Abstract
P38 mitogen-activated protein kinase (MAPK) is an important component of intracellular signaling cascades that initiate various inflammatory cellular responses. To determine the role of p38 MAPK in the procoagulant response to lipopolysaccharide (LPS), 24 healthy subjects were exposed to an intravenous dose of LPS (4 ng/kg), preceded 3 hours earlier by orally administered 600 or 50 mg BIRB 796 BS (a specific p38 MAPK inhibitor), or placebo. The 600-mg dose of BIRB 796 BS strongly inhibited LPS-induced coagulation activation, as measured by plasma concentrations of the prothrombin fragment F1 + 2. BIRB 796 BS also dose dependently attenuated the activation and subsequent inhibition of the fibrinolytic system (plasma tissue-type plasminogen activator, plasmin-α2-antiplasmin complexes, and plasminogen activator inhibitor type 1) and endothelial cell activation (plasma soluble E-selectin and von Willebrand factor). Activation of p38 MAPK plays an important role in the procoagulant and endothelial cell response after in vivo exposure to LPS.
Introduction
Activation of the coagulation system is an important manifestation of the systemic inflammatory response of the host to infection.1 Several in vivo models have been used to dissect the molecular mechanisms that contribute to coagulation activation by bacteria and bacterial products, including intravenous injection of low-dose lipopolysaccharide (LPS) into healthy humans.2 Tissue factor, a glycoprotein expressed on endothelial cells and monocytes upon stimulation, plays an essential role in coagulation activation induced by LPS or bacteria in vivo, as demonstrated by abolishment of this response by inhibitors of tissue factor.3, 4, 5, 6
Mitogen-activated protein kinases (MAPKs) participate in intracellular signaling cascades that mediate inflammatory responses to infectious and noninfectious stimuli.7,8 Recent in vitro studies have implicated one of these kinases, p38 MAPK, in the regulation of tissue factor expression on monocytes,9 endothelial cells,10,11 and smooth muscle cells.12 The role of p38 MAPK in activation of coagulation in vivo is not known. This prompted us to evaluate the effect of BIRB 796 BS, a specific and potent inhibitor of p38 MAPK,13 on the procoagulant response during human endotoxemia.
Study design
Design
This study was approved by the institutional scientific and ethics committees of the Academic Medical Center, Amsterdam, and written informed consent according to the Declaration of Helsinki was obtained from each subject prior to the start of the study. This study was a randomized, double-blind, placebo-controlled experiment performed simultaneously with a study examining the effect of BIRB 796 BS on LPS-induced cytokine release and neutrophil activation.14 Briefly, LPS (from Escherichia coli, lot G; United States Pharmacopeial Convention, Rockville, MD; 4 ng/kg body weight) was administered as a bolus intravenous injection to 24 healthy male volunteers (mean age, 22 years; range, 19-29 years). At 3 hours prior to infusion of LPS, the p38 MAPK inhibitor BIRB 796 BS (600 mg, n = 8; 50 mg, n = 8) or placebo (n = 8) was administered orally in 15 mL polyethylene glycol 400. BIRB 796 BS is a highly selective and potent p38 MAPK inhibitor developed by Boehringer Ingelheim Pharmaceuticals (Ridgefield, CT).13 Blood samples were obtained before administration of BIRB 796 BS or placebo (time [t] = – 3 hours), directly before LPS administration (t = 0 hours), then 0.5, 1.0, 1.5, 2, 3, 4, 5, 6, 8, 10, and 24 hours after LPS administration.
Assays
Blood was collected in siliconized vacutainer tubes (Becton Dickinson, Plymouth, England) containing 0.105 M sodium citrate in a 1:9 (vol/vol) anticoagulant-blood ratio. Enzyme-linked immunosorbent assays (ELISAs) were performed according to the instructions of the manufacturers: prothrombin fragment F1 + 2 (Dade Behring, Marburg GmbH, Germany); tissue-type plasminogen activator (tPA; Asserachrom tPA, Diagnostic Stago, Asnieres-sur-Seine, France); plasminogen activator inhibitor type 1 (PAI-1; Monozyme, Charlottelund, Denmark); von Willebrand factor (VWF; Dakopatts, Ävlsjö, Sweden); and soluble E-selectin (sE-selectin; Diaclone, Besancon Cedex, France). Plasmin-α2-antiplasmin complexes (PAPc) were determined by a radio immunoassay described elsewhere.15
Statistical analysis
All values are given as means ± SEM. Differences in response curves between the 3 treatment groups were tested by repeated measurements analysis of variance. A P value less than .05 was considered to represent a statistically significant difference.
Results and discussion
We recently demonstrated that BIRB 796 BS inhibited LPS-induced p38 MAPK activation in blood leukocytes of healthy humans in vivo, which was associated with a profound reduction in the release of cytokines and neutrophil activation.14 In the present study we sought to determine the role of p38 MAPK in the activation of the hemostatic mechanism in vivo.
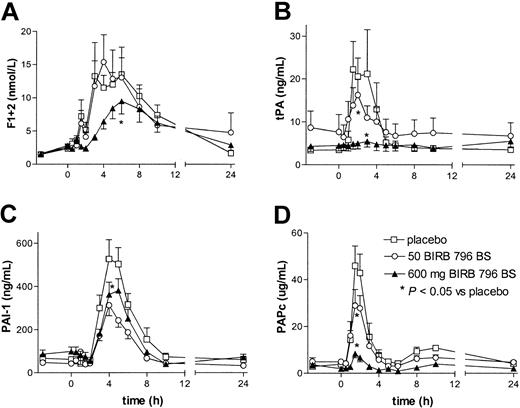
Intravenous injection of LPS was associated with activation of the coagulation system, as reflected by a rise in the plasma concentrations of the prothrombin fragment F1 + 2 (Figure 1). High-dose, but not low-dose, BIRB 796 BS inhibited LPS-induced coagulation activation, both delaying and diminishing the increase in plasma F1 + 2 concentrations (P < .05 vs placebo). The most likely explanation for the anticoagulant effect of BIRB 796 BS is inhibition of tissue factor expression. Indeed, tissue factor is essential for activation of the coagulation system in this model of low-grade endotoxemia,3,6 and p38 MAPK inhibition markedly diminishes LPS-induced tissue factor expression by monocytic cells in vitro.9 Moreover, p38 MAPK activation also mediates tissue factor expression by endothelial cells stimulated with thrombin or vascular endothelial growth factor, and by vascular smooth muscle cells stimulated with thrombin.10, 11, 12 Furthermore, interleukin-6 (IL-6) is an important mediator of LPS-induced coagulation activation in vivo,16 and BIRB 796 BS inhibited IL-6 release in a dose-dependent way.14 Thus, it is conceivable that in the high-dose BIRB 796 BS group, the reduction of IL-6 production had an additional inhibitory effect on the activation of the coagulation system.
BIRB 796 BS dose-dependently inhibits LPS-induced coagulation and fibrinolysis. Concentrations of F1 + 2 (A), tPA (B), PAI-1 (C), and PAPc (D) are shown. Subjects received an intravenous injection of LPS (4 ng/kg) at 0 hours, preceded by oral ingestion of placebo (□), 50 mg BIRB 796 BS (○), or 600 mg BIRB 796 BS (▴)at – 3 hours. Data are the mean ± SEM. Asterisks indicate a P value less than .05 versus placebo for the curve indicated, that is, high-dose BIRB 796 BS inhibited all parameters shown (all P < .05 vs placebo), and low-dose BIRB 796 BS inhibited tPA and PAPc (P < .05 vs placebo).
BIRB 796 BS dose-dependently inhibits LPS-induced coagulation and fibrinolysis. Concentrations of F1 + 2 (A), tPA (B), PAI-1 (C), and PAPc (D) are shown. Subjects received an intravenous injection of LPS (4 ng/kg) at 0 hours, preceded by oral ingestion of placebo (□), 50 mg BIRB 796 BS (○), or 600 mg BIRB 796 BS (▴)at – 3 hours. Data are the mean ± SEM. Asterisks indicate a P value less than .05 versus placebo for the curve indicated, that is, high-dose BIRB 796 BS inhibited all parameters shown (all P < .05 vs placebo), and low-dose BIRB 796 BS inhibited tPA and PAPc (P < .05 vs placebo).
BIRB 796 BS had an even stronger inhibitory effect on activation of the fibrinolytic system (Figure 1). The LPS-induced increases in the plasma levels of tPA and PAPc were dose-dependently reduced by BIRB 796 BS (both low and high dose P < .05 vs placebo), and almost completely prevented by the high dose of the p38 MAPK inhibitor. The high dose of BIRB 796 BS also modestly inhibited the release of PAI-1 (P < .05 vs placebo). Knowledge of the role of p38 MAPK in the production and release of fibrinolytic mediators is limited. The production of PAI-1 by bovine aortic endothelial cells exposed to hypoxia was not dependent on p38 MAPK.17 The strong inhibition of tumor necrosis factor α (TNF-α) release by BIRB 796 BS14 may in part be responsible for the observed reduction in tPA and PAPc levels, considering that complete neutralization of TNF-α in this model essentially prevents these responses.18,19 However, other mechanisms are also likely involved given that anti–TNF-α strategies also strongly inhibit LPS-induced PAI-1 release.18,19
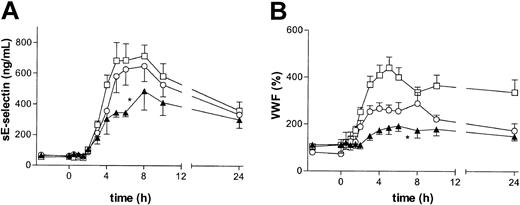
The effect of BIRB 769 BS on endothelial cell activation, as reflected by the plasma concentrations of sE-selectin and VWF,18, 19, 20 was also investigated. LPS administration elicited profound increases in the plasma levels of sE-selectin and VWF. These increases were attenuated by high-dose BIRB 796 BS (both P < .05 vs placebo; Figure 2). P38 MAPK may play a direct role in signaling inflammatory effects into the interior of the vascular endothelium,10,11,21 including the expression of E-selectin on stimulated endothelial cells.22,23 In addition, the reduced TNF-α levels in subjects treated with the p38 MAPK inhibitor14 may also have played a role in the attenuated endothelial cell activation, considering that elimination of TNF-α abolishes this response during endotoxemia.18,19 Thus, a combination of direct effects of p38 MAPK inhibition on endothelial cells and the inhibition of TNF-α release may explain the effect of BIRB 796 BS on the secretion of sE-selectin and VWF.
BIRB 796 BS dose-dependently inhibits LPS-induced endothelial cell activation. Subjects received an intravenous injection of LPS (4 ng/kg) at 0 hours, preceded by oral ingestion of placebo (□), 50 mg BIRB 796 BS (○), or 600 mg BIRB 796 BS (▴) at – 3 hours. Left panel: sE-selectin; right panel: VWF. Data are the mean ± SEM. Asterisks indicate P value less than .05 versus placebo for the curve indicated, that is, high-dose BIRB 796 BS inhibited both VWF and sE-selectin (P < .05 vs placebo).
BIRB 796 BS dose-dependently inhibits LPS-induced endothelial cell activation. Subjects received an intravenous injection of LPS (4 ng/kg) at 0 hours, preceded by oral ingestion of placebo (□), 50 mg BIRB 796 BS (○), or 600 mg BIRB 796 BS (▴) at – 3 hours. Left panel: sE-selectin; right panel: VWF. Data are the mean ± SEM. Asterisks indicate P value less than .05 versus placebo for the curve indicated, that is, high-dose BIRB 796 BS inhibited both VWF and sE-selectin (P < .05 vs placebo).
In this and our previous study14 we documented dose-dependent effects of BIRB 796 BS on various inflammatory responses. Nonetheless, the effect of BIRB 796 BS on p38 MAPK phosphorylation already seemed maximal at the lower dose of the inhibitor.14 Importantly, because the phosphorylation assays were done on blood leukocytes, this finding does not exclude a dose-dependent effect of BIRB 796 BS on p38 MAPK activation on inflammatory cells in tissues. The specificity of BIRB 796 BS for p38 MAPK was carefully documented earlier.13
We here demonstrate a role for p38 MAPK in activation of coagulation, fibrinolysis, and the vascular endothelium during human endotoxemia. These findings extend earlier observations of the inhibitory effect of p38 MAPK inhibitors such as BIRB 796 BS on activation of the cytokine network and neutrophils,14,24 and illustrate the broad role of this kinase in inflammation in vivo.
Prepublished online as Blood First Edition Paper, February 6, 2003; DOI 10.1182/blood-2002-11-3338.
Supported by grants from the Netherlands Organization of Scientific Research (NWO) to S.W., and Boehringer Ingelheim Pharma KG (Biberach, Germany).
A.G. is an employee of Boehringer Ingelheim.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal