was striking. In healthy donors the percentage of CD8+ cells with slow-rolling velocities was 14.6%, whereas the percentage of slow-rolling CD8+ cells from patients with MS was 52.4% (P < .01). Moreover, in healthy controls, the percentage of CD8+ T cells rolling at velocities greater than 40 μm/s was 2-fold higher than that of CD8+ T cells derived from MS patients, suggesting different molecular mechanisms mediating rolling interactions in the 2 CD8+ lymphocyte populations.
CD8+lymphocytes from patients with acute MS display increasedrolling and sticking in brain venules. CD8+ cells were isolated from 4 patients and 4 healthy controls. Experiments were performed in 4 mice. Seven venules were examined. (A) Rolling and arrest fractions were compared between healthy controls (□) and patients with acute MS (▪). Mean ± SD are presented. *P < .04. (B) Velocity histograms were generated as described in the legend to Figure 1B. Fifty-nine rolling cells were examined from healthy donors (□), and 64 rolling cells were considered from patients with acute MS (▪). (C) Adherent CD8+ T cells obtained from a healthy donor (Healthy) (CMFDA-labeled) and a patient with acute MS (MS) (CMTMR-labeled) in the same cerebral venule. Note the difference in the number of adherent cells between the 2 populations.
CD8+lymphocytes from patients with acute MS display increasedrolling and sticking in brain venules. CD8+ cells were isolated from 4 patients and 4 healthy controls. Experiments were performed in 4 mice. Seven venules were examined. (A) Rolling and arrest fractions were compared between healthy controls (□) and patients with acute MS (▪). Mean ± SD are presented. *P < .04. (B) Velocity histograms were generated as described in the legend to Figure 1B. Fifty-nine rolling cells were examined from healthy donors (□), and 64 rolling cells were considered from patients with acute MS (▪). (C) Adherent CD8+ T cells obtained from a healthy donor (Healthy) (CMFDA-labeled) and a patient with acute MS (MS) (CMTMR-labeled) in the same cerebral venule. Note the difference in the number of adherent cells between the 2 populations.
We next analyzed the sticking of CD8+ cells (Figure 3A,C). The percentage of CD8+ cells that firmly adhered was 1.1% ± 1% for healthy donors. In contrast, the mean percentage (± SD) of adherent cells was 3.2% ± 2.2% for MS CD8+ T cells (P < .04). These data clearly show that CD8+ cells from MS patients display increased adhesive capabilities in brain venules when compared with CD8+ cells from healthy controls.
VCAM-1 has an important role in the recruitment of CD4+ cells from patients with acute MS
We have previously observed that rapid adhesion of human T lymphocytes on murine purified VCAM-1 triggered by SDF-1 is blocked by the anti–human α4 antibody (HP2/1) and by the anti–murine VCAM-1 antibody (M.K. 2.7), indicating that human α4 integrins efficiently interact with murine VCAM-1 (C.L., unpublished observation, March 2001). Human PSGL-1 is also able to interact with endothelial selectins expressed by the murine endothelium.15 Taking advantage of the fact that human adhesion molecules expressed by leukocytes are able to efficiently interact with their endothelial ligands expressed by murine endothelium, we next endeavored to identify the molecular mechanisms involved in rolling of CD4+ T cells derived from patients with acute MS. Although these CD4+ cells express PSGL-1 at higher levels than CD4+ cells from healthy donors, a monoclonal antibody to PSGL-1 had no significant effect on rolling (Figure 4A). In contrast, 65% of rolling was blocked by an anti–VCAM-1 antibody (P < .01) (Figure 4A).
VCAM-1 is required for rolling and arrest of acute MS-derived CD4+T cells. (A) To determine the role of PSGL-1, CD4+ T cells were isolated from 4 patients with acute MS. Control cells received no treatment, whereas other cells were pretreated with 100 μg/mL anti–PSGL-1 mAb for 15 minutes at 25°C in a total volume of 300 μL and were injected through the right carotid catheter. A supplement of up to 100 μg mAb was administered, together with anti–PSGL-1 mAb-treated cells. Ten venules were analyzed. Venular and hemodynamic parameters (mean ± SD) during the experiments were: D = 40.6 ± 10.1 μm; Vm = 1857 ± 410 μm/s; WSS = 8.4 ± 2.2 dyne/cm2 for control cells; and D = 40.6 ± 10.1 μm; Vm = 2067 ± 393 μm/s; WSS = 10.2 ± 3.5 dyne/cm2 for antibody-treated cells. To determine the role of VCAM-1, CD4+ T cells were isolated from 3 patients with acute MS. Control cells were injected before mAb administration. Mice received intravenous administration of 100 μg mAb anti–VCAM-1. After 10 minutes, we injected the same number of cells used for the controls. Six venules were examined. Venular and hemodynamic parameters (mean ± SD) during the experiments were: D = 46.6 ± 21.8 μm; Vm = 2125 ± 448 μm/s; WSS = 10.5 ± 4.6 dyne/cm2 for control cells. After antibody treatment, the parameters were: D = 46.6 ± 21.8 μm; Vm = 2743 ± 629 μm/s; WSS = 12.5 ± 5.3 dyne/cm2. Bars depict rolling and arrest fractions as percentages of control cells that rolled and arrested in the same venule. □ indicates control; ▪, anti–VCAM-1; and ▦, anti–PSGL-1. Data are expressed as mean ± SEM. Groups were compared with control using the Kruskall-Wallis test followed by Bonferroni correction of P.*P < .01; **P < .001. (B) Velocity histograms were generated as described in the legend to Figure 1B. n indicates number of rolling cells examined.
VCAM-1 is required for rolling and arrest of acute MS-derived CD4+T cells. (A) To determine the role of PSGL-1, CD4+ T cells were isolated from 4 patients with acute MS. Control cells received no treatment, whereas other cells were pretreated with 100 μg/mL anti–PSGL-1 mAb for 15 minutes at 25°C in a total volume of 300 μL and were injected through the right carotid catheter. A supplement of up to 100 μg mAb was administered, together with anti–PSGL-1 mAb-treated cells. Ten venules were analyzed. Venular and hemodynamic parameters (mean ± SD) during the experiments were: D = 40.6 ± 10.1 μm; Vm = 1857 ± 410 μm/s; WSS = 8.4 ± 2.2 dyne/cm2 for control cells; and D = 40.6 ± 10.1 μm; Vm = 2067 ± 393 μm/s; WSS = 10.2 ± 3.5 dyne/cm2 for antibody-treated cells. To determine the role of VCAM-1, CD4+ T cells were isolated from 3 patients with acute MS. Control cells were injected before mAb administration. Mice received intravenous administration of 100 μg mAb anti–VCAM-1. After 10 minutes, we injected the same number of cells used for the controls. Six venules were examined. Venular and hemodynamic parameters (mean ± SD) during the experiments were: D = 46.6 ± 21.8 μm; Vm = 2125 ± 448 μm/s; WSS = 10.5 ± 4.6 dyne/cm2 for control cells. After antibody treatment, the parameters were: D = 46.6 ± 21.8 μm; Vm = 2743 ± 629 μm/s; WSS = 12.5 ± 5.3 dyne/cm2. Bars depict rolling and arrest fractions as percentages of control cells that rolled and arrested in the same venule. □ indicates control; ▪, anti–VCAM-1; and ▦, anti–PSGL-1. Data are expressed as mean ± SEM. Groups were compared with control using the Kruskall-Wallis test followed by Bonferroni correction of P.*P < .01; **P < .001. (B) Velocity histograms were generated as described in the legend to Figure 1B. n indicates number of rolling cells examined.
We then compared Vroll before and after treatment with anti–PSGL-1 and anti–VCAM-1 antibodies. No significant effect using anti–PSGL-1 antibody could be ascertained; thus, PSGL-1 did not participate in the strength of rolling interactions (Figure 4B). Vroll pooled in velocity classes after VCAM-1 mAb treatment showed an increase (30%-100%) in the number of cells in the velocity classes (more than 30 μm/s) and a decrease of 45% in cells with slow rolling (less than 10 μm/s) (Figure 4C).
Firm arrest was strongly blocked by treatment with anti–VCAM-1 mAb (inhibition of approximately 92%; P < .001) but not by anti–PSGL-1 antibody (Figure 4A). These findings suggest that VCAM-1/α4 integrins represent central molecules in the recruitment of CD4+ lymphocytes from patients with acute MS.
PSGL-1 is critical for the recruitment of CD8+ cells from patients with acute MS
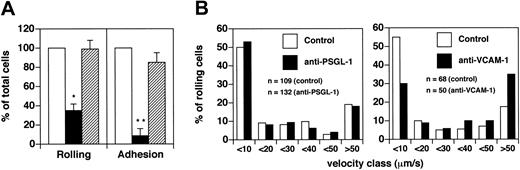
Flow cytometric data showed that in patients with acute MS, CD8+ T cells present a statistically significant increase in MFI of PSGL-1 compared with CD4+ T cells (P < .0002). Anti–PSGL-1 antibody blocked 85% of CD8+ T cell rolling (P < .001) (Figure 5A). Moreover, anti–VCAM-1 antibody blocked only 27% of rolling (P > .05) (Figure 5A). Similarly, the anti-α4 antibody HP2/1 did not show a statistically significant effect on rolling (data not shown). These results clearly contrast with data obtained using CD4+ cells derived from patients with acute MS, and they show that PSGL-1 expression on CD8+ T cells is critical for capture and rolling.
PSGL-1 is critical for the recruitment of acute MS-derived CD8+Tcells. (A) To determine the role of PSGL-1, CD8+ T cells were isolated from 3 patients with acute MS. Control cells received no treatment, but other cells were treated as described in the legend to Figure 4A. Seven venules were analyzed in total. Venular and hemodynamic parameters (mean ± SD) during the experiments were: D = 44.7 ± 10.9 μm; Vm = 1919 ± 749 μm/s; and WSS = 8.6 ± 2.5 dyne/cm2 for control cells. For anti–PSGL-1 mAb-treated cells, they were: D = 44.7 ± 10.9 μm; Vm = 1905 ± 682 μm/s; WSS = 8.6 ± 3.1 dyne/cm2. To determine the role of VCAM-1, CD8+ T cells were isolated from 3 patients with acute MS. Control cells were injected before mAb administration. Initially, mice received 100 μg mAb anti–VCAM-1 intravenously. After 10 minutes we injected the same number of cells as were injected for control. Six venules were examined. Venular and hemodynamic parameters (mean ± SD) during the experiments were: D = 50.8 ± 5.1 μm; Vm = 2017 ± 964 μm/s; WSS = 7.7 ± 3 dyne/cm2 for control cells. After antibody treatment they were: D = 50.8 ± 5.1 μm; Vm = 2256 ± 1022 μm/s; WSS = 8.6 ± 3.1 dyne/cm2. Bars depict rolling and arrest fractions as percentages of control cells that rolled and arrested in the same venule. □ indicates control; ▪, anti–VCAM-1; and ▦, anti–PSGL-1. Data are expressed as the mean ± SEM. Groups were compared with control using the Kruskall-Wallis test followed by Bonferroni correction of P.*P < .01. (B) Velocity histograms were generated as described in the legend to Figure 1B. n indicates number of rolling cells examined; □, control; and ▪, anti–VCAM-1.
PSGL-1 is critical for the recruitment of acute MS-derived CD8+Tcells. (A) To determine the role of PSGL-1, CD8+ T cells were isolated from 3 patients with acute MS. Control cells received no treatment, but other cells were treated as described in the legend to Figure 4A. Seven venules were analyzed in total. Venular and hemodynamic parameters (mean ± SD) during the experiments were: D = 44.7 ± 10.9 μm; Vm = 1919 ± 749 μm/s; and WSS = 8.6 ± 2.5 dyne/cm2 for control cells. For anti–PSGL-1 mAb-treated cells, they were: D = 44.7 ± 10.9 μm; Vm = 1905 ± 682 μm/s; WSS = 8.6 ± 3.1 dyne/cm2. To determine the role of VCAM-1, CD8+ T cells were isolated from 3 patients with acute MS. Control cells were injected before mAb administration. Initially, mice received 100 μg mAb anti–VCAM-1 intravenously. After 10 minutes we injected the same number of cells as were injected for control. Six venules were examined. Venular and hemodynamic parameters (mean ± SD) during the experiments were: D = 50.8 ± 5.1 μm; Vm = 2017 ± 964 μm/s; WSS = 7.7 ± 3 dyne/cm2 for control cells. After antibody treatment they were: D = 50.8 ± 5.1 μm; Vm = 2256 ± 1022 μm/s; WSS = 8.6 ± 3.1 dyne/cm2. Bars depict rolling and arrest fractions as percentages of control cells that rolled and arrested in the same venule. □ indicates control; ▪, anti–VCAM-1; and ▦, anti–PSGL-1. Data are expressed as the mean ± SEM. Groups were compared with control using the Kruskall-Wallis test followed by Bonferroni correction of P.*P < .01. (B) Velocity histograms were generated as described in the legend to Figure 1B. n indicates number of rolling cells examined; □, control; and ▪, anti–VCAM-1.
Vroll after PSGL-1 treatment was not determined because the number of cells that rolled was low. We asked whether there might be a significant contribution by α4 integrins to the strength of rolling CD8+ cells in brain microcirculation. We observed a 2-fold increase (from 11.3% to 22.9%) of the percentage of cells with a Vroll greater than 50 μm/s. Thus, VCAM-1/α4 integrins seem to be involved in the strength of rolling of a small subpopulation of CD8+ cells (Figure 5B).
Firm arrest was strongly blocked by treatment with anti–PSGL-1 mAb (inhibition of approximately 90%; P < .001). In contrast with the data obtained using CD4+ T cells, the percentage of CD8+ T cells that arrested after treatment with anti–VCAM-1 mAb was 68% ± 14% (mean ± SEM; P > .05) (Figure 5A). Similarly, the anti-α4 antibody HP2/1 did not show a statistically significant effect on sticking (data not shown). These findings show that VCAM-1 does not significantly contribute to the sticking of CD8+ cells from MS patients, suggesting that integrins other than VLA-4 are involved in this step.
CD4+ and CD8+ T-cell recruitment on P-selectin and VCAM-1 in vitro under flow
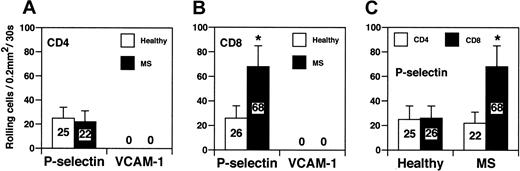
Finally, we studied tethering and rolling capacity of CD8+ and CD4+ lymphocytes in a reduced, well-controlled system in capillary tubes coated with human P-selectin or VCAM-1 under a physiologic shear of 2 dyne/cm2 (Figure 6). The number of CD4+ lymphocytes from healthy controls that rolled on P-selectin was comparable to the number of CD4+ T cells derived from MS patients (25 ± 11 vs 22 ± 9) (Figure 6A). In contrast, CD8+ cells from MS patients displayed a significant increase in rolling on P-selectin when compared with CD8+ cells from healthy donors (68 ± 17 vs 26 ± 10; P < .01) (Figure 6B). MS-derived CD8+ cells exhibited a 3-fold increase in tethering and rolling on P-selectin compared with MS-derived CD4+ cells (68 ± 17 vs 22 ± 9; P < .01) (Figure 6C). These results strongly indicate that CD8+, but not CD4+, cells from patients with acute MS have a higher capacity to roll on P-selectin, a ligand expressed by acute and subacute inflamed endothelium.12,28 VCAM-1 supported no rolling of either CD4+ or CD8+ cells derived from controls and MS patients (Figure 6A-B). This is in agreement with previous data showing that VLA-4/VCAM-1 alone was unable to mediate rolling because VLA-4/VCAM-1–mediated rolling requires a G-protein–linked signaling induced by a chemokine coexpressed with VCAM-1.29
CD8+cells from patients with acute MS exhibitincreased rolling on P-selectin in vitro. (A-C) CD4+ and CD8+ T cells were obtained from the blood of healthy donors and from patients with acute MS. Three patients were studied. Values are mean percentage of total interacting cells to an area of 0.2 mm2 in 30 seconds. Results are from 1 of 3 representative experiments. Error bars are standard deviation values from at least 10 separate areas on the same capillary tube. Single areas of 0.2 mm2 were recorded for at least 30 seconds. *P < .01.
CD8+cells from patients with acute MS exhibitincreased rolling on P-selectin in vitro. (A-C) CD4+ and CD8+ T cells were obtained from the blood of healthy donors and from patients with acute MS. Three patients were studied. Values are mean percentage of total interacting cells to an area of 0.2 mm2 in 30 seconds. Results are from 1 of 3 representative experiments. Error bars are standard deviation values from at least 10 separate areas on the same capillary tube. Single areas of 0.2 mm2 were recorded for at least 30 seconds. *P < .01.
Discussion
We show here that CD8+, but not CD4+, T cells from patients with RRMS in the acute phase of the disease have increased adhesion capabilities in brain vessels compared with cells from healthy donors. We also show that a large fraction of CD8+ T cells from patients with acute MS express surface markers characteristic of memory-effector cells. Furthermore, we document a fundamental dichotomy in the molecular mechanisms controlling the adhesion of CD8+ versus CD4+ lymphocytes from patients with acute MS, indicating the way for a potential selective blocking of the recruitment of lymphocyte subpopulations in RRMS.
Current international guidelines for RRMS therapy recommend early treatment—thus, the recruitment of patients not undergoing immunomodulatory therapy is becoming increasingly difficult.20,21 To our knowledge, we are the first to study the adhesiveness of lymphocyte subpopulations at the onset of a clinical attack of MS under physiologic conditions. The patients with RRMS in this study had never previously undergone immunomodulation therapy, and they donated blood within 24 hours of the onset of the first clinical symptom of neurologic dysfunction. Consequently, results obtained with CD4+ and CD8+ cells isolated from the blood of these patients have high relevance for the study of early inflammation in RRMS.
Seven-color FACS analysis was used for the first time for the characterization of the CD4+ and CD8+ lymphocytes derived from patients with RRMS at the beginning of the relapse phase. We showed that CD8+, but not CD4+, T cells from patients with acute MS have lower levels of L-selectin than those in healthy donors. Moreover, we observed that in patients in the acute phase of MS, the expression of L-selectin by CD8+ T cells is significantly lower than that of CD4+ T cells. It is known that L-selectin is down-regulated on T cells with the memory-effector phenotype and that cells that down-regulate L-selectin lose the ability to migrate to lymphoid organs, but they acquire the capacity to migrate to peripheral tissues and sites of inflammation.30-33 Thus, our data suggest that CD8+, but not CD4+, T cells from patients with acute MS have an increased ability to migrate to sites of inflammation. CD4+ and CD8+ T cells from patients with acute MS expressed increased levels of PSGL-1 compared with those in healthy donors. PSGL-1 is constitutively expressed by almost all lymphocytes, but it was shown that only memory/activated T cells express the functional PSGL-1 that is able to bind P-selectin.34-36 PSGL-1 becomes functional after posttranslational modifications by glycosyltransferases that are up-regulated in activated lymphocytes.36 We have previously shown that in vitro antigen-activated CD4+ T cells, in contrast to naive cells, express functional PSGL-1, which has a critical role in tethering and rolling on endothelial selectins in inflamed brain venules.12 However, the increased expression of PSGL-1 on CD4+ T cells derived from patients with acute MS was not functional, as shown by lymphocyte rolling in capillary tubes coated with P-selectin and by the lack of effect of anti–PSGL-1 antibodies on the recruitment in inflamed brain venules expressing P-selectin. In contrast, CD8+ T cells from patients with acute MS have increased expression of PSGL-1 and display a striking increase of rolling on P-selectin in vitro under flow. These data suggest that higher expression of PSGL-1 and improved posttranslational modifications might contribute to the increased recruitment of CD8+ T cells in inflamed brain venules in vivo. Recently, Weninger et al33 explored the migratory capacities of naive, central memory, and memory-effector CD8+ T cells in vivo. The study has shown that memory-effector CD8+ T cells are L-selectin—/low, possess high binding capacity to P-selectin, and efficiently migrate to sites of inflammation but not to lymphoid organs.33 Consistent with these data, our results show a decrease in the percentage of L-selectin–positive cells and a 3-fold increase in the capacity of cells to roll on P-selectin under flow, suggesting an increase of the memory-effector phenotype of CD8+ T cells in patients with acute MS. We also observed that CD8+ cells negative for L-selectin and CCR7 are responsible for the increased MFI of PSGL-1, suggesting that PSGL-1 expression is increased on memory-effector cells (data not shown). Thus, taken together our results support the hypothesis that at the beginning of a clinical relapse in patients with RRMS, CD8+, but not CD4+, T cells represent the effector arm of the immune response.
No study has yet distinguished the adhesive capacity of lymphocyte subpopulations in MS patients. Here we studied for the first time rolling and arrest of CD4+ and CD8+ T cells from acute, nontreated MS patients under physiologic conditions by using a novel method of intravital microscopy in mouse brain microcirculation and in vitro under flow assays to study rolling in capillary tubes coated with human P-selectin or VCAM-1. We first compared the adhesive capacities of T cells from patients with acute MS with those in healthy donors during intravital microscopy experiments performed in inflamed mouse brain venules. In our experimental model, brain endothelium expresses P- and E-selectin, ICAM-1, and VCAM-1, thus displaying the endothelial adhesion molecules involved in lymphocyte extravasation during inflammation.12,32,33 We observed a striking difference in adhesiveness between CD4+ and CD8+ T cells from patients with acute MS: MS-derived CD4+ T cells showed rolling and arrest fractions similar to those of CD4+ T cells from healthy donors. Moreover, analysis of rolling velocities showed no significant differences between velocity classes of CD4+ T cells from MS patients and control subjects. In contrast, the percentage of CD8+ T cells that rolled and arrested was significantly higher in patients with acute MS than in healthy subjects. Analysis of rolling velocities revealed a significant increase in the percentage of MS-derived CD8+ T cells with slow “inflammatory” rolling. Taken together, these results indicate that CD8+, but not CD4+, T cells isolated at the beginning of a clinical relapse are more activated and have increased adhesion capacities in inflamed brain venules.
We showed that anti–PSGL-1 antibodies blocked almost 90% of rolling of MS-derived CD8+ T cells, suggesting that PSGL-1 is critical for the recruitment of CD8+ T cells in inflamed brain vessels. In agreement with intravital microscopy data, CD8+ T cells showed a significant increase of rolling on P-selectin in vitro when compared with cells from healthy subjects. Importantly, we documented that CD8+ T cells from patients with acute MS have a 3-fold increase in rolling on P-selectin in capillary tubes compared with CD4+ T cells from the same patients. Thus, CD8+ T cells present an important characteristic required for the effector function—the capacity to bind P-selectin.33 Recent results obtained by Carrithers et al26 suggest that P-selectin, a PSGL-1 ligand, may be involved in the early recruitment of encephalitogenic lymphocytes to the brain. Moreover, Kerfoot and Kubes37 have recently shown that P-selectin mediates leukocyte–endothelial cell interactions controlling leukocyte trafficking in the brains of EAE mice. PSGL-1 binds E- and P-selectin in vivo, and we recently documented positivity for P- and E-selectin before the onset of clinical EAE.12,15 Moreover, E-selectin has been previously described in vessels from acute plaques of patients with MS.27 Antibodies to PSGL-1 inhibit interactions of leukocytes to areas of inflammation in other animal models.38,39 Furthermore, recombinant soluble forms of PSGL-1 inhibit selectin-mediated inflammatory responses in models of inflammation and thrombosis in vivo.40,41 Thus, PSGL-1, the molecule responsible for the preferential recruitment of CD8+ T cells from patients with acute MS, possibly represents a new pharmaceutical target that may be exploited to block the selective entrance of activated CD8+ cells into the brain in the early phases of brain inflammation.
Flow cytometry results also showed an increased expression of PSGL-1 on CD4+ T cells from patients with acute MS. In contrast to data obtained with CD8+ T cells, anti–PSGL-1 antibodies had no inhibitory effect on rolling of MS-derived CD4+ T cells, suggesting that PSGL-1 expressed on CD4+ T cells is not functional and does not expose the carbohydrate epitopes required for interaction with endothelial selectins. However, anti–VCAM-1 antibodies blocked approximately 65% of CD4+ cell rolling in brain venules. These results show that VCAM-1, but not PSGL-1, has an important role for capture and rolling and that the expression of α4 integrins on CD4+ cells from acute MS is required for efficient primary adhesion in brain venules. However, approximately 35% of CD4+ T-cell rolling was not blocked by antibodies to anti–VCAM-1, suggesting that other molecules, such as L-selectin, might participate in rolling. We have recently shown that PSGL-1 is critical for the rolling of antigen-stimulated mouse CD4+ T cells in inflamed brain venules. Flow cytometry results showed that CD4+ T cells have no increased capacity to bind P-selectin and that they display equivalent levels of L-selectin expression when compared with healthy donors. Taken together, these results suggest that patients with RRMS have no increase of CD4+ cells in the effector phase at the beginning of a clinical relapse.
Anti–VCAM-1 antibodies dramatically blocked the arrest of MS-derived CD4+ T cells, showing that α4 integrins/VCAM-1 control sticking of CD4+ T cells. In contrast, anti–VCAM-1 antibodies inhibited only approximately 30% of MS patient–derived CD8+ T cell arrest, leading us to speculate that LFA-1 may be critical in this step of extravasation.12 The diversity in the recruitment mechanisms of CD4+ and CD8+ lymphocytes from MS patients suggest that quantitative or qualitative expression of adhesion molecules on brain endothelium might orchestrate the preferential extravasation of lymphocyte subpopulations. Moreover, the notion that distinct adhesion mechanisms exist for CD8+ and CD4+ lymphocytes raises the potential for selective blocking of the recruitment of lymphocyte subpopulations in MS.
There is now convincing evidence in the literature that autoreactive CD8+ T cells contribute to the pathogenesis of organ-specific autoimmune diseases.42 A role for CD8+ T cells in MS is supported by evidence of overexpression of CD8+ T cells in the leading edge of MS plaques, by the oligoclonal expansion of CD8+ T cells in patients with MS, and by the presence of major histocompatibility complex (MHC) class 1, but not class 2, on the surfaces of oligodendrocytes, the myelinating cells of the CNS.8,43,44 Moreover, CD8+ T-cell clones specific for myelin antigens have been isolated from the periphery of MS patients, and myelin-specific CD8+ T cells lyse human HLA-matched oligodendrocytes.45 Recent evidence from 2 independent laboratories strongly supports a major role for CD8+ T cells in autoimmune demyelination in EAE, the animal model of MS.6,7 Interestingly, CD8-induced EAE explains some important results obtained in clinical trials of MS better than the CD4-induced models of EAE.7 It is widely known that the principal function of CD8+ T-effector cells is the defense against viral infections. Various data point to a viral pathogenesis of MS: geographic association of disease susceptibility with evidence of MS clustering, abnormal response to a variety of viruses, increase of disease exacerbations with viral infection, and analogy with animal models and other human diseases in which viruses can cause demyelinating diseases.46 Thus, given the central role of CD8+ T cells in viral infections, our findings of an increase in the frequency of cells displaying the effector phenotype within the CD8+ population and the display of an increased adhesive capacity in brain vessels by this cell subset further substantiate the hypothesis that viral infections or reactivations may trigger clinical exacerbations in RRMS.
In conclusion, our results support a role for CD8+ T cells in early inflammation in relapsing-remitting MS and, together with growing data from the literature, suggest that CD8+ T cells may represent major effectors in the autoimmune attack of the brain.
Prepublished online as Blood First Edition Paper, February 20, 2003; DOI 10.1182/blood-2002-10-3309.
Supported in part by grants from the Italian Ministry of Health (RF00177, 0095, 00103, 1AC/F2), Fondazione Cassa di Risparmio (Cariverona), Fondazione Italiana Sclerosi Multipla (2000/R/71, 2001/R/58), Italian National Research Council (G.C., L.B.), Italian Association for Cancer Research 2001 (C.L.), National Institutes of Health (GM56532; E.C.B.), and National Institutes of Health (HL65631; R.P.M.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal