Abstract
The BCR/ABL tyrosine kinase inhibitor imatinib mesylate (Gleevec, STI571; Novartis, Basel, Switzerland) has shown remarkable efficacy in the treatment of chronic myelogenous leukemia (CML), with a high proportion of patients achieving complete cytogenetic responses (CCRs). However, it is not clear whether remissions will be durable and whether imatinib mesylate can eliminate the malignant primitive progenitors in which the disease arises. We investigated whether residual BCR/ABL+ hematopoietic progenitors were present in patients who achieved CCRs with imatinib mesylate treatment. CD34+ progenitor cells were selected from bone marrow mononuclear cells (MNCs) and analyzed for the presence of the BCR/ABL fusion gene by fluorescence in situ hybridization (FISH). CD34+ cells were also plated in committed progenitor (colony-forming cell, or CFC) and primitive progenitor (long-term bone marrow culture-initiating cell, or LTCIC) cultures and resulting colonies analyzed for the presence of BCR/ABL+ cells by FISH. Using these assays, residual BCR/ABL+ progenitors were detected in all patients studied. Quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) analysis demonstrated increased levels of BCR/ABL mRNA in CD34+ cells compared with total MNCs. Evaluation of samples collected at different time points demonstrated persistence of BCR/ABL+ progenitors despite continued treatment with imatinib mesylate. Our results indicate that inhibition of BCR/ABL tyrosine kinase activity by imatinib mesylate does not eliminate malignant primitive progenitors in CML patients. Patients in CCR with imatinib mesylate treatment need to be followed carefully to assess for risk of relapse.
Introduction
Chronic myelogenous leukemia (CML) is a lethal hematologic disorder that results from malignant transformation of a very primitive hematopoietic cell. The disease usually has a triphasic course, presenting in an initial chronic phase (CP) but invariably progressing over time to an intermediate accelerated phase (AP) and a terminal blast crisis (BC). Cells of malignant clonal origin in CML are characterized by a balanced translocation between chromosomes 9 and 22, leading to the fusion of a portion of the ABL gene from chromosome 9 with the BCR gene on chromosome 22.1,2 The resulting BCR/ABL gene has been shown to play a critical role in the pathogenesis of CML.3-5 The c-ABL gene product is a tyrosine kinase with tightly regulated activity.6,7 The BCR/ABL gene product, p210BCR/ABL, demonstrates constitutive activation of tyrosine kinase activity with enhanced substrate phosphorylation compared with c-ABL.8-10 Abnormal kinase activity appears to be critical for BCR/ABL-induced transformation.8,11
Imatinib mesylate (Gleevec, STI571; Novartis, Basel, Switzerland) is a 2-phenylaminopyrimidine compound that has been demonstrated to be a potent inhibitor of all ABL tyrosine kinases (c-ABL, BCR/ABL, and Tel/ABL) as well as the platelet-derived growth factor (PDGF) receptor tyrosine kinase and c-kit tyrosine kinases.12 Other tyrosine kinases are not significantly inhibited. In preclinical studies, imatinib mesylate demonstrated high levels of activity against BCR/ABL-containing cells in vitro and in vivo.13,14 Subsequently phase 1 and phase 2 clinical trials have shown remarkable activity of imatinib mesylate in the treatment of CML.15-17 Almost all patients in CP achieve hematologic response, and a high proportion of patients achieve major and complete cytogenetic responses.17 The drug is also effective in patients with AP and BC CML although with a considerably lower rate of response.18-20
Although the success of this molecular therapeutic approach represents an exciting advance in targeted cancer therapy, it still needs to be determined whether responses to imatinib mesylate in CML patients will be durable. Recent reports indicate that BCR/ABL transcripts, although significantly reduced, can still be detected in patients in complete cytogenetic response (CCR) on imatinib mesylate and that molecular remissions are rare.21,22 In patients with CML in AP and BC, relapses are observed even after induction of complete cytogenetic response.18,19 Relapses have been infrequent in patients with CP disease, but follow-up is limited.17 It is not clear whether imatinib mesylate effectively targets and eliminates malignant primitive progenitors in which the disease originates. Persistent malignant progenitors could be a source of relapse in patients in CCR.
We have previously shown that in vitro exposure to imatinib mesylate results in inhibition of primitive progenitor growth. Suppression of primitive progenitor growth was partial and occurred mainly through inhibition of abnormally increased proliferation rather than through a selective increase in progenitor apoptosis.23 These observations suggest that BCR/ABL tyrosine kinase inhibition by imatinib mesylate may suppress but not eliminate all malignant progenitors in CML patients. On the other hand, it is possible that prolonged exposure to imatinib mesylate in vivo could have an increased suppressive effect on malignant primitive progenitors. In this study we investigated whether malignant primitive hematopoietic progenitors could be detected in bone marrow samples obtained from CML patients in complete cytogenetic remission following imatinib mesylate treatment. CD34+ cells selected from bone marrow mononuclear cells (MNCs) and colonies derived from in vitro assays of committed and primitive progenitors were analyzed for the presence of the BCR/ABL gene.
Patients, materials, and methods
Samples
Bone marrow samples were obtained from 15 CML patients who were identified to be in complete cytogenetic response (CCR) after imatinib mesylate treatment using guidelines approved by the Institutional Review Board of the City of Hope National Medical Center. CCR was defined as the complete absence of t(9;22) on karyotypic analysis or 6% or fewer BCR/ABL+ cells (normal background limits) on fluorescence in situ hybridization (FISH) analysis. For some patients 2 or more samples were obtained at different time points.24
MNCs were isolated by Ficoll-Hypaque (Sigma Diagnostics, St Louis, MO) density gradient separation (specific gravity 1.077) for 30 minutes at 400g. CD34+ cell–enriched populations were selected from MNCs using immunomagnetic column separation (Miltenyi Biotech, Auburn, CA).
Progenitor assays
Colony-forming cells. CD34+ cells were plated in semisolid methylcellulose progenitor culture for 14 to 18 days and assessed for the presence of granulocyte-macrophage colony-forming unit (CFU-GM) and erythroid burst-forming unit (BFU-E) colonies as previously described.25
Long-term culture-initiating cells (LTCICs). Cells were plated in long-term bone marrow culture (LTBMC) medium on M2-10B4 murine fibroblast feeders previously subcultured in 24-well plates. Cultures were maintained at 37°C in a humidified atmosphere with 5% CO2 and fed at weekly intervals by removal of half the medium from the wells and replacement with fresh medium. After 6 weeks all nonadherent and adherent cells were harvested, pooled, and plated in methylcellulose progenitor culture and the number of colony-forming cells (CFCs) evaluated after 14 days.26
Fluorescent in situ hybridization (FISH) analysis
FISH analysis was performed on freshly isolated total nucleated cells or selected CD34+ cells or on cells derived from colonies resulting from CFC and LTCIC assays, which were harvested and pooled. Cells were resuspended in hypotonic KCl solution at 37°C for 20 minutes, centrifuged, and fixed using Carnoy fixative (1 acetic acid–3 methanol) on ice for 1 hour. Just prior to slide preparation, fixed cells were resuspended in 5 to 10 μL Carnoy fixative. Hybridization using the LSI dual-labeled BCR/ABL DNA probe followed manufacturer's instruction (Vysis, Downers Grove, IL). Lymphocytes from a healthy individual were used as a BCR/ABL– control; SD-1 cell lines, derived from an acute lymphoblastic leukemia (ALL) patient, served as BCR/ABL+ control. A total of 200 nuclei were scored for each sample. A percentage less than or equal to 6% of cells with a BCR/ABL fusion signal is considered within background limits based on laboratory validation studies (sensitivity, 95.2%, with 95% confidence limits of 76.2%-99.9%; and specificity, 100.0%, with 95% confidence limits of 82.4%-100.0%).
Conventional cytogenetic analysis
Chromosome studies were performed according to standard methods on metaphase cells derived from 24- and 48-hour unstimulated and at least one culture supplemented with supernatant derived from a human bladder carcinoma cell line (ATCC no. 5637; American Type Culture Collection, Manassas, VA) known to contain hematopoietic growth factors.27-29 Chromosome abnormalities were described according to the International System for Human Cytogenetic Nomenclature.30 When available, a minimum of 20 metaphase cells per sample were analyzed.
Reverse transcriptase–polymerase chain reaction (RT-PCR) for BCR/ABL mRNA
A nested PCR approach was used to detect BCR/ABL mRNA in total MNC and CD34+ cells. Total cellular RNA was extracted using Trizol reagent (Life Technologies, Gaithersburg, MD) and cDNA generated by reverse transcription using oligo(dT) primers. PCR amplification was carried out using primers designed to specifically detect the transcripts from the breakpoint region of the p210 BCR-ABL fusion gene. Initially 30 cycles of PCR amplification were carried out using the BCR68 and ABL3 primers (Table 1). Subsequently a fraction of the first-stage product was subjected to an additional 30 cycles of PCR amplification using internal nested primers, BCRfwd and ABLrev. Amplified samples were size separated by gel electrophoresis, ethidium bromide stained, and photographed. In preliminary studies it was established that this assay could reliably detect 1 in 106 primary CML CD34+ cells diluted in normal MNCs. Cell lines K562 and Meg01 were used as positive controls and HL-60 as a negative control. In addition, negative (reagent only) controls from each stage of the assay were included in each run to exclude contamination. Each sample was also analyzed by nested PCR for the presence of the normal BCR gene as an internal control to confirm RNA integrity and loading (first-stage primers BCR68 and BCR15; second-stage primers BCRfwd and BCR20). Samples were considered to be negative for BCR/ABL if no BCR/ABL product was detected in 4 separate PCR assays.
Sequences of oligonucleotide primers and probes used for RT-PCR
Nested PCR | |
| First step | |
| BCR68 | 5′-AGAAGCTTCTCCCTGACATCCG-3′ |
| ABL3- | 5′-GGTACCAGGAGTGTTTCTCCAGACTG-3′ |
| BCR15 | 5′-CTCAGCTGTGTCCCTGTAGACG-3′ |
| Second step | |
| BCRfwd | 5′-TGAAACTCCAGACTGTCC-3′ |
| ABLrev | 5′-TCAGACCCTGAGGCTCAAAG-3′ |
| BCR20 | 5′-CTCCAGGGTGCAGTACAGA-3′ |
| Real-time quantitative PCR | |
| p210BCR/ABL | |
| Primer p210-F | 5′-CATTCCGCTGACCATCAATAA-3′ |
| Primer p210-R | 5′-AACGAGCGGCTTCACTCAGA-3′ |
| TaqMan probe | FAM-AGCGGCCAGTAGCATCTGACTTTGAGC-TAMRA |
| GAPDH | |
| Primer GAPDH-F | 5′-GAAGGTGAAGGTCGGAGTC-3′ |
| Primer GAPDH-R | 5′-GAAGATGGTGATGGGATTTC-3′ |
| TaqMan probe | FAM-CAAGCTTCCCGTTCTCAGCC-TAMRA |
Nested PCR | |
| First step | |
| BCR68 | 5′-AGAAGCTTCTCCCTGACATCCG-3′ |
| ABL3- | 5′-GGTACCAGGAGTGTTTCTCCAGACTG-3′ |
| BCR15 | 5′-CTCAGCTGTGTCCCTGTAGACG-3′ |
| Second step | |
| BCRfwd | 5′-TGAAACTCCAGACTGTCC-3′ |
| ABLrev | 5′-TCAGACCCTGAGGCTCAAAG-3′ |
| BCR20 | 5′-CTCCAGGGTGCAGTACAGA-3′ |
| Real-time quantitative PCR | |
| p210BCR/ABL | |
| Primer p210-F | 5′-CATTCCGCTGACCATCAATAA-3′ |
| Primer p210-R | 5′-AACGAGCGGCTTCACTCAGA-3′ |
| TaqMan probe | FAM-AGCGGCCAGTAGCATCTGACTTTGAGC-TAMRA |
| GAPDH | |
| Primer GAPDH-F | 5′-GAAGGTGAAGGTCGGAGTC-3′ |
| Primer GAPDH-R | 5′-GAAGATGGTGATGGGATTTC-3′ |
| TaqMan probe | FAM-CAAGCTTCCCGTTCTCAGCC-TAMRA |
Real-time quantitative RT-PCR (Q-PCR)
Q-PCR using TaqMan technology was used to quantitate BCR/ABL mRNA levels in MNC and CD34+ cells. Total cellular RNA was converted to cDNA by reverse transcription and used as the template for the PCR procedure using primers and probes designed to detect the p210 transcripts of the BCR/ABL fusion gene (Table 1). The PCR reaction exploits the 5′ nuclease activity of AmpliTaq Gold DNA polymerase to cleave a TaqMan probe containing a reporter dye at the 5′ end and a quencher dye at the 3′ end. The separation of the reporter dye from the quencher dye results in an increase of fluorescence of the reporter allowing real-time detection of target amplification during PCR. Forty-five amplification cycles were run using the ABI Prism 7700 Sequence Detection System (Applied Biosystems) with a standardized protocol. Cell lines K562 and SD-1 were used as positive controls with serial cell line dilutions and a known positive patient sample included in each run to confirm the linearity and reproducibility of the test. Internal control RNA amplification for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was also performed on each sample. A quantitative result was calculated using a formula based on the ratio of amplification of BCR/ABL to the internal control gene GAPDH. The procedure was initially tested on cell line dilutions to confirm the ability to accurately quantitate the result and could detect 1 in 104 to 105BCR/ABL+ cells.
Statistical analysis
Differences between groups were evaluated using the Student t test. Relationships between 2 sets of variables were evaluated using linear regression analysis.
Results
Fifteen patients who achieved complete cytogenetic response (CCR) following imatinib mesylate treatment were studied. CCR was defined as 100% Philadelphia chromosome–negative (Ph–) metaphases by conventional cytogenetics and/or BCR/ABL– by FISH analysis of bone marrow nucleated cells. Multiple samples were collected at different time points from some patients while in continued CCR. A total of 23 samples were studied. Table 2 shows characteristics of the patients studied and the samples collected. Twelve patients were in CP and 3 patients in AP at time of initiation of imatinib mesylate treatment. Eleven patients had received prior treatment with interferon or a combination of interferon and cytosine arabinoside, whereas 4 patients received imatinib mesylate as initial therapy (excluding hydroxyurea). The time from diagnosis of CML to start of imatinib mesylate treatment ranged from 1 to 61 months (median, 17 months). The time from initiation of imatinib mesylate treatment to the time when the first sample was obtained for the purpose of this study ranged from 3 to 18 months (median, 9 months). MNCs from 12 of 13 evaluable patients were BCR/ABL+ by a nested RT-PCR assay (18 of 19 samples).
Characteristics of patients and samples studied
. | . | . | . | . | . | Cytogenetic response status, total nucleated cells . | . | . | |
|---|---|---|---|---|---|---|---|---|---|
| UPN . | Phase at start of imatinib mesylate treatment . | Age, y/sex . | Months from diagnosis to start of imatinib mesylate . | Prior treatment . | Months of imatinib mesylate treatment . | Karyotyping, Ph+/total . | FISH, % BCR/ABL+ cells* . | PCR for BCR/ABL, MNCs . | |
| 144 | AP | 49/M | 7 | IFN/Ara-C | 16 | NE | Neg | NE† | |
| 23 | 0/1 | Neg | NE† | ||||||
| 177 | CP | 52/F | 27 | IFN | 9 | NA | Neg | NA | |
| 14 | 0/20 | Neg | Pos | ||||||
| 17 | 0/20 | Neg | Pos | ||||||
| 22 | 0/20 | Neg | Pos | ||||||
| 26 | 0/20 | Neg | Pos | ||||||
| 191 | AP | 52/F | 11 | IFN | 8 | 0/21 | Neg | NA | |
| 214 | CP | 35/M | 7 | IFN | 12 | 0/20 | Neg | Pos | |
| 222 | CP | 67/F | 57 | IFN | 3 | 0/20 | Neg | Pos | |
| 9 | 0/20 | Neg | Pos | ||||||
| 234 | CP | 45/F | 17 | IFN | 6 | NA | Neg | Pos | |
| 238 | CP | 64/M | 19 | IFN | 6 | 0/20 | Neg | Pos | |
| 12 | 0/20 | Neg | Pos | ||||||
| 248 | CP | 69/M | 2 | — | 11 | 0/20 | Neg | Neg | |
| 255 | CP | 65/M | 23 | IFN | 6 | 0/20 | Neg | Pos | |
| 9 | 0/20 | Neg | Pos | ||||||
| 256 | CP | 39/M | 1 | — | 10 | 0/20 | Neg | Pos | |
| 261 | AP | 47/F | 59 | IFN/Ara-C | 18 | 0/2 | Neg‡ | Pos | |
| 268 | CP | 56/F | 61 | IFN/Ara-C | 4 | 0/20 | Neg | Pos | |
| 276 | CP | 54/F | 51 | IFN | 6 | 0/20 | Neg | Pos | |
| 283 | CP | 61/M | 1 | — | 9 | 0/20 | NA | Pos | |
| 286 | CP | 65/M | 1 | — | 11 | 0/20 | Neg | Pos | |
. | . | . | . | . | . | Cytogenetic response status, total nucleated cells . | . | . | |
|---|---|---|---|---|---|---|---|---|---|
| UPN . | Phase at start of imatinib mesylate treatment . | Age, y/sex . | Months from diagnosis to start of imatinib mesylate . | Prior treatment . | Months of imatinib mesylate treatment . | Karyotyping, Ph+/total . | FISH, % BCR/ABL+ cells* . | PCR for BCR/ABL, MNCs . | |
| 144 | AP | 49/M | 7 | IFN/Ara-C | 16 | NE | Neg | NE† | |
| 23 | 0/1 | Neg | NE† | ||||||
| 177 | CP | 52/F | 27 | IFN | 9 | NA | Neg | NA | |
| 14 | 0/20 | Neg | Pos | ||||||
| 17 | 0/20 | Neg | Pos | ||||||
| 22 | 0/20 | Neg | Pos | ||||||
| 26 | 0/20 | Neg | Pos | ||||||
| 191 | AP | 52/F | 11 | IFN | 8 | 0/21 | Neg | NA | |
| 214 | CP | 35/M | 7 | IFN | 12 | 0/20 | Neg | Pos | |
| 222 | CP | 67/F | 57 | IFN | 3 | 0/20 | Neg | Pos | |
| 9 | 0/20 | Neg | Pos | ||||||
| 234 | CP | 45/F | 17 | IFN | 6 | NA | Neg | Pos | |
| 238 | CP | 64/M | 19 | IFN | 6 | 0/20 | Neg | Pos | |
| 12 | 0/20 | Neg | Pos | ||||||
| 248 | CP | 69/M | 2 | — | 11 | 0/20 | Neg | Neg | |
| 255 | CP | 65/M | 23 | IFN | 6 | 0/20 | Neg | Pos | |
| 9 | 0/20 | Neg | Pos | ||||||
| 256 | CP | 39/M | 1 | — | 10 | 0/20 | Neg | Pos | |
| 261 | AP | 47/F | 59 | IFN/Ara-C | 18 | 0/2 | Neg‡ | Pos | |
| 268 | CP | 56/F | 61 | IFN/Ara-C | 4 | 0/20 | Neg | Pos | |
| 276 | CP | 54/F | 51 | IFN | 6 | 0/20 | Neg | Pos | |
| 283 | CP | 61/M | 1 | — | 9 | 0/20 | NA | Pos | |
| 286 | CP | 65/M | 1 | — | 11 | 0/20 | Neg | Pos | |
Ara-C indicates cytosine arabinoside; NE, not evaluable; neg, negative; NA, not available; pos, positive; —, no prior treatment, excluding hydroxyurea; and UPN, unique patient number.
Upper limit of normal less than or equal to 6% BCR/ABL+ cells.
Variant BCR/ABL breakpoint, not amplified by the primers used.
7% cells had trisomy 8 but were BCR/ABL-.
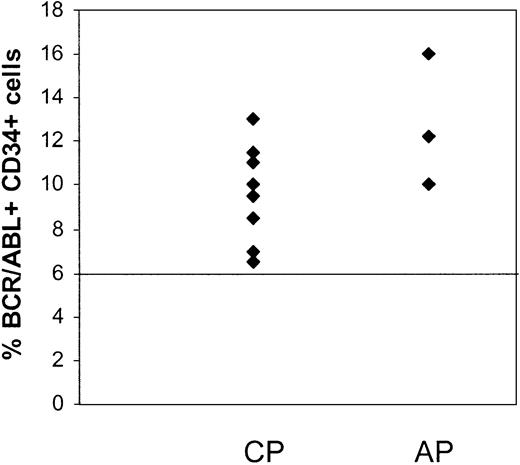
CD34+ cells were selected from MNCs of patients in CCR by immunomagnetic column separation and analyzed for the presence of BCR/ABL+ cells by FISH. Results are summarized in Table 3. Residual BCR/ABL+ cells were detected in the CD34+ cell fraction from the first sample studied from 11 of the 15 patients (Figure 1), including all 3 patients in AP and 8 of 12 patients in CP. Considerable variability in numbers of residual BCR/ABL+ CD34+ cells was observed between patients. There was a trend toward a higher percentage of BCR/ABL+ CD34+ cells in patients in AP (12.7% ± 1.8%) compared with CP (7.8% ± 1%) (P = .09). There was no significant correlation between the percentage of BCR/ABL+ CD34+ cells and time from diagnosis to start of imatinib mesylate treatment or the duration of imatinib mesylate treatment. The percentage of BCR/ABL+ CD34+ cells was also not significantly different between patients who had received prior interferon-based treatment or had received imatinib mesylate as initial therapy.
Results of progenitor analyses
. | Phase at start of imatinib mesylate treatment . | Months of imatinib mesylate treatment . | PCR for BCR/ABL, MNCs . | FISH, % BCR/ABL+ cells* . | . | . | Months of additional follow-up since last assessment . | Clinical status at last follow-up . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| UPN . | . | . | . | CD34+ cells . | CFCs . | LTCICs . | . | . | ||
| 144 | AP | 16 | NE† | 10 | 6.5 | NE | — | |||
| 23 | NE† | 13.2 | 6.9 | NE | 10 | Cytogenetic relapse (FISH) | ||||
| 177 | CP | 9 | NA | 8.5 | 9 | 7.5 | — | |||
| 14 | Pos | 9.5 | 7.5 | 8.5 | — | |||||
| 17 | Pos | 12.5 | NE | NE | — | |||||
| 22 | Pos | Neg | 10.8 | NE | — | |||||
| 26 | Pos | 7.5 | Neg | NE | 9 | CCR | ||||
| 191 | AP | 8 | NA | 12.2 | 11.5 | 8.5 | 6 | CCR | ||
| 214 | CP | 12 | Pos | 11 | 11 | Neg | 8 | CCR | ||
| 222 | CP | 3 | Pos | Neg | Neg | 8.5 | — | |||
| 9 | Pos | 13 | Neg | 10.5 | 8 | CCR | ||||
| 234 | CP | 6 | Pos | 6.5 | 9.7 | 19.5 | 10 | CCR | ||
| 238 | CP | 6 | Pos | 7 | 12 | 15.4 | — | |||
| 12 | Pos | Neg | 11 | NE | 6 | CCR | ||||
| 248 | CP | 11 | Neg | 13 | 6.5 | 8.5 | 7 | CCR | ||
| 255 | CP | 6 | Pos | Neg | Neg | Neg | — | |||
| 9 | Pos | Neg | Neg | 10 | 10 | CCR | ||||
| 256 | CP | 10 | Pos | 11.5 | 11.5 | NE | 6 | CCR | ||
| 261 | AP | 18 | Pos | 16 | 7.5 | 8 | 15 | Cytogenetic relapse (karyotype, FISH) | ||
| 268 | CP | 4 | Pos | Neg | 10.5 | Neg | 13 | CCR | ||
| 276 | CP | 6 | Pos | 9.5 | Neg | 7 | 10 | CCR | ||
| 283 | CP | 9 | Pos | Neg | Neg | 8.5 | 8 | CCR | ||
| 286 | CP | 11 | Pos | 10 | 8 | 8 | 6 | CCR | ||
. | Phase at start of imatinib mesylate treatment . | Months of imatinib mesylate treatment . | PCR for BCR/ABL, MNCs . | FISH, % BCR/ABL+ cells* . | . | . | Months of additional follow-up since last assessment . | Clinical status at last follow-up . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| UPN . | . | . | . | CD34+ cells . | CFCs . | LTCICs . | . | . | ||
| 144 | AP | 16 | NE† | 10 | 6.5 | NE | — | |||
| 23 | NE† | 13.2 | 6.9 | NE | 10 | Cytogenetic relapse (FISH) | ||||
| 177 | CP | 9 | NA | 8.5 | 9 | 7.5 | — | |||
| 14 | Pos | 9.5 | 7.5 | 8.5 | — | |||||
| 17 | Pos | 12.5 | NE | NE | — | |||||
| 22 | Pos | Neg | 10.8 | NE | — | |||||
| 26 | Pos | 7.5 | Neg | NE | 9 | CCR | ||||
| 191 | AP | 8 | NA | 12.2 | 11.5 | 8.5 | 6 | CCR | ||
| 214 | CP | 12 | Pos | 11 | 11 | Neg | 8 | CCR | ||
| 222 | CP | 3 | Pos | Neg | Neg | 8.5 | — | |||
| 9 | Pos | 13 | Neg | 10.5 | 8 | CCR | ||||
| 234 | CP | 6 | Pos | 6.5 | 9.7 | 19.5 | 10 | CCR | ||
| 238 | CP | 6 | Pos | 7 | 12 | 15.4 | — | |||
| 12 | Pos | Neg | 11 | NE | 6 | CCR | ||||
| 248 | CP | 11 | Neg | 13 | 6.5 | 8.5 | 7 | CCR | ||
| 255 | CP | 6 | Pos | Neg | Neg | Neg | — | |||
| 9 | Pos | Neg | Neg | 10 | 10 | CCR | ||||
| 256 | CP | 10 | Pos | 11.5 | 11.5 | NE | 6 | CCR | ||
| 261 | AP | 18 | Pos | 16 | 7.5 | 8 | 15 | Cytogenetic relapse (karyotype, FISH) | ||
| 268 | CP | 4 | Pos | Neg | 10.5 | Neg | 13 | CCR | ||
| 276 | CP | 6 | Pos | 9.5 | Neg | 7 | 10 | CCR | ||
| 283 | CP | 9 | Pos | Neg | Neg | 8.5 | 8 | CCR | ||
| 286 | CP | 11 | Pos | 10 | 8 | 8 | 6 | CCR | ||
NE indicates not evaluable; NA, not available; pos, positive; neg, negative.
Upper limit of normal less than or equal to 6% BCR/ABL+ cells.
Variant BCR/ABL breakpoint, not amplified by the primers used.
Persistence of BCR/ABL+CD34+cells in CML patients in CCR with imatinib mesylate treatment. CD34+ cells were selected from MNCs of CML patients in CCR (CP, n = 12; and AP, n = 3) by immunomagnetic column separation and analyzed for the presence of BCR/ABL+ cells by FISH. The percentage of BCR/ABL+ cells in the CD34+ cell fraction from the first sample studied is shown. A percentage less than or equal to 6% of cells with a BCR/ABL fusion signal is considered within background limits based on laboratory validation studies and is shown by the horizontal line. CD34+ cells from 4 patients in CP were BCR/ABL– by FISH.
Persistence of BCR/ABL+CD34+cells in CML patients in CCR with imatinib mesylate treatment. CD34+ cells were selected from MNCs of CML patients in CCR (CP, n = 12; and AP, n = 3) by immunomagnetic column separation and analyzed for the presence of BCR/ABL+ cells by FISH. The percentage of BCR/ABL+ cells in the CD34+ cell fraction from the first sample studied is shown. A percentage less than or equal to 6% of cells with a BCR/ABL fusion signal is considered within background limits based on laboratory validation studies and is shown by the horizontal line. CD34+ cells from 4 patients in CP were BCR/ABL– by FISH.
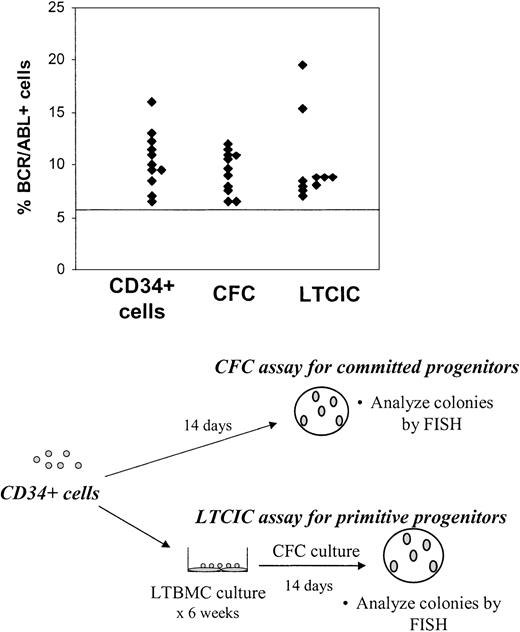
To determine whether BCR/ABL+ CD34+ cells had functional primitive and committed progenitor capacity, CD34+ cells were plated in CFC (committed progenitor) and LTCIC (primitive progenitor) assays and the resulting colonies were harvested and analyzed for BCR/ABL+ cells by FISH. BCR/ABL+ CFCs were present in the first sample studied from 11 of 15 patients and BCR/ABL+ LTCICs in 10 of 13 patients for whom results are available (Figure 2). Of the 4 patients in whom CD34+ cells were BCR/ABL– by FISH on initial examination, BCR/ABL+ CFCs were seen in 1 patient and BCR/ABL+ LTCICs in 2 patients. One of the patients with BCR/ABL– CD34+ cells but BCR/ABL+ LTCICs on first examination (no. 222) had detectable BCR/ABL+ CD34+ cells on analysis of a subsequent evaluation 6 months later. One patient (no. 255) did not have detectable BCR/ABL+ cells on evaluation of CD34+ cells, CFCs, or LTCICs on initial examination. However, BCR/ABL+ LTCICs were present in a sample obtained from this patient 3 months later. When the results for CD34+ cells, CFCs, and LTCICs are taken together, BCR/ABL+ progenitor cells were present in all patients included in this study.
Persistence ofBCR/ABL+cells with CFC and LTCIC capacity in CML patients in CCR with imatinib mesylate treatment. CD34+ cells were plated in CFC (committed progenitor, n = 15) and LTCIC (primitive progenitor, n = 13) assays, and the resulting colonies were harvested and analyzed for BCR/ABL+ cells by FISH as shown in the bottom half of the figure. Results from the first sample studied are shown and represent the percentage of BCR/ABL+ cells in colonies resulting from CFC and LTCIC assays. The percentage of CD34+ cells (n = 15) that were BCR/ABL+ is shown for comparison. A percentage less than or equal to 6% of cells with a BCR/ABL fusion signal is considered within background limits and is shown by the horizontal line. CD34+ cells from 4 patients, CFCs from 4 patients, and LTCICs from 3 patients were BCR/ABL– by FISH.
Persistence ofBCR/ABL+cells with CFC and LTCIC capacity in CML patients in CCR with imatinib mesylate treatment. CD34+ cells were plated in CFC (committed progenitor, n = 15) and LTCIC (primitive progenitor, n = 13) assays, and the resulting colonies were harvested and analyzed for BCR/ABL+ cells by FISH as shown in the bottom half of the figure. Results from the first sample studied are shown and represent the percentage of BCR/ABL+ cells in colonies resulting from CFC and LTCIC assays. The percentage of CD34+ cells (n = 15) that were BCR/ABL+ is shown for comparison. A percentage less than or equal to 6% of cells with a BCR/ABL fusion signal is considered within background limits and is shown by the horizontal line. CD34+ cells from 4 patients, CFCs from 4 patients, and LTCICs from 3 patients were BCR/ABL– by FISH.
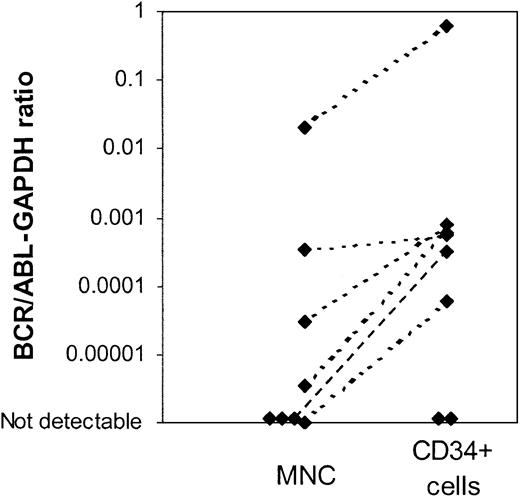
Using the nested PCR assay, BCR/ABL mRNA was detected in CD34+ cells from 9 of 13 evaluable patients (15 of 19 samples), including all patients with BCR/ABL– CD34+ cells on FISH analysis. Availability of only limited amounts of cells and low RNA yield may have compromised the sensitivity of the PCR assay for samples in which BCR/ABL message was not detectable. A real-time quantitative RT-PCR (Q-PCR) assay was used to compare BCR/ABL mRNA levels in MNC and CD34+ cells. This assay is less sensitive than the nested PCR assay but has the advantage of allowing quantitative comparison of results obtained from MNCs and CD34+ cells. Results are expressed as a ratio of BCR/ABL to the internal control gene GAPDH. Q-PCR results comparing BCR/ABL expression (BCR/ABL-GAPDH ratio) in CD34+ cells and MNCs are available for 8 patients (Figure 3). Increased levels of BCR/ABL mRNA were detected in CD34+ cells when compared with MNCs in 6 patients, consistent with the presence of an increased proportion of BCR/ABL+ cells in the CD34+ fraction. Two patients did not have detectable BCR/ABL message in either total nucleated cells or CD34+ cells using this assay, likely reflecting the lower sensitivity of the Q-PCR assay.
Real time quantitative PCR (Q-PCR) analysis of mononuclear cells (MNCs) and CD34+cells from patients in CCR with imatinib mesylate treatment.BCR/ABL mRNA levels in MNCs and CD34+ cells were measured using a Q-PCR assay. Results were expressed as a ratio of BCR/ABL to the internal control gene GAPDH. Q-PCR results of BCR/ABL expression in CD34+ cells and MNCs for 8 patients are shown. Two patients did not have detectable BCR/ABL message in either total nucleated cells or CD34+ cells using this assay.
Real time quantitative PCR (Q-PCR) analysis of mononuclear cells (MNCs) and CD34+cells from patients in CCR with imatinib mesylate treatment.BCR/ABL mRNA levels in MNCs and CD34+ cells were measured using a Q-PCR assay. Results were expressed as a ratio of BCR/ABL to the internal control gene GAPDH. Q-PCR results of BCR/ABL expression in CD34+ cells and MNCs for 8 patients are shown. Two patients did not have detectable BCR/ABL message in either total nucleated cells or CD34+ cells using this assay.
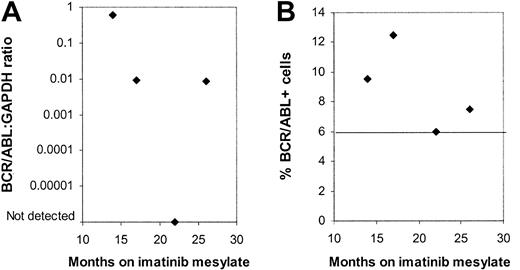
Four patients were evaluated on more than one occasion. Bone marrow (BM) samples obtained at 4 different time points from one patient showed no significant trend toward an increase or decrease in BCR/ABL+ CD34+ cells by FISH over the period from 9 to 26 months after initiation of imatinib mesylate treatment (Figure 4). BCR/ABL mRNA could be detected by nested PCR in CD34+ cells on all occasions. Q-PCR measurements indicated variable levels of BCR/ABL mRNA in CD34+ cells over time. Two other patients also showed persistent BCR/ABL+ progenitors without a definite trend toward an increase or decrease in the percentage of BCR/ABL+ progenitor cells. Finally, in one patient, also described earlier, CD34+ cells, CFCs, and LTCICs were all BCR/ABL– 6 months after start of treatment, whereas BCR/ABL+ LTCICs were detected at 9 months.
Persistence of BCR/ABL+progenitors in multiple samples obtained from a patient in continued CCR on imatinib mesylate treatment. Results for (A) BCR/ABL-GAPDH mRNA levels measured by Q-PCR and (B) the percentage of BCR/ABL+ CD34+ cells detected by FISH are shown for BM samples obtained from one patient from 12 to 26 months after initiation of imatinib mesylate treatment. A percentage less than or equal to 6% of cells with a BCR/ABL fusion signal is considered within background limits for the FISH assay and is shown by the horizontal line.
Persistence of BCR/ABL+progenitors in multiple samples obtained from a patient in continued CCR on imatinib mesylate treatment. Results for (A) BCR/ABL-GAPDH mRNA levels measured by Q-PCR and (B) the percentage of BCR/ABL+ CD34+ cells detected by FISH are shown for BM samples obtained from one patient from 12 to 26 months after initiation of imatinib mesylate treatment. A percentage less than or equal to 6% of cells with a BCR/ABL fusion signal is considered within background limits for the FISH assay and is shown by the horizontal line.
Thirteen of the 15 patients included in this study were in CCR at last follow-up, whereas 2 (nos. 144 and 261) have developed evidence of cytogenetic relapse. These 2 patients were both in AP when imatinib mesylate was started. For patient no. 144, results shown are based on analysis of samples obtained when the patient was in CCR (BCR/ABL FISH–), 16 and 21 months after initiation of imatinib mesylate. A subsequent bone marrow sample, obtained 27 months after initiation of imatinib mesylate, was BCR/ABL+ by FISH. For patient no. 261, results shown are for a sample obtained 18 months after initiation of imatinib mesylate, when the patient was Ph– and BCR/ABL FISH–. An unrelated clone with trisomy 8 but without t(9;22) was noted at that time. A subsequent bone marrow examination, performed 27 months after initiation of imatinib mesylate, showed evidence of cytogenetic relapse with 2 of 22 cells having the t(9;22) but without trisomy 8 on karyotypic analysis. Cytogenetic relapse was confirmed by BCR/ABL FISH. Two additional cells had trisomy 8 but did not have t(9;22), indicating persistence of the unrelated clone.
Discussion
We demonstrate here that residual BCR/ABL+ progenitors persist in CML patients in complete cytogenetic remission on imatinib mesylate therapy. Analysis of colonies generated in methylcellulose progenitor culture and long-term bone marrow cultures confirmed that BCR/ABL+ CD34+ cells retain functional committed and primitive progenitor capacity. Because BCR/ABL+ cells were not detected by conventional cytogenetic or FISH analysis of total nucleated cells, these results indicate selective persistence of malignant progenitor cells compared with more mature cells. Quantitative PCR assays also demonstrate that the level of BCR/ABL message was higher in the CD34+ cell population, consistent with enrichment of BCR/ABL+ cells in the progenitor fraction. BCR/ABL+ progenitor cells were not eliminated despite continued imatinib mesylate treatment for more than 2 years.
There was interindividual variability in the frequency of BCR/ABL+ CD34+ cells. A trend toward an increased frequency of BCR/ABL+ CD34+ cells was seen in patients in whom treatment was initiated in AP compared with CP. No association between levels of BCR/ABL+ progenitors and risk factors such as time from diagnosis, prior therapy, or length of time on imatinib mesylate treatment was apparent. Although 4 patients in CP did not have detectable CD34+ cells when first examined, residual BCR/ABL+ CFCs or LTCICs were found in 3 patients. The fourth patient did not have BCR/ABL+ CD34+ cells, CFCs, or LTCICs on initial examination, but BCR/ABL+ LTCICs were detected when the patient was restudied 3 months later.
These observations indicate that imatinib mesylate treatment of CML may not eliminate primitive hematopoietic cells in which the disease arises. Evaluation of samples obtained from patients at different times after initiation of treatment, including serial evaluation of samples from individual patients, suggests that the residual BCR/ABL+ progenitor population persists despite continued treatment with imatinib mesylate. Persistence of residual BCR/ABL+ progenitors raises the possibility that patients in CCR on imatinib mesylate therapy may be at risk for disease relapse. Studies in patients receiving allogeneic bone marrow transplantation (BMT) and interferon have shown that the level of residual malignant cells as determined by PCR is predictive of clinical outcome.31,32 Overall, BCR/ABL mRNA levels in marrow from patients in imatinib mesylate–induced remissions were significantly lower than those observed in patients with interferon-induced remissions.21,22 However, considerable variability in response was seen and BCR/ABL mRNA was still detectable in most patients, consistent with our own results. Higher levels of CD34+ cells in patients treated in AP may be consistent with clinical observations of a higher rate of relapse compared with CP CML patients.19 Factors such as frequency and type of residual BCR/ABL+ progenitors, and their trend toward increase or decrease over time, may better correlate with clinical outcomes.
Interestingly, some previous reports have suggested that persistence of small populations of BCR/ABL+ progenitors may still be compatible with long-term remission from leukemia. Residual BCR/ABL+ progenitors were detected in marrow samples from CML patients who have achieved prolonged cytogenetic remission, and even complete molecular remission (RT-PCR–), following interferon-α therapy. This suggests that the presence of a small population of Ph+ CML progenitor cells for a very long period of time is compatible with a durable remission and that interferon therapy may induce a state of tumor dormancy in CML.33,34 Similarly, Miyamoto et al have reported that AML1/ETO-expressing multipotent progenitors can be detected by RT-PCR in bone marrow of patients with acute myeloid leukemia (AML) with t(8;21) in remission after chemotherapy or autologous peripheral blood stem cell transplantation.35 However, this finding did not necessarily indicate impending relapse, because such progenitors could persist more than 10 years after achieving remission. Of the patients studied here, only 2 have developed evidence of clinical relapse on further follow-up. Both patients had accelerated-phase disease at initiation of imatinib mesylate treatment. Longer follow-up of a larger group of CML patients will be required to determine whether residual BCR/ABL+ progenitors are associated with risk of disease recurrence in a significant proportion on imatinib mesylate–responsive patients, especially those in chronic phase. It will also be of interest to investigate in the future whether BCR/ABL+ progenitors are eliminated in patients in long-term remission following allogeneic hematopoietic cell transplantation.
The mechanisms underlying persistence of malignant progenitors in patients in CCR are not known. Mechanisms such as BCR/ABL gene duplication, mutations in the ABL kinase domain, and acquisition of additional genetic abnormalities have been associated with primary or acquired resistance to imatinib mesylate in patients with advanced disease.36,37 Although the patients studied here were responsive to imatinib mesylate, it is possible that similar mechanisms could be active in a small subset of progenitors, resulting in their persistence. However, refractoriness to imatinib mesylate would be expected to allow rapid emergence of the resistant clone, whereas several patients studied several months apart did not show evidence of increasing BCR/ABL+ cells, arguing against this possibility. We did not observe multiple copies of the BCR/ABL fusion gene in residual BCR/ABL+ CD34+ cells by FISH in any of the samples studied. Therefore, other mechanisms may be responsible for persistence of malignant progenitors in patients responsive to imatinib mesylate. Normal hematopoietic stem cells demonstrate increased efflux pump activity, a characteristic that can be used to select primitive hematopoietic cell populations.38,39 It is possible that increased drug efflux in leukemic stem cells could lead to reduced intracellular levels of imatinib mesylate and be a mechanism of resistance to inhibition by imatinib mesylate.
Another potential mechanism for retention of BCR/ABL+ progenitors may be related to our previously reported observation that imatinib mesylate inhibits malignant primitive progenitor growth primarily through inhibition of their abnormally increased proliferation rather than selective induction of apoptosis.23 Consistent with this, Graham et al have also reported that primitive, quiescent Ph+ progenitor cells from CML patients are insensitive to imatinib mesylate in vitro.24 These results suggest that BCR/ABL kinase activity may be required for abnormal proliferation and expansion of BCR/ABL+ cells in CML but may not be essential for preservation of primitive malignant cells. BCR/ABL kinase inhibition by imatinib mesylate may therefore remove the proliferative advantage of BCR/ABL+ progenitors and their progeny cells and allow regrowth of coexisting BCR/ABL– cells but not eliminate all BCR/ABL+ primitive progenitors.
In conclusion, we demonstrate that BCR/ABL+ hematopoietic progenitors persist in the marrow of patients in CCR, indicating that malignant progenitors may be suppressed but not eliminated in the course of imatinib mesylate treatment. Future studies will examine potential mechanisms underlying persistence of malignant progenitors and the significance of residual malignant progenitors with respect to clinical outcomes.
Prepublished online as Blood First Edition Paper, February 6, 2003; DOI 10.1182/blood-2002-09-2780.
Supported in part by the Leukemia and Lymphoma Society Translational Research grant 6468 (R.B.), the American Cancer Society grant RPG-99-202-01-LBC (R.B.), and the Public Health Services grant CA33572 (M.L.S.). R.B. is a Clinical Scholar of the Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the following individuals for their excellent technical assistance: Feiyu Zhang for FISH analysis, Anna Arbitria and Helen Xu for PCR analysis, and Tinisha McDonald, Khristine Van Heijzen, Dana Miller, and Heidi Munthe for progenitor selection and assay. We also thank Allen Lin, Allison Sano, Debra Vasquez, and the physicians and staff in the Division of Hematology/BMT for assistance with patient samples.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal