Abstract
Factor XI deficiency, an injury-related bleeding disorder, is rare worldwide but common in Jews in whom 2 mutations, Glu117Stop (type II) and Phe283Leu (type III), prevail. Mean factor XI activities in homozygotes for Glu117Stop and for Phe283Leu are 1 and 10 U/dL, respectively. Inhibitors to factor XI in patients with severe factor XI deficiency have been reported in a small number of instances. This study was undertaken to determine the prevalence of acquired inhibitors against factor XI in patients with severe factor XI deficiency, discern whether these inhibitors are related to specific mutations, and characterize their activity. Clinical information was obtained from unrelated patients with severe factor XI deficiency, and blood was analyzed for factor XI activity, inhibitor to factor XI, and causative mutations. Immunoglobulin G purified from patients with an inhibitory activity was tested for binding to factor XI, effects on activation of factor XI by factor XIIa and thrombin, and activation of factor IX by exogenous factor XIa. Of 118 Israeli patients, 7 had an inhibitor; all belonged to a subgroup of 21 homozygotes for Glu117Stop who had a history of plasma replacement therapy. Three additional patients with inhibitors from the United Kingdom and the United States also had this genotype and were exposed to plasma. The inhibitors affected factor XI activation by thrombin or factor XIIa, and activation of factor IX by factor XIa. The results imply that patients with a very low factor XI level are susceptible to development of an inhibitor following plasma replacement.
Introduction
Factor XI is a zymogen that, upon activation, promotes coagulation by activating factor IX. In an activated partial thromboplastin time (aPTT)–based clotting assay used to measure factor XI activity, factor XIIa activates factor XI, but it is currently postulated that, in vivo, thrombin is the physiological activator of factor XI.1 The initial generation of thrombin by the tissue factor–factor VIIa pathway activates factor XI preferentially on the surface of platelets, which in turn leads to further thrombin generation even after the clot has formed.2 The additional amount of thrombin then activates thrombin-activatable fibrinolysis inhibitor giving rise to clot stabilization by augmenting resistance to fibrinolysis.2
Severe deficiency of factor XI is an injury-related bleeding disorder caused by mutations in the gene encoding factor XI. The deficiency is common in Ashkenazi and Iraqi Jews and has been observed sporadically in other populations3 and in Basques with low prevalence.4 In Ashkenazi Jews, 2 mutations prevail, a nonsense mutation Glu117Stop (termed type II) and a missense mutation Phe283Leu (termed type III), while in Iraqi Jews only type II mutation has been observed.5 Homozygotes for the genotype II/II and III/III have mean factor XI coagulant activity of approximately 1 U/dL and 10 U/dL, respectively, whereas compound heterozygotes for the type II and type III mutations have a mean level of 3 U/dL.6
The development of inhibitors to factor XI was reported in anecdotal cases with severe inherited factor XI deficiency following plasma infusion.7-15 Most such patients do not bleed spontaneously but can bleed seriously during or after surgery. The reported inhibitors were shown to bind to factor XI and interfere with factor XI and/or factor IX activation by factor XIa. The aim of this study was to determine the prevalence of acquired inhibitors against factor XI in Israeli patients with severe inherited factor XI deficiency, define the possible relation to the mutations causing the deficiency, and characterize the activity of the inhibitors.
Patients, materials, and methods
Study group
Known unrelated patients with severe factor XI deficiency (factor XI activity less than 15 U/dL) whose age was 16 years or older were asked for their consent to participate in the study. We defined 1 U/dL factor XI activity as the amount of factor XI present in 100 mL pooled normal plasma. Detailed clinical history was obtained, and particular attention was paid to spontaneous and trauma-related bleeding, previous treatment by plasma and/or factor XI concentrate, and previous detection of an inhibitor to factor XI. All available medical records of the patients were also reviewed. The Human Subject Ethics Committee of the Sheba Medical Center approved the study. Informed consent was provided according to the Declaration of Helsinki.
DNA samples from 4 additional patients with severe inherited factor XI deficiency in whom an inhibitor was detected (3 from the United Kingdom and 1 from the United States) were sent to the Israeli laboratory for examination. Because 2 of the patients from the United Kingdom were related, only one DNA sample, randomly chosen, was included in the analyses.
Sample collection, coagulation assays, and mutation identification
Blood was taken from each participant in 0.105 M buffered citrate for clotting assays and in EDTA (ethylenediaminetetraacetic acid) for DNA preparation and mutation identification. The citrated plasma was centrifuged once at 2500g for 5 minutes, and the separated plasma was centrifuged again at 14 000g for 5 minutes. Plasma was assayed immediately or kept frozen at —35°C before testing. The aPTT and factor XI activity levels were determined by standard methods, and factor XI inhibitor activity was determined by the Bethesda method. The molecular basis of factor XI deficiency was determined by analysis of the 4 previously described mutations in Jews (types I through IV)16,17 with the use of polymerase chain reactions (PCRs) and specific restriction enzymes. In 3 patients, additional mutations were detected. One patient with factor XI activity of less than 1 U/dL was found to be homozygous for a novel mutation, Gly555Glu.18 A second patient (factor XI activity of 2 U/dL) was a compound heterozygote for type II and a novel mutation, Tyr427Cys.18 A third patient (factor XI activity 9 U/dL) was a compound heterozygote for type III mutation and a previously reported mutation, Glu323Lys.19
Reagents
Purified human factors IX, X, XI, XIa, and XIIa and high–molecular-weight kininogen (HK) were purchased from Enzyme Research (South Bend, IN). Factor VIII Refacto concentrate was obtained from Genetic Institute (Planegg Martinsried, Germany) and human thrombin from Diagnostica Stago (Asniéres, France). Corn trypsin inhibitor (CTI) was obtained from Haematologic Technologies (Essex Junction, VT), and bovine serum albumin (BSA), dextran sulfate, hexadimethrine bromide (polybrene), hirudin, and the alkaline phosphatase substrate p-nitrophenyl phosphate were from Sigma (St Louis, MO). Cephalin and the chromogenic substrates S2366 and S2765 were purchased from Chromogenix (Milan, Italy). Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS) was composed of 0.150 M NaCl and 50 mM Tris, pH 7.5, and TBS-BSA contained 0.1% BSA in TBS unless otherwise indicated.
Purification of immunoglobulin G from plasma
Immunoglobulin G (IgG) was purified by commercial protein A column (Bio-Rad, Hercules, CA). First, 5 mL plasma was passed several times through the protein A column, which was then washed excessively with TBS to remove unbound proteins. IgG was eluted with 0.2 M glycine, pH 2.5, into tubes containing 1:10 volume of 1 M Tris to neutralize the low pH. The IgG was dialyzed in TBS and concentrated by centricon PM 30 (Millipore, Bedford, MA) to approximately 4 mg/mL, a concentration that is about a third of normal IgG concentration in plasma. IgG concentration was determined by reading the optical density (OD) at 280 nm with an extinction coefficient of 14.
Binding of patients' IgG to factor XI
Microtiter plates were coated overnight at 4°C with 100 μL TBS containing 3 μg/mL factor XI and were then blocked with 3% BSA in TBS. After washing, 100 μL control or patient's IgG diluted 1:12.5 to 1:200 in TBS-BSA were added. Bound human IgG was detected by 1:5000 diluted alkaline phosphatase goat antihuman IgG (Jackson, West Grove, PA), to which substrate p-nitrophenyl phosphate was added, and the OD was read at 405 nm.
To demonstrate whether the IgGs recognized the heavy chain or the light chain of factor XI, purified factor XIa, 0.2 μg per lane, was applied to 8% sodium dodecyl sulfate–polyacrylamide gels (SDS-PAGEs) under reducing conditions, electrophoresed, and transferred to polyvinylidene difluoride (PVDF) membrane (Millipore). Each lane was incubated with patient's IgG diluted 1:100 in TBS, and the immune complexes were visualized with the use of 1:1000 dilution of peroxidase-labeled antihuman IgG (Jackson) and the substrate DAB (Vector Laboratories, Burlingame, CA). Control IgG or IgG purified from plasma of a type II/II patient who did not have an inhibitor to factor XI served as a negative control. A positive control reaction was carried out by using purified factor XIa under the same conditions, incubating the blots with 1:1000 dilution of goat antihuman factor XI IgG (Enzyme Research), and detecting the immune complexes with peroxidase-labeled antigoat IgG (Jackson) and DAB.
The effect of patients' IgG on the binding of factor XI to HK
Microtiter plates were coated overnight at 4°C with 100 μL TBS containing 6 μg/mL HK and then blocked with 3% BSA in TBS. After washing the plates, we added 100 μL TBS-BSA that contained factor XI (6 nM) preincubated with control or patients' IgG (2 mg/mL) for 20 minutes at 22°C. Bound human IgG was detected by alkaline phosphatase goat antihuman IgG diluted 1:5000 (Jackson), to which p-nitrophenyl phosphate was added, and the OD was read at 405 nm. To confirm that the lack of patients' IgG binding was caused by its inhibitory effect on factor XI binding to HK, 100 μL of 6 nM factor XI was first added to plates coated with HK; the plates were subsequently washed, and then 100 μL control or patient's IgG (2 mg/mL) was added. Bound human IgG was detected as described. Addition of control or patients' IgG to plates coated with HK (no factor XI) served as negative control.
The effect of patients' IgG on the amidolytic activity of factor XIa
First, 40 μL control or patients' IgG was incubated for 20 minutes at 22°C with 40 μL factor XIa, 6 nM final concentration, followed by addition of 20 μL 1.25 mg/mL S2366 and OD reading at 405 nm.
The effect of patients' IgG on activation of factor XI by thrombin
We incubated 45 μL control or patients' IgG for 20 minutes at 22°C with 45 μL TBS-BSA containing 6 nM final concentration of factor XI. Thereafter, 10 μL TBS-BSA containing human thrombin (0.5 U/mL final concentration) and dextran sulfate (1 μg/mL final concentration) was added and the mixture was incubated for 30 minutes at 37°C. Factor XI activation was stopped by adding 6.6 U hirudin and 5 μg/mL polybrene in 10 μL TBS-BSA. The amount of generated factor XIa was then measured by adding 30 μL S2366 (1.25 mg/mL) and reading OD at 405 nm. Under these conditions, that is, the absence of thrombin and presence of dextran sulfate, no autoactivation of factor XI was demonstrable.
The effect of patients' IgG on activation of factor XI by factor XIIa
We analyzed the effects of patients' IgG on slow activation of factor XI by factor XIIa, and on fast activation by factor XIIa in the presence of dextran sulfate (with or without HK). We incubated 45 μL control or patients' IgG for 20 minutes at 22°C with 45 μL TBS-BSA containing 6 nM final concentration of factor XI, and then activation of factor XI was initiated by addition of 10 μL TBS-BSA solution containing factor XIIa (1 nM) and incubation at 37°C for 16 hours. The activity of factor XIIa was then blocked by addition of 10 μL TBS-BSA containing 2.5 μg CTI for 10 minutes. Activated factor XI was measured by adding 30 μL S2366 (1.25 mg/mL) and reading OD at 405 nm. In other experiments, dextran sulfate was added to factor XIIa at a final concentration of 1 μg/mL, either alone or with HK at a final concentration of 18 nM. These reactions were carried out at 37°C for 30 minutes and were stopped by addition of 10 μL TBS-BSA containing 2.5 μg CTI and 5 μg/mL polybrene for 10 minutes.
The effect of patients' IgG on activation of factor IX by factor XIa
We incubated 25 μL control or patients' IgG with 25 μL TBS-BSA containing 1 nM factor XIa for 20 minutes at 22°C. Then, 50 μL TBS-BSA containing 1 μM factor IX and 10 mM calcium chloride was added and the mixture was incubated for 60 seconds at 37°C. Factor IX activation by factor XIa was then quenched by adding 5 μL 0.5 M EDTA. Thereafter, 10 μL mixture was added to 50 μL TBS-BSA containing 8 U/mL factor VIII, 1 U/mL thrombin, 20% cephalin, and 10 mM calcium chloride and incubated for 60 seconds at 37°C. This was followed by adding 30 μL TBS-BSA with 450 nM factor X and incubation for 150 seconds. Thereafter, factor X activation was quenched by 5 μL 0.5 M EDTA. A subsample of 75 μL was mixed with 25 μL S2765 (1.25 mg/mL), and the change in OD at 405 nm was recorded.
Determination of HLA class II genotypes
Human leukocyte antigen (HLA) class II DRB1* and DQB1* was determined by sequence-specific oligonucleotide (SSO) probes and sequence-specific priming (SSP) technology from DNA samples by means of commercially available kits (DR-DQ SSP Combi Tray, DRB1*07 [01,02,03,04] [Genovison, Exton, PA], and DYNAL RELI SSO strips [Dynal Biotech, Bromborough, United Kingdom]).
In vitro correction of thrombin generation in patients' plasma by recombinant factor VIIa
Thrombin generation was measured as previously described.20 Briefly, 80 μL patients' plasma containing increasing concentrations of recombinant factor VIIa (Novonordisk, Copenhagen, Denmark) was placed in microtiter plates suitable for fluorescence measurements (Greiner Bio-1, Frickenhausen, Germany). Then, 40 μL HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)/BSA containing 12 μM phosphatidylserine-phosphatidyethanolamine-posphatidylcholine (Avanti Polar Lipids, Alabaster, AL) in a ratio of 1:1:1.25; 50 mM CaCl2; and 2.5 mM fluorogenic substrate Z-GGR-AMC (Bachem, Bubendorf, Switzerland) were added, and fluorescence was measured for 40 minutes by means of an excitation filter at 390 nM and an emission filter at 460 nM. Thrombin generation was expressed by endogenous thrombin potential (ETP) (the area under the thrombin curve), the lag time for initial thrombin formation, and the height of thrombin peak.
Statistical analysis
The aPTT values in subjects with and without inhibitors to factor XI were compared by the Wilcoxon rank sum test. The possible relation between HLA class II and an inhibitor was examined by means of the Fisher exact test. That test was also used to evaluate the relation between exposure to plasma and the development of factor XI inhibitor.
Results
Clinical presentation
Of 122 Israeli patients with severe factor XI deficiency enrolled during 3 years (1999-2001), 4 were excluded because no reliable information could be obtained regarding plasma replacement therapy. Of the remaining 118 patients, 6 harbored an inhibitor to factor XI. During the present study, no inhibitor could be detected in an additional patient, who was previously shown in our center to have an inhibitor (3 U/mL). This patient was included in the study, but characterization of his inhibitor could not be performed.
In 3 patients, a factor XI inhibitor was detected when they presented with excessive bleeding and failure to respond to plasma replacement therapy following surgery. In 4 patients, an inhibitor to factor XI was detected during tests performed prior to surgery (Table 1).
Laboratory findings in 7 patients with an inhibitor to factor XI
Patient*† . | Sex/age, y . | aPTT, sec . | Level of inhibitor, Bethesda units . | Circumstances at detection . | No. exposures to plasma . |
|---|---|---|---|---|---|
| 1 | M/77 | 99 | 25 | Bleeding | 1 |
| 2 | F/77 | 97 | 16 | Screening | 3 |
| 3 | M/64 | > 110 | 4 | Bleeding | 1 |
| 4 | M/68 | > 110 | 4 | Screening | 4 |
| 5 | M/87 | > 110 | 4 | Bleeding | 3 |
| 6 | M/54 | > 110 | 4 | Screening | 10 |
| 7 | M/51 | > 110 | 3‡ | Screening | 2 |
Patient*† . | Sex/age, y . | aPTT, sec . | Level of inhibitor, Bethesda units . | Circumstances at detection . | No. exposures to plasma . |
|---|---|---|---|---|---|
| 1 | M/77 | 99 | 25 | Bleeding | 1 |
| 2 | F/77 | 97 | 16 | Screening | 3 |
| 3 | M/64 | > 110 | 4 | Bleeding | 1 |
| 4 | M/68 | > 110 | 4 | Screening | 4 |
| 5 | M/87 | > 110 | 4 | Bleeding | 3 |
| 6 | M/54 | > 110 | 4 | Screening | 10 |
| 7 | M/51 | > 110 | 3‡ | Screening | 2 |
All patients were of the II/II genotype; II indicates an allele carrying type II (Glu117Stop) mutation in the factor XI gene.
All patients had less than 1 U/dL factor XI activity.
This titer was detected 3 years earlier; during the study, no inhibitory activity was detectable.
Of 118 patients, 64 had received plasma earlier in their life and 54 had not been exposed to plasma. All 7 patients with an inhibitor belonged to a subgroup of patients who had received plasma replacement therapy, and all were homozygous for type II mutation. Notable was the high prevalence of an inhibitor (33%) in patients with this genotype who were exposed to plasma (Table 2). In none of the remaining 43 patients who had other genotypes and were exposed to plasma was an inhibitor detectable (Table 2). All 3 unrelated patients from the United Kingdom and the United States with an inhibitor were also homozygous for the type II mutation, and each was previously treated with plasma.
Relation between factor XI genotype, exposure to plasma, and development of an inhibitor to factor XI
. | Received plasma . | . | Did not receive plasma . | . | ||
|---|---|---|---|---|---|---|
| Genotype* . | All patients . | Patients with an inhibitor . | All patients . | Patients with an inhibitor . | ||
| II/II | 21 | 7 | 13 | 0 | ||
| II/III | 27 | 0 | 28 | 0 | ||
| III/III | 14 | 0 | 10 | 0 | ||
| Other mutations† | 2 | 0 | 3 | 0 | ||
| Total | 64 | 7 | 54 | 0 | ||
. | Received plasma . | . | Did not receive plasma . | . | ||
|---|---|---|---|---|---|---|
| Genotype* . | All patients . | Patients with an inhibitor . | All patients . | Patients with an inhibitor . | ||
| II/II | 21 | 7 | 13 | 0 | ||
| II/III | 27 | 0 | 28 | 0 | ||
| III/III | 14 | 0 | 10 | 0 | ||
| Other mutations† | 2 | 0 | 3 | 0 | ||
| Total | 64 | 7 | 54 | 0 | ||
II represents alleles bearing Glu117Stop; III, alleles bearing Phe283Leu.
One patient (factor XI activity, less than 1 U/dL) was a compound heterozygote for type II and type I mutations; 1 patient (factor XI activity, 3 U/dL) was a compound heterozygote for type III and type IV mutations; and 3 patients bearing other genotypes were described in “Patients, materials, and methods.”
Laboratory features
Table 1 shows that all 7 patients with an inhibitor had less than 1 U/dL factor XI activity. The level of the inhibitor varied from 3 to 25 Bethesda units and was present for more than 5 years in 6 of 7 patients.
Interestingly, the aPTT values in patients with an inhibitor significantly exceeded the median value of 86 seconds observed in 27 patients who bore the same genotype but without an inhibitor (P = .002). A 1:1 mixture of patients' IgG or TBS with plasma from a type II homozygote without an inhibitor yielded similar prolongation of the aPTT after incubation at 37°C for 1 hour (data not shown). Hence, the increased prolongation of aPTT in plasma of patients with an inhibitor to factor XI did not seem to be related to the inhibitory activity and remains to be explained.
Characterization of inhibitors to factor XI
IgG was purified from plasma of 6 patients with an inhibitor to factor XI present at the time of the study. The IgG, which was concentrated to 4 mg/mL, had an inhibitory activity of 2 to 4 Bethesda units in 5 of the 6 patients. In the sixth patient, the purified IgG had an inhibitory activity of about 0.5 Bethesda units, and hence this inhibitor was not further characterized.
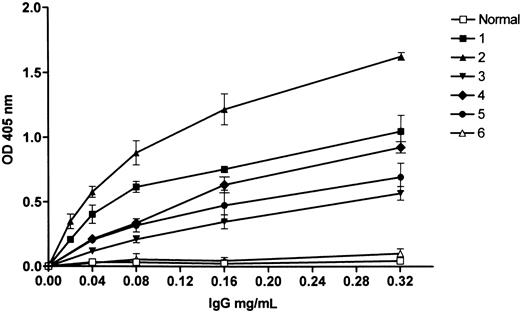
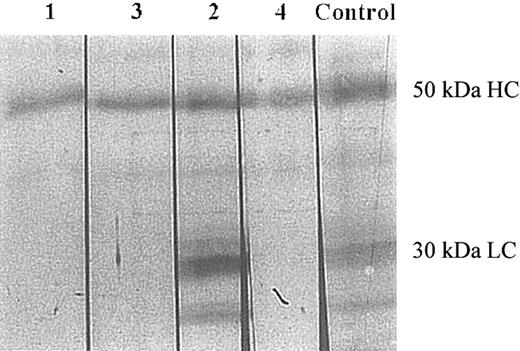
Binding of patients' IgG to factor XI. The patients' IgG bound to immobilized factor XI in a concentration-dependent manner (Figure 1), and to factor XI complexed with immobilized HK (Figure 2). The IgG examined from 4 patients recognized factor XIa by Western blotting (Figure 3). In 3 patients (nos. 1, 3, and 4), recognition of the IgG was restricted to the heavy chain, and in 1 patient (no. 2), the IgG bound to both heavy and light chains.
Patients' IgG binding to immobilized factor XI as detected by a secondary alkaline phosphatase goat antihuman IgG. “Normal” represents control IgG; 1 through 5 are IgGs of patients with an inhibitor to factor XI, and 6 is IgG of a type II homozygote without an inhibitor. Shown are mean ± standard deviation (SD) of triplicates.
Patients' IgG binding to immobilized factor XI as detected by a secondary alkaline phosphatase goat antihuman IgG. “Normal” represents control IgG; 1 through 5 are IgGs of patients with an inhibitor to factor XI, and 6 is IgG of a type II homozygote without an inhibitor. Shown are mean ± standard deviation (SD) of triplicates.
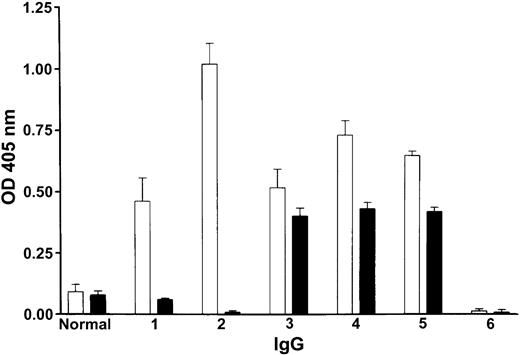
Effect of patients' IgG on factor XI binding to immobilized HK measured indirectly by binding of alkaline phosphatase goat antihuman IgG to patients' IgG complexed with factor XI. The white bars (□) show that the IgG of patients 1 through 5 bound to microtiter plates coated with HK to which purified factor XI was attached, in contrast to normal IgG or IgG of a type II homozygote with no inhibitor (no. 6), which did not bind. The black bars (▪) show that normal IgG or IgG of a type II homozygote with no inhibitor which was preincubated with purified factor XI (not expected to bind to each other) did not bind to the plates coated with HK. In patients 1 through 5 whose IgG was preincubated with purified factor XI (expected to bind to each other), the binding to HK was either totally blocked or reduced (compare black bars with white bars). Shown are mean ± SD of triplicates.
Effect of patients' IgG on factor XI binding to immobilized HK measured indirectly by binding of alkaline phosphatase goat antihuman IgG to patients' IgG complexed with factor XI. The white bars (□) show that the IgG of patients 1 through 5 bound to microtiter plates coated with HK to which purified factor XI was attached, in contrast to normal IgG or IgG of a type II homozygote with no inhibitor (no. 6), which did not bind. The black bars (▪) show that normal IgG or IgG of a type II homozygote with no inhibitor which was preincubated with purified factor XI (not expected to bind to each other) did not bind to the plates coated with HK. In patients 1 through 5 whose IgG was preincubated with purified factor XI (expected to bind to each other), the binding to HK was either totally blocked or reduced (compare black bars with white bars). Shown are mean ± SD of triplicates.
Analysis of binding of patients' IgG to factor XIa by Western blotting. Factor XIa was electrophoresed on 8% SDS-PAGE (0.2 μg per lane) under reducing conditions, followed by transfer to a PVDF membrane. Each lane was then incubated with the IgG of the patients (lanes 1-4), and one lane was incubated with goat antihuman factor XI IgG (control). The lanes were then processed as described in “Patients, materials, and methods.” The 50-kDa heavy chain (HC) and the 30-kDa light chain (LC) are depicted. Normal control IgG and IgG purified from a type II homozygote with no inhibitor failed to recognize factor XI (data not shown).
Analysis of binding of patients' IgG to factor XIa by Western blotting. Factor XIa was electrophoresed on 8% SDS-PAGE (0.2 μg per lane) under reducing conditions, followed by transfer to a PVDF membrane. Each lane was then incubated with the IgG of the patients (lanes 1-4), and one lane was incubated with goat antihuman factor XI IgG (control). The lanes were then processed as described in “Patients, materials, and methods.” The 50-kDa heavy chain (HC) and the 30-kDa light chain (LC) are depicted. Normal control IgG and IgG purified from a type II homozygote with no inhibitor failed to recognize factor XI (data not shown).
The effect of patients' IgG on amidolytic activity of factor XIa. IgG from all 5 patients did not inhibit the amidolytic activity of purified factor XIa measured by cleavage of S2366. Thus, 6 nM factor XIa in the presence of control or patients' IgG similarly cleaved the chromogenic substrate, yielding an absorbance range of 93 to 98 mOD per minute at 405 nm.
The effect of patients' IgG on activation of factor XI.Figure 4A demonstrates the effect of patients' IgG on activation of factor XI by thrombin, and Figure 4B-D demonstrates the effect of the IgGs on the activation of factor XI by factor XIIa in the presence or absence of dextran sulfate and of HK. Notably, the IgG of 5 patients inhibited the activation of factor XI by 75% to 90%. Because factor XI is bound to HK in plasma, we next examined the effect of patients' IgG on the binding of factor XI to HK. Purified factor XI was preincubated with either normal IgG or patients' IgG and then added to microtiter plates coated with HK. The extent of factor XI binding was indirectly assessed by adding goat antihuman IgG, expected to detect the patients' IgG, complexed with factor XI. As illustrated in Figure 2, the IgG of 2 patients (1 and 2) almost completely abolished binding of factor XI to HK, whereas the IgG from 3 patients (3, 4, and 5) had only a moderate effect.
The effect of the inhibitors on factor XI activation as measured by a chromogenic assay using S2366 and expressed as the percentage of factor XIa activity generated in the presence of normal IgG. (A) Activation of factor XI by thrombin in the presence of dextran sulfate. (B) Activation of factor XI by factor XIIa. (C) Activation of factor XI by factor XIIa in the presence of dextran sulfate. (D) Activation of factor XI by factor XIIa in the presence of HK and dextran sulfate. “Normal” represents control IgG; 1 through 5, IgGs of patients with an inhibitor to factor XI; and 6, IgG of a type II homozygote without an inhibitor. Shown are mean ± SD of triplicates.
The effect of the inhibitors on factor XI activation as measured by a chromogenic assay using S2366 and expressed as the percentage of factor XIa activity generated in the presence of normal IgG. (A) Activation of factor XI by thrombin in the presence of dextran sulfate. (B) Activation of factor XI by factor XIIa. (C) Activation of factor XI by factor XIIa in the presence of dextran sulfate. (D) Activation of factor XI by factor XIIa in the presence of HK and dextran sulfate. “Normal” represents control IgG; 1 through 5, IgGs of patients with an inhibitor to factor XI; and 6, IgG of a type II homozygote without an inhibitor. Shown are mean ± SD of triplicates.
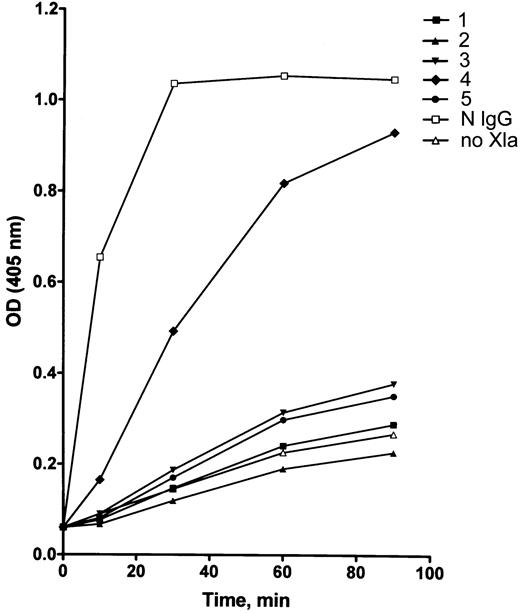
The effect of patients' IgG on activation of factor IX by factor XIa. Because all inhibitors examined failed to block the activity of factor XIa on a small substrate (S2366), it was intriguing to test whether factor IX activation by purified factor XIa would or would not be affected by the patients' IgG. As shown in Figure 5, in 4 of 5 patients, purified IgG profoundly inhibited factor IX activation.
The effect of the inhibitors on activation of factor IX. Purified factor XIa was incubated with patients' IgG prior to incubation with factor IX. Activation of factor IX was measured by its ability to activate factor X in the presence of factor VIII, thrombin, phospholipids, and calcium ions. Results express factor Xa activity measured by a chromogenic assay using S2765. N IgG designates normal IgG; no XIa indicates that factor XIa was not added; and nos. 1 through 5 represent patients' number. Mean of duplicates is displayed.
The effect of the inhibitors on activation of factor IX. Purified factor XIa was incubated with patients' IgG prior to incubation with factor IX. Activation of factor IX was measured by its ability to activate factor X in the presence of factor VIII, thrombin, phospholipids, and calcium ions. Results express factor Xa activity measured by a chromogenic assay using S2765. N IgG designates normal IgG; no XIa indicates that factor XIa was not added; and nos. 1 through 5 represent patients' number. Mean of duplicates is displayed.
The relation between HLA class II subtypes and presentation of an inhibitor to factor XI
HLA class II subtypes can affect the immune response by differential presentation of peptides from exogenously derived antigen by antigen-presenting cells in their interaction with helper T cells (CD4). We examined whether the occurrence of factor XI inhibitors was related to HLA class II subtypes. HLA class II genotypes of 10 unrelated patients with an inhibitor to factor XI (7 from Israel, 2 from the United Kingdom, and 1 from the United States) who were homozygous for type II mutation and had received plasma were compared with HLA class II genotypes of 13 patients who were also homozygous for type II mutation and had received plasma but did not develop an inhibitor. As shown in Table 3, no statistically significant differences in the distribution of DRB1* and DQB1* subtypes were found between patients with and without inhibitors.
. | Patients with an inhibitor, N = 10 . | . | Patients without an inhibitor, N = 13 . | . | . | ||
|---|---|---|---|---|---|---|---|
| HLA type . | No. . | % . | No. . | % . | P . | ||
| DRB1*01 | 1 | 10 | 2 | 15 | 1.00 | ||
| DRB1*03 | 1 | 10 | 2 | 15 | 1.00 | ||
| DRB1*04 | 6 | 60 | 5 | 38 | 0.41 | ||
| DRB1*0701 | 5 | 50 | 2 | 15 | 0.17 | ||
| DRB1*11 | 4 | 40 | 7 | 54 | 0.68 | ||
| DRB1*12 | 1 | 10 | 1 | 8 | 1.00 | ||
| DRB1*13 | 1 | 10 | 5 | 38 | 0.18 | ||
| DRB1*14 | 1 | 10 | 0 | 0 | 0.43 | ||
| DRB1*15 | 0 | 0 | 1 | 8 | 1.00 | ||
| DRB1*16 | 0 | 0 | 1 | 8 | 1.00 | ||
| DRB1*02 | 6 | 60 | 4 | 31 | 0.22 | ||
| DRB1*03 | 10* | 100 | 11† | 85 | 0.49 | ||
| DRB1*05 | 2 | 20 | 3 | 23 | 1.00 | ||
| DRB1*06 | 1 | 10 | 6 | 46 | 0.09 | ||
. | Patients with an inhibitor, N = 10 . | . | Patients without an inhibitor, N = 13 . | . | . | ||
|---|---|---|---|---|---|---|---|
| HLA type . | No. . | % . | No. . | % . | P . | ||
| DRB1*01 | 1 | 10 | 2 | 15 | 1.00 | ||
| DRB1*03 | 1 | 10 | 2 | 15 | 1.00 | ||
| DRB1*04 | 6 | 60 | 5 | 38 | 0.41 | ||
| DRB1*0701 | 5 | 50 | 2 | 15 | 0.17 | ||
| DRB1*11 | 4 | 40 | 7 | 54 | 0.68 | ||
| DRB1*12 | 1 | 10 | 1 | 8 | 1.00 | ||
| DRB1*13 | 1 | 10 | 5 | 38 | 0.18 | ||
| DRB1*14 | 1 | 10 | 0 | 0 | 0.43 | ||
| DRB1*15 | 0 | 0 | 1 | 8 | 1.00 | ||
| DRB1*16 | 0 | 0 | 1 | 8 | 1.00 | ||
| DRB1*02 | 6 | 60 | 4 | 31 | 0.22 | ||
| DRB1*03 | 10* | 100 | 11† | 85 | 0.49 | ||
| DRB1*05 | 2 | 20 | 3 | 23 | 1.00 | ||
| DRB1*06 | 1 | 10 | 6 | 46 | 0.09 | ||
All patients were exposed to plasma products.
One patient was homozygous for this allele.
Two patients were homozygous for this allele.
In vitro correction of thrombin generation by recombinant factor VIIa
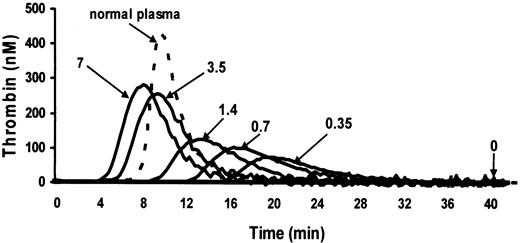
No thrombin generation was discernible when plasma from 5 patients with an inhibitor to factor XI was recalcified in the presence of phospholipids. Recombinant factor VIIa at final concentrations of 0.35 to 7 μg/mL corrected the thrombin generation in a dose-dependent manner, as illustrated in a representative patient (Figure 6). Notable are progressive shortening of the lag time until initial thrombin generation, increase in the height of thrombin peak, and progressive increase in ETP with increasing concentrations of recombinant factor VIIa. Correction of ETP to 50% of normal was obtained at a mean final concentration of 1 μg/mL recombinant factor VIIa in all 5 patients. Such a concentration is expected in plasma of patients who receive an infusion of 45 μg/kg recombinant factor VIIa.
Thrombin generation in a representative plasma with an inhibitor to factor XI in the absence and in the presence of increasing concentrations of recombinant factor VIIa. Thrombin generation was induced by recalcification in the presence of phospholipids. Numbers next to the plots denote final concentrations (μg/mL) of recombinant factor VIIa. The completely flat plot in the absence of recombinant factor VIIa is identical to the plots of thrombin generation in the plasma of homozygotes for type II mutation without an inhibitor (not shown).
Thrombin generation in a representative plasma with an inhibitor to factor XI in the absence and in the presence of increasing concentrations of recombinant factor VIIa. Thrombin generation was induced by recalcification in the presence of phospholipids. Numbers next to the plots denote final concentrations (μg/mL) of recombinant factor VIIa. The completely flat plot in the absence of recombinant factor VIIa is identical to the plots of thrombin generation in the plasma of homozygotes for type II mutation without an inhibitor (not shown).
Discussion
The presented data show that 7 of 118 unrelated patients with severe factor XI deficiency developed an inhibitor to factor XI. All 7 patients had received plasma replacement therapy and were homozygous for the nonsense type II mutation in the factor XI gene that is associated with extremely low levels of factor XI activity and antigenicity.6 The frequency of inhibitor formation among all individuals homozygous for type II mutation who received plasma was 7 of 21 (33%). No inhibitor to factor XI was detected in 84 patients with other genotypes whose factor XI activities ranged between 2 and 15 U/dL (except for 2 patients with factor XI activity of 1 U/dL). Of these 84 patients, 43 had received plasma. Three additional patients from the United Kingdom and the United States with an inhibitor to factor XI were also homozygous for type II mutation and had been previously exposed to plasma. These data indicate that the likelihood of developing an inhibitor is particularly high in patients with extremely low levels of plasma factor XI antigen who are exposed to exogenous factor XI. In accord with our findings are observations in patients with hemophilia A and B in whom null alleles of factor VIII and IX, respectively, were associated with a high frequency of inhibitor development.21,22
HLA class II subgroups were suggested to be a weak determinant for development of an inhibitor to factor VIII in hemophilia A.23 We therefore attempted to find such a relation in patients with factor XI deficiency and an inhibitor. However, the analysis of HLA DR and DQ polymorphisms did not reveal significant differences in the distribution of HLA class II genotypes between type II homozygotes with or without an inhibitor (Table 3).
Of the 14 previously described patients with inherited severe factor XI deficiency and an inhibitor,7-15 12 were of Jewish ancestry but in none was the mutation disclosed. Spontaneous bleeding was observed only in one of the reported patients8 and in none of the presently described patients. Exposure to plasma was probably the trigger for the development of inhibitors in all previously reported and currently described patients. An inhibitor to factor XI is usually detected in patients either during screening tests or following failure to achieve a significant rise of factor XI level following infusion of plasma or factor XI concentrate. In the present study, 4 of the 7 inhibitors were identified during screening tests, and 3 inhibitors were detected when no rise of factor XI level was observed after plasma infusion (Table 1).
Characterization of the factor XI inhibitors in the present study revealed that the IgG of the examined patients bound to immobilized factor XI and recognized factor XIa in Western blotting. In all patients, the IgG caused a decrease in binding of factor XI to HK, a markedly reduced activation of factor XI by factor XIIa in the presence or absence of dextran sulfate and HK, and profoundly reduced activation of factor XI by thrombin. Although the amidolytic activity of purified factor XIa was not impaired by the inhibitors, the activity of purified factor XIa on factor IX (the physiological substrate) was markedly reduced by the inhibitors in 4 patients. Similar findings were observed in previous reports, as follows: in 3 cases, binding of the inhibitor to factor XI was demonstrated8,13 ; in 3 cases, the inhibitor failed to decrease the amidolytic effect of factor XIa on a chromogenic substrate10,13 ;in2 cases, binding of factor XI to HK was impaired8,13 ; in 2 cases, the inhibitor impaired the activation of factor XI by factor XIIa8,13 ; and in 2 cases, the inhibitor impaired factor IX activation by factor XIa.8,13 Taken together, the data indicate that inhibitors to factor XI have several effects that are probably related to their binding to different epitopes of factor XI.
In summary, inhibitors to factor XI can develop in a relatively high proportion of patients whose factor XI is extremely low and who receive plasma replacement therapy. Because measurement of very low factor XI activity is not accurate, measurement of factor XI antigen and identification of the mutations involved can assist in defining which patients are susceptible to development of an inhibitor. Careful assessment of the need for plasma replacement therapy should be exercised during planning of surgery in such patients. Other treatment modalities such as antifibrinolytic agents and the use of fibrin glue should be considered as an alternative to plasma replacement therapy, particularly in minor procedures, for example, tooth extractions.24 If major surgery needs to be performed, use of plasma or a factor XI concentrate is unavoidable, but thereafter repeated testing for an inhibitor to factor XI should be carried out. If an inhibitor has developed, use of recombinant factor VIIa is an option, as was recently suggested.25 In the present study, it was shown that endogenous thrombin potential increased from 0% to 50% of normal in plasma of patients with an inhibitor to factor XI, even at a relatively low concentration of recombinant factor VIIa. Such a concentration is obtainable in vivo when recombinant factor VIIa is intravenously infused at a dose of 45 μg/kg. It remains to be demonstrated, however, that such a dose is hemostatically effective in patients with an inhibitor to factor XI who undergo surgery.
Prepublished online as Blood First Edition Paper, February 13, 2003; DOI 10.1182/blood-2002-09-2794.
O.S. and A.Z. contributed equally to the study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal