Abstract
Investigation of minimal residual disease (MRD) in acute leukemias by immunophenotyping and/or molecular techniques is proving to be increasingly valuable for disease monitoring. In acute lymphoblastic leukemia (ALL), most MRD studies have focused on children, whereas in contrast, information on the value of MRD on adult ALL is scanty, and almost exclusively restricted to polymerase chain reaction (PCR) studies. Early response to therapy is one of the most important prognostic factors in acute leukemia, which prompted us to investigate whether or not early immunophenotypic assessment of MRD could also be a valuable tool for predicting relapse in adult patients with ALL. For that purpose we have analyzed the level of MRD during the initial phase of treatment (induction phase) by multiparameter flow cytometry in a series of 102 adolescent (older than 14 years) and adult patients with ALL. Immunophenotypic evaluation of the bone marrow (BM) at day +35 showed that patients with low MRD levels (< 0.05% leukemia-associated phenotype [LAP+] cells) had a significantly longer relapse-free survival (RFS) than patients with high MRD levels, and this prognostic influence was retained when only those patients in morphologic complete remission (mCR) at day +35 were considered (median RFS: 42 months vs 16 months; P = .001). Moreover, immunophenotyping helped to identify a small subset of patients (n = 12) with negative or low MRD levels (< 0.03% LAP+ cells) by day +14, with an excellent prognosis (projected RFS of 90% at 5 years). The contrary is true of patients who achieved late mCR (after day +35), since immunophenotypic investigation of MRD showed that, in spite of the mCR, none of the cases with more than 0.1% LAP+ cells would be relapse-free after 2 years. Multivariate analysis showed that the immunologic evaluation of MRD at day +35 was the most relevant independent prognostic parameter for adult patients with ALL, and together with age, white blood cell (WBC) count at diagnosis, and presence of the Philadelphia (Ph) chromosome, represented the most informative combination of variables for predicting relapse-free survival.
Introduction
Investigation of minimal residual disease (MRD) by immunopheno-typing and/or molecular techniques is increasingly used in clinical practice for monitoring acute leukemias.1-30 In acute lymphoblastic leukemia (ALL) most MRD studies have focused on children, and they have shown that both immunophenotyping2-6,9,23,28 and polymerase chain reaction (PCR) investigation1,14-16,18,19,21,27 provide relevant prognostic information. In contrast, information on the value of MRD in adult ALL is scanty, and almost exclusively restricted to PCR analysis,10,11,15,17,20,21 since very few immunophenotypic studies, always based on a small number of cases, have been reported so far.4,5,7 Moreover, most MRD studies in ALL have been based on sequential analysis of follow-up samples, which are laborious and expensive.1-7,10,14,15,17,19,20,23,27 An alternative is to focus on therapeutically relevant time points at which the level of MRD may provide prognostic information, to help in the treatment decision-making process. It is well known that early response to therapy is a relevant prognostic factor in acute leukemia. Thus, we have recently communicated13 that early immunophenotypic evaluation of MRD in acute myeloid leukemia (AML) identifies different patient risk categories. In a similar way, in childhood ALL, it has been shown that morphologic evaluation of blast cells in peripheral blood (PB) or bone marrow (BM) during induction therapy,31-35 as well as molecular analysis of the BM on day +15,22 helps to identify different prognostic subgroups.
The prognosis of adult patients with ALL is dismal and early indicators of disease outcome would be particularly useful for the design of new experimental treatments. Despite this, no studies have been reported so far in which the prognostic impact of MRD as assessed by immunophenotypic techniques has been analyzed in a large series of adult patients with ALL.
We hypothesize that immunophenotypic evaluation of MRD during the initial phases of therapy (induction phase) might be of help in identifying prognostic categories in adolescent and adult patients with ALL. In order to contrast our hypothesis we have analyzed the levels of MRD in BM from 102 adolescent (older than 14 years) and adult patients with ALL using multiparametric flow cytometry. Our results show that the level of MRD in the BM obtained at day +14 and day +35 of therapy allows the discrimination between patients at high risk of relapse and those who have a relatively high chance of remaining in continuous complete remission (CR), thereby contributing to postinduction treatment stratification.
Patients, materials, and methods
Patients
A total of 102 adolescent (older than 14 years) and adult patients with ALL were included in the study. Eligibility criteria for entering the study were as follows: (1) to be consecutive ALL cases; (2) to have achieved morphologic complete remission (mCR) following induction therapy; and (3) availability of BM samples in mCR for inmunophenotypic investigation of MRD. There were 82 patients diagnosed and treated at the University Hospital of Salamanca, Spain, and 20 patients at the Portuguese Institute for Oncology, Lisbon, Portugal. As mentioned, one of the criteria for entering the study was to have achieved mCR; the 102 cases that fulfill this criteria derive from a total series of 129 patients with ALL immunophenotypically analyzed at diagnosis.
Of the 102 patients, 63 were male and 39 female, with a median age of 31 years (range: 14-82 years). According to the French-American-British (FAB) classification, 92 cases were L1 or L2 ALL and 10 corresponded to L3 cases. There were 73 cases with a B-lymphoid origin, and 29 cases with a T-lymphoid origin. Immunophenotypic classification36 was as follows: 13 cases were pro-B or BI; 51 were BII common ALL; 6 cases corresponded to pre-B (BIII) ALL; 3 were mature B (BIV) ALL; 4 cases were T-I ALL; 8 were T-II ALL; 4 were T-III ALL; and 13 corresponded to T-IV ALL.
Patients were treated with Berlin-Frankfurt-Munster (BFM)–oriented protocols slightly modified by the Spanish Pethema Cooperative group protocols.37,38 These protocols included a 5-week induction therapy with prednisone, vincristine, doxorubicin, and cyclophosphamide. Morphologic CR was defined by less than 5% blast cells in a regenerated BM aspirate, absence of extramedullary leukemia, and PB neutrophil and platelet counts of more than 1.5 × 109/L and more than 100 × 109/L, respectively.
mCR was achieved by 28 patients at day +14, 45 patients at day +35, and the remaining 29 patients after day +35 (median: 57 days; range, 45-75 days). In 65 cases, patients were treated only with chemotherapy, while the other 37 patients were consolidated with either autologous peripheral stem cell transplantation (14 cases) or allogeneic transplantation (23 patients). Selection criteria for transplantation included poor cytogenetics and late CR. The median times for relapse-free survival (RFS) and overall survival (OS) were 23 months (range, 3-130 months) and 34 months (range, 3.5-151 months), respectively. RFS and OS were not significantly different between patients who received transplants and those who received conventional chemotherapy (P = .6). At the time this study closed, the median follow-up was 29 months (range, 3-151 months), and 59 cases (58%) have already relapsed.
The following variables collected at diagnosis were included in the analysis: age, white blood cell (WBC) and platelet counts, hemoglobin (Hb) levels, percentage of blast cells in BM and absolute number in PB, days to achieve mCR, type of treatment received, morphologic FAB classification, immunophenotype, and cytogenetics. Karyotypic findings were as follows: the Philadelphia (Ph′) chromosome (with or without other aberrations) was found in 13 cases; 3 patients showed hypodiploid karyotypes, and hyperdiploidy was detected in another 3 cases; t(8;14) or t(8;22) in the presence or absence of additional abnormalities was detected in 5 cases, other aberrancies were found in 6 cases; a complex karyotype was found in 1 case; and a normal karyotype was found in 24 cases. The presence of the Ph' chromosome was excluded by fluorescence in situ hybridization (FISH) in 20 other Ph′ chromosome–negative cases. In the remaining 27 cases, karyotypic information was not available. The 2 patients with ALL who had either t(8;14) or t(8;22) and who did not show a mature (B-IV) B-cell phenotype were classified as pre-B (BIII).
Immunophenotypic investigation of MRD
Erythrocyte-lysed whole BM samples obtained at diagnosis were studied. All samples were stained with an identical panel of 3-color combinations of monoclonal antibodies (mAbs) aimed at the specific identification and immunophenotypic characterization of blast cells. Antigenic expression on blast cells at diagnosis was systematically analyzed by multiparametric flow cytometry (FACScan, Becton Dickinson Biosciences, San José, CA) according to previously described methods.4,13,24,39-42 The following triple-stainings (fluorescein isothiocyanate [FITC], phycoerythrin [PE], peridinin chlorophyll protein [PerCP], or phycoerythrin-cyanin 5 [PE-Cy5]) were used: CD4/CD8/CD3, CD7/CD5/CD3, CD19/CD34/CD45, CD10/CD13/CD19, CD5/CD33/CD20, CD65/CD2/HLA-DR, CD34/CD38/CD19, CD10/CD20/CD19, CD7/CD2/CD3, CD34/CD22/CD19, and TdT/CD10/CD19. In addition, in T-lineage ALL (T-ALL) and L3, a CD7/CD34/CD38 and a surface immunoglobulin (sIg) light chain sIg-Kappa/sIg-Lambda/CD19 triple-staining was used, respectively. All mAbs were purchased from Becton Dickinson, except for CD34 PE-Cy5 (Immunotech, Marseille, France), CD19 PE-Cy5, CD33 PE-Cy5, CD13 PE-Cy5, CD4 PE-Cy5, CD65 FITC, CD45 PE-Cy5, and CD20 PE-Cy5 (Caltag Laboratories, San Francisco, CA).
Using a 5-dimensional space formed by the 2 light scatter parameters— forward (FSC) and sideward light scatter (SSC)—and the 3 mAb-associated fluorescence emissions, we specifically identified the blast cells and defined their phenotypic characteristics at diagnosis, including the aberrant phenotypes. Based on our previous experience,4,13,39-42 4 main types of aberrant phenotypes were defined: (1) cross-lineage antigen expression; (2) asynchronous antigen expression; (3) antigen overexpression; and (4) ectopic phenotypes. At diagnosis, 89 cases were classified as having an aberrant phenotype and 13 cases were classified as having a minor phenotype (< 10—3 in normal adolescent [older than 14 years] and adult BM).
For the investigation of MRD, only those mAb combinations selected at diagnosis as informative for MRD studies were used. In order to increase the sensitivity of the analysis, data acquisition in the flow cytometer for follow-up samples was performed in 2 consecutive steps. Briefly, in the first step, acquisition of all cells present in the sample was performed and at this point at least 20 000 events per tube were measured. Subsequently, in a second step, a multiparametric live gate was used to acquire more data specifically on leukemic cells potentially present at low frequencies in the sample. For that purpose, acquisition through a low to intermediate SSC/CD19+ or CD7+ antigen live gate was performed and information was collected for at least 105 BM nucleated cells. Data analysis was based on the identification of cells with aberrant phenotypic features identical to those of leukemic cells at diagnosis.
Immunophenotyping study of MRD was investigated on day +14 in 63 of the 102 cases included in the study. It should be noted that this BM evaluation was not mandatory. From these 63 cases, 28 were already in CR while in the other 35 cases at least 5% blast cells persisted on morphologic grounds. As far as the BM sample on day +35 is concerned, from the total 102 patients included in the study, 73 were in mCR at this time point (this figure corresponds to the above mentioned 28 patients who had already achieved CR on day +14 plus 45 patients who achieved mCR on day +35) and all were screened for MRD. The remaining 29 patients achieved a late mCR (between day +45 and +75) and MRD was evaluated at this late time point. Nevertheless, 14 of these 29 patients had also been immunophenotypically analyzed on day +35, although they were not in mCR; therefore the total number of cases immunophenotypically studied at day +35 was 87 (73 in mCR plus 14 not in mCR). The remaining 15 late mCR were not referred for MRD evaluation at day +35, because the referral center considered that they were not candidates since overt leukemia (> 10% blast cells) still persisted on morphologic examination.
For data acquisition, either the LYSIS II or Cell-Quest software programs (Becton Dickinson Biosciences) were used. The PAINT-A-GATE PRO software program (Becton Dickinson Biosciences) with the polynomial SSC transformation capability was used for further data analysis. Analysis was performed on gated blast cells according to previously defined methods.13,39,42
Statistical analysis
The chi square and the Mann-Whitney U tests were used to estimate the statistical significance of differences observed between groups. Survival curves were plotted according to the method of Kaplan and Meier and comparison between the curves was performed using the log-rank and Breslow tests.43 In the univariate analysis for RFS, the following variables were tested: age, sex, WBC count, cytogenetics, days to achieve mCR (+14, +35), type of treatment, immunologic classification, and MRD levels at different time points after induction therapy. Subsequently, a multivariate analysis—stepwise regression44 —was performed to explore the independent effect of variables that showed a significant influence on disease-free survival in the univariate analysis (age, WBC count, cytogenetics, time to achieve mCR, and MRD levels at different time points after induction therapy). Quantitative variables were considered continuous as well as dicothomic.
All the statistical analyses were performed using the SPSS software, 10.0.1S version (SPSS Inc).
Results
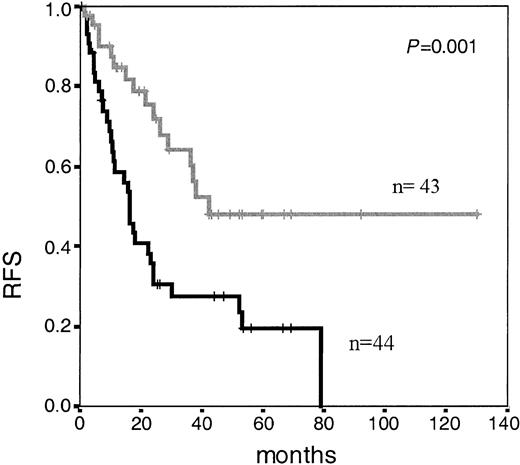
At day +35, immunophenotypic BM analysis was carried out in 87 patients. The number of residual blast cells showed that several threshold levels (ranging from 0.05% to 1% cells with a leukemia-associated phenotype [LAP+]) could be used for discrimination of different risk categories, with the 0.05% residual blast cells cut-off point showing the strongest discriminating power. Accordingly, patients with MRD levels less than 0.05% LAP+ cells (n = 43) had significantly longer RFS than patients with MRD levels of at least 0.05% (n = 44) (median: 42 months vs 16 months, respectively; P = .001) (Figure 1). Upon focusing exclusively on those patients who had achieved mCR by day +35 (n = 73), MRD levels were also found to have an impact on the risk of relapse: median RFS of 42 months versus 17 months for patients with low (n = 43) and high levels (n = 30) of MRD (< 0.05% and ≥ 0.05% LAP+ cells; P = .001). Upon comparing the clinical characteristics of patients who achieved low MRD status (< 0.05% LAP+ cells) to those who did not achieve this level (high MRD >= 0.05%), at day +35, the latter group was characterized by a higher median age, and a higher incidence of cases with adverse karyotype (Ph'-positive, complex, and hypodiploid) (Table 1).
Relapse-free survival in adolescent and adult patients with ALL according to immunophenotypic MRD level at day +35 of induction therapy. Median RFS of 42 months for patients with low MRD levels (< 0.05%; n = 43; gray line) versus 16 months for patients with high MRD levels (≥ 0.05%; n = 44; black line) (P = .001).
Relapse-free survival in adolescent and adult patients with ALL according to immunophenotypic MRD level at day +35 of induction therapy. Median RFS of 42 months for patients with low MRD levels (< 0.05%; n = 43; gray line) versus 16 months for patients with high MRD levels (≥ 0.05%; n = 44; black line) (P = .001).
Comparison of clinical characteristics of patients who achieve low MRD status (< 0.05% LAP+ cells) to those who did not achieve this level (high MRD: ≥ 0.05%) at day +35
. | Cases with MRD level < 0.05% at day +35 . | Cases with MRD level at least 0.05% at day +35 . | P . |
|---|---|---|---|
| Sex, male/female | 23/20 | 40/19 | .14 |
| Age, in years (range)* | 23 (15-74) | 35 (15-82) | .03 |
| WBC count, ×109/L* (range) | 11.9 (0.3-857) | 10.6 (0.9-295) | .93 |
| Hb level, g/dL* (range) | 9.5 (4.5-15) | 11.4 (4.8-19) | .24 |
| Platelets, ×106/L* (range) | 55 (3-224) | 54 (9-292) | .25 |
| Percent blast cells in PB* (range) | 59 (0-98) | 52 (0-100) | .74 |
| Percent blast cells in BM* (range) | 74 (30-98) | 80 (30-98) | .10 |
| Adverse karyotype: Ph′-positive, hypodiploid, complex (percent) | 5/29 (17%) | (12/46) (26%) | .06 |
| Inmmunophenotype | |||
| B-ALL (%) | 34 (79) | 39 (66) | .11 |
| T-ALL (%) | 9 (21) | 20 (34) | |
| Consolidation therapy | |||
| Chemotherapy (%) | 29 (66) | 36 (62) | .83 |
| Autologous BMT (%) | 5 (12) | 9 (16) | |
| Allogeneic BMT (%) | 10 (23) | 13 (22) |
. | Cases with MRD level < 0.05% at day +35 . | Cases with MRD level at least 0.05% at day +35 . | P . |
|---|---|---|---|
| Sex, male/female | 23/20 | 40/19 | .14 |
| Age, in years (range)* | 23 (15-74) | 35 (15-82) | .03 |
| WBC count, ×109/L* (range) | 11.9 (0.3-857) | 10.6 (0.9-295) | .93 |
| Hb level, g/dL* (range) | 9.5 (4.5-15) | 11.4 (4.8-19) | .24 |
| Platelets, ×106/L* (range) | 55 (3-224) | 54 (9-292) | .25 |
| Percent blast cells in PB* (range) | 59 (0-98) | 52 (0-100) | .74 |
| Percent blast cells in BM* (range) | 74 (30-98) | 80 (30-98) | .10 |
| Adverse karyotype: Ph′-positive, hypodiploid, complex (percent) | 5/29 (17%) | (12/46) (26%) | .06 |
| Inmmunophenotype | |||
| B-ALL (%) | 34 (79) | 39 (66) | .11 |
| T-ALL (%) | 9 (21) | 20 (34) | |
| Consolidation therapy | |||
| Chemotherapy (%) | 29 (66) | 36 (62) | .83 |
| Autologous BMT (%) | 5 (12) | 9 (16) | |
| Allogeneic BMT (%) | 10 (23) | 13 (22) |
Values represent means.
Upon analyzing the 29 patients who achieved late mCR (after day +35), it should be noted that none of the cases with more than 0.1% residual LAP+ cells (n = 15) was relapse-free at 24 months, versus 29% of patients with less than 0.1% LAP+ cells (n = 14) (P = .3). The overall survival of the total group of late mCR achievers was significantly poorer than that of patients in mCR on day +35 but not day +14 (median disease-free survival of 24 months vs 11 months, respectively; P = .04). Late mCR achievers showed an older age (mean: 40 years; range: 15-82 years) than patients in mCR at day +35 (mean 25; range: 15-74; P = .001), as well as a higher proportion of Ph′+ plus hypodiploid karyotypes as compared with patients in mCR at day +35 (38% vs 15%; P = .01). Other biologic characteristics (WBC count, Hb levels, platelet levels, percentage of blast cells in PB and BM, and FAB and immunophenotypic classification) were similar in both groups of patients.
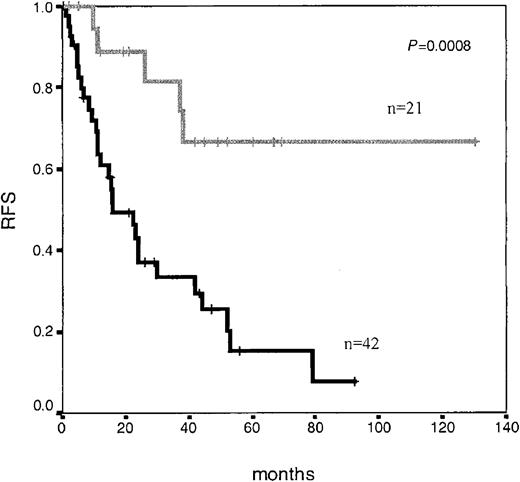
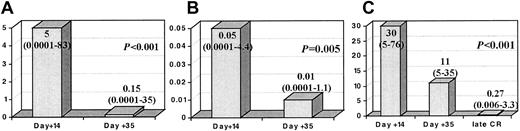
Interestingly, although it was not mandatory, we had the opportunity to perform immunophenotypic BM analysis on day +14 in 63 patients, of which 28 patients had less than 5% blast cells by morphology, and, as expected, these 28 patients showed significantly longer RFS (P < .001). Once again, several threshold points (0.01% to 1%) on a number of LAP+ cells at this time point discriminated different risk categories of patients (Figure 2). Moreover, immunophenotypic evaluation of MRD allowed the identification of a very good prognostic subgroup: 11 of the 12 (90%) patients who showed negative or very low MRD levels (< 0.03% LAP+ cells) by day +14 (n = 12) (which could be considered as early immunophenotypic remission) were relapse free at 5 years. As shown in Figure 3, in those cases that were sequentially analyzed, the number of LAP+ cells significantly decreased both from day +14 to day +35 (Figure 3A, mean: 5% at day +14 to 0.15% at day +35; Figure 3B, mean: 0.05% at day +14 to 0.01% at day +35) and from day +35 to late mCR evaluation (Figure 3C, mean: 30% at day +14 to 11% at day +35, and to 0.27% at late mCR).
Relapse-free survival in adolescent and adult patients with ALL according to immunophenotypic MRD level at day +14 of induction therapy. Median RFS not reached for patients with low MRD levels (< 0.5%; n = 21; gray line) versus 16 months for patients with high MRD levels (≥ 0.5%; n = 42; black line) (P = .0008).
Relapse-free survival in adolescent and adult patients with ALL according to immunophenotypic MRD level at day +14 of induction therapy. Median RFS not reached for patients with low MRD levels (< 0.5%; n = 21; gray line) versus 16 months for patients with high MRD levels (≥ 0.5%; n = 42; black line) (P = .0008).
Changes in LAP+cells throughout sequential studies. Columns represent the median (range) percentage as LAP+ cells. (A) The 63 cases tested at day +14 and day +35. (B) Patients in mCR at day +14 (28 cases). (C) The 14 patients who achieved a late mCR and had been tested at the 3 time points (day +14, day +35, and the late mCR evaluation). Please note that Y-axis scales are different between panels A, B, and C.
Changes in LAP+cells throughout sequential studies. Columns represent the median (range) percentage as LAP+ cells. (A) The 63 cases tested at day +14 and day +35. (B) Patients in mCR at day +14 (28 cases). (C) The 14 patients who achieved a late mCR and had been tested at the 3 time points (day +14, day +35, and the late mCR evaluation). Please note that Y-axis scales are different between panels A, B, and C.
Regarding other clinical and biologic variables influencing RFS, univariate analysis showed that time to achieve mCR had a significant influence. Thus, patients achieving mCR at day +14 had a significantly better RFS than patients achieving mCR at day +35 or later (median RFS: not reached, 24 months, and 12 months, respectively; P < .0001). Other disease characteristics with significant impact on RFS were age (discriminatory at 30 years and 60 years, with an RFS of 24 months vs 10 months, P = .002, for patients younger vs older than 60 years; and with an RFS of 37 months vs 16 months, P = .004, for patients younger vs older than 30 years), WBC count (discriminatory at 30 × 109/L, with an RFS of 28 months vs 12 months; P = .03), and the presence of Ph' chromosome (RFS: 10 months vs 38 months; P = .04). We did not find differences for RFS based on the type of consolidation therapy used (chemotherapy vs transplantation, either autologous or allogeneic), or on the type of aberrant phenotype present at diagnosis (cross-lineage antigen expression, asynchronous antigen expression, antigen overexpression, or ectopic phenotypes).
In order to explore whether or not the level of MRD was an independent prognostic factor for RFS among adolescent and adult patients with ALL, a multivariate analysis was conducted considering only those variables that had a prognostic influence in the univariate study (age, WBC count, Ph' chromosome, time to achieve mCR, and MRD level). In the whole series, variables having independent prognostic influence in the Cox model for RFS were level of MRD (P = .01), age (P = .05), and WBC count (P = .05). When we restricted the analysis to patients in mCR at day +35, only MRD level (P = .02) and the presence of Ph' chromosome (P = .04) had independent influence on RFS.
Discussion
Evidence supporting the value of MRD investigation in acute leukemias by immunophenotyping and/or molecular techniques has been increasingly reported over the last few years.1-28 The potential clinical value of these techniques is of particular interest for adolescent and adult ALL, since this group of patients shows a high incidence of relapse which, together with the lower complete remission rates observed among them as compared with childhood ALL, results in a very low percentage of long-term survivors.26,45,46 Therefore, complementary markers must be sought that would allow closer and more precise disease monitoring in order to establish alternative therapeutic strategies for those adolescent and adult patients with ALL at high risk of relapse.8,26,45,46
Several studies have been reported concerning the molecular investigation of MRD in adult patients with ALL. Mortuza et al,15 using semiquantitative immunoglobulin heavy chain (IgH) gene analysis in a series of 85 adult precursor B-lineage ALL (B-ALL), checked at 4 time points during the first 24 months of treatment, have shown that MRD positivity was associated with increased relapse rates. These data are concordant with the results reported by Brisco et al11 in a series of 27 patients. Radich et al10 have shown in a group of 36 Ph'-positive patients with ALL, of which 29 were adults, that persistence of BCR-ABL positivity was associated with a higher incidence of relapse, which is in line with the observation of Mitterbauer et al17 in a series of 20 Ph'-positive adult patients with ALL.
As far as immunophenotypic analysis of MRD in ALL is concerned, most investigations have focused on children,2-6,9,23,28 with no study specifically devoted to the analysis of adult patients with ALL. Coustan-Smith et al2,3 have analyzed the level of residual disease in bone marrow at different time points (weeks 14, 32, and 56, and at the end of therapy) in a large series of pediatric patients with ALL showing that the presence of detectable LAPs were associated with a high frequency of subsequent relapses, regardless of the time at which bone marrow samples were examined. In this study, patients with and without detectable leukemia after induction therapy had a 3-year cumulative incidence of relapse of 33% versus 7.5%. Dworzak et al,23 using immunophenotyping, have evaluated MRD at 4 follow-up points in a series of 108 children with ALL, showing that the best points for discriminating different risk group patients were day +33 and week 12. Although some series of immunophenotyping-based MRD studies have included both children and adults4,5,7 and suggest that this technique can also provide prognostic information for adult ALL, the number of adult cases was very limited to reach firm conclusions. In fact, to the best of our knowledge, this is the first report in which the clinical impact of MRD investigation by immunophenotyping is specifically analyzed in a large series of adolescent and adult patients with ALL using a standardized and reproducible approach.
Sequential follow-up MRD studies are expensive and time consuming and accordingly, identification of the most therapeutically relevant time points for MRD investigation would be of great value. Nowadays, it is well known that early response to therapy is one of the most important prognostic factors in acute leukemia. In AML, early response evaluated by conventional morphology47,48 or by immunophenotyping13 identifies different patient risk groups and it could be used for stratification of postinduction therapy. In childhood ALL, it has also been shown that morphologic evaluation of blast cells in PB or BM during induction therapy31-35 as well as molecular analysis of the BM on day +1522 helps to identify patients at different risk of relapse. In addition, in a recent communication Coustan-Smith et al28 reported that immunophenotypic evaluation of BM at day +19 of therapy is superior to that defined by morphologic studies, and is a valuable prognostic indicator in childhood ALL. Concerning molecular techniques, in a study based on 68 pediatric patients with ALL, Panzer-Grümayer et al22 evaluated residual disease at day +15 and day +35 by molecular methods, showing that all patients with negative or low MRD levels at day +15 (14 cases) had an excellent prognosis. In line with these findings, Mortuza et al15 found that MRD positivity detected at early time points in adults with ALL was also associated with an increased risk of relapse. This information provided by molecular studies prompted us to investigate whether or not early immunophenotypic assessment of MRD could also be a valuable tool for predicting relapse in adolescent and adult ALL. Our results show that immunophenotypic evaluation of MRD during the initial phase of therapy (induction phase), through the analysis of the BM obtained at day +14 and day +35 of therapy, allows the definition of different risk categories in adolescent and adult patients with ALL. Immunophenotypic evaluation of the BM at day +35 showed that patients with low MRD levels (< 0.05% LAP+ cells) had a significantly longer RFS than patients with high MRD levels, and that this prognostic influence was retained when only those patients in mCR at day +35 were considered. Upon comparing these results with the recent morphologic data reported by Sandlund et al,32 it can be observed that the threshold level provided by immunophenotyping for discrimination of different risk categories is significantly lower than that afforded by morphology (0.05% vs 1%). Moreover, immunophenotyping helped to identify a small subset of patients (n = 12) with negative or low MRD levels (< 0.03% LAP+ cells) by day +14, with an excellent prognosis (projected RFS of 90% at 5 years). The opposite situation applied to patients who achieved late mCR (after day +35), since immunophenotypic investigation of MRD showed that, in spite of the mCR, none of cases with more than 0.1% LAP+ cells would be relapse free after 2 years. In addition to the level of MRD, age, WBC count, time to achieve mCR, and the presence of the Ph' chromosome also showed a significant influence on RFS in our series. The prognostic influence of these latter variables has been previously reported in other series.26,45,46 By contrast, other variables such as the type of consolidation therapy, Hb level, FAB and immunophenotypic classification, did not show a prognostic impact on RFS. Interestingly, in the present series multivariate analysis showed that the immunologic evaluation of MRD at day +35 was the most relevant independent prognostic parameter for adolescent and adult patients with ALL, and together with age and WBC count at diagnosis represented the most informative combination of variables for predicting RFS. When we considered only patients who were in mCR at this follow-up time point, only the MRD levels and the presence of a Ph' chromosome had independent value for predicting RFS.
In summary, in the present study we show that multiparametric flow cytometry evaluation of MRD during induction therapy is a valuable tool for relapse prediction in adolescent and adult ALL, and that high MRD levels identify a cohort of patients with a poor prognosis, for whom new alternative therapeutic approaches are required.
Prepublished online as Blood First Edition Paper, February 13, 2003; DOI 10.1182/blood-2002-08-2613.
This work was integrated in the European BIOMED-1 Concerted Action (BMH-CMT 94-1675).
P.L., A.P., A.O., and J.F.S.-M. are members of the European BIOMED-1 Concerted Action (BMH-CMT 94-1675).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank all members of the BIOMED-1 Concerted Action (BMH-CMT 94-1675), particularly Jacques van Dongen for the fruitful discussions during the standardization of MRD studies. We would also like to thank M. Anderson and M. J. Rodrigo for their help with the English language.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal