Abstract
Myosin modulates the fibrinolytic process as a cofactor of the tissue plasminogen activator and as a substrate of plasmin. We report now that myosin is present in arterial thrombi and it forms reversible noncovalent complexes with fibrinogen and fibrin with equilibrium dissociation constants in the micromolar range (1.70 and 0.94 μM, respectively). Competition studies using a peptide inhibitor of fibrin polymerization (glycl-prolyl-arginyl-proline [GPRP]) indicate that myosin interacts with domains common in fibrinogen and fibrin and this interaction is independent of the GPRP-binding polymerization site in the fibrinogen molecule. An association rate constant of 1.81 × 102 M–1 · s–1 and a dissociation rate constant of 3.07 × 10–4 s–1 are determined for the fibrinogen-myosin interaction. Surface plasmon resonance studies indicate that fibrin serves as a matrix core for myosin aggregation. The fibrin clots equilibrated with myosin are stabilized against dissolution initiated by plasminogen and tissue-type plasminogen activator (tPA) or urokinase (at fibrin monomer-myosin molar ratio as high as 30) and by plasmin under static and flow conditions (at fibrin monomer-myosin molar ratio lower than 15). Myosin exerts similar effects on the tPA-induced dissolution of blood plasma clots. Covalent modification involving factor XIIIa does not contribute to this stabilizing effect; myosin is not covalently attached to the clot by the time of complete cross-linking of fibrin. Thus, our in vitro data suggest that myosin detected in arterial thrombi binds to the polymerized fibrin, in the bound form its tPA-cofactor properties are masked, and the myosinfibrin clot is relatively resistant to plasmin.
Introduction
The appropriate timing and localization of the proteolytic removal of the fibrin clots in the vascular bed is based on elaborate regulatory mechanisms that determine the availability of fibrinolytic proteases at the level of plasminogen activation1 and inactivation of the enzymes.2 Quantitative3,4 and morphologic5 data have revealed the importance of the structure of the fibrin substrate in determining the efficiency of fibrinolysis; the fiber diameter, the pore size of the gel, and the frequency of branching points profoundly affect the rate of fibrin dissolution. In experimental models it is rather convenient to modify the structural characteristics of the fibrin gel by changing the concentrations of thrombin and fibrinogen when fibrin is generated6,7 or the ionic strength at polymerization4,8 for evaluation of the efficiency of fibrinolysis on various fibrin substrates. In vivo, however, fibrinogen, the precursor of fibrin, circulates in blood surrounded by cells and proteins. Thus, when thrombin converts it to fibrin, the polymerization occurs in a milieu highly enriched in macromolecules. The fibrin assembly is definitely modified by the presence of plasma proteins (eg, albumin9 ), cellular elements (eg, erythrocytes10 ), or specific proteins of isolated compartments (eg, amyloid β-protein11 ). This modification can be attributed to the effect of volume occupancy on the catalytic and polymerization rates in solutions overcrowded with macromolecules.12 Fibrinogen, however, is not passively surrounded by other molecules; it circulates in weak noncovalent association with a number of plasma proteins.13 Thus, it can be predicted that when fibrinogen is converted to fibrin, microdomains of volume exclusion will be formed in the immediate vicinity of the fibrin fibers even at relatively low macromolecule concentrations in soluble phase due to the local accumulation of such interacting molecule species. Consequently, the catalytic processes occurring on the fibrin surface (eg, plasminogen activation, plasmin action) could be significantly modified. Identification of relevant macromolecules interfering with fibrinolysis in this way requires knowledge on their affinity to fibrin.
A potential fibrin modifier is myosin, which represents 5% of the total protein in platelets,14 and considering the average amount of protein and volume of platelets,15 this means that 7.5 mg/L myosin circulates in the blood within platelets. Recent data indicate that the platelet content of 10 mL whole blood is compacted in 400 μL arterial thrombi, whereas the fibrin content of the same thrombi corresponds to the fibrinogen concentration in blood plasma.16 Thus, within platelet-rich thrombi the concentrations of myosin and fibrin are in the micromolar range (approximately 0.5 and 5 μM, respectively). Some observations support the possibility for association of fibrin with the platelet cytoskeleton.17 Around the fibrin fibers platelets send out filopodia, in which thick myosin filaments are arranged.18 In 2 hours after the initiation of thrombus formation morphologic signs of platelet necrosis are detected. Beyond 6 hours platelet destruction is the predominant electron microscopic finding and through 12 hours there is no detectable fibrin degradation in arterial thrombi.16 When after activation the necrotic platelets release their cytosolic content and lose the majority of their phospholipid,19 the stage for interaction of fibrin and platelet-derived proteins is set up in the growing thrombi. We have already described some aspects of the impact of myosin on fibrinolysis: its action as a cofactor of plasminogen activation and its cleavage by plasmin.20 In the current report we present morphologic, kinetic, and equilibrium data on the interactions of myosin and fibrin related to the overall effect of myosin on the proteolytic dissolution of fibrin.
Materials and methods
Human plasma was collected from healthy volunteers. Human fibrinogen (plasminogen free), urokinase (human urine), and streptokinase were from Calbiochem (La Jolla, CA). The chromogenic plasmin substrate Spectrozyme-PL (H-D-norleucyl-hexahydrotyrosyl-lysine-p-nitroanilide) and the tissue-type plasminogen activator (tPA) were products of American Diagnostica (Hartford, CT) and Genentech (South San Francisco, CA), respectively. Lysine-Sepharose 4B, Sephadex G-25, and Sephacryl S-400 HR were from Pharmacia Biotech (Uppsala, Sweden). Human thrombin (1000 NIH U mg–1), glycyl-prolyl-arginyl-proline (GPRP), and p-nitrophenyl p′-guanidinobenzoate were the products of Sigma (St Louis, MO). Bovine serum albumin (BSA) was from Serva (Heidelberg, Germany), and lactoperoxidase and 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF) were from Boehringer (Mannheim, Germany). Flexible polyvinyl chloride (PVC) U-well microtiter plates were from Dynatech Deutschland (Denkendorf, Germany). Sensor chip CM5 and an amine immobilization kit containing N-hydroxysuccinimide, N-ethyl-N′-(3-diethylaminopropyl)-carbodiimide and 1 M ethanolamine-HCl, pH 8.5, were obtained from Biacore (Uppsala, Sweden). Human fibrinogen (plasminogen and factor XIII free) labeled with 125I according to the lactoperoxidase procedure21 was a kind gift from Dr István Mucha (Izinta, Budapest, Hungary). Fibrinogen was labeled with Eu-chelate (N1-(p-isothiocyanatobenzyl)-diethylenetriamine-N,1N,2N,3N4-tetraacetic acid chelated with Eu3+) according to the manufacturer's instructions (Wallac, Turku, Finland) with efficiency of 4 Eu/molecule protein (determined using a europium standard solution from Wallac). The Eu-label binds covalently to primary amino groups of the proteins. The detection sensitivity of the label is similar to that of radioisotopes.22 Factor XIII (FXIII) from human plasma was generously provided by Dr László Muszbek (University of Medicine, Debrecen, Hungary).
Published procedures were used for the isolation of myosin from bovine heart23 and of plasminogen from citrated human plasma,24 as well as for the generation of plasmin and determination of its active concentration.2 The fibrinogen preparations contain contaminant factor XIII, which following activation with 100 mM CaCl2 was inhibited by 10 mM iodoacetamide (after the inhibition the iodoacetamide and the CaCl2 were removed with dialysis).25 The inactivation procedure does not modify the functional properties of fibrinogen as checked with thrombin clotting time and turbidimetric fibrinolytic assay.26
Binding of labeled fibrinogen to immobilized myosin
Myosin was covalently attached to the microtiter plate using the procedure described for fibrinogen immobilization (by varying the concentration of myosin different levels of immobilized myosin could be achieved).27 The 125I-fibrinogen (100 μL 10 mM HEPES [N-2-hyddroxyethylpiperazine-N′-2-ethanesulfonic acid], 150 mM NaCl, pH 7.4, buffer) was added to the microtiter wells with covalently attached myosin. After the indicated binding times the fibrinogen solution was removed and the myosin surface was washed quickly (in < 20 seconds) 3 times with 300 μL 10 mM HEPES, 150 mM NaCl, pH 7.4, buffer. During this washing step the dissociation of the bound ligand is negligible taking into consideration the rates reported in “Results.” The wells were cut out, immersed into 3 mL scintillation cocktail (33.3% [vol/vol] Triton-X 100, 66.6% [vol/vol] toluene, 4.2 g/L 2,5-diphenyloxazole, 50 mg/L 1,4-bis-(5-phenyl-2-oxazolyl)benzene) and the bound radioactivity was measured in a Wallac 1410 liquid scintillation counter. For determination of the equilibrium-binding constants of fibrinogen and myosin, fibrinogen solutions (with volume of 100 μL) containing constant amount of 125I- or Eu-labeled fibrinogen (20 nM) and varying amounts of nonlabeled fibrinogen were applied in triplicate to the myosin-coated wells. The total concentration of fibrinogen was in the range of 0.02 to 9.35 μM with a constant amount of labeled protein. After incubation for 2 hours the myosin-bound radioactivity was measured as described. Following the same washing steps in the experiments with Eu-labeled fibrinogen, the myosin-bound Eu was released in 100 μL Enhancement Solution (Wallac). The emission fluorescence of Eu was measured with a time-resolved microplate fluorimeter Victor2 (Wallac; excitation wavelength 340 nm, emission wavelength 615 nm, counting delay 400 μsec). The binding data were fit by nonlinear least-squares regression analysis using the Levenberg-Marquardt algorithm to a model that assumed a single class of saturable binding sites and a nonsaturable (nonspecific) component described by
Biomolecular interaction analysis (BIA)
The interaction of fibrin(ogen) and myosin was evaluated with surface plasmon resonance (SPR) technology on Biacore X system (Biacore).31 Purified fibrinogen (plasminogen and factor XIIIa free) or myosin was immobilized covalently to the dextran matrix of the flow-cell 1 (Fc1) in a Sensor chip CM5 using the amine-coupling procedure.32 The surface concentration of the immobilized fibrinogen was 0.037 to 0.044 pmol/mm2 and that of myosin 0.002 pmol/mm2 calculated from the reported data that a SPR of 1000 relative units (RU) corresponds to 1 ng/mm2 protein independently of the nature of the protein.33 The immobilized fibrinogen was converted to fibrin monomers by a 1-hour treatment with 10 NIH U/mL thrombin (this treatment reduced the SPR of the fibrinogen surface by 400 RU). BSA was coupled to the flow-cell 2 (Fc2) of the Sensor chip CM5 at 0.04 pmol/mm2 concentration in the fibrin chip and at 0.004 pmol/mm2 concentration in the myosin chip and this surface was used as a reference in all measurements (the SPR responses are reported in RU as the difference Fc1–Fc2). Myosin (at concentrations in the range 1.06-4.25 μM in 10 mM HEPES pH 7.4 containing various concentrations of NaCl and 3 mM CaCl2) was injected over the surface at a flow rate of 5 μL/min at 25°C and the SPR response was recorded. The interaction of immobilized myosin with flow-phase fibrin (0.5-7 μM) was measured in 10 mM HEPES-NaOH, 150 mM NaCl, 3 mM CaCl2 in the presence of 5 mM Gly-Pro-Arg-Pro peptide, inhibitor of fibrin polymerization34 at 30 μL/min flow rate and 25°C. The equilibrium dissociation constant (Kd) for the myosin-fibrin interaction was calculated from the SPR response at equilibrium (Req) fitting the data to the equation
Turbidimetric assay of cross-linked fibrin dissolution
Factor XIII from human plasma (0.05 g/L in 25 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, 100 mM NaCl, 2.5 mM CaCl2, pH 7.4) was activated by 4 NIH U/mL thrombin for 10 minutes at room temperature immediately prior to the experiments. In a microtiter plate well 150 μL fibrinogen (8 μM) and plasminogen (0.5 μM) in 10 mM HEPES-NaOH buffer, pH 7.4, containing 100 mM NaCl, 3 mM CaCl2, and 50 μL myosin at various concentrations (in the range 1.6-13 μM) in 10 mM HEPES-NaOH buffer, pH 7.4, containing 250 mM NaCl, 3 mM CaCl2, were mixed and clotted with thrombin (1 NIH U/mL final concentration) for 3 hours. Cross-linked fibrin was prepared by clotting fibrinogen or mixtures of fibrinogen and myosin with 1 NIH U/mL thrombin in the presence of 5 μg/mL factor XIIIa. Cross-linking of fibrin was complete in 3 hours as evidenced by gel electrophoresis of reduced samples. For monitoring the fibrinolytic process, 100 μL 0.3 μM tPA or 0.15 μM urokinase was applied to the surface of preformed fibrin clots and the attenuance at 340 nm was measured in a Dynatech MR 5000 microplate reader as previously described.4 Evaluation of the fibrinolytic activity was based on the lysis time (t1/2, the time needed to reduce the turbidity of the clot to half-maximal value).26 Fibrinolysis in plasma was monitored with the same method. Plasma clots were prepared from equilibrated mixtures of human citrated blood plasma and myosin (100 μL plasma preincubated for 2 hours with 50 μL 0-12 μM myosin in 10 mM HEPES, 250 mM NaCl, pH 7.4, buffer) with the addition of 50 μL 6 NIH U/mL thrombin in 10 mM HEPES, pH 7.4, buffer containing 50 mM NaCl, 25 mM CaCl2, 0.01% Tween 20, and 10 nM tPA.
Monitoring of fibrinolysis under flow conditions
The experimental circuit previously described was used.35 Briefly, a 1.8-mL cylindrical fibrin clot with a longitudinal channel along its central axis was prepared from fibrinogen and myosin mixed as described, supplemented with plasmin, and clotted with thrombin (final concentrations: 6 μM fibrin, 2 μM myosin, 10 nM plasmin, 4.5 NIH U/mL thrombin in 10 mM HEPES-NaOH buffer, pH 7.4, containing 150 mM NaCl and 3 mM CaCl2). Thereafter 2 mL of the same buffer was perfused and continuously recirculated through the central channel with a peristaltic pump (Pharmacia LKB Pump-1) producing initial shear rate of 400 s–1 at the fibrin-fluid interface. The closed circuit passed through the flow cell (light path 2 mm) of Optical Unit UV-1 (Pharmacia LKB), which monitored the released protein degradation products on the basis of the absorbance at 280 nm.
Immunohistochemistry
Formalin-fixed, paraffin-embedded thrombi, removed from iliac artery by surgery were studied. The 5-μm sections were deparaffinized and digested with 0.1% trypsin for 10 minutes at 37°C. Endogenous peroxidase was blocked with 10% H2O2 for 30 minutes. To mask unspecific binding sites the slides were treated with 3% BSA and 5% (vol/vol) nonimmune horse serum in phosphate-buffered saline (PBS) for 30 minutes at 37°C. Polyclonal rabbit antimyosin antibody, raised against guinea pig muscle myosin (Calbiochem), was applied in 1:30 dilution, as suggested by the manufacturer. Vector Elite ABC kit (Vector, Burlingame, CA) was used to detect the binding of the primary antibody. The peroxidase reaction was visualized with 0.05 mg/mL 3-3′-diaminobenzidine-tetrahydrochloride. For control, the primary antibody was incubated with 1000-fold molar excess of myosin before use or replaced with nonimmune rabbit serum. The slides were counterstained by hematoxylin-eosin. Pictures were taken by digital camera, attached to Olympus Vanox microscope. For the immunofluorescence examination the thrombus specimen was deparaffinized and treated with 2 mg/mL glycine in PBS for 10 minutes to decrease the autofluorescence. Subsequently the specimen was blocked with 5% BSA in PBS for 30 minutes at 37°C. The primary antibodies were applied in 1:50 dilution for 30 minutes at room temperature (polyclonal rabbit antihuman myosin and monoclonal mouse antihuman fibrinogen clone 85D4 (Sigma), which recognizes an epitope common in fibrinogen and fibrin). After washing 3 times with PBS the sections were incubated for 30 minutes with fluorescein-isothiocyanate (FITC)–conjugated antirabbit and tetramethylrhodamine-isothiocyanate (TRITC)–conjugated antimouse antibodies (Sigma) in 1:100 dilution. After repeated washing with PBS the sections were mounted with antifade solution Fluoromount-G (Southern Biotechnology, Birmingham, AL) and studied with the Bio-Rad MRC 1024 confocal laser system (Bio-Rad, Hertfordshire, United Kingdom).
Results
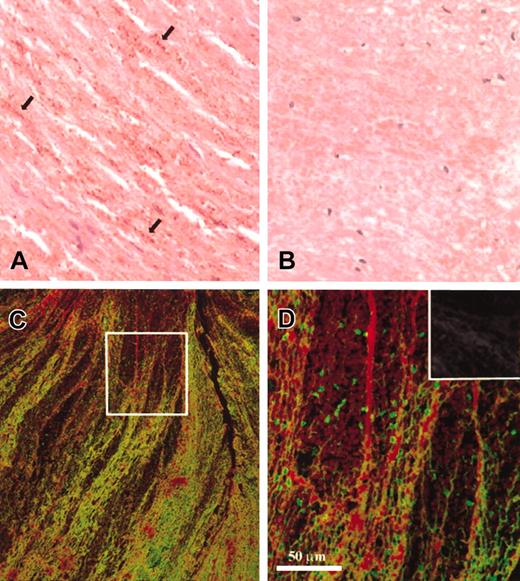
Immunohistochemical evaluation of thrombi from human iliac artery indicated massive presence of myosin in its structure (Figure 1A). The cellular source of the reactive material is difficult to decipher; the brown dots may correspond to aggregated platelets or free myosin forming a granular structure. Double fluorescent immunostaining was carried out to assess the amount of myosin relative to fibrin. Low- and high-resolution confocal images (Figure 1C-D) show interweaving myosin aggregates in the fibrin network of the thrombus. The ratio of myosin- and fibrin-positive immunofluorescence varies in different regions of the thrombus as illustrated by the selected areas (Figure 1C compared with D). Sections examined without primary antibodies (negative control) showed minimal autofluorescence (Figure 1D inset).
Myosin content of arterial thrombi. (A) The immunohistochemical detection of myosin (brown color) in a thrombus from human iliac artery (the reaction was carried out as described in “Materials and methods,” original magnification × 400). The antibody recognizes small dotlike deposits (arrows) running parallel with the erythrocytes. (B) Negative control, for which the primary antimyosin antibody has been preincubated with a 1000-fold molar excess of myosin (original magnification × 400). (C-D) Immunofluorescent confocal laser images of the same thrombus double-stained for myosin (green) and fibrin (red) as described in “Materials and methods.” The box in panel C indicates the area that is enlarged in panel D, whereas the inset in panel D shows a section not treated with antimyosin or antifibrin antibodies and inspected as indicated for this panel (autofluorescence). Original magnification × 20 for panel C and × 40 for panel D and its inset; the bar indicates the final magnification.
Myosin content of arterial thrombi. (A) The immunohistochemical detection of myosin (brown color) in a thrombus from human iliac artery (the reaction was carried out as described in “Materials and methods,” original magnification × 400). The antibody recognizes small dotlike deposits (arrows) running parallel with the erythrocytes. (B) Negative control, for which the primary antimyosin antibody has been preincubated with a 1000-fold molar excess of myosin (original magnification × 400). (C-D) Immunofluorescent confocal laser images of the same thrombus double-stained for myosin (green) and fibrin (red) as described in “Materials and methods.” The box in panel C indicates the area that is enlarged in panel D, whereas the inset in panel D shows a section not treated with antimyosin or antifibrin antibodies and inspected as indicated for this panel (autofluorescence). Original magnification × 20 for panel C and × 40 for panel D and its inset; the bar indicates the final magnification.
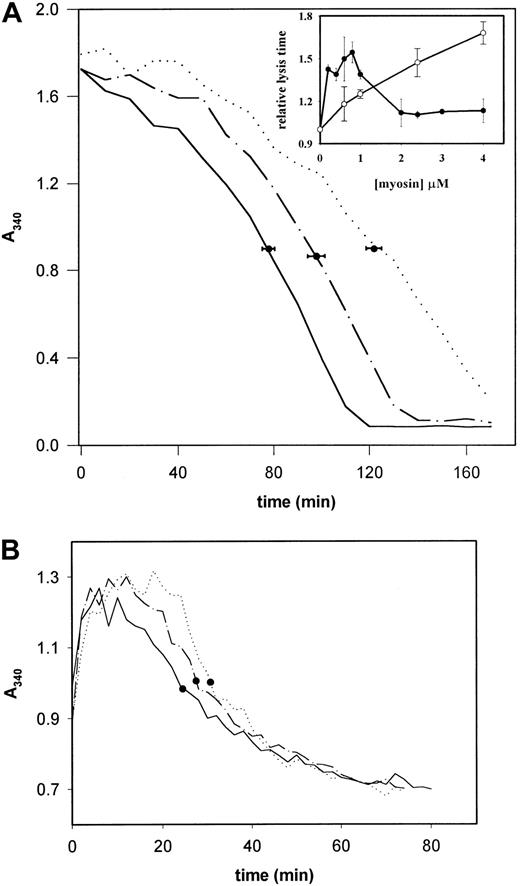
In vitro the dissolution of fibrin clots slowed down in the presence of myosin, when plasmin was formed by tPA within the fibrin gel containing plasminogen. In the presence of 0.2 μM myosin the lysis time increased from 78 ± 4.8 to 122 ± 5.7 minutes (P < .001 with Student t test), whereas at a 10-fold higher myosin concentration this inhibiting effect was moderated (t1/2 = 98 ± 7.2 minutes), but was still significant (P < .001; Figure 2A). The tPA-induced fibrinolysis was maximally retarded in the presence of myosin at concentrations in the range 0.6 to 1.0 μM (Figure 2A inset, •). When the plasminogen in the clot was activated with urokinase, which does not require a cofactor for its activity and in this case the plasminogen activation was not influenced by myosin, the inhibition of fibrinolysis was linearly dependent on the myosin concentration (Figure 2A inset, ○). The tPA-induced dissolution of clots prepared from citrated blood plasma was affected by myosin in a similar way (Figure 2B). Figure 2A suggests that myosin may have plural, opposing effects on fibrinolysis and that the known tPA-cofactor properties of myosin20 are expressed only after saturation of the binding sites on fibrin for myosin. Testing this hypothesis required direct evaluation of the plasmin-induced fibrin dissolution in the presence of myosin and the interaction of fibrin with myosin.
Plasminogen activator–induced dissolution of fibrin clots. (A) Fibrin clots containing plasminogen and varying amounts of myosin were prepared in microtiter plates and the activator-induced lysis was monitored as described in “Materials and methods.” The concentration of myosin in the clots was 0 (solid line), 0.2 μM (dotted line), or 2 μM (dashed and dotted line). The symbols indicate the mean of the t1/2 lysis time and its SE determined from 5 replicate measurements. The inset shows the lysis time in relative units (the t1/2 of fibrin without myosin is 1) determined under identical conditions for 0.3 μM tPA(•) and 0.15 μM urokinase (○) in quadruplicate samples. (B) Plasma clot formation and tPA-induced dissolution was monitored as described in “Materials and methods.” Representative curves are shown for plasma clots with no myosin (solid line), 0.5 μM myosin (dotted line), and 3 μM myosin (dashed and dotted line). • indicate the lysis time of the respective sample.
Plasminogen activator–induced dissolution of fibrin clots. (A) Fibrin clots containing plasminogen and varying amounts of myosin were prepared in microtiter plates and the activator-induced lysis was monitored as described in “Materials and methods.” The concentration of myosin in the clots was 0 (solid line), 0.2 μM (dotted line), or 2 μM (dashed and dotted line). The symbols indicate the mean of the t1/2 lysis time and its SE determined from 5 replicate measurements. The inset shows the lysis time in relative units (the t1/2 of fibrin without myosin is 1) determined under identical conditions for 0.3 μM tPA(•) and 0.15 μM urokinase (○) in quadruplicate samples. (B) Plasma clot formation and tPA-induced dissolution was monitored as described in “Materials and methods.” Representative curves are shown for plasma clots with no myosin (solid line), 0.5 μM myosin (dotted line), and 3 μM myosin (dashed and dotted line). • indicate the lysis time of the respective sample.
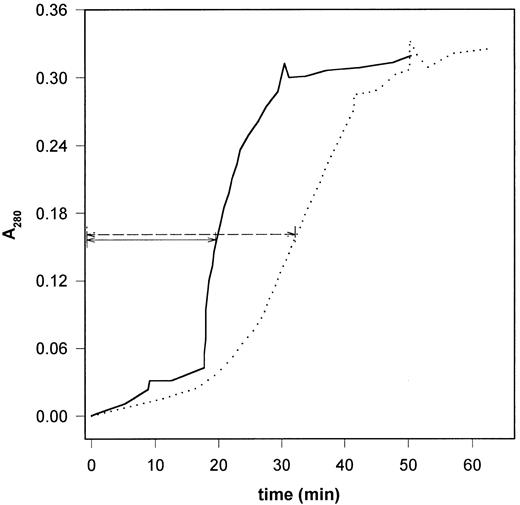
When fibrin was dissolved directly with plasmin under conditions in which fluid circulated over the surface of a fibrin clot with embedded plasmin (Figure 3), 2 stages could be distinguished in the time course of the fibrin dissolution: an initial linear increase in the release of soluble products followed by an abrupt disassembly.35 Myosin definitely modified the initial rate of solubilization: the rate of A280 change decreased from 0.14 h–1 (range, 0.132-0.145 h–1, n = 3) in the absence of myosin to 0.10 h–1 (range, 0.088-0.121 h–1, n = 3) in the presence of 2 μM myosin. The disassembly phase starts when a shear rate–dependent level of solubilization is reached.35 At shear rate of 400 s–1 in the absence of myosin the clot disassembly began when the protein concentration in the circulating phase reached 13% (range, 12.4%-13.5%) of the value for the completely solubilized clot. In the presence of myosin this occurred at 7.2% (range, 6.5%-8.2%) solubilization (Figure 3). Myosin modified also the second phase of clot disassembly: the slope of the abruptly ascending stage of the A280 curve in Figure 3 decreased in the presence of myosin. Thus, myosin prolonged the clot-disassembly time (defined as the time to reach half of the maximal A280) from 20 minutes (range, 19-22 minutes) to 33 minutes (range, 31-35 minutes). Altogether the fibrinolysis under flow conditions suggests a dual effect for myosin: impairment of the polymerization forces (lower threshold of disassembly) and retarded proteolysis (slower initial solubilization rate, prolonged disassembly time). Myosin competed efficiently with fibrin for the protease; when fibrin was digested with plasmin in the presence of equivalent amount of myosin (Figure 4), the rate of fibrin degradation was significantly reduced. 6-Aminohexanoate (at concentrations up to 5 mM) did not influence the rate and fragment pattern of myosin digestion with plasmin when evaluated with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (not shown).
Dissolution of fibrin clots containing myosin in a flow circuit. Cylindrical fibrin clot containing 10 nM plasmin was prepared as described in “Materials and methods” and buffer was perfused through its central longitudinal channel with an initial shear rate of 400 s–1. The attenuance of the soluble protein products released in the fluid phase was monitored at 280 nm. The median of triplicate experiments is shown as solid line (6 μM fibrin) and dotted line (6 μM fibrin containing 2 μM myosin). The arrows indicate the clot-disassembly time.
Dissolution of fibrin clots containing myosin in a flow circuit. Cylindrical fibrin clot containing 10 nM plasmin was prepared as described in “Materials and methods” and buffer was perfused through its central longitudinal channel with an initial shear rate of 400 s–1. The attenuance of the soluble protein products released in the fluid phase was monitored at 280 nm. The median of triplicate experiments is shown as solid line (6 μM fibrin) and dotted line (6 μM fibrin containing 2 μM myosin). The arrows indicate the clot-disassembly time.
Myosin and fibrin as substrates of plasmin. Fibrin (prepared from 3 μM fibrinogen) was digested with plasmin (10 nM) in the absence or in the presence of 2 μM myosin under the conditions described in “Materials and methods” for the turbidimetric assay. The reactions were stopped at the indicated times by dissolving the clots in 100 mM Tris-HCl, pH 8.2, buffer containing 100 mM NaCl, 8 M urea, and 2% sodium dodecyl sulfate and boiling. The samples were analyzed with electrophoresis on 10% polyacrylamide gel under nonreducing conditions.
Myosin and fibrin as substrates of plasmin. Fibrin (prepared from 3 μM fibrinogen) was digested with plasmin (10 nM) in the absence or in the presence of 2 μM myosin under the conditions described in “Materials and methods” for the turbidimetric assay. The reactions were stopped at the indicated times by dissolving the clots in 100 mM Tris-HCl, pH 8.2, buffer containing 100 mM NaCl, 8 M urea, and 2% sodium dodecyl sulfate and boiling. The samples were analyzed with electrophoresis on 10% polyacrylamide gel under nonreducing conditions.
Several sources of evidence support the existence of noncovalent interaction between myosin and fibrin(ogen). Ultracentrifugation (2 hours at 105g in a 10 mM HEPES, pH 7.4, buffer containing 150 mM NaCl) almost completely removed myosin from the soluble phase, whereas under the same conditions fibrinogen and fibrin monomers (maintained in soluble form by 5 mM GPRP-peptide) formed a concentration gradient in the solution. When pre-equilibrated myosin-fibrin(ogen) mixtures were centrifuged, the protein content in the upper layer of the solution decreased by 20% to 25% indicating acceleration of the fibrin(ogen) sedimentation, presumably caused by intermolecular complex formation. The sedimentation rate changes in the fibrinogen-myosin mixtures were not modified by 3 mM CaCl2 or 5 mM GPRP-peptide (data not shown).
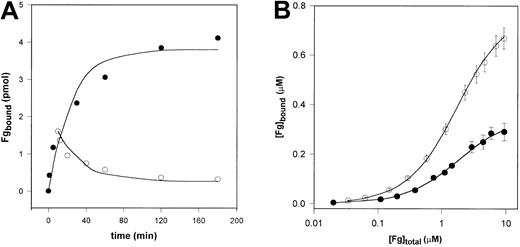
Fibrinogen bound reversibly to immobilized myosin and both the association and the dissociation phase of the interaction were slow: equilibrium was achieved in approximately 2 hours (Figure 5A). Using a bimolecular binding model and nonlinear regression analysis of the experimental data an association rate constant of 180.6 M–1 · s–1 (90% CI, 141.4-289.0 M–1 · s–1) and a dissociation rate constant of 3.07 × 10–4 s–1 (90% CI, 2.40 × 10–4-4.91 × 10–4 M–1 · s–1) was calculated for the fibrinogen-myosin interaction. Evaluation of the equilibrium-binding data (Figure 5B) on 2 different myosin surfaces gave a dissociation constant Kd = 1.70 μM (90% CI, 1.28-2.29 μM) with 125I-fibrinogen and Kd = 1.35 μM (90% CI, 1.17-1.49 μM) with Eu-fibrinogen for a single class of binding sites.
Interaction of fibrinogen and immobilized myosin. (A) Time course of fibrinogen (Fg) binding to and dissociation from myosin. 125I-labeled fibrinogen (250 nM) was applied in a 100-μL volume to myosin immobilized on microtiter plates. For the follow-up of the binding (•), at the indicated times the fibrinogen solution was removed and after washing the bound radioactivity was measured. For monitoring the dissociation (○), after a binding phase of 10 minutes the fibrinogen solution was discarded and 100 μL 10 mM HEPES 150 mM NaCl, pH 7.4, buffer was applied. At the indicated times the solution was removed and the residual bound radioactivity was measured (symbols). The lines show the modeled time course of the process using the rate constants gained with nonlinear fit to the experimental points. (B) Equilibrium-binding data. Mixtures of 125I-fibrinogen (•) or Eu-fibrinogen (○) and varying amounts of nonlabeled fibrinogen were incubated in myosin-coated microtiter plates for 2 hours. After washing the myosin-bound labeled fibrinogen was measured as described in “Materials and methods.” Results are presented as mean measured bound fibrinogen (symbols), its SD (bars), and the best fit (solid line), which is obtained for a single class of binding sites as described in “Materials and methods.”
Interaction of fibrinogen and immobilized myosin. (A) Time course of fibrinogen (Fg) binding to and dissociation from myosin. 125I-labeled fibrinogen (250 nM) was applied in a 100-μL volume to myosin immobilized on microtiter plates. For the follow-up of the binding (•), at the indicated times the fibrinogen solution was removed and after washing the bound radioactivity was measured. For monitoring the dissociation (○), after a binding phase of 10 minutes the fibrinogen solution was discarded and 100 μL 10 mM HEPES 150 mM NaCl, pH 7.4, buffer was applied. At the indicated times the solution was removed and the residual bound radioactivity was measured (symbols). The lines show the modeled time course of the process using the rate constants gained with nonlinear fit to the experimental points. (B) Equilibrium-binding data. Mixtures of 125I-fibrinogen (•) or Eu-fibrinogen (○) and varying amounts of nonlabeled fibrinogen were incubated in myosin-coated microtiter plates for 2 hours. After washing the myosin-bound labeled fibrinogen was measured as described in “Materials and methods.” Results are presented as mean measured bound fibrinogen (symbols), its SD (bars), and the best fit (solid line), which is obtained for a single class of binding sites as described in “Materials and methods.”
The interaction of fibrin and myosin was characterized with BIA technology (Figure 6). When myosin was injected over the surface of immobilized fibrin, no binding was detected in a milieu of high ionic strength (NaCl concentration > 300 mM). At 250 mM NaCl a slowly progressing increase of the SPR response was detected on the fibrin-coated surface, whereas no protein attachment was seen on the control albumin-coated surface (Figure 6A reports the difference in the SPR response of the fibrin and albumin surfaces). At this ionic strength the dissociation was extremely slow; the attached myosin could be removed only with 4 M urea indicating that the curves of Figure 6A show fibrin-initiated myosin aggregation rather than simply myosin-fibrin association. When soluble fibrin was injected over a surface with immobilized myosin (Figure 6B), the SPR response produced the square pulse signals typical of weak ligand interaction.36,37 It is noteworthy that when in the latter BIA setting the analyte was prevented in its aggregation, the interaction occurred in the time scale of seconds, strengthening the interpretation of the plasmon resonance curves in Figure 6A as fibrin-initiated aggregation. The equilibrium dissociation constant calculated for the interaction of this soluble fibrin species and myosin is 0.94 μM (Figure 6B inset).
Binding of myosin and fibrin monomers measured by SPR. (A) Myosin (at the indicated concentrations in 10 mM HEPES, 250 mM NaCl, 3 mM CaCl2, pH 7.4) was applied to fibrin immobilized on the surface of the sensor chip and the relative SPR response (subtracting the response on the control BSA-coated surface) was recorded in a Biacore X system. (B) Fibrin prepared in the presence of 5 mM GPRP (in 10 mM HEPES, 150 mM NaCl, 3 mM CaCl2, pH 7.4) was applied at the concentrations indicated in the inset to myosin immobilized on the surface of the sensor chip. The relative SPR response was recorded. The primary Req values were plotted (inset) and fitted to the equation described in “Materials and methods.”
Binding of myosin and fibrin monomers measured by SPR. (A) Myosin (at the indicated concentrations in 10 mM HEPES, 250 mM NaCl, 3 mM CaCl2, pH 7.4) was applied to fibrin immobilized on the surface of the sensor chip and the relative SPR response (subtracting the response on the control BSA-coated surface) was recorded in a Biacore X system. (B) Fibrin prepared in the presence of 5 mM GPRP (in 10 mM HEPES, 150 mM NaCl, 3 mM CaCl2, pH 7.4) was applied at the concentrations indicated in the inset to myosin immobilized on the surface of the sensor chip. The relative SPR response was recorded. The primary Req values were plotted (inset) and fitted to the equation described in “Materials and methods.”
Because both myosin38,39 and fibrin25 are substrates of FXIIIa, we checked the possibility for myosin being covalently cross-linked to fibrin by FXIIIa. According to the evaluation of the electrophoretic pattern of cross-linked fibrin with autoradiography and silver staining (data not shown), the presence of equimolar amounts of myosin did not influence the time course and the final pattern of cross-linking of fibrin with FXIIIa. Thus, myosin is a rather poor substrate of FXIIIa; at concentrations of the enzyme, at which fibrin is almost completely cross-linked, no covalent modification of myosin is seen.
Discussion
Because myosin is present in aged arterial thrombi (Figure 1), it is of practical interest to characterize its effect on fibrin dissolution. The previously described tPA-cofactor and plasmin-substrate properties of myosin20 cannot provide a straightforward explanation for the behavior of fibrinolysis in the presence of myosin (Figure 2). The maximal stabilization of the clot against tPA-induced fibrinolysis is seen in the presence of 0.6 to 1.0 μM myosin (Figure 2 inset), which coincides with the range of the measured Kd for the myosin-fibrin(ogen) interaction (Figures 5, 6). The fact that the fibrin-stabilizing effect is moderated at higher myosin concentrations when the ratio of free to bound myosin increases strongly implies that only free myosin (and not complexed with fibrin) contributes as a cofactor to plasminogen activation. The role of myosin as a cofactor in the tPA-induced plasminogen activation is underscored by the comparison with the effects of myosin on the urokinase-induced fibrinolysis (Figure 2 inset). The urokinase-dependent plasminogen activation does not require a cofactor and thus the observed linear inhibition of fibrinolysis by myosin can be attributed solely to the effects of myosin on the action of the generated plasmin. Under flow conditions (Figure 3) myosin retards the dissolution of fibrin with embedded plasmin, but at the same time it exerts some effects that favor this process. The fibrin clot containing myosin starts disassembling at a lower degree of plasmin digestion than the pure fibrin clot. This implies that the presence of myosin weakens the interactions of fibrin degradation products, which still polymerize. This increased solubility of the partially degraded fibrin-myosin clot, however, cannot compensate for the slower degradation of the fibrin (illustrated by Figure 4); the overall lysis is delayed (Figure 3). Thus, altogether the presence of myosin is antifibrinolytic in the flow model.
Seeking the background of the observed complex effect of myosin on the fibrinolytic system, we investigated the interactions of myosin with the substrate of the fibrinolytic enzymes, the fibrin gel. Because the efficiency of fibrin degradation depends on the structure of the fibrin clot4 and the latter can be influenced by the presence of macromolecules or cellular elements, we examined the interactions of myosin with fibrinogen, which is the soluble precursor of fibrin. The association of fibrinogen and myosin is supported by several independent experimental approaches: ultracentrifugation, kinetic, and equilibrium-binding studies (Figure 5). The interaction of myosin and fibrinogen can be defined as reversible binding characterized with dissociation constant of 1.35 to 1.70 μM (the established equilibrium constant is within the expected in vivo concentration range of the interacting molecules).
For the characterization of the myosin-fibrin(ogen) interaction we used GPRP-peptide, which prevents fibrin polymerization due to binding to fibrinogen at a site in the molecule that is responsible for the association of 2 fibrin monomers.34,40 Its application allowed us to exclude the involvement of this polymerization site in the fibrinogen-myosin interaction and to examine the sedimentation behavior of fibrin monomers kept in soluble form by the presence of GPRP. Altogether the sedimentation data support the conclusion that myosin interacts with domains common on the surface of the fibrinogen and fibrin molecule and this interaction is not dependent on the GPRP-acceptor pocket in the γ-chain of the fibrinogen molecule.
Because myosin interacts with fibrinogen and fibrin, a consequent change in the fibrin gel architecture may modify the fibrin as a substrate of the fibrinolytic enzymes. The relevance of such an effect can be estimated from the relative kinetics of the fibrin(ogen)-myosin and fibrin-fibrin interactions. According to our data there is a difference of 4 orders of magnitude between the rate constant of the myosin-fibrinogen binding (1.81 × 102 M–1 · s–1) and that of the fibrin-monomer association (2.5 × 106 M–1 · s–1).41 Thus, due to the low rate of the interaction, myosin cannot influence the formation of the fibrin clot. Although we cannot preclude the formation of multilayered myosin surface in our solid-phase binding assay, which may slow down the establishment of equilibrium, the physiologic relevance of the measured binding parameters is supported by the identical clot turbidity in the presence and absence of myosin (initial A340 values in Figure 2). The turbidity of the clot, which is a good indicator of the fibrin structure,10 is expected to be changed, if myosin binding proceeds at a rate comparable with the rate of fibrin polymerization. Our kinetic data suggest that complete association of myosin and fibrinogen at micromolar concentrations occurs in the time scale of hours and so this phenomenon is relevant not to the formation but to the maturation of the fibrin clots. The fibrin network may serve as a matrix core that initiates the aggregation of myosin (Figure 6A). In solution, myosin self-associates (aggregates), which is dependent on ionic strength and pH.42 At physiologic ionic strength (0.1-0.15 M) and pH (around 7) myosin starts to self-associate by forming first “bipolar fragments” aligning and attaching to each other along their long helical tail. These “antiparallel” myosin dimers are the start of a larger aggregate formation of the myosin. The fibrin surface is an ideal initiator of the start of this process attracting/concentrating the myosin from the broken platelets or other sources.
The square SPR signals of Figure 6B indicate that fibrin binds to and dissociates from myosin much faster than fibrinogen (Figure 5A), but because of the low affinity of the interacting molecules the exact association and dissociation rate constants could not be determined as experienced in other similar systems.36,37 The acceleration in the interaction kinetics again raises the possibility for interference of myosin with the formation of the fibrin gel. Despite the accelerated association rate of myosin and fibrin instead of fibrinogen, however, the higher affinity of the self-aggregating fibrin monomers (Kd = 0.156 μM)43 dominates and apparently the fibrin architecture is not affected as previously mentioned. The equilibrium dissociation constant estimated from the BIA experiments (Figure 6B) for the fibrin-myosin interaction is definitely in the same range as the value for the fibrinogenmyosin interaction (Figure 5B). Thus, the nature of the interacting molecule species (fibrinogen or fibrin monomers) does not affect the fraction of free myosin in the final equilibrium mixture. As discussed, withdrawal of the latter in the form of fibrin-complex could be an important factor in the stabilization of fibrin clots against dissolution initiated with plasminogen and tPA (Figure 2 inset).
A well-known aspect of the stabilization of fibrin against dissolution is its covalent cross-linking by FXIIIa.44 Although myosin may be also a substrate of FXIIIa,38,39 this transglutaminase does not contribute to the described stabilization of fibrin in the presence of myosin; neither is myosin covalently attached to fibrin, nor does it influence the time course of the fibrin cross-linking at biologically relevant FXIIIa concentrations.
Finally, we can conclude that in arterial thrombi myosin binds to the already polymerized fibrin; in the bound form its tPA-cofactor properties are masked and the myosin-fibrin clot is relatively resistant to plasmin (competing effect of myosin or a conformation of fibrin in the complex with myosin that is less susceptible to plasmin). Because similarly to myosin blood plasma proteins are also occluded in the generated fibrin clot when fibrinogen is converted to fibrin in vivo,45 it is tempting to suggest a more general function of fibrin as a scavenger of waste proteins in the vascular bed at sites of vessel wall injury with consequent effects on fibrinolysis.
Prepublished online as Blood First Edition Paper, January 23, 2003; DOI 10.1182/blood-2002-10-3227.
Supported by grants from Hungarian Scientific Research Fund (T031891), Hungarian Ministry of Health (ETT 287 and 288/2000), Hungarian Ministry of Education (FKFP 0013/99), Wellcome Trust (069520/Z/02/Z), and National Institutes of Health (ROIAR39288) and by the Mayo Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Györgyi Oravecz and Krisztina Egedy for their technical assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal